Abstract
We evaluated the effects of moderate-to-severe impairment of the estimated glomerular filtration rate (eGFR: 15 to 59 mL/min per 1.73 m2) and of proteinuria on the central hemodynamics and the pulse wave velocity (PWV) in 2244 middle-aged healthy Japanese men who were not receiving any medications for cardiovascular diseases or cardiovascular risk factors. The adjusted value of the radial augmentation index was higher in the subjects with proteinuria than in those without proteinuria. On the other hand, this value was similar between the subjects with and without moderate-to-severe impairment of the eGFR. Not only proteinuria but also moderate-to-severe impairment of the eGFR was associated with increase in the adjusted value of the brachial-ankle PWV. Thus, proteinuria was found to be an independent risk factor for abnormal central hemodynamics and increased stiffness of the large- to middle-sized arteries, while moderate-to-severe impairment of the eGFR was associated with an increase of the arterial stiffness, but not with abnormality of the central hemodynamics.
1. Introduction
Chronic kidney disease (CKD) is an independent risk factor for cardiovascular disease [1–3]. Assessment of the severity of CKD is employed in general health checkups for cardiovascular risk stratification [1–3]. Estimated glomerular filtration rate (eGFR) (mL/min per 1.73 m2) and presence/absence of proteinuria are the key components measured during assessment of the severity of CKD. A recent meta-analysis demonstrated that the central hemodynamics (i.e., augmentation index (AI) and central blood pressure (CBP)) and pulse wave velocity (PWV), a marker of the stiffness of large arteries, are independent risk factors for cardiovascular disease and that these variables may reflect different facets of the pathophysiological abnormalities related to arterial stiffness [4, 5]. Several studies have demonstrated abnormal central hemodynamics and increased PWV in subjects with end-stage renal disease (i.e., eGFR <15 mL/min per 1.73 m2) [6, 7]. On the other hand, it has not yet been clearly revealed whether moderate-to-severe impairment of the eGFR (15 to 59 mL/min per 1.73 m2) and proteinuria may also be associated with impairment of the central hemodynamics and increase of the PWV [8, 9]. One of the reasons for the inconclusiveness of this issue may be related to the medications prescribed to the study subjects, because the medications prescribed for the cardiovascular risk factors are known to potentially affect the central hemodynamics and PWV [10].
Recently, we reported several findings of clinical studies conducted by us on the arterial stiffness [11, 12]. Our study subjects mostly comprised middle-aged healthy Japanese men and revealed gender differences in the central hemodynamic variables [13]. The present study was conducted to evaluate the effects of moderate-to-severe impairment of the eGFR (15 to 59 mL/min per 1.73 m2) and proteinuria on the central hemodynamics and PWV in middle-aged healthy Japanese men who were not receiving any medications for cardiovascular disease or the cardiovascular risk factors.
2. Methods
2.1. Study Cohort
The study protocol has been described in detail in a previous report [11, 12]. The study subjects were all employees of a construction company who underwent annual health checkup plus the measurements of the brachial-ankle PWV and of the radial augmentation index (radial AI) in 2008. The annual health checkup was conducted in the morning hours after the subjects had fasted overnight and included physical examinations, blood pressure measurement (2 times), blood and urine examinations, electrocardiography, chest roentgenography, upper gastrointestinal roentgenography, audiometry, and vision testing. In addition, measurements of the brachial-ankle PWV and radial augmentation index (radial AI) were also conducted during this series of examinations. The following subjects were excluded from the study: (1) subjects with conditions in which the reliability of the brachial-ankle PWV measurement is considered to be questionable [11, 12] (atrial fibrillation; ankle/brachial systolic blood pressure index of less than 0.95; undergoing regular hemodialysis); (2) subjects in whom the standard deviation of the radial AI, calculated from ten radial pressure waveforms, was over 6%; (3) subjects with serum C-reactive protein levels ≥10 mg/L; (4) subjects receiving medications for heart disease, stroke, or risk factors for cardiovascular disease (i.e., hypertension, hypercholesterolemia, and/or diabetes mellitus). Verbal informed consent was obtained from all of the participants prior to the measurements. The study was conducted with the approval of the Ethics Guidelines Committee of Tokyo Medical University.
2.2. Measurements
Subjects were randomly allocated to measurement of the brachial-ankle PWV first, followed by that of the radial AI, or to measurement of the radial AI first, followed by that of the brachial-ankle PWV. The procedural details of both measurements are described elsewhere.
2.2.1. Augmentation Index
The measurements of the blood pressure and radial AI were conducted after the subjects had rested for 5 minutes in the sitting position, in an air-conditioned room (24–26°C) earmarked exclusively for this purpose. The blood pressure was measured in the right upper arm by the oscillometric method (HEM-907; Omron Healthcare Co., Ltd., Kyoto, Japan). Immediately after this measurement, the left radial arterial waveform was recorded using an arterial applanation tonometry probe equipped with an array of 40 micropiezoresistive transducers (HEM-9000AI; Omron Healthcare Co., Ltd. Kyoto, Japan) [12, 13]. The HEM-9000AI device is programmed to automatically determine the pressure against the radial artery to yield the optimal radial arterial waveforms, and 10 radial arterial pressure waveforms were recorded. Then, the first and second peaks of the peripheral systolic pressure (SBP1 and SBP2) and radial diastolic pressure (DBP) were automatically detected, and the radial AI was calculated using the equation (SBP2 − DBP)/(SBP1 − DBP) × 100(%). The waveform data were confirmed by well-trained technicians, and the data of subjects with a standard deviation of the radial AI of over 6% were excluded from the analysis. The radial AI values were adjusted to a heart rate of 75 beats/minute (radial AI75).
2.3. Pulse Wave Velocity
The brachial-ankle PWV was measured using a volume-plethysmographic apparatus (Form/ABI, OMRON Colin Co. Ltd., Kyoto, Japan) [11, 12]. In brief, electrocardiographic electrodes were placed on both wrists, and a microphone for the phonocardiogram was fixed to the left chest. Occlusion cuffs, which were connected to both the plethysmographic and oscillometric sensors, were tied around both upper arms and both ankles, with the subjects lying supine. The brachial and posttibial arterial pressures were measured using the oscillometric sensor. The brachial and posttibial arterial pressure waveforms determined by the plethysmographic sensor and recorded for 10 seconds were stored. The measurements were conducted after the subjects had rested for at least 5 minutes in the supine position in an air-conditioned room (24–26°C) designated exclusively for this purpose.
2.4. Laboratory Measurements
The serum levels of total cholesterol (TC), high-density lipoprotein cholesterol (HDL), and triglycerides (TG) and the fasting plasma glucose (FPG) were measured enzymatically (FALCO Biosystems Co. Ltd, Tokyo, Japan). The serum CRP level was determined by the latex-aggregation method (Eiken Co., Tokyo, Japan), which is a high-sensitivity assay method with a detection threshold of <0.1 mg/L. All the blood and urine samples were obtained in the morning after the patients had fasted overnight.
The severity of the renal function impairment was determined based on measurement of the eGFRcr using the Modification of Diet in Renal Disease (MDRD) equation for Japanese subjects [3]: eGFRcr = 194 × Crnn−1.094 × Age−0.287 (in females, ×0.739).
A urinary dipstick test result for proteinuria of >1+ (urinary protein concentration ≥30 mg/dl) was defined as proteinuria [2, 3, 12].
2.5. Statistical Analysis
The data were represented as mean ± SD. The basic covariates used for the adjustments were the age, height, waist circumference, smoking status (non- or past smoker: 0, present smoker: 1), alcohol intake status (nondrinker: 0, light-to-moderate drinker (ethanol consumption, 1–29 g/day): 1, and heavy drinker (ethanol consumption, over 30 g/day): 2) TC, HDL, TG, and FPG.
For assessment of the differences in each variable among the groups, an unpaired t-test for continuous variables and the chi-squared test for categorical variables were applied. Furthermore, for assessment of the differences in the brachial-ankle PWV, radial AI, and SBP2 among the groups, a general linear model (GLM) analysis with adjustments was applied. All the analyses were conducted using the SPSS software for Windows, version 11.0J (SPSS, Chicago, Ill, USA); P values < .05 were considered to denote statistical significance.
3. Results
Initially, 2740 male subjects were enrolled in the present study. However, 496 subjects were excluded because they had atrial fibrillation (n = 13), had an ABI of less than 0.95 (n = 14), were undergoing regular hemodialysis (n = 2), had a standard deviation of the radial AI of over 6% (n = 68), had a serum CRP level >10 mg/L (n = 31), were receiving medications for heart disease (n = 26), stroke (n = 12), or risk factors for cardiovascular disease (i.e., hypertension (n = 250), hypercholesterolemia (n = 46), and/or diabetes mellitus (n = 44)). Finally, the data of 2244 subjects were included for the analysis in this study.
Table 1 shows the clinical characteristics of the entire study population. In the entire study population, 48 subjects had an eGFR of 15 to 59 mL/min per 1.73 m2, and 65 subjects had proteinuria. Table 2 summarizes the clinical characteristics in the subjects with eGFR ≥ 60 mL/min per 1.73 m2/eGFR = 15 to 59 mL/min per 1.73 m2 with/without proteinuria. The body mass index, brachial-ankle PWV, SBP2, TC, TG, and FBG were higher, and the percentage of current smokers and blood pressure were lower in the subjects with an eGFR = 15 to 59 mL/min per 1.73 m2 than in those with an eGFR ≥ 60 mL/min per 1.73 m2. Age, body mass index, percentage of current smokers, blood pressure, brachial-ankle PWV, radial AI, and SBP2 were higher and the serum HDL was lower in the subjects with proteinuria than in those without proteinuria.
Table 1.
Clinical characteristics of the entire study population.
| Parameter | |
|---|---|
| number | 2244 |
| Age (y) | 44 ± 9 |
| Height (cm) | 172 ± 6 |
| BMI | 23.9 ± 3.0 |
| WC (cm) | 84 ± 8 |
| Smoking (%) | 707 (32) |
| ALC (N/LM/H) (%) | 291/1386/567 (13/62/25) |
| SBP (mmHg) | 123 ± 13 |
| DBP (mmHg) | 76 ± 11 |
| Pulse rate (bpm) | 68 ± 10 |
| baPWV (m/s) | 12.9 ± 1.8 |
| radial AI (%) | 71 ± 13 |
| SBP 2 (mmHg) | 111 ± 16 |
| TC (mmol/L) | 5.4 ± 0.9 |
| HDL (mmol/L ) | 1.6 ± 0.4 |
| TG (mmol/L) | 1.4 ± 1.0 |
| FPG (mmol/Ll) | 4.9 ± 0.6 |
| eGFR (mL/min per 1.73 m2) | 82 ± 12 |
| eGFR < 60 mL/min per 1.73 m2 (%) | 48 (2.1) |
| Proteinuria (%) | 65 (2.9) |
Abbreviations. BMI: body mass index; WC: waist circumference; smoking: number of subjects who were smoking; ALC: (N: nondrinker, LM: light-to-moderate drinker (ethanol intake: 1–29 g/day), and H: heavy drinker (ethanol intake: ≥30 g/day)); SBP: systolic blood pressure; DBP: diastolic blood pressure; baPWV: brachial-ankle pulse wave velocity; radial AI: radial augmentation index; SBP2: second peak of the radial pressure waveform; TC: serum total cholesterol; HDL: serum high-density lipoprotein cholesterol; TG: serum triglycerides; FPG: fasting plasma glucose; eGFR: estimated glomerular filtration rate; eGFR < 60 mL/min per 1.73 m2: number of subjects with eGFR < 60 mL/min per 1.73 m2; proteinuria: number of subjects with proteinuria.
Table 2.
Clinical characteristics of the study subjects classified by the presence/absence of proteinuria.
| Pro (+) | Pro (−) | P-value | eGFR < 60 | eGFR ≥ 60 | P value | |
|---|---|---|---|---|---|---|
| Number | 65 | 2179 | 48 | 2196 | ||
| Age (y) | 43 ± 10 | 44 ± 9 | .69 | 54 ± 8 | 44 ± 9 | <.01 |
| Height (cm) | 172 ± 6 | 172 ± 6 | .21 | 172 ± 6 | 172 ± 6 | .92 |
| BMI | 25.1 ± 4.1 | 23.9 ± 3.0 | <.05 | 24.6 ± 2.6 | 23.9 ± 3.0 | <.05 |
| WC (cm) | 87 ± 11 | 84 ± 8 | <.05 | 86 ± 6 | 84 ± 8 | .09 |
| Smoking (%) | 30 (46) | 677 (31) | <.01 | 7 (15) | 700 (32) | <.01 |
| ALC (N/LM/H)(%) | 12/34/19 (18/52/30) | 279/1352/548 (13/62/25) | .25 | 5/32/11 (10/67/23) | 286/1354/556 (13/62/25) | .76 |
| SBP (mmHg) | 126 ± 15 | 122 ± 14 | <.05 | 130 ± 15 | 122 ± 14 | <.01 |
| DBP (mmHg) | 79 ± 12 | 76 ± 11 | <.05 | 84 ± 12 | 76 ± 11 | <.01 |
| Pulse rate (bpm) | 71 ± 9 | 68 ± 10 | <.05 | 71 ± 9 | 68 ± 10 | .07 |
| baPWV (m/s) | 13.7 ± 2.1 | 12.9 ± 1.8 | <.01 | 14.5 ± 2.3 | 12.9 ± 1.8 | <.01 |
| radial AI (%) | 74 ± 10 | 71 ± 13 | <.05 | 73 ± 14 | 71 ± 13 | .12 |
| SBP 2 (mmHg) | 117 ± 17 | 111 ± 16 | <.01 | 120 ± 19 | 111 ± 16 | <.01 |
| TC (mmol/L) | 5.5 ± 1.0 | 5.3 ± 0.9 | .19 | 5.9 ± 1.0 | 5.3 ± 0.9 | <.01 |
| HDL (mmol/L ) | 1.4 ± 0.4 | 1.6 ± 0.4 | <.01 | 1.5 ± 0.4 | 1.6 ± 0.4 | .16 |
| TG (mmol/L) | 1.6 ± 1.7 | 1.4 ± 1.0 | .31 | 1.8 ± 1.0 | 1.4 ± 1.0 | <.01 |
| FPG (mmol/Ll) | 5.2 ± 0.5 | 4.9 ± 0.5 | .12 | 5.1 ± 0.5 | 4.9 ± 0.5 | <.01 |
| eGFR (mL/min per 1.73 m2) | 85 ± 15 | 82 ± 12 | .10 | 56 ± 4 | 82 ± 12 | <.01 |
| eGFR <60 (%) | 1 (1.5) | 64 (2.9) | .59 | — | — | — |
| Proteinuria (%) | — | — | — | 1 (2.0) | 47 (2.1) | .75 |
Abbreviations. Pro (+): subjects with proteinuria; Pro (−): subjects without proteinuria; eGFR < 60: subjects with eGFR of 15 to 59 mL/min per 1.73 m2; eGFR ≥ 60: subjects with eGFR ≥ 60 mL/min per 1.73 m2; BMI: body mass index; WC: waist circumference; smoking: number of subjects who were smoking; ALC: (N: nondrinker, LM: light-to-moderate drinker (ethanol intake: 1–29 g/day), and H: heavy drinker (ethanol intake: ≥30 g/day)); SBP: systolic blood pressure; DBP: diastolic blood pressure; baPWV: brachial-ankle pulse wave velocity; radial AI: radial augmentation index; SBP2: second peak of the radial pressure waveform; TC: serum total cholesterol; HDL: serum high-density lipoprotein cholesterol; TG: serum triglycerides; FPG: fasting plasma glucose; eGFR: estimated glomerular filtration rate; eGFR < 60 mL/min per 1.73 m2: number of subjects with eGFR < 60 mL/min per 1.73 m2; proteinuria: number of subjects with proteinuria.
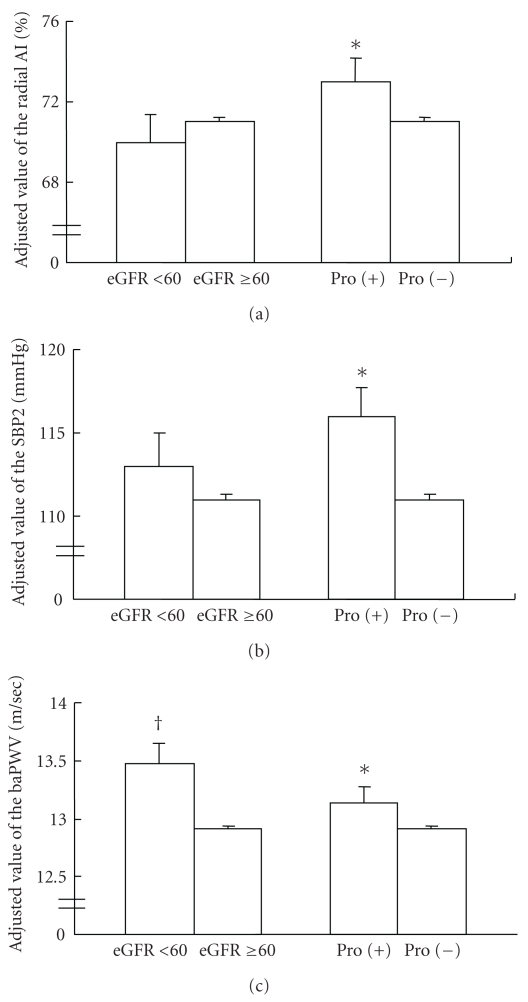
After the adjustments (basic covariates plus eGFR, mean blood pressure, and pulse rate obtained at the time of measurement of the radial AI), the value of the radial AI was higher in the subjects with proteinuria than in those without proteinuria (Figure 1(a)). However, the adjusted (basic covariates plus presence/absence proteinuria, mean blood pressure, and pulse rate obtained at the time of measurement of the radial AI) value was similar between the subjects with an eGFR of 15 to 59 mL/min per 1.73 m2 and those with an eGFR ≥ 60 mL/min per 1.73 m2. Furthermore, the adjusted (basic covariates plus eGFR, pulse rate obtained at the measurement of radial AI) value of SBP2 was also higher in the subjects with proteinuria than in those without proteinuria (Figure 1(b)). On the other hand, after the adjustments (basic covariates plus eGFR, mean blood pressure, and pulse rate obtained at the time of measurement of brachial-ankle PWV), the value of the brachial-ankle PWV was higher in the subjects with proteinuria than in those without proteinuria. The adjusted (basic covariates plus presence/absence proteinuria, mean blood pressure, and pulse rate obtained at the measurement of brachial-ankle PWV) value of the brachial-ankle PWV was also higher in the subjects with eGFR of 15 to 59 mL/min per 1.73 m2 than in those with eGFR ≥ 60 mL/min per 1.73 m2 (Figure 1(c)).
Figure 1.
Adjusted values of the radial augmentation index, second peak of the radial pressure waveform, and brachial-ankle pulse wave velocity in subjects classified by the presence/absence of proteinuria or eGFR ≥ 60 mL/min per 1.73 m2/<60 mL/min per 1.73 m2. Abbreviations. radial AI: radial augmentation index; SBP2: second peak of the radial pressure waveform; baPWV: brachial-ankle pulse wave velocity; *P < .05 versus subjects without proteinuria; †P < .05 versus subjects with eGFR ≥ 60 mL/min per 1.73 m2.
4. Discussion
The present study was conducted to examine the effect of proteinuria and that of moderate-to-severe impairment of the eGFR (15 to 59 mL/min per 1.73 m2) on the central hemodynamics and PWV in healthy subjects (i.e., those who were not receiving any medication for cardiovascular disease or cardiovascular risk factors).
Central hemodynamics and PWV are markers reflecting the pathophysiological abnormalities related to arterial stiffness [4, 5, 14]. Vascular damage of the systemic arterial tree affects the central hemodynamics, while vascular damage of the large- to middle-sized arteries are major determinants of the brachial-ankle PWV [4, 5, 14]. Few studies have simultaneously examined the effects of impaired GFR and proteinuria on the central hemodynamics and arterial stiffness, especially central arterial stiffness [8, 9]. The Framingham study demonstrated that the presence of microalbuminuria, but not the eGFR, was related to an increased AI and increased carotid-femoral PWV, the golden standard as a marker of the central arterial stiffness [8]. The Hoon study demonstrated that while microalbuminuria was related to increased AI and increased carotid arterial stiffness, impaired eGFR was related to only increased carotid arterial stiffness [9]. However, in both studies, around 30–40% of the subjects were receiving medication for hypertension [8, 9], which potentially affects the AI and PWV [10]. Thus, the present study was conducted in subjects who were not receiving any medications for either cardiovascular disease or for risk factors and demonstrated that proteinuria was associated with an increased brachial-ankle PWV, radial AI, and SBP2, whereas impaired eGFR was associated with only increase of the brachial-ankle PWV. Therefore, proteinuria may be associated with vascular damage of the central and peripheral arteries, and impaired GFR may be associated with vascular damage only of the large- to middle-sized arteries in healthy subjects.
Recent prospective studies have demonstrated that increased stiffness of the larger arteries, as assessed by the carotid-femoral PWV, or of the large- to middle-sized arteries, as assessed by the brachial-ankle PWV, is a risk factor for the progressive decline of the GFR or the new onset of microalbuminuria [8, 15–17]. On the other hand, only the Framingham study has been conducted to evaluate the clinical significance of abnormal central hemodynamics in predicting the future incidence of CKD or microalbuminuria; however, no clinical significance of these variables could be found in this study [8]. However, kidney has luxury blood flow because of the low resistance to blood flow, therefore, small arterial vessels in the kidney are exposed to high-pressure fluctuations, especially in cases with elevated central blood pressure [18]. Thus, a further prospective study is proposed to examine whether abnormal central hemodynamics might be a predictor of the progression of glomerular damage, new onset of proteinuria, and/or the aggravation of proteinuria.
The present study had some limitations. First, persistent proteinuria is one of the criteria for the diagnosis of CKD, but in the present study, only the results of spot proteinuria as assessed by the urinary dipstick test were available. Second, while the urinary microalbumin-to-creatinine excretion ratio is another recommended parameter for estimating the severity of renal dysfunction [8, 16], we did not measure this parameter. Third, brachial-ankle PWV is primarily a marker of stiffness of the aorta and middle-sized arteries and closely associated with not only the aortic PWV, as assessed by a direct catheter method, but also with the carotid-femoral PWV [11, 19], the most well-established index for the measurement of aortic stiffness [4, 5, 8].
5. Conclusion
In middle-aged healthy Japanese subjects, who were not receiving medications for cardiovascular diseases or their risk factors, proteinuria was found to be an independent risk factor for abnormal central hemodynamics and increased stiffness of the large- to middle-sized arteries. On the other hand, moderate-to-severe impairment of the eGFR (15 to 59 mL/min per 1.73 m2) was only associated with increased arterial stiffness.
Acknowledgment
This study was supported in part by a fund awarded by OMRON Health Care Company (Kyoto, Japan) to Professor A. Yamashina.
References
- 1.Brosius FC, III, Hostetter TH, Kelepouris E, et al. Detection of chronic kidney disease in patients with or at increased risk of cardiovascular disease: a science advisory from the American Heart Association Kidney and Cardiovascular Disease Council; the Councils on High Blood Pressure Research, Cardiovascular Disease in the Young, and Epidemiology and Prevention; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;114(10):1083–1087. doi: 10.1161/CIRCULATIONAHA.106.177321. [DOI] [PubMed] [Google Scholar]
- 2.Imai E, Horio M, Watanabe T, et al. Prevalence of chronic kidney disease in the Japanese general population. Clinical and Experimental Nephrology. 2009;13(6):621–630. doi: 10.1007/s10157-009-0199-x. [DOI] [PubMed] [Google Scholar]
- 3.Imai E, Horio M, Iseki K, et al. Prevalence of chronic kidney disease (CKD) in the Japanese general population predicted by the MDRD equation modified by a Japanese coefficient. Clinical and Experimental Nephrology. 2007;11(2):156–163. doi: 10.1007/s10157-007-0463-x. [DOI] [PubMed] [Google Scholar]
- 4.Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. European Heart Journal. 2010;31(15):1865–1871. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- 5.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness. A systematic review and meta-analysis. Journal of the American College of Cardiology. 2010;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 6.London GM, Blacher J, Pannier B, Guérin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38(3):434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 7.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99(18):2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 8.Upadhyay A, Hwang SJ, Mitchell GF, et al. Arterial stiffness in mild-to-moderate CKD. Journal of the American Society of Nephrology. 2009;20(9):2044–2053. doi: 10.1681/ASN.2009010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermans MMH, Henry R, Dekker JM, et al. Estimated glomerular filtration rate and urinary albumin excretion are independently associated with greater arterial stiffness: the Hoorn study. Journal of the American Society of Nephrology. 2007;18(6):1942–1952. doi: 10.1681/ASN.2006111217. [DOI] [PubMed] [Google Scholar]
- 10.Asmar R. Effects of pharmacological intervention on arterial stiffness using pulse wave velocity measurement. Journal of the American Society of Hypertension. 2007;1(2):104–112. doi: 10.1016/j.jash.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Tomiyama H, Hashimoto H, Tanaka H, et al. Continuous smoking and progression of arterial stiffening: a prospective study. Journal of the American College of Cardiology. 2010;55(18):1979–1987. doi: 10.1016/j.jacc.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 12.Odaira M, Tomiyama H, Matsumoto C, et al. Association of serum cystatin C with pulse wave velocity, but not pressure wave reflection, in subjects with normal renal function or mild chronic kidney disease. American Journal of Hypertension. 2010;23(9):967–973. doi: 10.1038/ajh.2010.100. [DOI] [PubMed] [Google Scholar]
- 13.Tomiyama H, Yamazaki M, Sagawa Y, et al. Synergistic effect of smoking and blood pressure on augmentation index in men, but not in women. Hypertension Research. 2009;32(2):122–126. doi: 10.1038/hr.2008.20. [DOI] [PubMed] [Google Scholar]
- 14.Tomiyama H, Yamashina A. Non-invasive vascular function tests: their pathophysiological background and clinical application. Circulation Journal. 2010;74(1):24–33. doi: 10.1253/circj.cj-09-0534. [DOI] [PubMed] [Google Scholar]
- 15.Tomiyama H, Tanaka H, Hashimoto H, et al. Arterial stiffness and declines in individuals with normal renal function/early chronic kidney disease. Atherosclerosis. 2010;212(1):345–350. doi: 10.1016/j.atherosclerosis.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 16.Munakata M, Miura Y, Yoshinaga K. Higher brachial-ankle pulse wave velocity as an independent risk factor for future microalbuminuria in patients with essential hypertension: the J-TOPP study. Journal of Hypertension. 2009;27(7):1466–1471. doi: 10.1097/HJH.0b013e32832b4740. [DOI] [PubMed] [Google Scholar]
- 17.Ford ML, Tomlinson LA, Chapman TPE, Rajkumar C, Holt SG. Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension. 2010;55(5):1110–1115. doi: 10.1161/HYPERTENSIONAHA.109.143024. [DOI] [PubMed] [Google Scholar]
- 18.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46(1):200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka H, Munakata M, Kawano Y, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. Journal of Hypertension. 2009;27(10):2022–2027. doi: 10.1097/HJH.0b013e32832e94e7. [DOI] [PubMed] [Google Scholar]