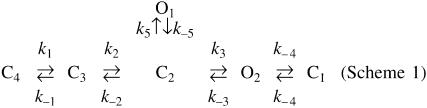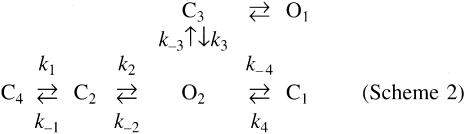Abstract
ATP-sensitive potassium (KATP) channels regulate insulin secretion, vascular tone, heart rate and neuronal excitability by responding to transmitters as well as the internal metabolic state. KATP channels are composed of four pore-forming α-subunits (Kir6.2) and four regulatory β-subunits, the sulfonylurea receptor (SUR1, SUR2A or SUR2B). Whereas protein kinase A (PKA) phosphorylation of serine 372 of Kir6.2 has been shown biochemically by others, we found that the phosphorylation of T224 rather than S372 of Kir6.2 underlies the catalytic subunits of PKA (c-PKA)- and the D1 dopamine receptor-mediated stimulation of KATP channels expressed in HEK293 cells. Specific changes in the kinetic properties of channels treated with c-PKA, as revealed by single-channel analysis, were mimicked by aspartate substitution of T224. The T224D mutation also reduced the sensitivity to ATP inhibition. Alteration of channel gating and a decrease in the apparent affinity for ATP inhibition thus underlie the positive regulation of KATP channels by PKA phosphorylation of T224 in Kir6.2, which may represent a general mechanism for KATP channel regulation in different tissues.
Keywords: phosphorylation/PKA/potassium channel/single-channel/transfection
Introduction
ATP-sensitive potassium (KATP) channels, widely distributed in the central and peripheral nervous system, pituitary, cardiac muscle, smooth muscle, skeletal muscle and pancreatic β-cells, serve a variety of cellular functions (Babenko et al., 1998). Not only are they modulated by transmitters, but KATP channels are inhibited by intracellular ATP and stimulated by Mg–ADP and thus couple cellular metabolic status to changes in transmembrane potassium fluxes and cellular excitability. In the brain, KATP channels contribute to the glucose-sensing mechanism involved in appetite control (Ashford et al., 1990). They also protect central neurons and cardiac myocytes under metabolic stress (Mercuri et al., 1994; Roeper and Ashcroft, 1995; Stanford and Lacey, 1995; Watts et al., 1995; Seutin et al., 1996; Isomoto et al., 1997; Nichols and Lopatin, 1997; Yokoshiki et al., 1998). In pancreatic β-cells, KATP channels regulate insulin secretion in response to glucose metabolism (Ashcroft and Rorsman, 1989). In smooth muscles, transmitter-mediated modulation of KATP channels controls vascular tone: vasodilators stimulate KATP channels whereas vasoconstrictors inhibit them (Ashcroft, 1996; Isomoto et al., 1997; Nichols and Lopatin, 1997; Quayle and Nelson, 1997; Seino et al., 1997; Babenko et al., 1998; Yokoshiki et al., 1998). Indeed, KATP channels have become the therapeutic targets for a variety of diseases including angina, hypertension and diabetes (Nichols and Lopatin, 1997).
The KATP channel is an octamer (Clement et al., 1997; Inagaki et al., 1997; Shyng and Nichols, 1997; Babenko et al., 1998). It is composed of four pore-forming α–subunits (Kir6.2) (Inagaki et al., 1995; Sakura et al., 1995) and four regulatory β-subunits (SUR1 or SUR2) (Aguilar-Bryan et al., 1995; Inagaki et al., 1996; Isomoto et al., 1996). Partial complexes with fewer than eight subunits do not reach the cell membrane because of the exposure of an endoplasmic reticulum (ER) retention/retrieval signal that is present in each subunit (Zerangue et al., 1999). Deleting the last 36 amino acids containing this ER retention/retrieval signal therefore permits functional expression of Kir6.2ΔC36 channels in the absence of SUR (Tucker et al., 1997).
Native KATP channels in different tissues comprise the same Kir6.2 (α) subunits, which form a weakly inwardly rectifying potassium channel, but different SUR (β) subunits. KATP channels in pancreatic β-cells and some central neurons contain Kir6.2 and SUR1 (Inagaki et al., 1995; Aguilar-Bryan et al., 1998). Mutations of either Kir6.2 or SUR1 cause persistent hyperinsulinemic hypoglycemia of infancy (PHHI), a disease associated with unregulated insulin secretion (Thomas et al., 1995). Cardiac and skeletal muscle KATP channels are composed of Kir6.2 and SUR2A (Inagaki et al., 1996; Okuyama et al., 1998). KATP channels in smooth muscle and certain central neurons in the substantia nigra (SN) comprise Kir6.2 and SUR2B instead (Isomoto et al., 1996; Yamada et al., 1997; Liss et al., 1999a). The sites mediating ATP inhibition are located in the Kir6.2 subunits, but SUR may modulate the ATP sensitivity further (Nichols et al., 1996; Gribble et al., 1997; Shyng et al., 1997b; Trapp et al., 1997; Tucker et al., 1997; John et al., 1998). Moreover, the SUR subunits, members of the ATP-binding cassette (ABC) superfamily, mediate channel inhibition by sulfonylurea drugs and stimulation by Mg–ADP and potassium channel openers (KCOs) (Ashcroft, 1996; Isomoto et al., 1997; Nichols and Lopatin, 1997; Seino et al., 1997; Tucker et al., 1997; Babenko et al., 1998). Compared with SUR2A- or SUR2B-containing KATP channels, SUR1-containing KATP channels exhibit higher affinity for sulfonylurea drugs such as tolbutamide (Isomoto and Kurachi, 1997; Babenbo et al., 1998; Gribble et al., 1998) and higher sensitivity to metabolic stress (Ashcroft and Gribble, 1998; Liss et al., 1999a).
Protein kinases play important roles in the physiological regulation of KATP channels, typically by mediating the effects of transmitters. The induction of ischemic pre-conditioning in the heart may involve activation of KATP channels via protein kinase C (PKC) (Lawson and Downey, 1993; Light et al., 1995; Light, 1996). Vasodilators and vasoconstrictors modulate smooth muscle KATP via protein kinase A (PKA) (Quayle et al., 1994) and PKC (Hatakeyama et al., 1995), respectively. Vasodilatation of the renal artery may be mediated by activation of the D1 dopamine receptor (D1R) (Goldberg et al., 1978) that is coupled to G-αs and stimulates adenylate cyclase and hence PKA (Missale et al., 1988). Whether the D1R action is due to KATP channel activation has not been determined. In the mammalian brain, D1R is widely distributed (Civelli et al., 1993) and participates in a number of dopamine-mediated functions, including locomotor activity, cognition, emotion, positive reinforcement, food intake and endocrine regulation (Missale et al., 1998). Dopaminergic neurons as well as γ-aminobutyric acid (GABA)ergic neurons in the SN express not only D1-like receptors but also KATP channels (Gerfen et al., 1990; Le Moine et al., 1991; Nicola et al., 1996; Liss et al., 1999a). In the weaver mouse, a model for neurodegeneration, the surviving subpopulation of SN dopaminergic neurons contain Kir6.2 and SUR1 exclusively, in contrast to wild-type dopaminergic neurons, which express Kir6.2, SUR1 and/or SUR2B (Liss et al., 1999a,b). It is hence suggested that KATP channels may confer resistance to neurodegeneration in the SN, origin of the nigrostriatal dopaminergic system. Whether D1R can activate KATP channels in these and other central neurons is therefore an interesting open question.
To determine whether D1R stimulates KATP channels and whether such stimulation involves PKA phosphorylation of KATP channels, and to identify the channel subunit(s) and the specific residue(s) responsible for such PKA modulation of KATP channels, it is necessary to use heterologous expression systems. By examining HEK293 cells transiently transfected with various combinations of mutant or wild-type KATP channel subunits and D1R, we found that D1R activation by dopamine caused an increase in KATP channel activities. Moreover, application of c-PKA, the catalytic subunit of PKA, to the cytoplasmic side of inside-out membrane patches also stimulated KATP channels. To test whether the positive c-PKA effect is mediated by phosphorylation of Kir6.2, we expressed Kir6.2ΔC36 alone without SUR1. Indeed, these tetrameric channels were stimulated by c-PKA. The involvement of SUR1 or SUR2A in channel stimulation by PKA appears to be negligible, since c-PKA failed to stimulate KATP channels containing mutant Kir6.2 devoid of PKA consensus sites. The Kir6.2 sequence contains two putative phosphorylation sites: T224 and S372. Only the former site is present in Kir6.2ΔC36 channels. Mutation of a single residue in Kir6.2, T224, abolished channel stimulation by either c-PKA or D1R, indicating that D1R activates KATP channels via PKA.
A recent biochemical study has shown persistent PKA phosphorylation of S372 but not T224 of Kir6.2 (Béguin et al., 1999). We found, however, that T224 rather than S372 was necessary for PKA stimulation of channel activities: mutations of T224 but not S372 abolished the ability of c-PKA and D1R to enhance channel activities. Moreover, the T224D mutation mimicked c-PKA-mediated channel activation by increasing the opening frequency and decreasing the mean closed duration of the Kir6.2ΔC36 channel. The elevated activity of the T224D mutant was persistent, in contrast to the transient stimulation of wild-type channels following a brief application of c-PKA, making it feasible to determine whether the presence of a negatively charged group at position 224 of Kir6.2 affects the ATP inhibition of the channel. The physiological significance of PKA phosphorylation affecting KATP channel gating and ATP sensitivity, as well as the intriguingly different effects of PKA phosphorylation of T224 and S372 of Kir6.2, will be discussed.
Results
c-PKA enhanced the single-channel currents of Kir6.2ΔC36 channels in transiently transfected HEK293 cells
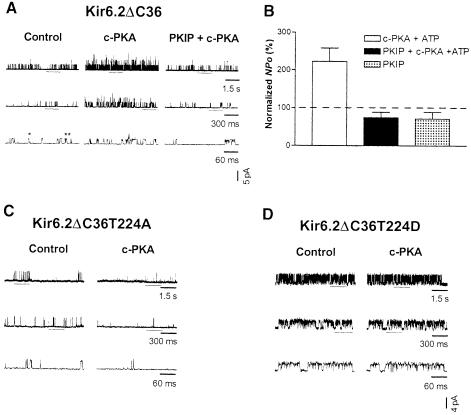
The KATP channels in different tissues differ in their SUR subunits (e.g. SUR1 in pancreas, SUR2A in myocardium, SUR2B in smooth muscle and SUR1 or SUR2 isoforms in brain), but share the same inwardly rectifying channel subunit, Kir6.2 (Karschin et al., 1997). Thus, we first examined the PKA effects on Kir6.2 channels. The expression of full-length Kir6.2 does not result in functional channels in the absence of SUR due to the ER retention/retrieval signal in the C–terminus of Kir6.2 (Zerangue et al., 1999). Truncation of the last 36 amino acids removes the retention signal, thereby allowing functional expression of Kir6.2ΔC36 channels in the absence of SUR (Tucker et al., 1997). We therefore expressed the Kir6.2ΔC36 channels in HEK293 cells by transient transfection. Channel activities were recorded from excised, inside-out patches that were exposed to 140 mM potassium solutions on both sides and voltage clamped at –60 mV. Single-channel openings, most frequently at a conductance level of ∼70 pS (chord conductance between –30 and –100 mV), appear as upward deflections at –60 mV (Figure 1). At increasing temporal resolution (Figure 1A, control, top to bottom), single-channel openings of Kir6.2ΔC36 channels exposed to ATP-free solutions could be resolved into singular openings (*) and bursts of openings (**) that were separated by closures shorter than a critical time (burst terminator) determined in individual patches (see Materials and methods). After recording the basal activity (Figure 1A, control), 30 μl or less of c-PKA (50 μg/ml) and Mg–ATP (1 mM) were applied to the patch through a glass capillary by pressure ejection (see Materials and methods). The c-PKA application lasted for 30–60 s; subsequent mixing with the 2 ml solution in the bath would reduce the c-PKA concentration to <25 U/ml. The apparent opening frequency was increased during c-PKA application (Figure 1A, c-PKA), and gradually reverted to control levels in a few minutes. This c-PKA effect was reproducible; a second application of c-PKA caused a similar stimulation (data not shown). The increase in the apparent opening frequency resulted in simultaneous openings of 2–3 channels (Figure 1A, c-PKA), which were only observed infrequently prior to c-PKA application (Figure 1A, control). The conductance level was not affected by c-PKA (Figure 1A). When the inhibitory peptide specific for PKA, PKIP (200 μg/ml), was applied together with c-PKA (50 μg/ml) and Mg–ATP (1 mM), there was no effect on single-channel conductance or opening frequency (Figure 1A, PKIP + c-PKA). As shown in Figure 1B, the normalized open probability (NPo) during c-PKA application was increased to 222.3 ± 35.2% (mean ± SEM, 12 patches; Table I, p <0.05, paired t–test), whereas the application of PKIP plus c-PKA and Mg–ATP (75.0 ± 14.1%, six patches) or PKIP alone (71.9 ± 17.6%, four patches) failed to increase channel activity (not significantly different, paired t-test). c-PKA also exerted significant effects on other single-channel properties of the channel, such as opening frequency, mean closed duration and bursting frequency (Table I, details described later in the text). Because these effects were abolished when PKIP was applied with c-PKA, the channel stimulation is most probably due to PKA phosphorylation rather than to a non-specific effect of the kinase.
Fig. 1. Enhancement of Kir6.2ΔC36 channel activity by PKA phosphorylation of residue T224. The (mouse) Kir6.2ΔC36 channel was expressed in HEK293 cells in the absence of SUR subunit. Recordings were performed in symmetrical potassium solutions at room temperature and voltage was clamped at –60 mV. (A) Single-channel current traces of a Kir6.2ΔC36 channel obtained from an inside-out patch prior to c-PKA treatment (left, control), during c-PKA (50 μg/ml) treatment (center, c-PKA) and during c-PKA plus PKIP (200 μg/ml) application (right). Upward deflections represent openings from closed states. No ATP was included in the bath solution. Segments of raw recordings underlined are shown in successive traces at increasing temporal resolution, revealing singular openings (*) and bursts of openings (**). (B) Normalized open probability (NPo) of Kir6.2ΔC36 channel currents obtained during application of c-PKA (open column), c-PKA plus PKIP (filled column) or PKIP alone (hatched column). ATP (1 mM) was included in the drug solutions. NPo was normalized to the corresponding control in an ATP-free bath (taken as 100%) in individual patches. Dotted lines indicate control levels. Data are presented as the mean ± SEM of 4–12 patches. (C) Single-channel current traces of a Kir6.2ΔC36T224A channel in an inside-out patch prior to and during c-PKA application illustrated at increasing time resolution. T224 is the only putative PKA phosphorylation site in the truncated Kir6.2 channel. Alanine was introduced to disrupt this PKA site. (D) Single-channel current traces of a Kir6.2ΔC36T224D channel in an inside-out patch before and during c-PKA treatment. Aspartate was introduced to mimic the charge effect of protein phosphorylation (Li et al., 1993).
Table I. Effects of c-PKA on normalized open, closed and burst properties of mouse Kir6.2ΔC36 channels expressed in acutely transfected HEK293 cells.
| Properties | Control | PKA | PKIP + PKA |
|---|---|---|---|
| Open probability (%) | 100 | 222.3 ± 35.2* | 75.0 ± 14.1 |
| Opening frequency (%) | 100 | 205.1 ± 30.8** | 81.7 ± 6.1 |
| Mean open duration (%) | 100 | 108.1 ± 2.5 | 86.7 ± 13.2 |
| Mean closed duration (%) | 100 | 58.3 ± 8.9** | 133.3 ± 17.0 |
| Mean burst duration (%) | 100 | 124.4 ± 13.8 | 82.2 ± 16.8 |
| Bursting frequency (%) | 100 | 176.9 ± 18.5* | 91.3 ± 5.7 |
| Opening per burst (%) | 100 | 113.7 ± 11.5 | 90.5 ± 8.4 |
Single-channel recordings of Kir6.2ΔC36 channels in inside-out patches were obtained at –60 mV in symmetrical 140 mM K+ solutions. Control recordings were obtained in an ATP-free bath prior to drug application. c-PKA (50 μg/ml, 12 patches) or c-PKA plus PKIP (200 μg/ml, six patches) was applied to the cytoplasmic surface of patches via pressure ejection. ATP (1 mM) was included in both drug solutions. Data were normalized to the corresponding controls obtained in individual patches (taken as 100%), averaged and are presented as the mean ± SEM (as a percentage). Significance levels are: *p <0.05; **p <0.01 (paired t-tests).
c-PKA failed to enhance single-channel currents when the T224 residue of Kir6.2ΔC36 channel was substituted with alanine
To test whether c-PKA increased Kir6.2ΔC36 channel function directly by phosphorylation of the channel protein, we substituted the only PKA consensus site of the Kir6.2ΔC36 channel, threonine (T) 224, with alanine (A). The single-channel conductance level of Kir6.2ΔC36T224A was similar to that of Kir6.2ΔC36. However, unlike with Kir6.2ΔC36, treatment with c-PKA (50 μg/ml) in the presence of Mg–ATP (1 mM) did not stimulate the single-channel currents of Kir6.2ΔC36T224A (Figure 1C, control versus c-PKA). The averaged absolute NPo of Kir6.2ΔC36T224A was 0.8 ± 0.3% in ATP-free control and 0.3 ± 0.2% during application of c-PKA and Mg–ATP (two patches, Table II). In the absence of SUR1, Kir6.2ΔC36 channels are inhibited by millimolar ATP (Tucker et al., 1997). The modest reduction in the apparent opening frequency and increase in the mean closed duration of Kir6.2ΔC36T224A during c-PKA application (Table II) were probably due to the ability of ATP, which was applied together with c-PKA, to inhibit the channel. The abolition of a positive c-PKA effect by T224A mutation indicates that T224 is the phosphorylation site through which PKA modulates Kir6.2ΔC36 channel function.
Table II. Abolition of c-PKA effects on the single-channel kinetic properties of Kir6.2ΔC36T224A and Kir6.2ΔC36T224D channels.
| Properties | Control | c-PKA |
|---|---|---|
| Kir6.2ΔC36T224A | ||
| Open probability (%) | 0.8 ± 0.3 | 0.3 ± 0.2 |
| Opening frequency (s–1) | 8.4 ± 0.7 | 4.3 ± 2.6 |
| Mean open duration (ms) | 0.9 ± 0.3 | 0.4 ± 0.1 |
| Mean closed duration (ms) | 112.1 ± 13.7 | 404.6 ± 249.8 |
| Kir6.2ΔC36T224D | ||
| Open probability (%) | 37.5 ± 18.8 | 23.9 ± 15.6 |
| Opening frequency (s–1) | 125.8 ± 58.2 | 74.4 ± 48.9 |
| Mean open duration (ms) | 2.3 ± 0.5 | 2.3 ± 0.6 |
| Mean closed duration (ms) | 29.3 ± 23.2 | 71.2 ± 45.0 |
Recordings were performed at –60 mV in symmetrical K+ solutions. Single-channel open and closed properties were determined from records obtained before (control) and during c-PKA (50 μg/ml) application in patches expressing Kir6.2ΔC36T224A (two patches) or Kir6.2ΔC36T224D (four patches). ATP (1 mM) was included in the drug solution. Data were averaged and are presented as the mean ± SEM. Paired t-tests of data pairs (control and c-PKA-treated) obtained in the same patch revealed no significant differences.
Aspartate substitution of the T224 residue in Kir6.2ΔC36 increased basal single-channel activity, but eliminated the c-PKA effects
To mimic the effect of phosphorylation, we used aspartate (D) to replace T224. Compared with wild-type channels, Kir6.2ΔC36T224D exhibited similar single-channel conductance, but increased channel activity (Figure 1D). Significant differences were detected for open probability, opening frequency and mean closed duration between Kir6.2ΔC36 and Kir6.2ΔC36T224D channels (Table III). In addition to these parameters, the mean open duration was also significantly different between Kir6.2ΔC36T224A and Kir6.2ΔC36T224D channels (Table III). Treatment with c-PKA and Mg–ATP did not increase further the single-channel currents of Kir6.2ΔC36T224D channels (Figure 1D, control versus c–PKA; Table II, paired t-test); the averaged NPo of Kir6.2ΔC36T224D during application of c-PKA and Mg–ATP was 53.9 ± 19.2% of control (four patches). The inability of c-PKA treatment to stimulate the T224D mutant channels indicates again that the T224 residue is responsible for the c-PKA-mediated modulation of Kir6.2ΔC36 channel function. Furthermore, the T224D mutation mimicked c-PKA stimulation of Kir6.2ΔC36 by increasing the opening frequency as well as reducing the mean closed duration (Tables I and III), indicating that the PKA stimulatory effect arises from the attachment of a negatively charged group to the residue at position 224.
Table III. Changes in basal single-channel properties by aspartate or alanine substitution at T224 of the Kir6.2 subunit.
| Properties | Kir6.2ΔC36 | Kir6.2ΔC36T224A | Kir6.2ΔC36T224D |
|---|---|---|---|
| Open probability (%) | 3.83 ± 0.91a | 0.59 ± 0.13b | 31.59 ± 15.73 |
| Opening frequency (s–1) | 22.63 ± 3.53a | 4.73 ± 1.07c | 108.20 ± 48.36 |
| Mean open duration (ms) | 1.47 ± 0.11 | 1.29 ± 0.30d | 2.26 ± 0.41 |
| Mean closed duration (ms) | 78.39 ± 20.39e | 339.10 ± 114.40b | 28.36 ± 17.98 |
| No. of openings | 70 938 | 2137 | 33 620 |
| No. of patches | 21 | 7 | 5 |
Recordings were made on inside-out patches in an ATP-free bath at –60 mV. Comparisons were made by performing one-way analysis of variance (ANOVA) on data obtained from Kir6.2ΔC36, Kir6.2ΔC36T224A and Kir6.2ΔC36T224D channels, followed by Bonferroni's multiple comparison tests. Data are presented as the mean ± SEM. Significance levels are: aKir6.2ΔC36 versus Kir6.2ΔC36T224D (p <0.001); bKir6.2ΔC36T224A versus Kir6.2ΔC36T224D (p <0.01); cKir6.2ΔC36T224A versus Kir6.2ΔC36T224D (p <0.001); dKir6.2ΔC36T224A versus Kir6.2ΔC36T224D (p <0.05); and eKir6.2ΔC36 versus Kir6.2ΔC36T224D (p <0.01).
PKA enhanced currents of recombinant Kir6.2/SUR1 channel, a neuronal/pancreatic isoform of KATP
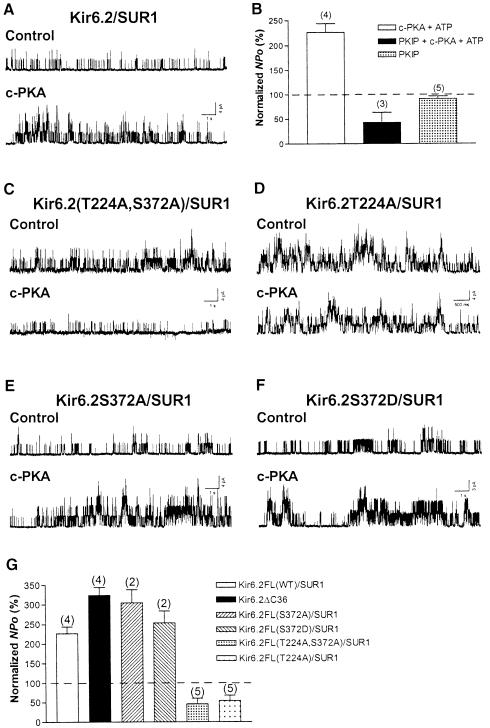
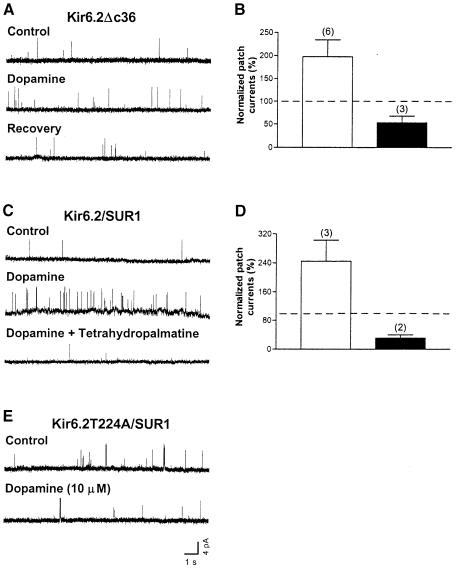
Owing to the higher sensitivity of Kir6.2/SUR1 channels to ATP inhibition than that of Kir6.2ΔC36 channels, a lower concentration of Mg–ATP (0.3 mM instead of 1 mM) was co-applied with c-PKA. Application of c-PKA (50 μg/ml) and Mg–ATP (0.3 mM) to the cytoplasmic surface of inside-out patches of HEK293 cells transfected with Kir6.2 and SUR1 did not alter the single-channel conductance, but caused an increase in the apparent opening frequency (Figure 2A, control versus c-PKA). c-PKA significantly increased the averaged NPo of the Kir6.2/SUR1 channel to 226.6 ± 17.2% of control (four patches; p <0.01, one-sample t-test). No significant stimulation was observed for co-application of c-PKA with PKIP (200 μg/ml) and Mg–ATP (0.3 mM) (43.1 ± 20.5%, three patches, one-sample t-test; Figure 2B), or application of PKIP alone (91.5 ± 5.2%, five patches, one-sample t-test; Figure 2B). KATP channels containing SUR1 and Kir6.2 are highly sensitive to ATP inhibition (Nichols et al., 1996; Gribble et al., 1997; Shyng et al., 1997b; Trapp et al., 1997; Tucker et al., 1997). Exposure to 0.3 mM Mg-free ATP would reduce NPo to 1.9 ± 1.0% (seven patches, Figure 5). KATP channel activity is also reactivated or refreshed by a brief application of a high concentration (mM) of Mg–ATP (Ohno-Shosaku et al., 1987). This ‘refreshment’ effect does not require the nucleotide-binding domains (NBDs) of SUR1 (Gribble et al., 1997) and becomes apparent when the inhibitory effects of ATP subside following removal of Mg–ATP (for a review, see Babenko et al., 1998). In our hands, the refreshment effect on channels became evident after terminating the application of 30 μl of 0.3 mM Mg–ATP to the patch and was long-lasting. It was therefore essential to examine the PKA effect during the 30–60 s period of c-PKA application. As evident from the controls, within this period of pressure ejection the inhibitory effects of ATP outweighed the refreshment effect of Mg–ATP. Moreover, the stimulatory effects of Kir6.2/SUR1 channel activity obtained during c-PKA application were abolished by PKIP, indicating a specific PKA phosphorylation effect.
Fig. 2. Enhancement of Kir6.2/SUR1 channel activity by PKA phosphorylation of the T224 site of Kir6.2. Currents were obtained from HEK293 cells co-expressing mouse Kir6.2 (full-length) and hamster SUR1 subunits. (A) Single-channel current traces of a Kir6.2/SUR1 channel in an inside-out patch prior to (upper trace, control) and during c-PKA treatment (lower trace). (B) NPo of Kir6.2/SUR1 channels obtained during application of c-PKA (open column), c-PKA plus PKIP (filled column) or PKIP alone (stippled column). Owing to the greater sensitivity of Ki6.2/SUR channels to ATP inhibition, 0.3 mM ATP (instead of 1 mM) was included in c-PKA and c-PKA plus PKIP solutions. Concentrations of c-PKA and PKIP were the same as in Figure 1. Data are presented as the mean ± SEM of 3–5 patches. (C–F) Single-channel current traces of Kir6.2(T224A,S372A)/SUR1 (C), Kir6.2T224A/SUR1 (D), Kir6.2S372A/SUR1 (E) and Kir6.2S372D/SUR1 (F) channels in inside-out patches before and during c-PKA application. T224 and S372 are the two putative PKA phosphorylation sites in full-length Kir6.2. The PKA effect was compared with control recordings of the same patch for each channel construct. (G) NPo of wild-type and mutant Kir6.2/SUR1 channels obtained in the presence of c-PKA. The PKA effect obtained from a Kir6.2ΔC36 channel is included for comparison. NPo was normalized in individual patches as described in Figure 1B. One-way ANOVA followed by Bonferroni's multiple comparison tests was performed (Fig. 2G).
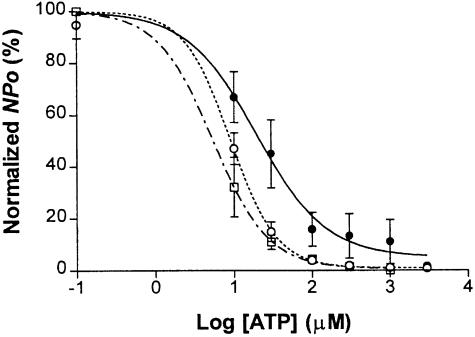
Fig. 5. Effect of phosphorylation of T224 on the ATP sensitivity of reconstituted KATP channels. The relationship of ATP concentration to normalized open probability was determined in Kir6.2/SUR1 (○, seven patches), Kir6.2T224D/SUR1 (•, seven patches) and Kir6.2T224A/SUR1 (□, three patches) channels in inside-out patches excised from HEK293 cells. NPo was normalized to the control at zero ATP in individual patches and is presented as the mean ± SEM. Solid and dashed curves represent non-linear regression fits to the averaged NPo.
Alanine substitution of Kir6.2T224 of the Kir6.2/SUR1 channel resulted in insensitivity of the channels to c-PKA
While Kir6.2ΔC36 contains only one putative PKA phosphorylation site, T224, another PKA phosphorylation site, S372, is located in the C–terminus of Kir6.2 (Inagaki et al., 1995) and there are at least three consensus phosphorylation sites for PKA on SUR1 (Aguilar-Bryan et al., 1995). Biochemical studies have revealed PKA phosphorylation of S372 and one site on human SUR1 (S1571), but not on SUR1 from other species or on SUR2 (Béguin et al., 1999). We thus examined Kir6.2T224A/SUR1, Kir6.2(T224A,S372A)/SUR1, Kir6.2S372A/SUR1 and Kir6.2S372D/SUR1 channels in inside-out patches to look for possible effects of PKA mediated through Kir6.2 or SUR1. Application of c-PKA (50 μg/ml) and Mg–ATP (0.3 mM) failed to increase the single-channel currents of Kir6.2(T224A,S372A)/SUR1 (Figure 2C) and Kir6.2T224A/SUR1 (Figure 2D) channels; the channel activities appeared to be reduced, probably due to the ATP inhibition of the channel. By contrast, the apparent opening frequency of Kir6.2S372A/SUR1 (Figure 2E) and Kir6.2S372D/SUR1 (Figure 2F) was enhanced by the same treatment. Treatment with c-PKA reduced the NPo to 54.0 ± 13.8% for Kir6.2T224A/SUR1 (five patches) and to 46.0 ± 14.2% for Kir6.2(T224A,S372A)/SUR1 (five patches), but increased the NPo to 304.3 ± 34.1% for Kir6.2S372A/SUR1 (two patches) and to 253.5 ± 30.1% for Kir6.2S372D/SUR1 (two patches), respectively. In comparison, c-PKA increased the NPo to 226.6 ± 17.3% of control for Kir6.2/SUR1 (four patches, Figure 2B) and to 323.8 ± 20.8% for Kir6.2ΔC36 channels (four patches, Figure 2G). Bonferroni's multiple comparison tests following one-way ANOVA revealed significant differences when Kir6.2(T224A,S372A)/SUR1 was compared with Kir6.2/SUR1, Kir6.2S372A/SUR1 or Kir6.2S372D (p <0.001 in each pairwise comparison). Significant differences were also found when Kir6.2T224A/SUR1 was compared with Kir6.2/SUR1, Kir6.2S372A/SUR1 or Kir6.2S372D (p <0.001 in each case). These data demonstrate that T224 is primarily responsible for the PKA stimulation of the KATP channel.
PKA enhanced currents of recombinant Kir6.2/SUR2A channel, a cardiac/skeletal muscle isoform of KATP
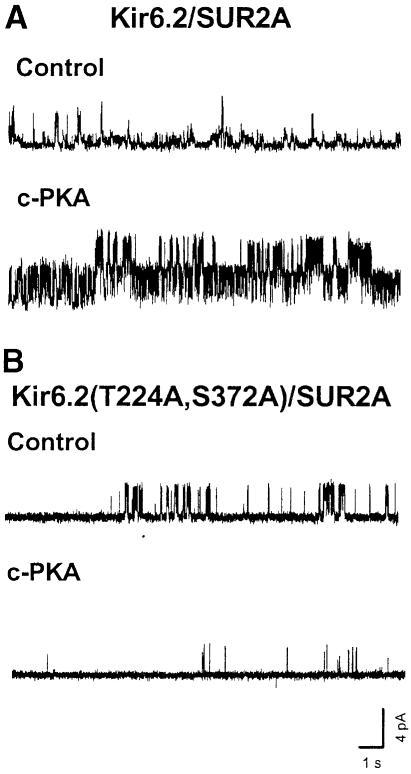
Application of c-PKA (50 μg/ml) and Mg–ATP (0.3 mM) to the cytoplasmic surface of inside-out patches excised from HEK293 cells transiently transfected with SUR2A and Kir6.2 increased the apparent opening frequency (Figure 3A, control versus c-PKA) without altering the single-channel conductance level. The NPo during c-PKA treatment was 280.4 ± 109.4% (two patches). A significant c-PKA effect on the opening frequency was detected for Kir6.2/SUR2A channels (317.4 ± 170.3%; p <0.025, paired t-test), but not mutant Kir6.2(T224A,S372A)/SUR2A channels (Figure 3B), implicating PKA phosphorylation of Kir6.2 as the cause of the modulation of Kir6.2/SUR2A channels. Given that SUR2A and SUR2B differ only in the C–terminal sequences due to alternative splicing and that no PKA phosphorylation site was identified in either subtype (Béguin et al., 1999), it seems likely that the primary cause of PKA stimulation of KATP channels containing SUR2 isoforms is due to phosphorylation of Kir6.2 at T224.
Fig. 3. Enhancement of Kir6.2/SUR2A channel activity by c-PKA. Currents were recorded from HEK293 cells co-expressing mouse Kir6.2 (full-length) and rat SUR2A subunits. (A) Single-channel current traces of a Kir6.2/SUR2A channel in an inside-out patch prior to (upper trace, control) and during c-PKA treatment (50 μg/ml) (lower trace). (B) Single-channel current traces of a Kir6.2(T224A,S372A)/SUR2A channel in an inside-out patch before and during c-PKA treatment. The bath was ATP free, and ATP (0.3 mM) was included in the drug solution.
Dopamine increased currents of Kir6.2ΔC36 channels in cell-attached patches
The D1R, which is coupled to G-αs and activates PKA through adenylate cyclase stimulation (Missale et al., 1988), and KATP channels containing SUR1 and/or SUR2B are found in dopaminergic SN neurons as well as in other central neurons (Gerfen et al., 1990; Le Moine et al., 1991; Nicola et al., 1996; Missale et al., 1998; Liss et al., 1999a). It is therefore of interest to examine Kir6.2 channel modulation by D1R. Dopamine was applied through a glass capillary by pressure ejection to the medium bathing HEK293 cells co-expressing D1R and Kir6.2ΔC36. Channel activity obtained in cell-attached patches was typically low due to the high ATP concentration inside the cell (∼5 mM; Dunne et al., 1989) (Figure 4A, control). However, dopamine (10 μM) was capable of producing a reversible increase in the apparent opening frequency (Figure 4A). The NPo of Kir6.2ΔC36 was significantly increased to 197.6 ± 36.5% in the presence of dopamine (six patches, p <0.05, one-sample t-test; control taken as 100%). In contrast, the NPo was 52.9 ± 14.8% when dopamine was applied with a D1/D2 receptor antagonist tetrahydropalmatine (100 μM) (three patches, Figure 4B; not significantly different from control, one-sample t-test). These results indicate that the Kir6.2ΔC36 channel is functionally modulated through a Gs-coupled pathway, presumably by activation of endogenous PKA.
Fig. 4. Increase in the activity of Kir6.2ΔC36 and Kir6.2/SUR1 channels in cell-attached patches by D1R stimulation. D1R was co-expressed with the channels in HEK293 cells. (A) Single-channel current traces of a Kir6.2ΔC36 channel obtained from a cell-attached patch before, during and after exposing the cell to dopamine (10 μM). (B) NPo of Kir6.2ΔC36 channel currents obtained during application of dopamine (open column) and during co-application of dopamine and the D1/D2R antagonist tetrahydropalmatine (100 μM, filled column). NPo was normalized to the corresponding control recordings from the same patch (taken as 100%) before drug application in individual patches. Data are presented as the mean ± SEM of six and three patches, respectively. (C) Single-channel current traces of a Kir6.2/SUR1 channel obtained from a cell-attached patch in the control, during dopamine application and during co-application of dopamine and tetrahydropalmatine. (D) NPo of Kir6.2/SUR1 channel currents obtained during application of dopamine (open column) and during co-application of dopamine and tetrahydropalmatine (filled column). Data are presented as the mean ± SEM of three and two patches, respectively. (E) Single-channel current traces of a Kir6.2T224A/SUR1 channel in a cell-attached patch prior to and during dopamine application.
Dopamine increased Kir6.2/SUR1 channel currents in cell-attached patches
To reconstitute one possible physiological mechanism for D1R modulation of KATP channels in the brain, D1R was co-expressed with Kir6.2 and SUR1 in HEK293 cells. Dopamine (10 μM) increased the apparent opening frequency (Figure 4C), and the increase was abolished when dopamine (10 μM) was administered with the antagonist tetrahydropalmatine (100 μM) (Figure 4C). The NPo of Kir6.2/SUR1 was 244.3 ± 58.6% (three patches) in the presence of dopamine and 31.0 ± 9.0% (two patches; not significantly different from control, one-sample t-test) when dopamine and tetrahydropalmatine were co-applied (Figure 4D). Moreover, dopamine failed to increase channel activity (NPo) in patches containing Kir6.2T224A/SUR1 channels (Figure 4E; 45.6 ± 21.2%, five patches; not significantly different from control, one-sample t-test). Taken together, these results indicate that stimulation of KATP channels by D1R is mediated by diffusible second messengers and is likely to involve PKA phosphorylation of T224 in Kir6.2.
PKA may alter the ATP sensitivity of Kir6.2/SUR1 channels expressed in HEK cells: Kir6.2T224D/SUR1 channels exhibited a decrease in ATP sensitivity
The Kir6.2ΔC36T224D channels resembled the phosphorylated channel in single-channel properties (Figure 1A and D; Tables I, III and IV), except that the elevated channel activity persists in the aspartate mutant but not in the wild-type channel following the c-PKA treatment. Therefore, to determine whether PKA might have an effect on the ATP sensitivity of KATP channels, we compared the dose–response curves for ATP inhibition of Kir6.2/SUR1, Kir6.2T224A/SUR1 and Kir6.2T224D/SUR1 channels in inside-out patches. The averaged Ki or IC50 for ATP and Hill coefficients were 10.5±1.9 μM and 2.25 ± 0.39 for Kir6.2/SUR1 channels (six patches), 39.0 ± 13.2 μM and 1.72 ± 0.57 for Kir6.2T224D/SUR1 channels (five patches) and 7.2 ± 1.4 μM and 2.14 ± 0.57 for Kir6.2T224A/SUR1 channels (three patches), respectively. It thus appeared that the Kir6.2T224D/SUR1 channels (filled circles, Figure 5) were less sensitive to ATP than wild-type (open circles, Figure 5) or Kir6.2T224A/SUR1 channels (open squares, Figure 5), whereas the Hill coefficients were not significantly changed. The difference in Ki between Kir6.2/SUR1 and Kir6.2T224D/SUR1 channels was statistically significant (p <0.05, unpaired t–test). Assuming that Kir6.2T224D/SUR1 is functionally equivalent to a phosphorylated channel, these data indicate that PKA might exert its positive modulatory effect at least in part by reducing the apparent ATP sensitivity of the Kir6.2/SUR1 channel.
Table IV. Effect of c-PKA on the closed duration distributions of Kir6.2ΔC36 channels expressed in HEK293 cells.
| Closed components | Control | PKA | PKIP + PKA |
|---|---|---|---|
| τ1 | 0.36 ± 0.02 | 0.37 ± 0.02 | 0.36 ± 0.01 |
| a1 | 0.48 ± 0.02 | 0.50 ± 0.01 | 0.51 ± 0.03 |
| τ2 | 23.93 ± 6.09 | 13.00 ± 2.00 | 8.13 ± 1.52 |
| a2 | 0.23 ± 0.06 | 0.18 ± 0.01 | 0.19 ± 0.06 |
| τ3 | 93.10 ± 31.26 | 54.33 ± 11.19 | 63.32 ± 26.13 |
| a3 | 0.27 ± 0.05 | 0.28 ± 0.01 | 0.22 ± 0.05 |
| τ4 | 881.90 ± 477.90 | 267.10 ± 108.20 | 1069.00 ± 838.10 |
| a4 | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.08 ± 0.05 |
The time constants (τ, in ms) and relative areas (a) of four exponential closed components in the duration histograms were obtained by fitting the distributions of duration in individual patches. Data from four inside-out patches were averaged and are presented as the mean ± SEM. Estimates of exponential areas and time constants were obtained using the method of maximal likelihood estimation.
Kinetic mechanism of Kir6.2ΔC36 channel activation by PKA
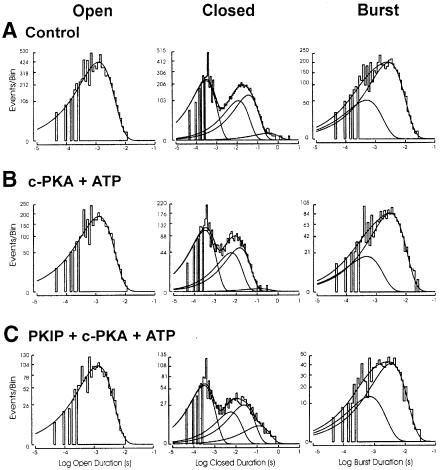
Channel function and its modulation are determined by the conformational changes that the channel undergoes to enable opening of the pore and ensuing ion permeation, as reflected by the number of open and closed states it exhibits and the rates of transitions between different states. To understand how PKA modulates these conformational changes of KATP, it is important to determine whether the c-PKA effects resulted from more frequent entry into the open state (i.e. increase in opening frequency), prolonged stay in the open state (i.e. increase in open duration), decreased dwelling time in the closed states (decreased closed duration) or any combination of the above. These kinetic studies require patch recording of a single channel and could be achieved more readily with Kir6.2ΔC36 channels because they exhibited much lower surface expression than Kir6.2/SUR1. We therefore carried out single-channel analysis of Kir6.2ΔC36 channel currents in inside-out patches that were exposed to c-PKA or PKIP together with c-PKA, and compared the kinetic properties with those of the same patches prior to the treatment with c-PKA or c-PKA plus PKIP.
Channel openings. The open duration frequency histograms under all three conditions were best fitted by one exponential function [Figure 6, left column; top to bottom: control (A), c-PKA (B), PKIP + c-PKA (C)], and the corrected mean open duration was not significantly altered by c-PKA (Table I, paired t-test). By contrast, the NPo and opening frequency were increased 2-fold during c–PKA treatment (Table I; Figure 1B, p <0.05 and <0.01, respectively, paired t-test).
Fig. 6. The frequency histograms of the open, closed and burst duration distributions of the Kir6.2ΔC36 channel obtained from an inside-out patch. Frequency histograms arrayed from top to bottom are open duration distribution (left column), closed duration distribution (center column) and burst duration distribution (right column), respectively. Frequency histograms of duration distributions fitted from events obtained (A) prior to c-PKA treatment, (B) during c-PKA (50 μg/ml) treatment and (C) during co-application of PKIP (200 μg/ml) plus c-PKA (50 μg/ml). Duration histograms were constructed as described in Materials and methods. See the text for details.
Channel closures. The mean closed duration was significantly reduced by c-PKA (Table I; p <0.01, paired t–test). The frequency histograms of the closed duration of Kir6.2ΔC36 channels were best fitted by a sum of four exponential functions under all three conditions [Figure 6, center column; top to bottom: control (A), c-PKA (B), PKIP + c-PKA (C)]. The time constant of the shortest closed state (designated C1) was not changed, while the time constants of the other three states (designated C2, C3 and C4) were decreased during c-PKA treatment (four patches, Table IV; see also Materials and methods for details). These changes in the time constants largely account for the reduction in mean closed duration during c-PKA treatment.
Bursts of channel openings. The burst duration distribution was best fitted with the sum of two or three exponential functions [Figure 6, right column; top to bottom: control (A), c-PKA (B), PKIP + c-PKA (C)]. The distribution of burst duration was not changed by c-PKA. The time constants were 0.63 ± 0.07 ms (B1) and 3.63 ± 0.49 ms (B2), respectively (seven patches). The shorter burst state (B1) had a time constant briefer than the open time constant fitted from open duration histograms. The possible implication of these brief bursts will be discussed later. The averaged corrected mean burst duration was not altered by c-PKA treatment, nor was the number of openings within a burst (Table I). Part of the c-PKA stimulation arises from a significant increase in the bursting frequency to 176.9 ± 18.3% of the control (12 patches, Table I; p <0.05).
As a control, co-application of PKIP and c-PKA did not produce changes in NPo, corrected mean open duration, closed duration, burst duration, opening or bursting frequency (Table I), or the duration distributions of openings, closures and bursts (Figure 6; Table IV). Kir6.2ΔC36T224A channels resembled Kir6.2ΔC36 channels in some single-channel properties (mean open duration and unit conductance), but had a lower activity level (Table III) and could not be stimulated by c-PKA (Table II). In contrast, Kir6.2ΔC36T224D channels, with the negative charge introduced by the aspartate substitution for T224, had an increased channel activity level and altered single-channel kinetic behavior (Table III). These alterations due to the T224D mutation mimicked the functional effect of PKA phosphorylation (Table I).
Discussion
This study examines the mechanism of PKA stimulation of KATP channels, by first identifying the T224 residue of the Kir6.2 channel subunit as the PKA phosphorylation site responsible for channel stimulation and by then studying the functional consequences of T224 phosphorylation. We summarize here our findings and then compare these observations with previously reported studies of KATP channel modulation.
Channel stimulation by PKA is mediated via T224 of Kir6.2
Mutations of T224 of the Kir6.2 subunit abolish c-PKA stimulation of octameric KATP channels containing SUR1 or SUR2A (Figures 2 and 3), KATP channel stimulation by D1R (Figure 4), as well as c-PKA- and D1R-mediated stimulation of the tetrameric Kir6.2ΔC36 channels without SUR (Figures 1 and 4; Table II). The stimulatory effects of c-PKA are eliminated by an inhibitory peptide of PKA, PKIP, therefore confirming that the kinase activity of c–PKA is responsible for the observed channel stimulation (Figures 1, 2 and 6; Tables I and IV). Furthermore, the T224D mutation mimicked c-PKA-mediated channel activation by increasing the opening frequency and decreasing the mean closed duration of the channel (Figure 1; Tables I and III), indicating that PKA stimulation of channel activity is due to the attachment of a negatively charged phosphate group to T224 of Kir6.2. These findings strongly suggest that PKA stimulation of channel activity results from phosphorylation of T224 in Kir6.2.
Functional consequences of T224 phosphorylation by PKA
Mechanistically, our single-channel analysis reveals that c-PKA-treated Kir6.2ΔC36 channels (but not Kir6.2T224AΔC36 channels) open more frequently and close for shorter periods of time than channels not exposed to c-PKA (Figures 1 and 6; Tables I, II and IV). As well as altering the relative stability of the various closed and open states of the channel (see below), PKA could conceivably alter the channel's sensitivity to ATP inhibition. We examined this possibility by characterizing ATP inhibition of the T224D mutant channel, taking advantage of a persistent rather than transient increase in channel activity caused by the T224D mutation that mimics the kinetic changes caused by c-PKA. The apparent affinity of ATP inhibition is reduced in Kir6.2T224D/SUR1 (Figure 5), analogous to the stimulatory effect of phosphoinositides (Baukrowitz et al., 1998; Shyng and Nichols, 1998; Fan and Makielski, 1999). In comparison, PKC exerts its effects by reducing the cooperativity of ATP-mediated inhibition of the cardiac KATP channel (Light et al., 1995). It is possible that the gating effects of the T224D mutation or T224 phosphorylation contribute to the reduction of apparent ATP sensitivity for channel inhibition. In any case, these data suggest that channel stimulation by PKA phosphorylation of T224 could arise from reduced ATP inhibition of the channel and altered channel gating properties.
PKA alters KATP channel gating in specific ways. Previous studies indicate that the burst kinetics of the Kir6.2/SUR1 channel expressed in COS cells include one open state and three closed states (Alekseev et al., 1997). In our study of the Kir6.2ΔC36 tetrameric channel, we could resolve one open component, four closed components and two burst components (B1 0.63 ± 0.07 ms; B2 3.63 ± 0.49 ms, seven patches). The shorter burst component has a time constant smaller than the time constant of the single open state detected (1.34 ± 0.07 ms, seven patches), indicating that there may actually be two open states, one relatively brief and rare (O1) and the other longer lasting and more frequent (O2). We suggest, therefore, that the Kir6.2ΔC36 channel has at least two open states (O1 and O2), four closed states (C1, C2, C3 and C4) and two burst states (B1 and B2). The stimulation of channel activity by c-PKA appears to result from a combination of increased opening frequency and bursting frequency, reduced dwelling time in longer but not shorter closed states, and decreased mean closed duration (Tables I and IV; Figure 6).
The single-channel kinetics of Kir6.2ΔC36 in ATP-free solutions could be accounted for by the following kinetic scheme of Kir6.2 channel gating (Scheme 1).
Scheme 1.
In this scheme, O is the open state, C represents the closed state (C1 the shortest, C4 the longest), and the rate constants (k) correspond to microscopic transition rates between adjacent states. PKA phosphorylation of the Kir6.2 channel appears to increase transition rates from the longest closed state C4 to C3 (k1) and from C3 to C2 (k2), the opening rate from C2 to O2 (k3), and the closing rate from O2 to C1 (k–4). PKA phosphorylation also decreases the closing rate from O2 to C2 (k–3). The transition rates from C2 to O1 (k5), O1 to C2 (k–5) and C1 to O2 (k4) may be unaltered.
The observed single-channel kinetics are also compatible with an alternative kinetic scheme in which the two open states O1 and O2 are connected to separate extra-burst closed states (Scheme 2). The transition rates of several steps in this scheme would also be expected to increase, including the forward rates leading to opening of O2 (k1 and k2) and the closing rate from O2 to intra-burst closed state C1 (k–4). In contrast, the closing rate from O2 to C2 (k–2) is decreased. Here we also suggest that the transition rate between O1 and state C3 (k5 and k–5) and the opening rate from C1 to O2 (k4) are not affected.
Scheme 2.
Independently of the specific schemes used to model the single-channel kinetics, phosphorylation of T224 by PKA appears to stabilize the open conformation or, alternatively, destabilize the closed states, by impairing the transitions to long(er) closed states.
Differences between two PKA phosphorylation sites of Kir6.2
Two potential PKA phosphorylation sites, T224 and S372, are present in Kir6.2. A recent biochemical study of octameric KATP channels shows phosphorylation of S372 but not T224 of Kir6.2 when oocyte homogenates or COS-1 cells are treated with cAMP analogs and when Gs-coupled receptors are activated (Béguin et al., 1999). The lack of detectable phosphorylation of Kir6.2S372A mutant channel subunits due to the activity of the PKA added to the oocyte homogenates conceivably could result from high basal phosphorylation of T224. The biochemical experiments with COS-1 cells, however, indicate that phosphorylation of S372 but not of T224 is sustainable in the (potential) presence of protein phosphatases and through the hours-long microsome preparation and immunoprecipitation procedures. These investigators further conclude that S372 phosphorylation accounts for the PKA stimulation of channel activity, because the S372A mutation blocks the PKA stimulation whereas the S372D mutation causes a 2- to 4-fold increase in open probability (Béguin et al., 1999). How might the discrepancy between this previous study and our study be reconciled?
We observed channel stimulation while ⩽30 μl of solution containing c-PKA (50 μg/ml, or ∼2000 U/ml) and Mg–ATP (1 mM for the tetrameric channel and 0.3 mM for the octameric channel) were applied to the membrane patch over a period of 30–60 s. Béguin et al. (1999), on the other hand, used a much lower concentration of c–PKA (100 U/ml) and ATP (10 μM) in the bath solution and waited for 6–10 min before measuring the stimulatory effects of PKA. Using analogous conditions, we observed channel rundown, probably due to the inability of 10 μM Mg–ATP and 1.39 mM free magnesium in the bath solution to maintain KATP channel activity (Ohno-Shosaku et al., 1987). The Kir6.2T224A/SUR1 channel activities declined to 30.7 ± 12.3% (four patches) following 6–8 min exposure of the excised patch to 100 U/ml c-PKA and 10 μM Mg–ATP, but the Kir6.2/SUR1, Kir6.2S372A/SUR1 and Kir6.2S372D/SUR1 channel activities showed less decline. Thus, channel stimulation due to PKA phosphorylation of T224 may have counteracted the channel rundown. It should be noted that that our experimental conditions are similar but not exactly identical to those used by Béguin et al. (1999). It thus remains possible that under conditions where channel rundown is prevented, prolonged exposure to PKA may result in phosphorylation of S372 and consequently stimulation of channel activity.
It is also important to note that the octameric KATP channel activity could increase due to ‘refreshment’ by ATP (Ohno-Shosaku et al., 1987; Babenko et al., 1998), which is applied together with the kinase in these experiments. This refreshment effect was evident and long-lasting in our experiments after the inhibitory effects of pressure-ejected ATP subsided and the ATP concentration was diluted to 20 μM due to subsequent mixing with the 2 ml of bath solution. Channel stimulation due to PKA phosphorylation, but not channel refreshment by ATP, should be blocked specifically by PKA inhibitors. Béguin et al. (1999) showed that the PKA inhibitor H-89 prevents PKA phosphorylation of S372 of Kir6.2 in their biochemical experiments. It will be of interest to test the effect of PKA inhibitors on the functional stimulation of channels that they observed following prolonged exposure to c–PKA, and to explore any possible involvement of S372 in KATP channel refreshment. The ability of the PKA inhibitory peptide PKIP to antagonize the PKA effects in our experiments indicates that the channel stimulation observed within the first minute of c-PKA application is due to the kinase activity of PKA (Figures 1, 2 and 6; Tables I and IV). This PKA stimulation is abolished by the T224A and T224D mutations, but not by S372A and S372D, or deletion of S372 (Figures 1, 2 and 4), implicating T224 as the phosphorylation site for channel stimulation.
Evidently, both T224D and S372D mutations increase channel open probability, as does channel exposure to c–PKA (Béguin et al., 1999; this study). In our single-channel analysis, we show that the same kinetic parameters are altered in a similar fashion by T224D mutation and by c-PKA treatment of Kir6.2ΔC36 channels (Tables I and III). These kinetic changes are different from those caused by mutations of several Kir6.2 residues that increase channel activity (Shyng et al., 1997a; Trapp et al., 1998), suggesting a distinct kinetic mechanism for PKA phosphorylation to modulate KATP channel activity. It will be important to determine whether the transitions between closed states and open states are modified in similar ways by S372D and by PKA phosphorylation of Kir6.2. These and other experiments may determine whether T224 and S372 exhibit different affinities for PKA, different susceptibility to subsequent dephosphorylation, different basal levels of phosphorylation in different cells and different functional effects on KATP channels.
Dopamine receptor stimulates KATP channels via T224 of Kir6.2
The ability of c-PKA and D1R to stimulate KATP channels and Kir6.2ΔC36 channels, but not mutant channels with alanine substituting for Thr224 (Figures 1, 2 and 4; Tables I and II), indicates that T224 of Kir6.2 is a target of endogenous PKA upon transmitter stimulation of receptors that are coupled to G-αs. Our study thus strongly suggests that PKA phosphorylation of T224 of Kir6.2 is primarily responsible for KATP channel activation upon D1R stimulation, even though this phosphorylation may be transient and not readily detectable in biochemical studies (Béguin et al., 1999). We have shown that PKA phosphorylation of the common Kir6.2 subunit exerts its functional effects by destabilizing the closed states relative to the open states and by decreasing the apparent ATP sensitivity for channel inhibition. These stimulatory effects may serve as a general mechanism to accomplish functional modulation of KATP channels by G-αs-coupled receptors in the central nervous system and in smooth muscles.
Physiological significance of PKA stimulation of KATP channels
Understanding the mechanism of PKA stimulation of KATP channels is of physiological relevance in vivo. Arterial muscle tone and blood flow are controlled by KATP channels, which are activated not only during hypoxia (Nelson and Quayle, 1995) but also by vasodilators such as calcitonin gene-related peptide (CGRP), adenosine and endothelin isopeptides via PKA (Wellman et al., 1998). KATP channels in renal epithelium (Wang and Giebisch, 1991) and follicular cells (Wibrand et al., 1992) are also stimulated by PKA. Moreover, KATP channels in rat ventromedial hypothalamic neurons are positively regulated by phosphorylation, since the channel activity is decreased by the non-specific kinase inhibitor H7 but increased by the non-specific phosphatase inhibitor microcystin (Routh et al., 1997). We have used HEK293 cells as a heterologous expression system, in order to examine the effects of PKA on Kir6.2 expressed in the presence or absence of SUR subunits, and the consequences of specific channel mutations (Figures 1–5). These studies identify the target of PKA for KATP channel stimulation as T224 of Kir6.2, the common pore-forming subunit of native KATP channels whose SUR subunits vary with the cell type. The mechanisms uncovered in our study therefore most probably underlie the PKA stimulatory effects of KATP channels in vivo.
Materials and methods
Mutagenesis and construction of cDNAs
A stop codon was introduced into the mouse Kir6.2 cDNA using site-directed mutagenesis to remove the last 36 amino acids at the C–terminus. The cDNA encoding Kir6.2ΔC36 was modified further to replace Thr224 with either alanine or aspartate by site-directed mutagenesis using an overlap PCR strategy. In addition, mutations T224A/D and/or S372A/D were introduced into full-length Kir6.2 cDNA in the same way. To reconstitute the pancreatic/neuronal-type and the cardiac/skeletal muscle-type KATP channels, we used sulfonylurea receptor cDNAs SUR1 (hamster clone) and SUR2A (rat clone), respectively. All cDNA constructs were verified by DNA sequencing and subcloned into expression vector pcDNA3 (Invitrogen), except for the cDNA encoding D1R, which was in a pcDM6 vector. Plasmids prepared using Qiagen maxipreps were used for transient transfection.
Cell culture and transient transfection
HEK293 cells (from the American Type Culture Collection) were maintained in Dulbecco's modified Eagle's medium DMEM/F12 (Mediatech; supplemented with 2 mM l-glutamine, 10% fetal bovine serum, 100 IU/ml penicillin and 100 IU/ml streptomycin) at 37°C in humidified 5% CO2. Cells were transiently transfected using a lipofectamine reagent (Gibco-BRL) or the FuGENE™ 6 reagent (Roche) mixed with expression plasmids containing cDNAs of interest in serum-free medium. A marker gene encoding the green fluorescent protein (pEGFP-1; Clontech) was co-transfected with the cDNA of interest in a ratio of 1:10. Transfection was carried out according to the manufacturer's protocols. The cells were re-plated the following day at a density of 5000–20 000 cells/dish either onto 35 mm culture dishes or onto 12 mm glass coverslips pre-coated with 1 μg/ml fibronectin (Sigma) for recording 24–48 h later.
Electrodes, recording solutions and single-channel recordings
The recording electrodes were pulled from thin-walled borosilicate glass with an internal filament (MTW150F-3; World Precision Instruments) using a P-87 Flaming Brown puller (Sutter Instrument), and were fire-polished to a resistance of 5–10 MΩ. Glass capillaries used for drug application were pulled from the same type of glass and the tip diameter was ∼30–50 μm. The intracellular solution consisted of (in mM): KCl 110, MgCl2 1.44, KOH 30, EGTA 10, HEPES 10, pH to 7.2. The extracellular solution consisted of (in mM): KCl 140, MgCl2 1.2, CaCl2 2.6, HEPES 10, pH to 7.4. For inside-out as well as cell-attached patch recordings, the recording chamber (or dish) was filled with the intracellular solution (the bath solution) and the pipet was filled with the extracellular solution. The equilibrium potential for potassium ions was 0 mV. Inside-out or cell-attached single-channel recordings (Hamill et al., 1981) were performed at room temperature 48–72 h after transfection. All patches were voltage clamped at –60 mV intracellularly. Single-channel currents were recorded with an Axopatch 200A patch clamp amplifier (Axon Instruments) and were low-pass filtered (3 dB, 1 kHz) with an 8-pole Bessel filter (Frequency Devices). Single-channel data were acquired and digitized at 20 kHz (or at 5 kHz for multiple-channel currents) on-line using Clampex 7 software (Axon) via a 16-bit A/D converter (Digidata acquisition board 1200A; Axon). Simultaneously, data were stored on VHS tapes using a JVC HR-J400U VCR and a PCM converter system (VR-10B; Instrutech). Channel activity in inside-out patches exhibited a fast rundown immediately after membrane excision in the ATP-free bath solution and channel activity then reached a relatively steady-state level, as indicated by similar channel activities in three consecutive recordings lasting for 1 min each. Immediately following these control recordings, c-PKA plus ATP, with or without PKIP, or PKIP alone, was applied to the patch as described below.
Preparations of drugs
The vials containing c-PKA (Promega) were stored at –80°C. Once thawed, c-PKA was kept at 4°C and used within a week. The stock of PKIP (rabbit sequence; Sigma) was stored at –20°C. Both were diluted with bath solution to 50 μg/ml for c-PKA and 200 μg/ml for PKIP prior to use. The dipotassium salt of ATP (0.3 or 1 mM) was added to both drug solutions. The solutions containing c-PKA or PKIP plus c-PKA were kept on ice and applied to the cytoplasmic surface of patches by pressure ejection via a glass capillary positioned adjacent to the patch. A picospritzer (General Valve) was used to control the ejection pressure and the duration of drug application (30–60 s). In a different set of experiments, 10 μM dopamine (Calbiochem) and 100 μM D1/D2R antagonist tetrahydropalmatine (Calbiochem) were applied to the cell via a pressure ejection system as described above. The chemicals were prepared in extracellular solution prior to use from frozen stocks of dopamine (10 mM) and tetrahydropalmatine (13.5 mM). Working drug solutions were put on ice and kept away from light. The channel activities from cell-attached patches were monitored. In experiments determining the ATP concentration–response curves, a small recording chamber (RC-25F; Warner) was used. ATP (potassium salt) at 5–3000 μM was made up from 0.8 M stocks into Mg2+-free intracellular solution and was applied through a perfusion system.
Data analysis
Single-channel currents. Digitized single-channel records were detected using Fetchan 6.05 (events list) of pCLAMP (Axon) using a 50% threshold crossing criterion and analyzed with Interval5 (Dr Barry S.Pallotta). Analysis was performed at the main conductance level (∼70 pS). Only patches with infrequent multiple-channel activity were used for analysis. Duration histograms were constructed as described by Sigworth and Sine (1987) and estimates of exponential areas and time constants were obtained using the method of maximal likelihood estimation. The number of exponential functions required to fit the duration distribution was determined by fitting increasing numbers of functions until additional components could not improve the fit significantly (Horn, 1987; McManus and Magleby, 1988). Events with a duration <1.5 times the system dead time were displayed in the histogram but were not included in the fit. Bursts were defined as groups of openings surrounded by closures greater than a critical closed time. A critical gap (burst terminator) was determined for each patch individually from the closed time distribution to equalize the proportion of misclassified events (Colquhoun and Sakmann, 1985). Only the shortest component C1 was designated to be the closure within a burst (intra-burst closure), because the time constants of other closed states (C2–C4) were too long to be classified as intra-burst closures (Tables IV). Mean durations were corrected for missed events by taking the sum of the relative area (a) of each exponential component in the duration frequency histogram multiplied by the time constant (τ) of the corresponding component. Data were normalized to the corresponding controls obtained in individual patches (taken as 100%) whenever mentioned.
Multiple-channel currents. Digitized current records were analyzed using Fetchan 6.05 (browse) of pCLAMP to integrate currents in 30–60 s segments. The integrated current values were then normalized to the corresponding controls in the same patches to obtain NPo (control as 100%). The ATP sensitivity curves and the IC50 of KATP to ATP were obtained by fitting the dependence of NPo on [ATP] using the formula: NPo = NPo(max)/{1 + ([ATP]/Ki)n]}, where NPo is the normalized open probability in varying concentrations of ATP relative to the maximal Po [Po(max)] in the absence of ATP, Ki is the half-maximal inhibitory [ATP] (or inhibition constant) and n is the slope factor or Hill coefficient.
Statistics
Data were averaged and presented as the mean ± SEM. Statistical comparisons were made using Student's two-tailed paired t-tests (for absolute data), unpaired t-tests, one-sample t-test (for normalized data) or one-way ANOVA followed by Bonferroni's multiple comparison tests. Significance was assumed when p <0.05. The majority of these comparisons were performed using Prism (GraphPad Software).
Acknowledgments
Acknowledgements
We thank Dr Susumu Seino for discussions of different PKA effects. We are also grateful to Drs Susumu Seino and Joseph Bryan for the kind gifts of cDNA clones of hamster SUR1, rat SUR2A and mouse Kir6.2. The cDNA for D1R was a kind gift from Dr Bruce Conklin. The single-channel analysis program Interval5 was generously provided by Dr Barry Pallotta. The site-directed mutagenesis of Kir6.2 was carried out with the kind assistance of Ms Mei Yu. Y.N.J. and L.Y.J. are HHMI investigators. This study was supported by NIH grant NS15693.
References
- Aguilar-Bryan L., et al. (1995) Cloning of the β-cell high-affinity sulphonylurea receptor: a regulator of insulin secretion. Science, 268, 423–426. [DOI] [PubMed] [Google Scholar]
- Aguilar-Bryan L., Clement, J.P., IV, Gonzalez, G., Kunjilwar, K., Babenko, A. and Bryan, J. (1998) Toward understanding the assembly and structure of KATP channels. Physiol. Rev., 78, 227–245. [DOI] [PubMed] [Google Scholar]
- Alekseev A.E., Kennedy, M.E., Navarro, B. and Terzic, A. (1997) Burst kinetics of co-expressed Kir6.2/SUR1 clones: comparison of recombinant with native ATP-sensitive K+ channel behaviour. J. Membr. Biol., 159, 161–168. [DOI] [PubMed] [Google Scholar]
- Ashcroft F.M. (1996) Mechanisms of the glycaemic effects of sulfonylureas. Horm. Metab. Res., 28, 456–463. [DOI] [PubMed] [Google Scholar]
- Ashcroft F.M. and Gribble, F.M. (1998) Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci., 21, 288–294. [DOI] [PubMed] [Google Scholar]
- Ashcroft F.M. and Rorsman, P. (1989) Electrophysiology of the pancreatic β-cell. Prog. Biophys. Mol. Biol., 54, 87–143. [DOI] [PubMed] [Google Scholar]
- Ashford M.L., Boden, P.R. and Treherne, J.M. (1990) Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflügers Arch., 415, 479–483. [DOI] [PubMed] [Google Scholar]
- Babenko A.P., Aguilar-Bryan, L. and Bryan, J. (1998) A view of SUR/Kir6.X, KATP channels. Annu. Rev. Physiol., 60, 667–687. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T., Schulte, U., Oliver, D., Herlitze, S., Krauter, T., Tucker, S.J., Ruppersberg, J.P. and Fakler, B. (1998) PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science, 282, 1141–1144. [DOI] [PubMed] [Google Scholar]
- Béguin P., Nagashima, K., Nishimura, M., Gonoi, T. and Seino, S. (1999) PKA-mediated phosphorylation of the human KATP channel: separate roles of Kir6.2 and SUR1 subunit phosphorylation. EMBO J., 18, 4722–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O., Bunzow, J.R. and Grandy, D.K. (1993) Molecular diversity of the dopamine receptors. Annu. Rev. Pharmacol. Toxicol., 32, 281–307. [DOI] [PubMed] [Google Scholar]
- Clement J.P. IV, Kunjilwar, K., Gonzalez, G., Schwanstecher, M., Panten, U., Aguilar-Bryan, L. and Bryan, J. (1997) Association and stoichiometry of KATP channel subunits. Neuron, 18, 827–838. [DOI] [PubMed] [Google Scholar]
- Colquhoun D. and Sakmann, B. (1985) Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J. Physiol., 369, 501–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne M.J., West-Jordan, J.A., Abraham, R.J., Edwards, R.H.T. and Peterson, O.H. (1989) Cromakalim (RBL 34915) and diazoxide activate ATP-regulated potassium channels in insulin-secreting cells. J. Membr. Biol., 104, 165–177. [Google Scholar]
- Fan Z. and Makielski, J.C. (1999) Phosphoinositides decrease ATP sensitivity of the cardiac ATP-sensitive K+ channel. A molecular probe for the mechanism of ATP-sensitive inhibition. J. Gen. Physiol., 114, 251–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen C.R., Engber, T.M., Mahan, L.C., Susel, Z., Chase, T.N., Mosma, F.J., Jr and Sibley, D.R. (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science, 250, 1429–1432. [DOI] [PubMed] [Google Scholar]
- Goldberg L.I., Volkman, P.H. and Kohli, J.D. (1978) A comparison of the vascular dopamine receptors with other dopamine receptors. Annu. Rev. Pharmacol. Toxicol., 18, 57–79. [DOI] [PubMed] [Google Scholar]
- Gribble F.M., Tucker, S.J. and Ashcroft, F.M. (1997) The essential role of the Walker A motifs of SUR1 in K-ATP channel activation by Mg–ADP and diazoxide. EMBO J., 16, 1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble F.M., Tucker, S.J., Haug, T. and Ashcroft, F.M. (1998) MgATP activates the β cell KATP channel by interaction with its SUR1 subunit. Proc. Natl Acad. Sci. USA, 95, 7185–7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O.P., Marty, A., Neher, E., Sakmann, B. and Sigworth, F.J. (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch., 391, 85–100. [DOI] [PubMed] [Google Scholar]
- Hatakeyama N., Wang, Q., Goyal, R.K. and Akbarali, H.I. (1995) Muscarinic suppression of ATP-sensitive K+ channel in rabbit esophageal smooth muscle. Am. J. Physiol., 268, C877–C885. [DOI] [PubMed] [Google Scholar]
- Horn R. (1987) Statistical methods for model discrimination. Applications to gating kinetics and permeation of the acetylcholine receptor channel. Biophys. J., 51, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N., Gonoi, T., Clement, J.P., IV, Namba, N., Inazawa, J., Gonzalez, G., Aguilar-Bryan, L., Seino, S. and Bryan, J. (1995) Reconstitution of IKATP: an inward rectifier subunit plus the sulphonylurea receptor. Science, 270, 1166–1169. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Gonoi, T., Clement, J.P., IV, Wang, C.Z., Aguilar-Bryan, L., Bryan, J. and Seino, S. (1996) A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron, 16, 1011–1017. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Gonoi, T. and Seino, S. (1997) Subunit stoichiometry of the pancreatic β-cell ATP-sensitive K+ channel. FEBS Lett., 409, 232–236. [DOI] [PubMed] [Google Scholar]
- Isomoto S., Kondo, C., Yamada, M., Matsumoto, S., Higashiguchi, O., Horio, Y., Matsuzawa, Y. and Kurachi, Y. (1996) A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J. Biol. Chem., 271, 24321–24324. [DOI] [PubMed] [Google Scholar]
- Isomoto S., Kondo, C. and Kurachi, Y. (1997) Inwardly rectifying potassium channels: their molecular heterogeneity and function. Jpn J. Physiol., 47, 11–39. [DOI] [PubMed] [Google Scholar]
- John S.A., Monck, J.R., Weiss, J.N. and Ribalet, B. (1998) The sulphonylurea receptor SUR1 regulates ATP-sensitive mouse Kir6.2 K+ channels linked to the green fluorescent protein in human embryonic kidney cells (HEK293). J. Physiol., 510, 333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschin C., Ecke, C., Ashcroft, F.M. and Karschin, A. (1997) Overlapping distribution of KATP channel-forming Kir6.2 subunit and the sulfonylurea receptor SUR1 in rodent brain. FEBS Lett., 401, 59–64. [DOI] [PubMed] [Google Scholar]
- Lawson C.S. and Downey, J.M. (1993) Precondition: state of the art myocardial protection. Cardiovasc. Res., 27, 542–550. [DOI] [PubMed] [Google Scholar]
- Le Moine C., Normand, E. and Bloch, B. (1991) Phenotypical characterization of the rat striatal neurons expressing the D1 dopamine receptor gene. Proc. Natl Acad. Sci. USA, 88, 4205–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., West, J.W., Numann, R., Murphy, B.J., Scheuer, T. and Catterall, W.A. (1993) Convergent regulation of sodium channels by protein kinase C and cAMP-dependent protein kinases. Science, 261, 1439–1442. [DOI] [PubMed] [Google Scholar]
- Light P. (1996) Regulation of ATP-sensitive potassium channels by protein phosphorylation. Biochim. Biophys. Acta, 1286, 65–73. [DOI] [PubMed] [Google Scholar]
- Light P.E., Allen, B.G., Walsh, M.P. and French, R.J. (1995) Regulation of adenosine triphosphate-sensitive potassium channel from rabbit ventricular myocytes by protein kinase C and type 2A protein phosphatase. Biochemistry, 34, 7252–7257. [DOI] [PubMed] [Google Scholar]
- Liss B., Bruns, R. and Roeper, J. (1999a) Alternative sulfonylurea receptor expression defines metabolic sensitivity of K-ATP channels in dopaminergic midbrain neurons. EMBO J., 18, 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss B., Neu, A. and Roeper, J. (1999b) The weaver mouse gain-of-function phenotype of dopaminergic midbrain neurons is determined by coactivation of wvGirk2 and K-ATP channels. J. Neurosci., 19, 8839–8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O.B. and Magleby, K.L. (1988) Kinetic states and modes of single large-conductance calcium activated potassium channels in cultured rat skeletal muscle. J. Physiol., 402, 79–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri N., Bonci, A., Johnson, S., Stratta, F., Calibresi, P. and Bernadi, G. (1994) Effects of anoxia on rat midbrain dopamine neurons. J. Neurophysiol., 71, 1165–1173. [DOI] [PubMed] [Google Scholar]
- Missale C., Castelletti, L., Memo, M., Carruba, M.O. and Spano, P.F. (1988) Identification of postsynaptic D1 and D2 dopamine receptors in the cardiovascular system. J. Cardiovasc. Pharmacol., 11, 643–650. [DOI] [PubMed] [Google Scholar]
- Missale C., Nash, S.R., Bobinson, S.W., Jaber, M. and Caron, M.G. (1998) Dopamine receptors: from structure to function. Physiol. Rev., 78, 189–225. [DOI] [PubMed] [Google Scholar]
- Nelson M.T. and Quayle, J.M. (1995) Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol., 268, C799–C822. [DOI] [PubMed] [Google Scholar]
- Nichols C.G. and Lopatin, A.N. (1997) Inward rectifier potassium channels. Annu. Rev. Physiol., 59, 171–191. [DOI] [PubMed] [Google Scholar]
- Nichols C.G., Shyng, S.-L., Nestorowicz, A., Glaser, B., Clement, J.P., IV, Gonzalez, G., Aguilar-Bryan, L., Permutt, M.A. and Bryan, J. (1996) Adenosine diphosphate as an intracellular regulator of insulin secretion. Science, 272, 1785–1787. [DOI] [PubMed] [Google Scholar]
- Nicola S.M., Kombian, S.B. and Malenka, R.C. (1996) Psychostimulants depress excitatory synaptic transmission in the nucleus accumbens via presynaptic D1-like dopamine receptors. J. Neurosci., 16, 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T., Zünkler, B.J. and Trube, G. (1987) Dual effects of ATP on K+ currents of mouse pancreatic β-cells. Pflügers Arch., 408, 133–138. [DOI] [PubMed] [Google Scholar]
- Okuyama Y., et al. (1998) The effects of nucleotides and potassium channel openers on SUR2A/Kir6.2 complex K+ channel expressed in a mammalian cell line, HEK293T cells. Pflügers Arch., 435, 595–603. [DOI] [PubMed] [Google Scholar]
- Quayle J.M. and Nelson, M.T. (1997) ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol. Rev., 77, 1165–1232. [DOI] [PubMed] [Google Scholar]
- Quayle J.M., Bonev, A.D., Brayden, J.E. and Nelson, M.T. (1994) Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. J. Physiol., 475, 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeper J. and Ashcroft, F.M. (1995) Metabolic inhibition and low central ATP activate K-ATP channels in rat dopaminergic substantia nigra neurons. Pflügers Arch., 430, 44–54. [DOI] [PubMed] [Google Scholar]
- Routh V.H., McArdle, J.J. and Levin, B.E. (1997) Phosphorylation modulates the activity of the ATP-sensitive K+ channel in ventromedial hypothalamic nucleus. Brain Res., 778, 107–119. [DOI] [PubMed] [Google Scholar]
- Sakura H., Ämmälä, C., Smith, P.A., Gribble, F.M. and Ashcroft, F.M. (1995) Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel expressed in pancreatic β-cells, brain, heart and skeletal muscle. FEBS Lett., 377, 338–344. [DOI] [PubMed] [Google Scholar]
- Seino S. et al. (1997) Molecular basis of functional diversity of ATP-sensitive K+ channel. Jpn J. Physiol. Suppl. 1, 47, S3–S4. [PubMed] [Google Scholar]
- Seutin V., Shen, K.-Z., North, R. and Johnson, S. (1996) Sulphonylurea-sensitive potassium current evoked by sodium loading in rat midbrain dopamine neurons. Neuroscience, 71, 709–719. [DOI] [PubMed] [Google Scholar]
- Shyng S.-L. and Nichols, C.G. (1997) Octameric stoichiometry of KATP channel complex. J. Gen. Physiol., 110, 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng S.L. and Nichols, C.G. (1998) Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science, 282, 1138–1141. [DOI] [PubMed] [Google Scholar]
- Shyng S.L., Ferrigni, T. and Nichols, C.G. (1997a) Control of rectification and gating of KATP channels by the Kir6.2 subunit. J. Gen. Physiol., 110, 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng S.L., Ferrigni, T. and Nichols, C.G. (1997b) Regulation of KATP channel activity by diazoxide and MgADP: distinct functions of the two nucleotide binding folds of the sulphonylurea receptor. J. Gen. Physiol., 110, 643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F.J. and Sine, S.M. (1987) Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys. J., 52, 1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford I. and Lacey, M. (1995) Regulation of potassium conductance in rat midbrain dopamine neurons by intracellular adenosine triphosphate (ATP) and the sulphonylureas tolbutamine and glibenclamide. J. Neurosci., 15, 4651–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P.M., Cote, G.J., Wohllk, N., Haddad, B., Mathew, P.M., Rabl, W., Aguilar-Bryan, L., Gagel, R.F. and Bryan, J. (1995) Mutations in the sulphonylurea receptor gene in familial persistent hyperinsulinaemic hypoglycaemia of infancy. Science, 268, 425–429. [DOI] [PubMed] [Google Scholar]
- Trapp S., Tucker, S.J. and Ashcroft, F.M. (1997) Activation and inhibition of KATP currents by guanine nucleotides is mediated by different channel subunits. Proc. Natl Acad. Sci. USA, 94, 8872–8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S., Proks, P., Tucker, S.J. and Ashcroft, F.M. (1998) Molecular analysis of ATP-sensitive K channel gating and implications for channel inhibition by ATP. J. Gen. Physiol., 112, 333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker S.J., Gribble, F.M., Zhao, C., Trapp, S. and Ashcroft, F.M. (1997) Truncation of Kir6.2 produces ATP-sensitive K-channels in the absence of the sulphonylurea receptor. Nature, 387, 179–183. [DOI] [PubMed] [Google Scholar]
- Wang W.H. and Giebisch, G. (1991) Dual modulation of renal ATP-sensitive K+ channel by protein kinases A and C. Proc. Natl Acad. Sci. USA, 88, 9722–9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts A., Hicks, G. and Henderson, G. (1995) Putative postnatal pre- and postsynaptic ATP-sensitive potassium channels in the rat substantia nigra in vitro. J. Neurosci., 15, 3065–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman G.C., Quayle, J.M. and Standen, N.B. (1998) ATP-sensitive K+ channel activation by calcitonin gene-related peptide and protein kinase A in pig coronary arterial smooth muscle. J. Physiol., 507, 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibrand F., Honore, E. and Lazdunski, M. (1992) Opening of glibenclamide-sensitive K+ channels in follicular cells promotes Xenopus oocyte maturation. Proc. Natl Acad. Sci. USA, 89, 5133–5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Isomoto, S., Matsumoto, S., Kondo, C., Shindo, T., Horio, Y. and Kurachi, Y. (1997) Sulphonylurea receptor 2B and Kir 6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J. Physiol., 499, 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoshiki H., Sunagawa, M., Seki, T. and Sperelakis, N. (1998) ATP-sensitive K+ channels in pancreatic, cardiac, and vascular smooth muscle cells. Am. J. Physiol., 274, C25–C37. [DOI] [PubMed] [Google Scholar]
- Zerangue N., Schwappach, B., Jan, Y.N. and Jan, L.Y. (1999) A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron, 22, 537–548. [DOI] [PubMed] [Google Scholar]