Abstract
Purpose
Electrical stimulation of pudendal afferents can evoke reflex bladder contractions with relaxation of the external urethral sphincter in cats. This voiding reflex is mediated by pudendal sensory fibers innervating the penile and prostatic urethra that engage either a spinal or spinobulbospinal micturition pathway, respectively. However, the clinical translation of this potential therapy in persons with spinal cord injury (SCI) is limited by the lack of evidence demonstrating analogous reflex mechanisms in humans.
Materials and Methods
We investigated the presence of excitatory pudendal-to-bladder reflexes in seven individuals with chronic SCI. The isovolumetric bladder pressure and perineal electromyogram (EMG) were recorded in response to intraurethral (IU) electrical stimulation at varying amplitudes and frequencies.
Results
Selective electrical stimulation of the proximal and distal segments of the urethra evoked sustained reflex bladder contractions in different subsets of participants: 24.9 ± 13.9 cmH2O (n = 3) and 23.3 ± 16.1 cmH2O (n = 3), respectively. In contrast, the corresponding reflex perineal EMG exhibited a differential activation pattern between proximal and distal IU stimulation (normalized EMG, p < 0.05): 1.3 ± 0.2 and 0.3 ± 0.1, respectively.
Conclusions
This study presents the first clinical evidence of two independent excitatory pudendal-to-bladder reflex pathways that can, in turn, differentially modulate efferent pudendal output. Both reflex mechanisms involve a complex interaction of multiple sensory inputs and may provide a neural substrate for restoring micturition following SCI.
Keywords: sensory pudendal nerve, spinal reflex, urinary function, spinal cord injury, intraurethral stimulation
Introduction
Spinal cord injury (SCI) between the brainstem and the lumbosacral spinal cord can result in neurogenic detrusor overactivity and bladder-external urethral sphincter dyssynergia.1 The disruption of descending supraspinal activity leads to changes in the synergic modulation of spinal autonomic (parasympathetic and sympathetic) and somatic activity that regulates the lower urinary tract.2 Despite treatment with anticholinergic medications and intermittent catheterization, bladder dysfunction in persons with SCI remains a significant problem with substantial adverse impact on quality of life.3
Independent of the brainstem, the spinal cord can mediate reflexes that have clinical relevance for restoring urinary function. For example, activity in afferents of the dorsal genital branch (DGN) of the pudendal nerve can activate a bladder inhibitory reflex. Studies in both cats4, 5 and persons with SCI6–8 demonstrate robust inhibition of bladder contractions and increased cystometric capacity by low frequency (3 to 20 Hz) stimulation of DGN fibers.
However, our understanding of a complementary excitatory pudendal-to-bladder reflex in human remains incomplete. Experimental evidence in cats demonstrates that activity in pudendal afferents can generate reflex bladder contractions and voiding4, 9, 10, but evidence of an analogous reflex in humans is limited11, 12. Further, the inability to identify the stimulated nerve fibers and incomplete knowledge of somatic sensory innervation of the lower urinary tract13, 14 make it difficult to interpret the effects of electrically stimulating different afferent pathways on bladder reflexes. Recent work in cats demonstrates that the innervation of the proximal (prostatic) and distal urethra is via two independent branches of the pudendal nerve: the cranial sensory (CSN) and the DGN nerve, respectively.15 Further, selective electrical stimulation of each sensory branch revealed that reflex excitation of the bladder required different frequencies of stimulation and was mediated by either spinal (DGN) or supraspinal (CSN) networks.10
We sought to translate these preclinical findings to a cohort of persons with chronic SCI. We measured changes in bladder pressure and perineal muscle activity in response to intraurethral stimulation of the proximal and distal segments of the urethra, corresponding to the CSN and DGN components of the pudendal nerve, respectively. In contrast to what was observed in the acute spinal cat10, electrical stimulation of CSN and DGN afferents revealed that both excitatory pudendal-to-bladder reflex pathways remained intact in persons with SCI.
Methods
The responses of the bladder and striated perineal muscle to selective intraurethral (IU) electrical stimulation were measured in seven participants with chronic SCI. The experimental protocols were approved by the Institutional Review Board of Duke University Medical Center, and all participants provided written informed consent. The inclusion criteria included a neurologically stable SCI (> 1 year post injury) at or above T12, neurogenic bladder confirmed via cystometry, and the absence of potential confounding factors (e.g., urethral stricture/obstruction or urinary tract infection).
Experimental Setup
Instrumentation in each participant included urethral insertion of a 12F Foley catheter (Bardex Foley, C.R. Bard, Inc.) to adjust bladder volume and a 4F PVC catheter (BPC-4L, Life-Tech Inc., Stafford, TX) to measure bladder pressure (Pbladder); and rectal insertion of a 9F balloon catheter (RPC-9, Life-Tech Inc.) to measure abdominal pressure (Pabdomen). The latter two catheters were connected in series with pressure transducers (Deltran I, Utah Medical Products, Inc.). Electromyographic (EMG) activity of transverse perineal muscles was recorded by pairs of stainless steel wires (1512A-M, Life-Tech Inc.) inserted percutaneously through the perineum. Both pressure and EMG signals were filtered (DC – 40 Hz, 3 Hz – 1 kHz), amplified (gain = 1000, ETH-255, iWorx/CB Sciences, Inc.), and sampled digitally at 5 kHz on a computer.
Baseline Measurement of Bladder Function
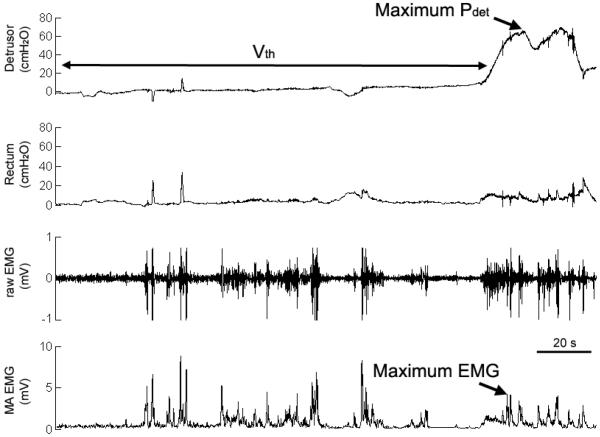
At the start of each experiment the detrusor pressure (Pdet, defined as Pbladder – Pabdomen) and perineal EMG activity were measured as the bladder was filled with room temperature saline at 20–40 ml/min (Figure 1). The volume threshold (Vth) at which a distension-evoked (DE) bladder contraction (Pdet exceeded 30 cmH2O for at least 10 s) occurred was denoted as the bladder capacity. Infusion was discontinued if fluid leaked around the urethral catheter, signs of dysreflexia were visible, or maximum cystometric capacity (500 ml) was reached.
Figure 1.
A distension evoked bladder contraction during the initial urodynamic fill confirmed intact detrusor muscle and pelvic nerve function. The peak detrusor pressure, bladder pressure (not shown) minus the rectal pressure, was defined as maximum Pdet. The recorded perineal electromyogram (raw EMG) was rectified and averaged to determine the maximum EMG activity evoked during a sustained bladder contraction. The bladder volume at which a sustained bladder contraction occurred was defined as the threshold (Vth).
Instrumentation for Intraurethral (IU) Stimulation
An 8-electrode stimulating catheter (diameter = 1.4 mm, electrode length = 3 mm and inter-electrode separation = 7 mm) was inserted in the urethra. In males (urethral length ± SD = 23.8 ± 4.2 cm), the active electrode was positioned 4 to 6 cm distal to the bladder neck for stimulation of the proximal urethra, and 3 to 5 cm proximal to the urethral meatus for stimulation of the distal urethra. In the female participant, the active electrode was positioned 1 cm distal to the bladder neck (proximal urethra) and 1 cm proximal to the urethral meatus (distal urethra). A single return electrode (Empi 5000, 2.75 × 4 in. rectangle) was placed on the upper hip. The electrodes were connected to a battery-powered electrical stimulator (300PV, Empi) that delivered charge-balanced asymmetric biphasic current pulses (pulse width = 200 μs).
With the bladder empty, the stimulation threshold (T) to elicit a pudendal-topudendal reflex (bulbocavernosus reflex (BCR), Table 1) was measured by increasing the amplitude of current pulses delivered at 2 Hz until a triggered EMG response or anal twitch was observed. The threshold was measured individually for the stimulating contacts positioned in the proximal (Tp) and distal (Td) urethra (Table 1). If no BCR was elicited, a default Tp or Td of 15 mA was used.
Stimulation Protocol
The bladder was filled to approximately 80 % of Vth (500 ml, if no DE bladder contraction), and 30-s trains of current pulses were applied at multiples (2T and 4T) of the threshold current needed to evoke a BCR. A randomized sequence of 6 stimulation trials was conducted that combined two stimulus amplitudes (2 and 4 times Tp or Td) and three frequencies (2, 10 and 35 Hz). In two experiments (Exp 1 and 4), additional stimulation trials (20 Hz, amplitude = T to 4T) were conducted with the active electrode located at the bladder neck.
Data Analysis
The baseline was the Pdet averaged over 2 s prior to electrical stimulation. An evoked response was characterized by the peak Pdet normalized to the maximum Pdet measured during each initial urodynamic fill (Figure 1). An excitatory bladder response was defined as a minimum increase in detrusor pressure of 8 cmH2O above baseline during stimulation that also was at least a 10% increase in the normalized Pdet.
The mean perineal EMG activity evoked during each 30-s stimulus train was calculated by first removing the stimulus artifact (6 ms window) from the raw EMG and averaging over the duration of stimulation. The effects of this blanking window on the overall measured EMG activity was negligible considering that (1) IU stimulation did not directly activate the recorded perineal muscle and (2) this window was shorter than the typical latency for evoking BCR activity. All EMG activity was normalized to the peak dyssynergic EMG activity evoked during each initial urodynamic fill (Figure 1).
All data were averaged across multiple experiments and expressed as a mean ± standard error, unless otherwise specified. Statistical analysis was conducted using a oneway ANOVA followed by a Tukey multiple comparison test (S-PLUS, Insightful Corp.).
Results
The participants were predominantly male (6 males, 1 female) with a mean age (± SD) of 54 ± 15 years (range = 26 to 67 years) and were 23 ± 12 years (range = 9 to 42 years) post injury. The level of injury ranged from C5 to T12, with 4 participants diagnosed with complete SCI. Two participants regularly used anticholinergic medication, but stated that medication was discontinued for at least 24 hours prior to the experiment.
I. Urodynamic Evaluation
Distention evoked (DE) reflex bladder contractions were observed in 6 of 7 individuals during the initial urodynamic fill, where spontaneous bladder and perineal activity was observed below the volume threshold for a DE contraction (Vth, Figure 1). Both the volume threshold for DE contractions (Vth, range = 19 to 300 ml) and peak detrusor pressure (Pdet, range = 34 to 113 cmH2O) were highly variable among 6 participants. Failure to evoke a DE bladder contraction in one individual (Exp7, Table 1) suggested the possible residual effects of anticholinergic medication, and this individual was excluded from further analysis.
II. Electrical Activation of Urethral-to-Perineal Reflexes
With an empty bladder, intraurethral (IU) stimulation evoked reflex perineal EMG responses (i.e., bulbocavernosus reflex) in 5 of 6 individuals (Table 1). The stimulation amplitude required to evoke this reflex was consistent between proximal (14.4 ± 2.09 mA) and distal (12 ± 2.0 mA) segments of the urethra. Other than a `tapping' sensation reported in Exp 1, electrical stimulation did not elicit any apparent sensations.
III. Reflex Bladder Activation by IU Stimulation
At bladder volumes above 80% of Vth, excitatory bladder responses were evoked in 4 of the 6 participants by 30-s epochs of electrical stimulation applied to either the proximal or distal urethra. Post-stimulus bladder contractions were observed in some trials, but were not considered as responses to stimulation. The continued increase in Pdet beyond the end of the stimulation train indicated a possible stimulation-evoked reduction in Vth16 or a triggered DE contraction17.
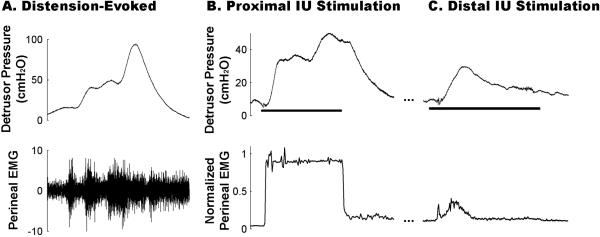
Stimulation at different locations along the urethra resulted in qualitatively different bladder responses to IU stimulation. In 3 of 6 participants, electrical stimulation of the proximal urethra evoked a sustained bladder contraction (Figure 2B) with an average peak detrusor pressure above baseline of 29.7 ± 11.6 cmH2O (range = 17.6 to 52.9 cmH2O). This corresponded to a normalized peak Pdet of 0.27 ± 0.06 (range = 0.16 to 0.53, Figure 3A). This excitatory bladder response was elicited independent of the severity of spinal injury or presence of a BCR response.
Figure 2.
Distension- and stimulation-evoked bladder contractions and perineal EMG activity in persons with spinal injury. (A) Robust distension-evoked bladder contractions were consistently accompanied by dyssynergic activity of the perineal musculature. (B) Proximal intraurethral (IU) stimulation (10 Hz, 4T, Exp 1) evoked sustained increases in detrusor pressure and high levels of perineal EMG activity that persisted throughout stimulation. (C) In contrast, electrical stimulation of the distal urethra (35 Hz, 2T, Exp 1) elicited a transient bladder contraction and low levels of perineal EMG.
Figure 3.
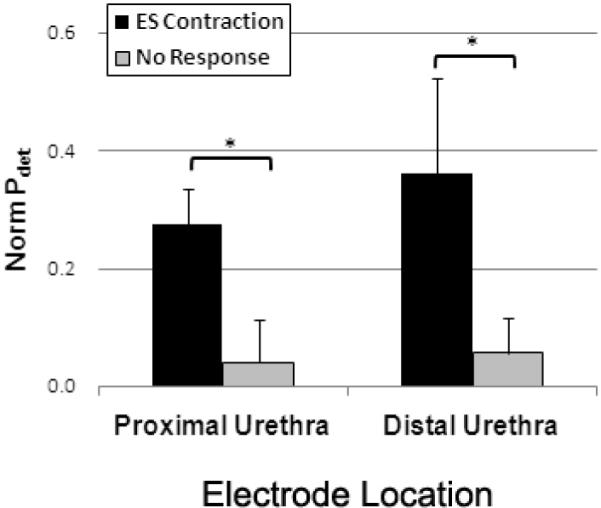
Peak magnitude of bladder contractions evoked by proximal and distal intraurethral (IU) stimulation in persons with SCI. Electrical stimulation of the proximal urethra evoked sustained bladder contractions in 3 of 6 individuals with spinal cord injury. Stimulation of the distal urethra generated reflex bladder contractions in 3 of 6 participants. In both groups, the electrically evoked Pdet were significantly greater than non-responses (*, p<0.05), but there was no difference between proximal and distal IU stimulation (p = 0.55).
In 3 of 6 participants, electrical stimulation of the distal urethra evoked transient bladder contractions during the 30-s stimulus train (Figure 2C). The evoked contractions exhibited an average peak detrusor pressure of 23.3 ± 9.28 cmH2O (range = 9.3 to 40.9 cmH2O), with a corresponding normalized peak Pdet of 0.36 ± 0.16 (range = 0.2 to 0.52, Figure 3B). Similar to proximal IU stimulation, the response was independent of both the presence of a BCR response and the severity of spinal injury. Excitatory bladder responses were evoked at both locations in only one individual (Exp 1, Table 1).
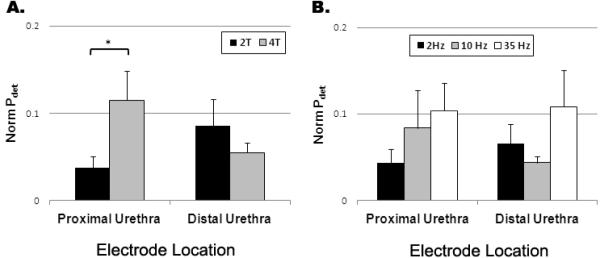
The peak evoked Pdet was dependent on the stimulation intensity during proximal IU stimulation, but not for distal IU stimulation (Figure 4A). At both locations, there was an increase in the evoked detrusor pressure at higher stimulation frequencies (Figure 4B), but no statistical difference was observed (p > 0.27).
Figure 4.
The effect of stimulation (A) amplitude and (B) frequency on the normalized peak detrusor pressure (Pdet). (A) Higher stimulus amplitudes applied to the proximal urethra resulted in significantly larger detrusor pressures (p < 0.05); whereas contractions evoked by stimulation of the distal urethra were not sensitive to the stimulus amplitude (p = 0.33). (B) Bladder contractions evoked by electrical stimulation of either the proximal (p = 0.43) or distal (p = 0.25) urethra were not dependent on stimulation frequency.
In two experiments (Figure 5), the feasibility of directly activating the bladder was tested by applying 30-second 20 Hz pulse trains at the most proximal electrode (i.e., bladder neck). Instead of generating robust bladder contraction, as might be expected from direct activation of the pelvic nerve, the responses were identical to those evoked by proximal IU stimulation (Figure 5, Table 1): sustained bladder contraction (Pdet = 0.41) in Exp1, and no response in Exp 4.
Figure 5.
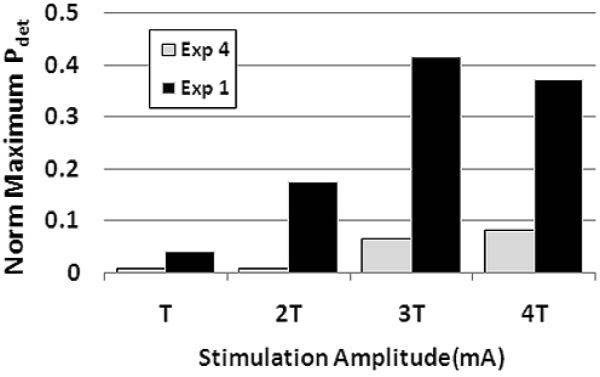
Electrical stimulation of the bladder neck in two participants that both exhibited distension evoked bladder contractions. Thirty-second trains of stimulation (frequency = 20 Hz, amplitude = T to 4T) failed to evoke directly sustained increases in bladder pressures that were comparable to distension evoked responses. The peak evoked detrusor pressures (Pdet) in experiments 1 (T = 15 mA), and 4 (T = 20 mA), were 41 cmH2O and 4 cmH2O, respectively.
IV. Differential Modulation of Perineal EMG Activity
The level of perineal EMG activity measured during IU stimulation was dependent on both the location of stimulation and whether or not a bladder contraction was evoked. Compared to the “no stimulation” condition (Figure 6), the perineal EMG measured during “no response” trials increased significantly to approximately half the level of dyssynergic activity observed during the initial urodynamic fill (columns 2 and 3, Figure 6): proximal (0.6 ± 0.1) and distal (0.5 ± 0.1) IU stimulation. However, in contrast to the further increase in perineal EMG activity observed during contractions generated by proximal urethra stimulation (column 1, 1.3 ± 0.2), the level of reflex EMG activity during “contraction” trials evoked by distal urethra stimulation (column 4, 0.3 ± 0.1) remained comparable to that for the “no response” trials.
Figure 6.
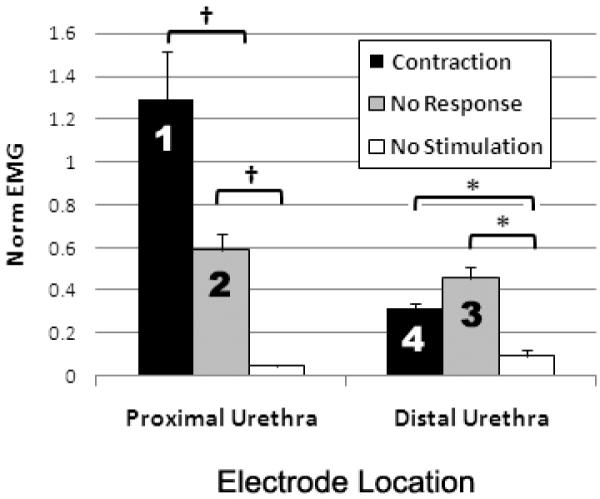
Reflex perineal EMG activity elicited by electrical stimulation of the proximal and distal urethra. The average normalized perineal EMG (norm EMG) evoked during bladder contractions generated by proximal IU stimulation (column 1, n = 3 participants) was significantly greater than the norm EMG measured both during distal IU stimulation evoked bladder contractions (column 4, n = 3 participants) and stimulation trials that failed to generate a bladder response (columns 2 and 3, “no response”). [*, p < 0.05; †, p < 0.001]
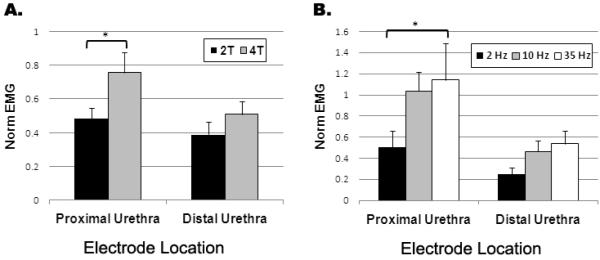
Consistent with the reflex bladder contractions evoked by proximal IU stimulation, the perineal EMG activity was also dependent on the stimulation amplitude (Figure 7A) and frequency (Figure 7B). In contrast, the perineal EMG activity during distal IU stimulation did not exhibit any dependence on stimulation amplitude (p = 0.33) or frequency (p = 0.27).
Figure 7.
The effect of intraurethral (IU) stimulation amplitude and frequency on the normalized perineal EMG. The normalized perineal EMG evoked by proximal IU stimulation was dependent on the amplitude and frequency of stimulation (*, p < 0.05). In contrast, there was no significant effect of stimulation amplitude (p = 0.33) or frequency (p = 0.25) on the evoked reflex EMG activity during distal IU stimulation.
Discussion
The results of this study bridge our current understanding of pudendal-to-bladder reflexes from the cat to persons with chronic SCI. This is the first demonstration of bladder contractions evoked by electrical stimulation of either the proximal or distal segments of the urethra in persons with SCI. These results present a striking parallel to responses generated by CSN and DGN stimulation in the cat.10 However, in contrast to cats, this study shows that proximal intraurethral (IU) stimulation in SCI human can elicit sustained bladder contractions in the absence of supraspinal inputs. Concomitant measurement of perineal EMG activity during stimulation-evoked bladder contractions revealed that efferent pudendal nerve activity was dependent on the location of stimulation, as well as the presence of stimulation evoked bladder contractions. The activation of reflex bladder activity in individuals with either complete or incomplete SCI indicated that these reflexes are mediated by spinal mechanisms.
Bladder Contractions are Evoked by a Spinal Mechanism
The results of this study support the presence of a spinal mechanism, similar to that demonstrated in cats,5, 10, 18 which generates reflex bladder contractions via spinal convergence of pelvic and pudendal afferents. First, the electrical activation of bladder contractions was strongly dependent on pelvic afferent activity and required bladder volumes of at least 80% of the distension evoked volume threshold. Second, as demonstrated in the cat12, 19 and rat20, electrical activation of bladder contractions required stimulation amplitudes greater than or equal to the threshold to elicit a pudendalto-pudendal reflex (i.e., BCR). This stimulation intensity suggested that the pudendal-to-bladder reflex in humans also involved the activation of smaller diameter myelinated fibers.12 And finally, the dependence of response magnitude on stimulation frequency exhibited a pattern similar to that in cats.9, 10, 21
The Role of Pudendal Afferents
The proximal urethra of the cat exhibits a dual innervation pattern by branches of the pudendal nerve, where the CSN provides sensory innervation to the intraluminal surface and a perineal branch of the pudendal nerve provides mostly efferent input to the external urethral sphincter.15 Although a CSN branch has not been identified in humans,13, 14 the results of this study suggest that is an analogous pudendal sensory branch innervating the proximal urethra. This would explain the requisite minimum bladder volume12 and the absence of any change in the evoked bladder response between proximal IU and bladder neck stimulation (Figure 5), at amplitudes and frequencies reported to elicit direct activation of parasympathetic fibers.22, 23 The role of putative CSN afferents in electrically evoked reflex bladder contractions is further supported by an excitatory urethrovesical reflex in humans,24 which is mediated by sensory fibers innervating the proximal (or prostatic) urethra.25 The results of this study extend previous knowledge of this reflex11 to include sustained reflex bladder contractions that occur during each stimulation trial.
Electrical stimulation of the distal urethra also evoked robust bladder contractions in 3 of 6 participants. The durations of these evoked responses were notably longer than the transient responses evoked by percutaneous stimulation of the pudendal nerve trunk,26 which likely co-activated bladder inhibitory reflexes mediated by the tibial nerve or anal nerve afferents.27, 28 Despite these excitatory responses, the clinical utility of distal IU stimulation in persons with SCI may be tempered by the robust bladder inhibitory effects of DGN afferents.6, 8 This confounding effect is reflected in the comparatively smaller bladder responses evoked at frequencies ≤ 10 Hz (Figure 4B). The clinical translation of this DGN pathway may also be limited by the absence in humans of the neonatal reflex,29 which is an excitatory bladder reflex evoked by perigenital stimulation in cats. This physiological difference may explain the discrepancy between the DGN mediated bladder contractions evoked in this study and those in the cat.5
Location of Stimulation Affects Reflex Perineal EMG
Detrusor sphincter dyssynergia remains a significant obstacle to restoring micturition in persons with SCI, and electrical stimulation of the pudendal nerve may exacerbate this condition. Given the absence of any flow or distension related urethral afferent activity during bladder contractions (i.e., isovolumetric bladder contractions), it was possible to identify the influence of the different sensory pathways on reflex activation of perineal muscles. Stimulation of the proximal and distal urethra that failed to evoke a bladder contraction (“no response”) revealed EMG activity associated with the guarding and bulbocavernosus reflexes, respectively. While bladder contractions evoked by proximal IU stimulation appeared to exacerbate the dyssynergic effects of pelvic afferents sensitive to changes in bladder pressure,30 the decreased perineal EMG activity during bladder contractions evoked by distal IU stimulation suggests a potential inhibitory mechanism that may suppress dyssynergic activity of the pudendal nerve (Figure 3). Further work towards understanding this mechanism may offer a clinical tool for achieving efficient micturition.
Limitation of Study: Intra-individual Variability
There were no definitive physical (e.g., gender or SCI level) or physiological (e.g., bulbocavernosus reflex) attributes that predicted whether an individual participant would respond to either proximal or distal urethral stimulation. Based on the consistent presence of an excitatory urethrovesical reflex in healthy humans,24 this variability in the evoked bladder responses may be a result of plastic changes in spinal cord circuitry following injury. This is particularly relevant to the excitatory bladder responses evoked by distal IU stimulation, as the dominant response to electrical stimulation of genital afferents is reflex inhibition of the bladder. The precise factor(s) underlying this variable pattern of reflex bladder contractions remains to be identified.
Acknowledgements
The authors thank Jean Maynor, RN, John Beaudry, PAC, and Betty O'Neal, NA, for their assistance in these experiments. Financial support was provided by the Craig H Neilsen Foundation and the National Institutes of Health (R01 NS050514).
References
- 1.Benevento BT, Sipski ML. Neurogenic bladder, neurogenic bowel, and sexual dysfunction in people with spinal cord injury. Phys Ther. 2002;82:601. [PubMed] [Google Scholar]
- 2.de Groat WC, Araki I, Vizzard MA, et al. Developmental and injury induced plasticity in the micturition reflex pathway. Behav Brain Res. 1998;92:127. doi: 10.1016/s0166-4328(97)00185-x. [DOI] [PubMed] [Google Scholar]
- 3.Shingleton WB, Bodner DR. The development of urologic complications in relationship to bladder pressure in spinal cord injured patients. J Am Paraplegia Soc. 1993;16:14. doi: 10.1080/01952307.1993.11735878. [DOI] [PubMed] [Google Scholar]
- 4.Tai C, Wang J, Wang X, et al. Bladder inhibition or voiding induced by pudendal nerve stimulation in chronic spinal cord injured cats. Neurourol Urodyn. 2007;26:570. doi: 10.1002/nau.20374. [DOI] [PubMed] [Google Scholar]
- 5.Woock JP, Yoo PB, Grill WM. Activation and inhibition of the micturition reflex by penile afferents in the cat. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1880. doi: 10.1152/ajpregu.00029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen J, Media S, Nohr M, et al. Treatment of neurogenic detrusor overactivity in spinal cord injured patients by conditional electrical stimulation. J Urol. 2005;173:2035. doi: 10.1097/01.ju.0000158160.11083.1b. [DOI] [PubMed] [Google Scholar]
- 7.Horvath EE, Yoo PB, Amundsen CL, et al. Conditional and continuous electrical stimulation increase cystometric capacity in persons with spinal cord injury. Neurourol Urodyn. 2010;29:401. doi: 10.1002/nau.20766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wheeler JS, Jr., Walter JS, Zaszczurynski PJ. Bladder inhibition by penile nerve stimulation in spinal cord injury patients. J Urol. 1992;147:100. doi: 10.1016/s0022-5347(17)37145-8. [DOI] [PubMed] [Google Scholar]
- 9.Boggs JW, Wenzel BJ, Gustafson KJ, et al. Frequency-dependent selection of reflexes by pudendal afferents in the cat. J Physiol. 2006;577:115. doi: 10.1113/jphysiol.2006.111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoo PB, Woock JP, Grill WM. Bladder activation by selective stimulation of pudendal nerve afferents in the cat. Exp Neurol. 2008;212:218. doi: 10.1016/j.expneurol.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gustafson KJ, Creasey GH, Grill WM. A catheter based method to activate urethral sensory nerve fibers. J Urol. 2003;170:126. doi: 10.1097/01.ju.0000070821.87785.14. [DOI] [PubMed] [Google Scholar]
- 12.Yoo PB, Grill WM. Minimally-invasive electrical stimulation of the pudendal nerve: a pre-clinical study for neural control of the lower urinary tract. Neurourol Urodyn. 2007;26:562. doi: 10.1002/nau.20376. [DOI] [PubMed] [Google Scholar]
- 13.Karam I, Droupy S, Abd-Alsamad I, et al. The precise location and nature of the nerves to the male human urethra: histological and immunohistochemical studies with three-dimensional reconstruction. Eur Urol. 2005;48:858. doi: 10.1016/j.eururo.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Zvara P, Carrier S, Kour NW, et al. The detailed neuroanatomy of the human striated urethral sphincter. Br J Urol. 1994;74:182. doi: 10.1111/j.1464-410x.1994.tb16583.x. [DOI] [PubMed] [Google Scholar]
- 15.Yoo PB, Woock JP, Grill WM. Somatic innervation of the feline lower urinary tract. Brain Res. 2008;1246:80. doi: 10.1016/j.brainres.2008.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bycroft JA, Craggs MD, Sheriff M, et al. Does magnetic stimulation of sacral nerve roots cause contraction or suppression of the bladder? Neurourol Urodyn. 2004;23:241. doi: 10.1002/nau.20009. [DOI] [PubMed] [Google Scholar]
- 17.Gustafson KJ, Creasey GH, Grill WM. A urethral afferent mediated excitatory bladder reflex exists in humans. Neurosci Lett. 2004;360:9. doi: 10.1016/j.neulet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Boggs JW, Wenzel BJ, Gustafson KJ, et al. Spinal micturition reflex mediated by afferents in the deep perineal nerve. J Neurophysiol. 2005;93:2688. doi: 10.1152/jn.00978.2004. [DOI] [PubMed] [Google Scholar]
- 19.Shefchyk SJ, Buss RR. Urethral pudendal afferent-evoked bladder and sphincter reflexes in decerebrate and acute spinal cats. Neurosci Lett. 1998;244:137. doi: 10.1016/s0304-3940(98)00155-4. [DOI] [PubMed] [Google Scholar]
- 20.Peng CW, Chen JJ, Cheng CL, et al. Role of pudendal afferents in voiding efficiency in the rat. Am J Physiol Regul Integr Comp Physiol. 2008;294:R660. doi: 10.1152/ajpregu.00270.2007. [DOI] [PubMed] [Google Scholar]
- 21.Woock JP, Yoo PB, Grill WM. Intraurethral Stimulation Evokes Bladder Responses via 2 Distinct Reflex Pathways. J Urol. 2009 doi: 10.1016/j.juro.2009.02.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brindley GS, Polkey CE, Rushton DN. Sacral anterior root stimulators for bladder control in paraplegia. Paraplegia. 1982;20:365. doi: 10.1038/sc.1982.65. [DOI] [PubMed] [Google Scholar]
- 23.Walter JS, Wheeler JS, Fitzgerald MP, et al. A spinal cord injured animal model of lower urinary tract function: observations using direct bladder and pelvic plexus stimulation with model microstimulators. J Spinal Cord Med. 2005;28:246. [PubMed] [Google Scholar]
- 24.Shafik A, el-Sibai O, Ahmed I. Effect of urethral dilation on vesical motor activity: identification of the urethrovesical reflex and its role in voiding. J Urol. 2003;169:1017. doi: 10.1097/01.ju.0000046384.71563.51. [DOI] [PubMed] [Google Scholar]
- 25.Todd JK. Afferent Impulses in the Pudendal Nerves of the Cat. Q J Exp Physiol Cogn Med Sci. 1964;49:258. doi: 10.1113/expphysiol.1964.sp001730. [DOI] [PubMed] [Google Scholar]
- 26.Yoo PB, Klein SM, Grafstein NH, et al. Pudendal nerve stimulation evokes reflex bladder contractions in persons with chronic spinal cord injury. Neurourol Urodyn. 2007;26:1020. doi: 10.1002/nau.20441. [DOI] [PubMed] [Google Scholar]
- 27.Amarenco G, Ismael SS, Even-Schneider A, et al. Urodynamic effect of acute transcutaneous posterior tibial nerve stimulation in overactive bladder. J Urol. 2003;169:2210. doi: 10.1097/01.ju.0000067446.17576.bd. [DOI] [PubMed] [Google Scholar]
- 28.McGuire EJ, Zhang SC, Horwinski ER, et al. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129:78. doi: 10.1016/s0022-5347(17)51928-x. [DOI] [PubMed] [Google Scholar]
- 29.Thor KB, Hisamitsu T, de Groat WC. Unmasking of a neonatal somatovesical reflex in adult cats by the serotonin autoreceptor agonist 5-methoxy-N,N-dimethyltryptamine. Brain Res Dev Brain Res. 1990;54:35. doi: 10.1016/0165-3806(90)90062-4. [DOI] [PubMed] [Google Scholar]
- 30.Rudy DC, Awad SA, Downie JW. External sphincter dyssynergia: an abnormal continence reflex. J Urol. 1988;140:105. doi: 10.1016/s0022-5347(17)41499-6. [DOI] [PubMed] [Google Scholar]