Abstract
Productive rearrangement of the T–cell receptor (TCR) β gene and signalling through the pre–TCR–CD3 complex are required for survival, proliferation and differentiation of T–cell progenitors (pro–T cells). Here we identify a role for death receptor signalling in early T–cell development using a dominant-negative mutant of the death receptor signal transducer FADD/MORT1 (FADD–DN). In rag–1–/– thymocytes, which are defective in antigen receptor gene rearrangement, FADD–DN bypassed the requirement for pre–TCR signalling, promoting pro–T–cell survival and differentiation to the more mature pre–T stage. Surprisingly, differentiation was not accompanied by the proliferation that occurs normally during transition to the pre–T stage. Consistent with a role for FADD/MORT1 in this cell division, FADD–DN rag–1–/– pro–T cells failed to proliferate in response to CD3ε ligation. Concomitant signalling through the pre–TCR and death receptors appears to trigger pro–T cell survival, proliferation and differentiation, whereas death receptor signalling in thymocytes that lack a pre–TCR induces apoptosis. Later in life all FADD–DN rag–1–/– mice developed thymic lymphoma, indicating that FADD/MORT1 can act as a tumour suppressor.
Keywords: apoptosis/FADD/MORT1/pre-TCR/thymocyte
Introduction
T lymphocytes of the T–cell receptor (TCR) α/β lineage develop in the thymus from bone marrow- or fetal liver–derived multi-potential stem cells. Glycoproteins expressed at the cell surface and the rearrangement status of the TCRα and TCRβ gene loci identify distinct stages of thymocyte differentiation (Rodewald and Fehling, 1998). Early T–cell progenitors (here called pro–T cells) are CD4–8– and can be subdivided into four populations according to expression of CD25 [interleukin-2 (IL-2) receptor α-chain] and CD44 (Pgp–1). The presently accepted developmental sequence is CD25–44+ (pro–T1) → CD25+44+ (pro–T2) → CD25+44– (pro–T3) → CD25–44– (pro–T4) (Godfrey and Zlotnik, 1993). Signals through the IL-7 receptor (IL-7R)/γc, c-Kit (SCF receptor) and Flk2 are essential for cell proliferation and survival during the pro–T1 to pro–T3 stages of development (Rodewald and Fehling, 1998). Rearrangement of TCRβ genes is initiated during transition from the pro–T2 to the pro–T3 stage (Capone et al., 1998). A productive rearrangement and expression of a pre–TCR composed of the TCRβ chain, the pTα chain and CD3 signal transducing proteins (Groettrup et al., 1993) results in progression to the pro–T4 and CD4+8+ pre–T stages (Rodewald and Fehling, 1998). Thymocytes that survive the pre–TCR checkpoint proliferate and differentiate to yield the numerically dominant CD4+8+ population. Maturation to the pre–T stage coincides with TCRα gene rearrangement (Petrie et al., 1993), and thymocytes expressing a complete TCRα/β–CD3 complex become subject to immunological selection based on their TCRα/β specificity (von Boehmer, 1994).
Mutant scid mice and mice lacking either of the recombinase-activating genes, rag–1 or rag-2, are defective in antigen receptor gene rearrangement. These mice do not produce pro–T4 cells, pre–T cells or mature TCRα/β T cells because differentiation is arrested at the pro–T3 stage (Habu et al., 1987; Shores et al., 1990; Mombaerts et al., 1992a; Shinkai et al., 1992). The pro–T3 cells have a lifespan of ∼3–4 days and appear to die from a lack of signalling through the pre–TCR (Penit et al., 1995). A requirement for CD3ɛ in signal transduction at the pre–TCR checkpoint is demonstrated by the lack of pro–T4, pre–T and mature T cells in CD3ɛ-deficient mice (Malissen et al., 1995). Furthermore, ligation of surface-bound CD3ɛ with cross-linking antibodies is sufficient to trigger pre–T–cell generation in rag–1–/–, rag-2–/–, pTα–/– or TCRβ–/– mice (Levelt et al., 1993; Jacobs et al., 1994; Shinkai and Alt, 1994; Fehling et al., 1997).
The mechanism by which pre–TCR signalling controls survival and proliferation of thymocytes remains unclear. Overexpression of the anti-apoptotic protein Bcl–2 rescues pro–T cells from a lack of IL–7R signalling (Akashi et al., 1997; Maraskovsky et al., 1997), but it does not promote survival of pre–TCR-deficient pro–T3 cells in scid (Strasser et al., 1994a) or rag–1–/– mice (Maraskovsky et al., 1997). Some CD4+8+ pre–T cells were observed in scid, rag–1–/– and rag-2–/– mice lacking the tumour suppressor p53 (Bogue et al., 1996; Guidos et al., 1996; Jiang et al., 1996; Nacht et al., 1996; Nacht and Jacks, 1998). There was large variation between individual animals in the number of CD4+8+ thymocytes produced and most mice rapidly developed lymphoid malignancy (Nacht et al., 1996). p53 deficiency also restored normal pre–T–cell content in CD3γ–/– mice (Haks et al., 1999). A recent report describing CD4+8+ pre–T cells and even mature CD3+4+8– and CD3+4–8+ thymocytes in scid mice homozygous for the Fas (also called CD95 or APO–1) loss-of-function lpr mutation (Yasutomo et al., 1997) implied that Fas may deliver the death signal to pro–T3 cells that lack a pre–TCR.
Fas belongs to a subgroup of the tumour necrosis factor receptor (TNF–R) family whose members have a cytoplasmic death domain (Ashkenazi and Dixit, 1998). This domain mediates protein–protein interactions between ligated death receptors and death domain-containing cytoplasmic adaptor proteins such as FADD (also called MORT1) and TRADD (Boldin et al., 1995; Chinnaiyan et al., 1995; Hsu et al., 1995). Apoptosis is signalled when recruited FADD uses its death effector domain to bind one of the death effector domains in pro-caspase-8 (Boldin et al., 1996; Muzio et al., 1996). Aggregation of caspase-8 zymogens facilitates their autocatalytic activation and this triggers the proteolytic cascade that leads to apoptosis (Martin et al., 1998; Muzio et al., 1998; Yang et al., 1998). Some death receptors can also signal activation of Jun kinase and/or the NF-κB family of transcription factors, and in certain settings they promote cell growth and differentiation rather than cell death (Ashkenazi and Dixit, 1998).
Inhibition of FADD function by gene targeting or by expression of a dominant interfering mutant of FADD (here called FADD–DN) renders T cells resistant to apoptosis induced by Fas ligand (FasL), and surprisingly it also inhibits mitogen-induced T–cell proliferation (Newton et al., 1998; Zhang et al., 1998). Thus, FADD participates not only in the transduction of an apoptotic signal, but is also essential for transmitting growth and/or survival signals. Here we examine FADD–DN rag–1–/– and lpr rag–1–/– mice to determine whether FADD or Fas act at the pre–TCR checkpoint. Our data are consistent with a model in which FADD signals apoptosis in pro–T cells with non-productive TCRβ gene rearrangements and promotes proliferation of pro–T cells expressing a pre–TCR. Evidence that FADD can act as a tumour suppressor is also presented.
Results
Pro–T cells express genes involved in death receptor signalling
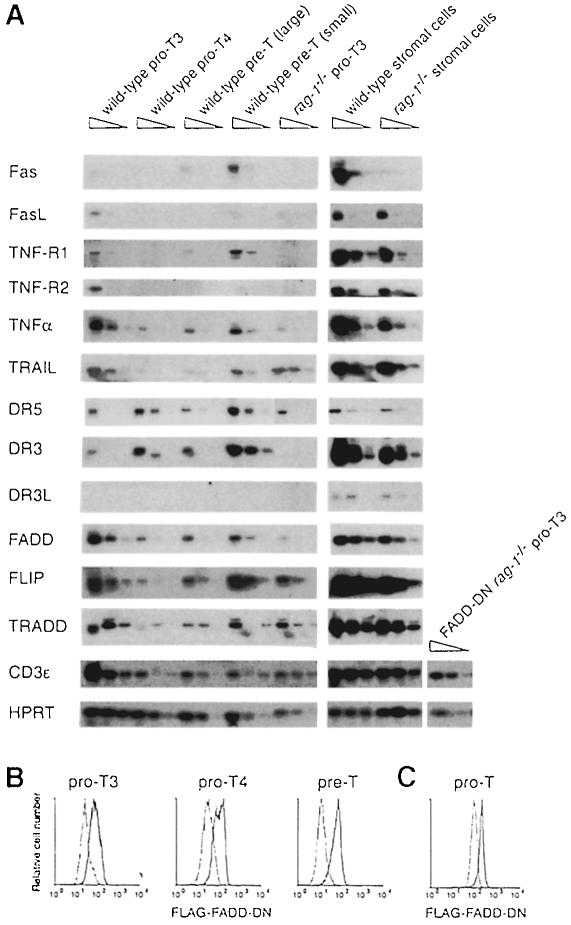
To investigate whether death receptor signalling might regulate early T–cell development, we first assayed expression of death receptors in pro–T3, pro–T4 and pre–T cells from wild-type and rag–1–/– mice (Figure 1A). Transcripts for DR3 and DR5 (also called TRAIL-R2 or KILLER) were detected in pro–T3, pro–T4 and pre–T cells, while TNF–R1 transcripts were detected in pro–T3 and pre–T cells. Expression of TNF-R2, which lacks a death domain, was detected only in pro–T3 cells. Consistent with flow cytometric analyses of Fas expression (Nishimura et al., 1995; Ogasawara et al., 1995), we detected Fas mRNA in pre–T cells but not in pro–T cells. Expression of the ligands for these receptors was also examined. DR3L mRNA was identified in a population enriched for thymic stromal cells, but not in purified pro–T or pre–T cells. A similar result was obtained for FasL, although this mRNA could be detected at low levels in pro–T3 cells. TNFα and TRAIL mRNAs were detected in pro–T3, pro–T4 and pre–T cells, and in the stromal cell-enriched fraction. Transcripts for the cytoplasmic adaptor proteins TRADD and FADD were also detected in pro–T3, pro–T4 and pre–T cells, and FLIP, an inhibitor of death receptor signalling (Irmler et al., 1997), had a similar pattern of expression. Since death receptors and their cytoplasmic adaptors are expressed in developing T cells, while their ligands are expressed in thymocytes or thymic stromal cells, death receptors are candidates for regulating apoptosis at the pre–TCR checkpoint.
Fig. 1. Expression of death receptors, death ligands and their signalling molecules in thymocyte subsets. (A) Pro–T3, pro–T4, pre–T (divided into large and small cells on the basis of their forward light scatter) and thymic stromal cells from 6- to 10-week-old rag–1–/– and FADD–DN rag–1–/– mice were analysed for the presence of specific RNA transcripts by RT–PCR. PCR was performed on 5–fold serial dilutions of cDNA and the amplification products were probed with a 32P–labelled internal oligonucleotide. (B and C) Pro–T3, pro–T4 and pre–T cells from FADD–DN rag–1+/+ mice (B) and pro–T cells from FADD–DN rag–1–/– mice (C) were analysed for FADD–DN expression by cytoplasmic immunofluorescence and flow cytometry using anti-FLAG antibody. Solid lines represent FADD–DN transgenic cells and dotted lines represent wild-type (B) or rag–1–/– (C) cells. Profiles are representative of analyses of three mice of each genotype.
FADD–DN prevents the death of pro–T cells that fail to express TCR chains
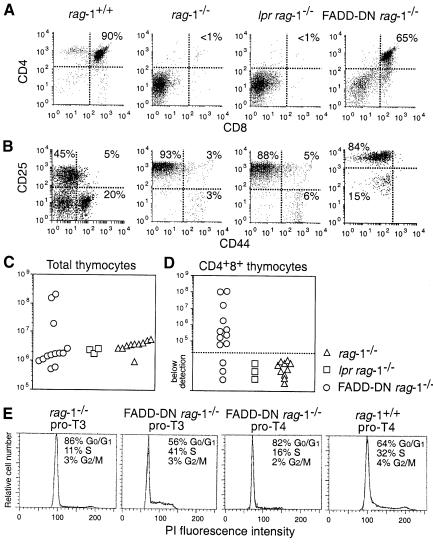
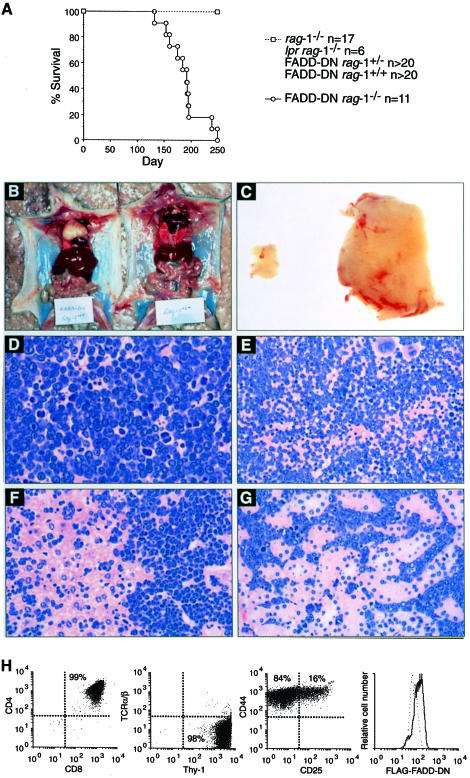
The mechanism for eliminating pro–T3 cells that lack a pre–TCR can be studied in rag-deficient mice since all their pro–T cells have this fate. Ablation of the mouse FADD gene results in embryonic lethality at a time that precludes analysis of T–cell development in FADD–/– rag–1–/– mice (Yeh et al., 1998). Therefore, we used FADD–DN rag–1–/– mice to investigate whether death receptors deliver an apoptotic signal to pro–T3 cells lacking a pre–TCR. The identical phenotypes of FADD–DN transgenic T cells and FADD–/– T cells prove that FADD–DN is a specific inhibitor of FADD (Newton et al., 1998; Zhang et al., 1998). Western blot and flow cytometric analysis confirmed that FADD–DN protein is expressed in pro–T3 cells in both FADD–DN rag–1+/+ and FADD–DN rag–1–/– mice (Figure 1B and C; data not shown). Thymi from FADD–DN rag–1–/–, Fas-deficient lpr rag–1–/– and control rag–1–/– mice were analysed at 5–13 weeks of age for total cellularity and the presence of the various thymocyte subsets. Contrary to a report describing CD4+8+ thymocytes in young lpr scid mice (Yasutomo et al., 1997), we observed no CD4+8+ pre–T cells in lpr rag–1–/– mice. T–cell development in these animals was arrested at the CD25+44– pro–T3 stage (Figure 2A and B), and thymus cellularity was comparable to that in rag–1–/– mice, at 1–3 × 106 cells per thymus (Figure 2C). In contrast, 10 of 13 FADD–DN rag–1–/– mice analysed had CD25–44– pro–T4 and CD4+8+ pre–T cells (Figure 2A and D). The absolute number of CD4+8+ cells varied considerably among individual FADD–DN rag–1–/– mice, ranging from 2.9 × 103 to 1.1 × 108 (Figure 2D). Older mice had the most CD4+8+ cells. Total thymocyte numbers in the 5- to 9-week-old FADD–DN rag–1–/– mice were as low as those in rag–1–/– littermate controls, and CD4+8+ pre–T cells in these animals were similar in size and surface marker expression to pre–T cells from wild-type mice. However, three mice aged between 10 and 13 weeks showed a 10- to 100–fold increase in thymic cellularity (Figure 2C). The CD4+8+ pre–T cells in these animals were abnormally large, indicating that they might be in the process of neoplastic transformation. CD4+8+25–/+ thymocytes from one mouse were transplanted into histocompatible recipients, but these cells were not fully transformed because they did not produce tumours.
Fig. 2. FADD–DN promotes development of pro–T4 and pre–T cells in rag–1–/– mice. (A and B) Flow cytometric analysis of thymocytes from 5- to 13-week-old rag–1+/+, rag–1–/–, lpr rag–1–/– and FADD–DN rag–1–/– mice. Profiles in (A) show expression of CD4 and CD8. Profiles in (B) show expression of CD25 and CD44 after gating on Thy–1+CD3–4–8– cells. CD25 staining varies due to different instrument settings. Total thymic cellularity (C) and CD4+8+ pre–T–cell content (D) were determined by cell counting and flow cytometric analysis of thymocytes stained with antibodies to CD4 and CD8. Each symbol represents one mouse. (E) Purified pro–T3 and pro–T4 cells from rag–1+/+, rag–1–/– and FADD–DN rag–1–/– mice were analysed for their cell cycle distribution.
A productive TCRβ gene rearrangement and expression of a pre–TCR by wild-type pro–T3 cells coincides with their entry into the cell cycle (Hoffman et al., 1996). Consistent with FADD–DN overcoming the block in T–cell development imposed by rag deficiency, a much greater proportion of FADD–DN rag–1–/– pro–T3 cells were observed in the S, G2 and M phases of the cell cycle when compared with rag–1–/– pro–T3 cells (Figure 2E). We conclude from these results that (i) FADD signals apoptosis in pro–T3 cells lacking a pre–TCR and (ii) Fas is not essential for this death.
FADD function is required for CD3ε ligation-induced proliferation of rag–1–/– pro–T cells
Transition of pro–T3 cells to the pre–T stage of thymocyte development is normally accompanied by 6–10 population doublings (Penit et al., 1995). Hence ∼1 × 106 pro–T cells typically give rise to ∼1 × 108 CD4+8+ pre–T cells. Therefore, it was surprising that most FADD–DN rag–1–/– animals did not show increased thymus cellularity (Figure 2C). The pro–T4 cells that developed spontaneously in FADD–DN rag–1–/– mice were cycling less than rag–1+/+ pro–T4 cells (Figure 2E), suggesting that signals from FADD or the missing pre–TCR were needed for efficient pro–T–cell proliferation. Mature T cells require FADD for mitogen-induced proliferation as well as death receptor-induced apoptosis (Newton et al., 1998; Zhang et al., 1998), so we examined whether FADD is needed for proliferation at the pre–TCR checkpoint. This was investigated by injecting FADD–DN rag–1–/– mice with cross-linking anti-CD3ɛ antibody. Previous studies with rag–1–/–, rag-2–/–, pTα–/– and TCRβ–/– mice have shown that this treatment circumvents the need for a pre–TCR and results in proliferation of thymocytes plus differentiation to the CD4+8+ pre–T stage (Levelt et al., 1993; Jacobs et al., 1994; Shinkai and Alt, 1994; Fehling et al., 1997).
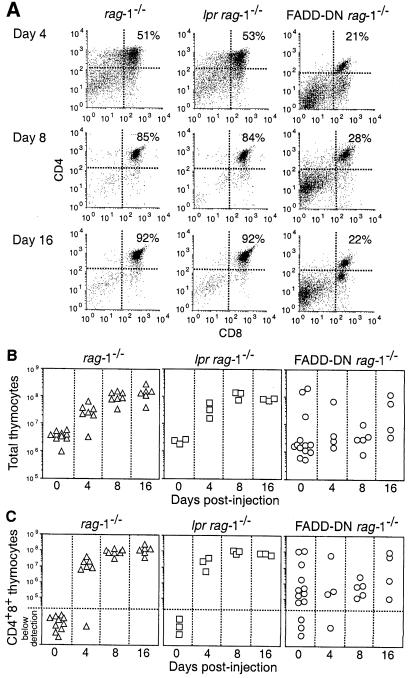
One to two weeks after injection with anti-CD3ɛ antibody, each lpr rag–1–/– and control rag–1–/– mouse had a thymus that was similar in size and CD4+8+ pre–T–cell content to a wild-type thymus (Figure 3). At 4 days post-injection their thymi had ∼2 × 107 CD4+8+ pre–T cells and at 8 days post-injection the average number of pre–T cells had increased to 9 × 107 and pro–T cells accounted for <8% of thymocytes. Flow cytometric analysis indicated that most of the CD4+8+ pre–T cells were small and CD25–44– (data not shown). Pre–T–cell production was a specific effect of CD3ɛ ligation because CD4+8+ cells were not observed in rag–1–/– or lpr rag–1–/– mice injected with an isotype-matched control antibody. The rapid and synchronous transition from the pro–T3 to the pre–T stage and the accompanying increase in thymus cellularity induced by anti-CD3ɛ antibody were not observed in FADD–DN rag–1–/– mice (Figure 3). The thymus in FADD–DN rag–1–/– mice treated with anti-CD3ɛ antibody resembled that in untreated FADD–DN rag–1–/– mice (compare Figure 3B and C with Figure 2C and D) or FADD–DN rag–1–/– mice injected with an isotype-matched control antibody (data not shown). FADD–DN rag–1–/– mice injected with anti-CD3ɛ antibody had on average 100- to 1000–fold fewer CD4+8+ pre–T cells than similarly treated rag–1–/– or lpr rag–1–/– mice (Figure 3C). CD3ɛ mRNA was present at normal levels in FADD–DN rag–1–/– pro–T cells (Figure 1A), so failure of these cells to respond to CD3ɛ ligation is unlikely to be due to the absence of CD3ɛ protein at the cell surface. These results are consistent with a critical role for FADD in signalling cell proliferation at the pro–T3 to pre–T–cell transition. Indeed, given the spontaneous development of CD4+8+ thymocytes in most untreated or control antibody-injected FADD–DN rag–1–/– mice (Figure 2), the inhibitory effect of FADD–DN on pro–T– and/or pre–T–cell proliferation is probably understated in these experiments. Our results also indicate that Fas is not essential for proliferation and differentiation of thymocytes at the pre–TCR checkpoint.
Fig. 3. FADD–DN diminishes CD3ɛ ligation-induced production of CD4+8+ pre–T cells in rag–1–/– mice. (A) Flow cytometric analysis of thymocytes from 5- to 13–week-old rag–1–/–, lpr rag–1–/– and FADD–DN rag–1–/– mice after intraperitoneal injection with 100 μg of 145-2C11 hamster anti-mouse CD3ɛ monoclonal antibody. Total thymic cellularity (B) and CD4+8+ pre–T–cell content (C) were determined by cell counting and flow cytometric analysis of thymocytes stained with antibodies to CD4 and CD8. Each symbol represents one mouse.
CD4+8+ pre–T cells are produced in lpr rag–1–/– or FADD–DN rag–1–/– mice exposed to γ–irradiation
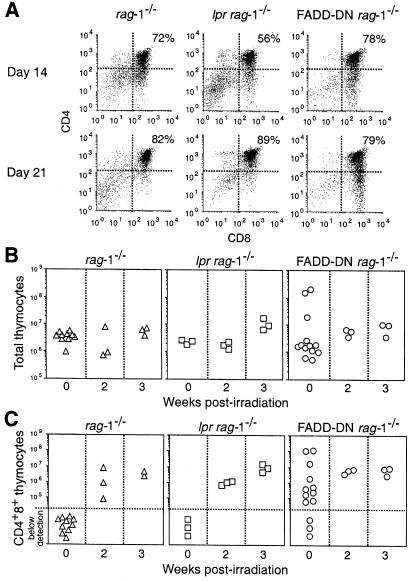
We also investigated a role for FADD and Fas during transition from the pro–T3 to the pre–T stage of thymocyte development by subjecting FADD–DN rag–1–/– and lpr rag–1–/– mice to sublethal γ–irradiation. Like CD3ɛ ligation, γ–irradiation promotes the production of CD4+8+ pre–T cells in mutant scid, rag–1–/– or rag-2–/– mice (Danska et al., 1994; Murphy et al., 1994; Zúñiga-Pflücker et al., 1994; Guidos et al., 1995). Both FADD–DN rag–1–/– and lpr rag–1–/– mice responded to 5 Gy of γ-irradiation like control rag–1–/– mice. CD4+8+ cells were detected in γ-irradiated mice after 2 weeks and these cells represented ∼75% of all thymocytes (Figure 4A). In contrast to the variation among individual FADD–DN rag–1–/– mice treated with anti-CD3ɛ antibody (Figure 3B and C), all γ-irradiated FADD–DN rag–1–/– mice possessed similar numbers of thymocytes and CD4+8+ pre–T cells (Figure 4B and C). No CD4+8+ pre–T cells were detected in rag–1–/– mice 1 week after γ-irradiation even using a dose of 7.5 Gy (data not shown), indicating that γ-irradiation is less efficient than CD3ɛ ligation at inducing pre–T–cell production. Consistent with this notion and in agreement with previous observations (Guidos et al., 1995), γ-irradiation produced less thymocyte proliferation than injection of anti-CD3ɛ antibody. Mice examined 3 weeks after γ–irradiation displayed only a 2- to 3–fold increase in thymus cellularity (Figure 4B). The fact that γ–irradiation promotes pro–T–cell differentiation without significant thymocyte proliferation probably explains why FADD–DN rag–1–/– mice responded similarly to rag–1–/– mice. Our interpretation of these results is that FADD is needed for efficient proliferation of cells at the pre–TCR checkpoint but is dispensable for cell differentiation. Fas appears to be dispensable for both processes.
Fig. 4. FADD–DN does not affect γ-irradiation-induced production of CD4+8+ pre–T cells in rag–1–/– mice. (A) Flow cytometric analysis of thymocytes from 5- to 10-week-old rag–1–/–, lpr rag–1–/– and FADD–DN rag–1–/– mice exposed to 5 Gy of γ-irradiation. Total thymic cellularity (B) and CD4+8+ pre–T–cell content (C) were determined by cell counting and flow cytometric analysis of thymocytes stained with antibodies to CD4 and CD8. Each symbol represents one mouse.
FADD–DN rag–1–/– mice develop thymic lymphoma
The abnormal size and surface marker profiles of pre–T cells from three older FADD–DN rag–1–/– mice with very large thymi (Figure 2C) prompted us to investigate whether loss of FADD function might be oncogenic. Indeed, all older (>16 weeks) FADD–DN rag–1–/– mice, which did not acquire the terminal heart disease that affects some rag–1–/– mice in our colony, developed thymic lymphoblastic lymphoma that could be transplanted into histocompatible hosts (Figure 5). Not a single rag–1–/–, lpr rag–1–/–, FADD–DN rag–1+/– or FADD–DN rag–1+/+ mouse developed a tumour during this time (Figure 5). Thus, the combination of impaired FADD signalling and rag–1 deficiency was required for neoplastic transformation, and signals from Fas alone could not account for this function of FADD.
Fig. 5. FADD–DN rag–1–/– mice develop thymic lymphoma. (A) The incidence of thymic lymphoma in rag–1–/–, lpr rag–1–/–, FADD–DN rag–1–/+, FADD–DN rag–1+/+ and FADD–DN rag–1–/– mice that did not develop heart disease. (B) A 19-week-old FADD–DN rag–1–/– mouse with thymic lymphoma (left) and a healthy rag–1–/– littermate (right). (C) Thymus from a FADD–DN rag–1–/– mouse with thymic lymphoma (right) and from a healthy rag–1–/– littermate (left). (D–G) Histological sections of the thymus (D), spleen (E), liver (F) and kidney (G) from a FADD–DN rag–1–/– mouse with thymic lymphoma were stained with haematoxylin and eosin. Photographs were taken at 200× (D) or 100× (E–G) magnification. (H) Flow cytometric analysis of cells from a typical FADD–DN rag–1–/– thymoma.
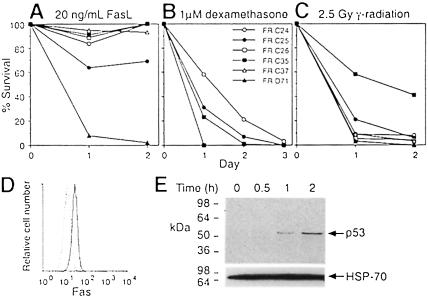
In addition to a swollen thymus, tumour-burdened FADD–DN rag–1–/– mice had pale kidneys and a very large spleen and liver. Histological sections of these organs revealed extensive tumour cell infiltration (Figure 5D–G). Flow cytometric analysis showed that the FADD–DN rag–1–/– tumour cells were CD4+8+25–/+44–/+, Thy–1+ and TCRα/β– (Figure 5H), and therefore represented neoplastic counterparts of the pre–T cells that develop spontaneously in these animals (Figure 2A). Almost all of the thymomas expressed FADD–DN protein at a level detectable by flow cytometric analysis (Figure 5H). Cultured cell lines were established from six of the FADD–DN rag–1–/– thymomas and they all expressed Fas (Figure 6D). Four of the cell lines were highly resistant to apoptosis induced by cross-linked soluble FasL (Figure 6A), presumably due to FADD–DN expression. As expected, not all pathways to apoptosis were blocked in the FasL-resistant tumour cells, because they were highly sensitive to dexamethasone and γ–irradiation (Figure 6B and C). Spontaneous development of CD4+8+ thymocytes and lymphoid malignancy has also been observed in p53–/– rag–1–/– mice (Nacht and Jacks, 1998), so the p53 status of the FADD–DN rag–1–/– tumour cell lines was investigated. Western blot analysis using monoclonal antibodies specific to wild-type p53 revealed that γ–irradiated FADD–DN rag–1–/– tumour cells contained more p53 protein than untreated cells (Figure 6E). Moreover, viable γ–irradiated cells did not accumulate in the G2 phase of the cell cycle (data not shown) as has been observed for p53–deficient thymic lymphoma cells (Strasser et al., 1994b). These results demonstrate that the FADD–DN rag–1–/– tumours retained wild-type p53 function. Overall, these observations show that blocking FADD function can cause lymphoid malignancy.
Fig. 6. The response of FADD–DN rag–1–/– thymoma cell lines to FasL and γ–irradiation. (A–C) FADD–DN rag–1–/– thymoma cell lines were cultured with 20 ng/ml FLAG-tagged FasL cross-linked with 1 μg/ml anti-FLAG monoclonal antibody (A), 1 μM dexamethasone (B), or were exposed to 2.5 Gy γ-radiation (C). Cell survival was determined by flow cytometric analysis of PI-stained cells. Survival is shown as a percentage of that in culture medium without treatment. Cells cultured with anti-FLAG antibody alone were indistinguishable from cells cultured in plain medium. (D) FR D71 cells were examined for surface expression of Fas. The solid line represents cells stained with Jo2 anti-Fas monoclonal antibody, and the dotted line represents cells stained with an isotype-matched control antibody. Similar results were obtained with cell lines derived from five other FADD–DN rag–1–/– mice. (E) Western blot analysis of p53 expression in FR C37 cells after a 5 Gy dose of γ-radiation (2.5 × 106 cell equivalents/well). The blot was probed with a cocktail of monoclonal antibodies to wild-type p53, or as a loading control, with a monoclonal antibody that recognizes mouse HSP-70. Similar results were obtained with the cell line FR C24.
Discussion
Death receptors have pleiotropic actions and can promote cell death, proliferation or differentiation. Several death receptors are regulators of mature lymphocyte homeostasis. For example, signalling from Fas, most likely through FADD-mediated activation of pro-caspase-8, is responsible for the removal of autoreactive and chronically stimulated effector lymphocytes in peripheral lymphoid organs (Nagata and Golstein, 1995). The work presented here demonstrates that signalling through FADD is also critical for regulating apoptosis of T–cell progenitors at the pre–TCR checkpoint. Surprisingly, FADD was found to have a second function during thymocyte development, mediating signals required for efficient proliferation during transition from the pro–T3 to the pre–T stage. Expression of FADD–DN in rag–1–/– mice promoted survival and differentiation of TCR-negative pro–T3 cells (Figure 2), but it inhibited proliferation of pro–T3 cells that were stimulated through CD3 (Figure 3). In addition, our data reveal that FADD constitutes a barrier against neoplastic transformation (Figure 5).
Regulation of cell death, proliferation and differentiation at the pre–TCR checkpoint
Developing thymocytes are selected on the basis of TCR expression and specificity to generate a functional repertoire of mature T cells. The first round of selection occurs at the pro–T3 stage following rearrangement of the TCRβ chain loci. Most pro–T3 cells that fail to express a functional TCRβ chain do not progress to the pro–T4 stage because they undergo apoptosis (von Boehmer et al., 1999). The presence of cycling pro–T3 cells and the development of pro–T4 and pre–T cells in FADD–DN rag–1–/– mice (Figure 2) indicate that FADD transmits the apoptotic signal within the doomed pro–T3 cells. To date, FADD has only been implicated in signalling by death receptors (Ashkenazi and Dixit, 1998). It is therefore likely that one or several of these receptors deliver the death signal to pro–T3 cells lacking a pre–TCR. Candidate receptors that are expressed in mouse pro–T cells are DR3, DR5 and TNF-R1. Fas is dispensable for this killing, since T–cell development is arrested at the pro–T3 stage in Fas-deficient lpr rag–1–/– mice (Figure 2). This finding is consistent with expression of Fas being restricted to pre–T cells (Figure 1; Nishimura et al., 1995; Ogasawara et al., 1995). Development of pre–T and mature T cells in lpr scid mice (Yasutomo et al., 1997) may have resulted because the scid mutation imposes only a partial block on antigen receptor gene rearrangement (Bosma and Carroll, 1991).
We speculate that stimulation of death receptors on pro–T cells is achieved by membrane-anchored death ligands on thymic stromal cells. However, death ligands such as TRAIL and TNFα were also expressed by the thymocytes themselves (Figure 1A), so autocrine and/or paracrine apoptosis signalling between lymphoid cells is also a possibility. Crossing animals that lack individual death receptors or death ligands with rag–1–/– mice may identify which receptor initiates the suicide of pro–T cells lacking a pre–TCR. However, pro–T cells express several death receptors, so their role in early T lymphopoiesis might only be revealed by deletion of multiple death receptors, or as we have shown using FADD–DN, by inhibition of a common signal transducer.
Small numbers of pre–T cells in young FADD–DN rag–1–/– mice may reflect the involvement of additional, FADD-independent pathways to apoptosis. We prefer the idea that FADD–DN rag–1–/– mice have a small thymus because thymocyte proliferation is impaired. Diminished proliferation of pro–T cells in FADD–DN rag–1–/– mice injected with anti-CD3ɛ antibody (Figure 3) and reduced pro–T4 cells in FADD–DN mice (Walsh et al., 1998; data not shown) are consistent with a role for FADD in thymocyte production. We think it unlikely that variation between the thymocyte populations in rag–1–/– and FADD–DN rag–1–/– mice at the time of injection accounted for their different responses to CD3ɛ ligation. TCRβ–/– or pTα–/– mice also contain CD4+8+ pre–T cells (Mombaerts et al., 1992b; Fehling et al., 1995), but their pro–T cells can proliferate and differentiate like rag–1–/– pro–T cells in response to CD3ɛ ligation (Levelt et al., 1993; Fehling et al., 1997). Given that pro–T3 cells in FADD rag–1–/– mice are actively cycling (Figure 2E), FADD might be required for sustained proliferation of pro–T and pre–T cells. Chimeric mice generated from rag–1–/– blastocysts injected with FADD–/– embryonic stem cells have pro–T and pre–T cells at birth, although overall thymic cellularity is reduced, but by 5 weeks of age the mice have virtually no pre–T cells (Zhang et al., 1998). However, in this system it is impossible to deduce whether the defect in T–cell production is cell autonomous or due to FADD deficiency in the thymic microenvironment.
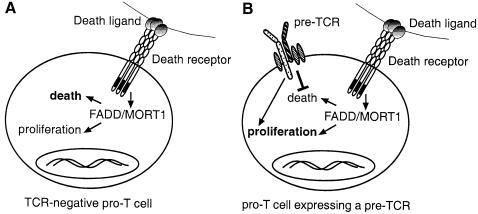
Death receptors can signal proliferation in certain settings (Ashkenazi and Dixit, 1998), so we postulate that death receptors use FADD to regulate proliferation as well as survival of pro–T and pre–T cells. A model of how death receptors and the pre–TCR might interact to determine whether a pro–T cell dies, or alternatively, survives, divides and differentiates is shown in Figure 7. In this model, pro–T3 cells express death receptors that are engaged continuously by ligands on neighbouring stromal cells or thymocytes. Signals transduced in the absence of a pre–TCR lead to apoptosis. One function of the pre–TCR is to block apoptosis signalling by death receptors, thereby allowing death receptor signalling pathways that contribute to pro–T4 and pre–T–cell production to have an impact. Like mature T–cell proliferation, which requires TCR activation plus co-stimulatory signals, efficient pro–T–cell proliferation may require signals from the pre–TCR and from death receptors. Since pro–T4 and pre–T cells develop spontaneously in FADD–DN rag–1–/– mice (Figure 2), neither FADD nor the pre–TCR appear to be essential for differentiation. Sustained pro–T–cell survival may be sufficient for implementation of the differentiation programme, although pre–TCR- and FADD-independent signals might also be involved.
Fig. 7. Model for death receptor-controlled apoptosis and cell production at the pre–TCR checkpoint. Death receptors on CD4–8–25+44– pro–T3 cells can signal apoptosis or proliferation depending on the presence or absence of signals from the pre–TCR. (A) In the absence of a signal from the pre–TCR, such as in cells with non-productive TCRβ gene rearrangements, pro–T3 cells die by apoptosis. (B) Expression of a pre–TCR blocks the apoptotic signal from death receptors, thereby allowing cell proliferation and differentiation. Efficient pro–T4 and pre–T–cell generation probably requires signals from death receptors and the pre–TCR.
p53–/– rag–1–/– and FADD–DN rag–1–/– mice have remarkably similar thymi (Nacht and Jacks, 1998). Mice of both strains show large variation in pre–T–cell numbers (Figure 2). There are two possible links between p53 and death receptors. p53 might signal apoptosis downstream of death receptors, but this seems unlikely because Fas-induced apoptosis is normal in p53–/– lymphocytes (Boehme and Lenardo, 1996; O'Connor and Strasser, 1999). Furthermore, p53-induced cell death can be blocked by Bcl-2 (Strasser et al., 1994b), which is a poor inhibitor of death receptor-induced apoptosis (Vanhaesebroeck et al., 1993; Strasser et al., 1995). We prefer the notion that pro–T cells require p53 for expression of a critical death receptor, ligand or signalling molecule. For example, it was reported that DR5 gene expression is induced by p53 (Wu et al., 1999). The death receptors, ligands and signalling molecules that were expressed in wild-type pro–T cells (Figure 1) were also detected in pro–T cells from p53–/– mice (data not shown). These results could indicate that p53 regulates expression of a yet to be characterized death receptor or ligand that uses FADD to trigger apoptosis in pre–TCR-deficient pro–T3 cells.
The role of FADD in suppressing tumorigenesis
The spontaneous development of thymic lymphomas in FADD–DN rag–1–/– mice (Figure 5) underscores the importance of abnormalities in cell death control in neoplasia (Strasser et al., 1997), and demonstrates for the first time that FADD has a tumour suppressive function. Although Fas deficiency can elicit plasmacytoid tumours, particularly when mice also lack T cells (Peng et al., 1996; Davidson et al., 1998), the lpr rag–1–/– mice in our study did not develop tumours (Figure 5). Therefore, Fas is not the only death receptor to suppress tumour development through FADD. Since both rag–1- and FADD-deficiency were necessary for tumorigenesis, there can be co-operation between mutations in cell death control and mutations that inhibit cell differentiation. Inhibition of FADD blocks apoptosis but also inhibits cell production, and in this regard resembles oncogenes that promote as well as inhibit cell growth. For instance, c-myc promotes cell cycle entry but predisposes cells to apoptosis when growth factors are limiting. Another example is bcl-2, which inhibits apoptosis but slows transition between the quiescent and cycling states (Evan and Littlewood, 1998). FADD therefore represents a tumour suppressor that can have positive and negative effects on cell growth.
Materials and methods
Mice
FADD–DN rag–1–/– and lpr rag–1–/– mice were obtained by crossing rag–1–/– mice (Maraskovsky et al., 1997) with lck-FADD–DN strain 64 mice (Newton et al., 1998) or lpr mice (Cohen and Eisenberg, 1993). All animals had a C57BL/6J genetic background. Genotyping of FADD–DN and lpr mice has been described (Singer and Abbas, 1994; Newton et al., 1998). rag–1–/– mice lack B220+IgM+ cells and were identified by flow cytometric analysis of peripheral blood cells stained with anti-IgM and anti-B220 antibodies. Mice at 5–13 weeks of age were injected intraperitoneally with 100 μg of protein G–Sepharose (Pharmacia) purified 145-2C11 hamster anti-mouse CD3ɛ antibody, or with the isotype-matched control antibody, 3F11 hamster anti-mouse Bcl-2. γ-irradiated mice received a dose of 5 Gy from a 60Co source. Dispersed cells (1–5 × 106) from organs suspected of containing tumour cells were transplanted into the peritoneal cavity of C57BL/6J mice. Transplant recipients were killed when unwell or after 6 months.
Immunofluorescence staining and flow cytometry
Thymocytes were stained with monoclonal antibodies diluted in 2.4G2 anti-mouse FcγRII hybridoma supernatant plus 1% rat serum. Antibodies RA3-6B2 anti-B220, KT3 anti-CD3, GK1.5 anti-CD4, H129.19 anti-CD4, YTS 169 anti-CD8, 53.6.72 anti-CD8, PC61 anti-CD25, 5.1 anti-IgM, 11-26C anti-IgD, T24.31.2 anti–Thy–1, IM781 anti-CD44, 8C5 anti-Gr–1, MI/70 anti-Mac–1 and Ter119 anti-erythroid cell surface marker were purified on protein G–Sepharose and conjugated with Cy5, fluorescein isothiocyanate (FITC) or biotin (Molecular Probes). Surface Fas expression was determined using Jo2 hamster anti-mouse Fas antibody (PharMingen) revealed by FITC-conjugated anti-hamster IgG antibodies (PharMingen). 6C8.28 hamster anti-human Bcl-2 antibody served as an isotype-matched control antibody. A total of 5000–10 000 viable cells [not stained by propidium iodide (PI)] were analysed in a FACScan (Becton Dickinson). Expression of FLAG-tagged FADD–DN protein was determined by cytoplasmic immunofluorescence staining (Newton et al., 1998).
Cell sorting
Wild-type and FADD–DN pro–T cells were isolated using a depletion step followed by cell sorting. Thymocytes were stained with antibodies to B220, CD3, CD4, CD8, Gr–1, IgM, IgD, Mac–1 and Ter119, and cells expressing these surface markers were removed using magnetic beads coated with anti-rat IgG antibodies (Paesel and Lorei). Depletion was never complete so contaminating cells were identified with R-PE-conjugated anti-rat IgG antibodies (Caltag). A separate staining was then performed with FITC-conjugated anti-CD44, Cy5-conjugated anti–Thy–1 and biotinylated anti-CD25 antibody. Rat serum (2%) was included in the antibody cocktail to absorb R-PE-conjugated anti-rat IgG antibodies left over from the previous stain. Biotinylated anti-CD25 antibody was revealed with TRI-COLOR streptavidin. Viable Cy5+FITC–PE–TRI+ pro–T3 and Cy5+FITC–PE–TRI– pro–T4 cells were sorted in a MoFlo sorter (Cytomation). CD4+8+ pre–T cells were sorted after staining with R-PE-conjugated H129.19 anti-CD4 (PharMingen) plus FITC-conjugated anti-CD8 antibodies. rag–1–/– and FADD–DN rag–1–/– thymocytes were stained with Cy5-conjugated anti–Thy–1, FITC-conjugated anti-CD4 plus anti-CD8, R-PE-conjugated PC61 anti-CD25 (Caltag) and biotinylated anti-CD44 antibodies. Biotinylated antibody was detected with TRI-COLOR streptavidin. Cy5+FITC–PE+TRI– pro–T3, Cy5+FITC–PE–TRI– pro–T4 and Cy5+FITC+PE–TRI– pre–T cells were sorted.
Cell cycle and cell survival analysis
Cells were fixed in 70% ethanol and stained in 38 mM sodium citrate (pH 7.4) that contained 69 μM PI and 5 μg/ml RNase A. A total of 10 000–20 000 cells were analysed in a FACScan and their cell cycle distribution was determined using ‘CellFit’ Software (Becton Dickinson). Thymoma-derived cell lines were cultured in the high-glucose version of Dulbecco's modified Eagle's medium supplemented with 13 μM folic acid, 250 μM l-asparagine, 50 μM 2-mercaptoethanol and 10% fetal calf serum (Biosciences). Cells were treated with 20 ng/ml soluble FLAG-tagged human FasL (Apotech) cross-linked with 1 μg/ml anti-FLAG monoclonal antibody (Sigma), 1 μM dexamethasone (Sigma) or 2.5 Gy of γ–radiation from a 60Co source. Cell viability was determined by flow cytometric analysis of PI-stained cells in a FACScan.
cDNA synthesis and RT–PCR
Total RNA was isolated from pro–T or pre–T cells using Trizol (Gibco-BRL). To obtain a population enriched for thymic stromal cells, thymocytes were squeezed from the thymus with forceps and RNA was extracted from the shell that remained. RNA samples were treated with DNase I (Boehringer Mannheim) and oligo(dT)-primed first-strand cDNA synthesis was performed using AMV reverse transcriptase (Promega). PCR amplification was performed using the following primers.
CD3ɛ: CTCCTAGCTGTTGGCACTTG; GCTCCTTGTTTTGCCCTCTG; Fas: GCTCACAGTTAAGAGTTCATACTCAAGG; AAGTGGAATTAACAAAACAAGGATG;
FasL: ATTAGGAATGTATCAGCTCTTCCACC; AGCACTGGTAAGATTGAATACTGCC;
DR3: AGAAGAGGTATGGCCCGTTTTGTT; GAGAGATGGGCAGTCTGTGGTGAG;
DR3L: TACCTTTCTTGGAACAACTAGTCCG; GAAAGAGTCCAAAGTAGGTTAGGAAGG;
DR5: GAGCCTCCAGGACCCAGCACGCC: CCCGTTCACAGCCTCTTTTTATAC;
FADD: CGCGAGCTGAAGGTGTCTG; CTGAGGAAGACACAGTTGAATC;
FLIP: TGTGTAGAGATGTGACTGAGAACCTG; TGGATCTTTGTGTTCAAGTCTATTCTG;
HPRT: GCTGGTGAAAAGGACCTCT; CACAGGACTAGAACACCTGC;
TNFα: CCTATGTCTCAGCCTCTTCTCATTC; GTTGACTTTCTCCTGGTATGAGATAGC;
TNF-R1: AAGGAACCTACTTGGTGAGTGACTG; GTACTGGAGACAGGAGAACTAAAGCC;
TNF-R2: CCAATATGTGAAACATTTCTGCAAC; AACAATCAGACCAATTGGAAGAGAG;
TRADD: CAGGGTGGAGCCATACAGGTAG; GCTAGACTAGTGAGTTCGTTCTC;
TRAIL: TTACTCCAAAATTGGACTAGCTTGC; ACTTGTAGATGTACTGCACCAGCTG.
Five-fold serial dilutions of each sample were amplified in 20 μl using 30 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 1 min. Reactions contained 200 μM dNTPs, 1.5 mM MgCl2, 0.5 μM primers and 1.25 U of Taq polymerase (Boehringer Mannheim). PCR products resolved by agarose gel electrophoresis were transferred to a nitrocellulose filter by Southern blotting and detected with the following 32P-labelled oligonucleotides.
CD3ɛ: ACGTCTGCTACACACCAGCCTCAA;
Fas: CATGGCTCAAGGGTTCCATGTTCACACG;
FasL: TGGTTGTTGCAAGACTGACCCCGGAAGT;
DR3: TGACCAGAGACAACCACTTTAAGACTGACT;
DR3L: AGCCTTAAGATGAGCCCAGGGGAGGGTG;
DR5: TATTCTCTGCACAGTCTGTAAGGAAG;
FADD: CTTCTCAGCATTCTTCCAGACTTTC;
FLIP: CAGACAAAGCAACCGTGGAGGACCACCT;
HPRT: GGATACAGGCCAGACTTTGT;
TNFα: GTAGTCGGGGCAGCCTTGTCCCTTGAAG;
TNF-R1: GGCAACAGCACCGCAGTACCTGAGTCCT;
TNF-R2: GATGGCACTTAGAGTTGGGGACTCGGGC;
TRADD: CAGACGTTTGCGCGCTCGGTGGGTC;
TRAIL: GCCCTTTCCGAGAGGACTCCCAGGATTC.
Western blots
Cell lysates were prepared in RIPA buffer [phosphate-buffered saline (PBS), 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS] containing 2 μM pefabloc, 1 μg/ml aprotinin and 1 μg/ml leupeptin. Proteins resolved by SDS–PAGE were transferred to nitrocellulose membranes by electroblotting and non-specific binding sites were blocked in PBS containing 5% skim milk and 0.1% Tween-20. Membranes were incubated with 1 μg/ml of PAB 296 and PAB 481 anti-wild-type p53 antibodies or N27 anti-HSP70 antibody (ascites fluid diluted 1:10 000). Bound antibody was detected with peroxidase-conjugated, sheep anti-mouse Ig antibodies (Silenus) and ECL reagent (Amersham).
Acknowledgments
Acknowledgements
We thank A.Elefanty for reagents and advice on RT–PCR, M.Degli-Esposti for mouse DR3 sequence, L.Corcoran for rag–1–/– mice and J.Tschopp for FasL. We are grateful to A.Naughton, E.Shomali, K.Gray and A.Mifsud for animal husbandry, F.Battye, D.Kaminaris, V.Lapatis and J.Parker for operating the MoFlo, J.Birtles for editorial assistance, and K.Shortman, W.Heath and L.Wu for review of the manuscript. K.N. has a Melbourne Research Scholarship and A.S. is a Scholar of the Leukemia Society of America and a recipient of a Clinical Investigator Award from the Cancer Research Institute (New York). This work was supported by grants from the NHMRC (Canberra), the Dr Josef Steiner Cancer Foundation (Bern) and the Anti-Cancer Council of Victoria (Melbourne).
References
- Akashi K., Kondo, M., von Freeden-Jeffry, U., Murray, R. and Weissman, I.L. (1997) Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell, 89, 1033–1041. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A. and Dixit, V.M. (1998) Death receptors: signaling and modulation. Science, 281, 1305–1308. [DOI] [PubMed] [Google Scholar]
- Boehme S.A. and Lenardo, M.J. (1996) TCR-mediated death of mature T lymphocytes occurs in the absence of p53. J. Immunol., 156, 4075–4078. [PubMed] [Google Scholar]
- Bogue M.A., Zhu, C., Aguilar-Cordova, E., Donehower, L.A. and Roth, D.B. (1996) p53 is required for both radiation-induced differentiation and rescue of V(D)J rearrangement in scid mouse thymocytes. Genes Dev., 10, 553–565. [DOI] [PubMed] [Google Scholar]
- Boldin M.P., Varfolomeev, E.E., Pancer, Z., Mett, I.L., Camonis, J.H. and Wallach, D. (1995) A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J. Biol. Chem., 270, 7795–7798. [DOI] [PubMed] [Google Scholar]
- Boldin M.P., Goncharov, T.M., Goltsev, Y.V. and Wallach, D. (1996) Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO–1- and TNF receptor-induced cell death. Cell, 85, 803–815. [DOI] [PubMed] [Google Scholar]
- Bosma M.J. and Carroll, A.M. (1991) The scid mouse mutant: definition, characterization and potential uses. Annu. Rev. Immunol., 9, 323–350. [DOI] [PubMed] [Google Scholar]
- Capone M., Hockett, R.D., Jr and Zlotnik, A. (1998) Kinetics of T cell receptor β, γ and δ rearrangements during adult thymic development: T cell receptor rearrangements are present in CD44+CD25+ Pro–T thymocytes. Proc. Natl Acad. Sci. USA, 95, 12522–12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnaiyan A.M., O'Rourke, K., Tewari, M. and Dixit, V.M. (1995) FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell, 81, 505–512. [DOI] [PubMed] [Google Scholar]
- Cohen P.L. and Eisenberg, R.A. (1993) The lpr and gld genes in systemic autoimmunity: life and death in the Fas lane. Immunol. Today, 13, 427–428. [DOI] [PubMed] [Google Scholar]
- Danska J.S., Pflumio, F., Williams, C.J., Huner, O., Dick, J.E. and Guidos, C.J. (1994) Rescue of T cell-specific V(D)J recombination in SCID mice by DNA-damaging agents. Science, 266, 450–455. [DOI] [PubMed] [Google Scholar]
- Davidson W.F., Giese, T. and Fredrickson, T.N. (1998) Spontaneous development of plasmacytoid tumors in mice with defective Fas–Fas ligand interactions. J. Exp. Med., 187, 1825–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. and Littlewood, T. (1998) A matter of life and cell death. Science, 281, 1317–1321. [DOI] [PubMed] [Google Scholar]
- Fehling H.J., Krotkova, A., Saint-Ruf, C. and von Boehmer, H. (1995) Crucial role of the pre–T–cell receptor α gene in development of α-β but not γ-δ T cells. Nature, 375, 795–798. [DOI] [PubMed] [Google Scholar]
- Fehling H.J., Iritani, B.M., Krotkova, A., Forbush, K.A., Laplace, C., Perlmutter, R.M. and von Boehmer, H. (1997) Restoration of thymopoiesis in pTα–/– mice by anti-CD3e antibody treatment or with transgenes encoding activated Lck or Tailless pTα. Immunity, 6, 703–714. [DOI] [PubMed] [Google Scholar]
- Godfrey D.I. and Zlotnik, A. (1993) Control points in early T–cell development. Immunol. Today, 14, 547–553. [DOI] [PubMed] [Google Scholar]
- Groettrup M., Ungewiss, K., Azogui, O., Palacios, R., Owen, M.J., Hayday, A.C. and von Boehmer, H. (1993) A novel disulfide-linked heterodimer on pre–T cells consists of the T cell receptor β chain and a 33 kd glycoprotein. Cell, 75, 283–294. [DOI] [PubMed] [Google Scholar]
- Guidos C.J., Williams, C.J., Wu, G.E., Paige, C.J. and Danska, J.S. (1995) Development of CD4+CD8+ thymocytes in RAG-deficient mice through a T cell receptor β chain-independent pathway. J. Exp. Med., 181, 1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidos C.J., Williams, C.J., Grandal, I., Knowles, G., Huang, M.T.F. and Danska, J.S. (1996) V(D)J recombination activates a p53-dependent DNA damage checkpoint in scid lymphocyte precursors. Genes Dev., 10, 2038–2054. [DOI] [PubMed] [Google Scholar]
- Habu S., Kimura, M., Katsuki, M., Hioki, K. and Nomura, T. (1987) Correlation of T cell receptor gene rearrangements to T cell surface antigen expression and to serum immunoglobulin level in scid mice. Eur. J. Immunol., 17, 1467–1471. [DOI] [PubMed] [Google Scholar]
- Haks M.C., Krimpenfort, P., van den Brakel, J.H.N. and Kruisbeek, A.M. (1999) Pre–TCR signaling and inactivation of p53 induces crucial cell survival pathways in pre–T cells. Immunity, 11, 91–101. [DOI] [PubMed] [Google Scholar]
- Hoffman E.S., Passoni, L., Crompton, T., Leu, T.M.J., Schatz, D.G., Koff, A., Owen, M.J. and Hayday, A.C. (1996) Productive T–cell receptor β-chain gene rearrangement: coincident regulation of cell cycle and clonality during development in vivo. Genes Dev., 10, 948–962. [DOI] [PubMed] [Google Scholar]
- Hsu H., Xiong, J. and Goeddel, D.V. (1995) The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell, 81, 495–504. [DOI] [PubMed] [Google Scholar]
- Irmler M., et al. (1997) Inhibition of death receptor signals by cellular FLIP. Nature, 388, 190–194. [DOI] [PubMed] [Google Scholar]
- Jacobs H., Vandeputte, D., Tolkamp, L., de Vries, E., Borst, J. and Berns, A. (1994) CD3 components at the surface of pro–T cells can mediate pre–T cell development in vivo. Eur. J. Immunol., 24, 934–939. [DOI] [PubMed] [Google Scholar]
- Jiang D., Lenardo, M.J. and Zúñiga-Pflücker, J.C. (1996) p53 prevents maturation to the CD4+CD8+ stage of thymocyte differentiation in the absence of T cell receptor rearrangement. J. Exp. Med., 183, 1923–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt C.N., Mombaerts, P., Iglesias, A., Tonegawa, S. and Eichmann, K. (1993) Restoration of early thymocyte differentiation in T–cell receptor β-chain-deficient mutant mice by transmembrane signaling through CD3ɛ. Proc. Natl Acad. Sci. USA, 90, 11401–11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen M., Gillet, A., Ardouin, L., Bouvier, G., Trucy, J., Ferrier, P., Vivier, E. and Malissen, B. (1995) Altered T cell development in mice with a targeted mutation of the CD3-ɛ gene. EMBO J., 14, 4641–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraskovsky E., O'Reilly, L.A., Teepe, M., Corcoran, L.M., Peschon, J.J. and Strasser, A. (1997) Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag–1–/– mice. Cell, 89, 1011–1019. [DOI] [PubMed] [Google Scholar]
- Martin D.A., Siegel, R.M., Zheng, L. and Lenardo, M.J. (1998) Membrane oligomerization and cleavage activates the caspase-8 (FLICE/MACHα1) death signal. J. Biol. Chem., 273, 4345–4349. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini, J., Johnson, R.S., Herrup, K., Tonegawa, S. and Papaioannou, V.E. (1992a) RAG–1-deficient mice have no mature B and T lymphocytes. Cell, 68, 869–877. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., et al. (1992b) Mutations in T–cell antigen receptor genes α and β block thymocyte development at different stages. Nature, 360, 225–231. [DOI] [PubMed] [Google Scholar]
- Murphy W.J., et al. (1994) Induction of T cell differentiation and lymphomagenesis in the thymus of mice with severe combined immune deficiency (SCID). J. Immunol., 153, 1004–1014. [PubMed] [Google Scholar]
- Muzio M., et al. (1996) FLICE, a novel FADD homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/Apo–1) death-inducing signaling complex. Cell, 85, 817–827. [DOI] [PubMed] [Google Scholar]
- Muzio M., Stockwell, B.R., Stennicke, H.R., Salvesen, G.S. and Dixit, V.M. (1998) An induced proximity model for caspase-8 activation. J. Biol. Chem., 273, 2926–2930. [DOI] [PubMed] [Google Scholar]
- Nacht M. and Jacks, T. (1998) V(D)J recombination is not required for the development of lymphoma in p53-deficient mice. Cell Growth Diff., 9, 131–138. [PubMed] [Google Scholar]
- Nacht M., Strasser, A., Chan, Y.R., Harris, A.W., Schlissel, M., Bronson, R.T. and Jacks, T. (1996) Mutations in the p53 and SCID genes cooperate in tumorigenesis. Genes Dev., 10, 2055–2066. [DOI] [PubMed] [Google Scholar]
- Nagata S. and Golstein, P. (1995) The Fas death factor. Science, 267, 1449–1456. [DOI] [PubMed] [Google Scholar]
- Newton K., Harris, A.W., Bath, M.L., Smith, K.G.C. and Strasser, A. (1998) A dominant interfering mutant of FADD/Mort1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J., 17, 706–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Ishii, A., Kobayashi, Y., Yamasaki, Y. and Yonehara, S. (1995) Expression and function of mouse Fas antigen on immature and mature T cells. J. Immunol., 154, 4395–4403. [PubMed] [Google Scholar]
- O'Connor L. and Strasser, A. (1999) Fas, p53 and apoptosis. Science, 284, 1430. [Google Scholar]
- Ogasawara J., Suda, T. and Nagata, S. (1995) Selective apoptosis of CD4+CD8+ thymocytes by the anti-Fas antibody. J. Exp. Med., 181, 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S.L., Robert, M.E., Hayday, A.C. and Craft, J. (1996) A tumor-suppressor function for Fas (CD95) revealed in T cell-deficient mice. J. Exp. Med., 184, 1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penit C., Lucas, B. and Vasseur, F. (1995) Cell expansion and growth arrest phases during the transition from precursor (CD4–8–) to immature (CD4+8+) thymocytes in normal and genetically modified mice. J. Immunol., 154, 5103–5113. [PubMed] [Google Scholar]
- Petrie H.T., Livak, F., Schatz, D.G., Strasser, A., Crispe, N.I. and Shortman, K. (1993) Multiple rearangements in TCR-α chain genes maximize the production of useful thymocytes. J. Exp. Med., 178, 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald H.-R. and Fehling, H.J. (1998) Molecular and cellular events in early thymocyte development. Adv. Immunol., 69, 1–112. [DOI] [PubMed] [Google Scholar]
- Shinkai Y. and Alt, F.W. (1994) CD3 ɛ-mediated signals rescue the development of CD4+CD8+ thymocytes in RAG-2–/– mice in the absence of TCR β chain expression. Int. Immunol., 6, 995–1001. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., et al. (1992) RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangements. Cell, 68, 855–867. [DOI] [PubMed] [Google Scholar]
- Shores E.W., Sharrow, S.O., Uppenkamp, I. and Singer, A. (1990) T cell receptor-negative thymocytes from SCID mice can be induced to enter the CD4/CD8 differentiation pathway. Eur. J. Immunol., 20, 69–77. [DOI] [PubMed] [Google Scholar]
- Singer G.G. and Abbas, A.K. (1994) The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity, 1, 365–371. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris, A.W., Corcoran, L.M. and Cory, S. (1994a) bcl-2 expression promotes B but not T lymphoid development in scid mice. Nature, 368, 457–460. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris, A.W., Jacks, T. and Cory, S. (1994b) DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell, 79, 329–339. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris, A.W., Huang, D.C.S., Krammer, P.H. and Cory, S. (1995) Bcl-2 and Fas/APO–1 regulate distinct pathways to lymphocyte apoptosis. EMBO J., 14, 6136–6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Huang, D.C.S. and Vaux, D.L. (1997) The role of the bcl-2/ced-9 gene family in cancer and general implications of defects in cell death control for tumourigenesis and resistance to chemotherapy. Biochim. Biophys. Acta, 1333, F151–F178. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B., Reed, J.C., de Valck, D., Grooten, J., Miyashita, T., Tanaka, S., Beyaert, R., van Roy, F. and Fiers, W. (1993) Effect of bcl–2 proto-oncogene expression on cellular sensitivity to tumor necrosis factor-mediated cytotoxicity. Oncogene, 8, 1075–1081. [PubMed] [Google Scholar]
- von Boehmer H. (1994) Positive selection of lymphocytes. Cell, 76, 219–228. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Aifantis, I., Feinberg, J., Lechner, O., Saint-Ruf, C., Walter, U., Buer, J. and Azogui, O. (1999) Pleiotropic changes controlled by the pre–T–cell receptor. Curr. Opin. Immunol., 11, 135–142. [DOI] [PubMed] [Google Scholar]
- Walsh C.M., Wen, B.G., Chinnaiyan, A.M., O'Rourke, K., Dixit, V.M. and Hedrick, S.M. (1998) A role for FADD in T cell activation and development. Immunity, 8, 439–449. [DOI] [PubMed] [Google Scholar]
- Wu G.S., Burns, T.F., Zhan, Y., Alnemri, E.S. and el-Deiry, W.S. (1999) Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res., 59, 2770–2775. [PubMed] [Google Scholar]
- Yang X., Chang, H.Y. and Baltimore, D. (1998) Autoproteolytic activation of pro-caspases by oligomerization. Mol. Cell, 1, 319–325. [DOI] [PubMed] [Google Scholar]
- Yasutomo K., Maeda, K.-I., Hisaeda, H., Good, R.A., Kuroda, Y. and Himeno, K. (1997) The Fas-deficient SCID mouse exhibits the development of T cells in the thymus. J. Immunol., 158, 4729–4733. [PubMed] [Google Scholar]
- Yeh W.C., et al. (1998) FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science, 279, 1954–1958. [DOI] [PubMed] [Google Scholar]
- Zhang J., Cado, D., Chen, A., Kabra, N.H. and Winoto, A. (1998) Fas-mediated apoptosis and activation-induced T–cell proliferation are defective in mice lacking FADD/Mort1. Nature, 392, 296–300. [DOI] [PubMed] [Google Scholar]
- Zúñiga-Pflücker J.C., Jiang, D., Schwartzberg, P.L. and Lenardo, M.J. (1994) Sublethal γ-radiation induces differentiation of CD4–/CD8– into CD4+/CD8+ thymocytes without T cell receptor β rearrangement in recombinase activation gene 2–/– mice. J. Exp. Med., 180, 1517–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]