Abstract
Objective
To evaluate the feasibility of improving active ankle dorsiflexion with contralaterally controlled neuromuscular electrical stimulation (CCNMES).
Design
CCNMES dorsiflexes the paretic ankle with a stimulation intensity that is directly proportional to the degree of voluntary dorsiflexion of the unimpaired contralateral ankle, which is detected by an instrumented sock. Three subjects with chronic (>6-mo poststroke) dorsiflexor paresis participated in a 6-wk CCNMES treatment, which consisted of self-administering CCNMES-assisted ankle dorsiflexion exercises at home daily and practicing an ankle motor control task in the research laboratory twice a week.
Results
For subjects 1 and 2, respectively, maximum voluntary ankle dorsiflexion increased by 13 and 17 degrees, ankle movement tracking error decreased by ~57% and 57%, and lower limb Fugl-Meyer score (maximum score is 34) increased by 4 and 5 points. Subject 3 had no appreciable improvement in these measures. Both subjects 1 and 2 maintained their performance in ankle movement tracking through the 3-mo follow-up; subject 2 also maintained the gains in maximum ankle dorsiflexion and Fugl-Meyer score.
Conclusions
These results suggest that CCNMES may have a positive effect on ankle motor impairment in some stroke survivors. Further investigation of the effect of CCNMES on gait is warranted.
Keywords: Stroke Rehabilitation, Hemiplegia, Contralaterally Controlled Neuromuscular Electrical Stimulation, Footdrop, Ankle
Hemiparesis of the lower limb is one of the most common impairments resulting from stroke. Approximately 70% of the >780,000 strokes that occur annually in the United States results acutely in the loss of ability to walk household distances independently.1 Of those stroke survivors who have walking impairment acutely, ~65% remains either nonambulatory or require personal assistance to walk despite intensive rehabilitation.2 A major contributor to impaired ambulation is the inability to dorsiflex the ankle during the swing phase of gait, which causes the foot to drag and results in inefficient and unsafe ambulation or nonambulation. Many patients are prescribed an ankle-foot-orthosis (AFO), the standard of care, which by design limits the mobility of the ankle during ambulation. Hence, although an AFO may assist stroke survivors’ ambulation in the short-term, it is possible that it inhibits recovery of ankle movement in the long term. This study investigates a new rehabilitation treatment that aims to reduce lower limb motor impairment and improve gait in stroke survivors by improving voluntary ankle dorsiflexion.
The most effective stroke rehabilitation therapies will likely be those that facilitate the ability of the brain to recover motor control. Basic science and clinical studies on central motor neuroplasticity have demonstrated that active, repetitive, goal-oriented movement training plays a major role in motor recovery after stroke, even in the chronic phase.3–5 Among several advanced rehabilitation, strategies for restoring gait that rely on activity-dependent neuroplasticity are body weight-supported treadmill training5,6 and robot-assisted gait training.7–9 Both of these techniques engage the participant in active (patient-initiated), repetitive, goal-oriented movement of the paretic lower limb. Although functional gains with these therapies have been reported, and some studies have even shown the improvements to be correlated with changes in the brain,5 the time and physical demands on therapists have so far prevented body weight-supported treadmill training from wide clinical acceptance. Robotic devices, such as the Lokomat, still require skilled personnel, are expensive, and may not be as effective as conventional gait training.8,9 Thus, there remains a need for motor retraining interventions that are effective, applicable to patients with severe and moderate motor impairments, and easy to implement (perhaps even by the patients themselves at home).
Functional electrical stimulation (FES) has been used to correct footdrop in stroke patients by stimulating the peroneal nerve during gait.10–12 FES footdrop systems produce ankle dorsiflexion during the swing phase of gait, thereby allowing the stroke survivor to clear his or her toes, reducing the need to use compensatory strategies (e.g., hip hiking), reducing the risk of falling, conserving energy, and eliminating the need for an AFO. With FES footdrop systems, the user does not consciously control the timing of the stimulation; rather, stimulation is timed to the swing phase with a tilt sensor worn around the paretic lower leg,11 or a heel switch worn in the shoe of the paretic foot.10 Therefore, the cognitive investment is relatively low. Although this is analogous to normal walking, where one does not consciously control movement at the hip, knee, and ankle joints, a rehabilitation therapy that requires greater cognitive investment and motor intention may be necessary to produce neuroplastic changes leading to motor recovery. Then, if recovery of conscious volitional ankle dorsiflexion is achieved, it may be possible with gait training to progress toward more normal subconscious lower limb control during gait.
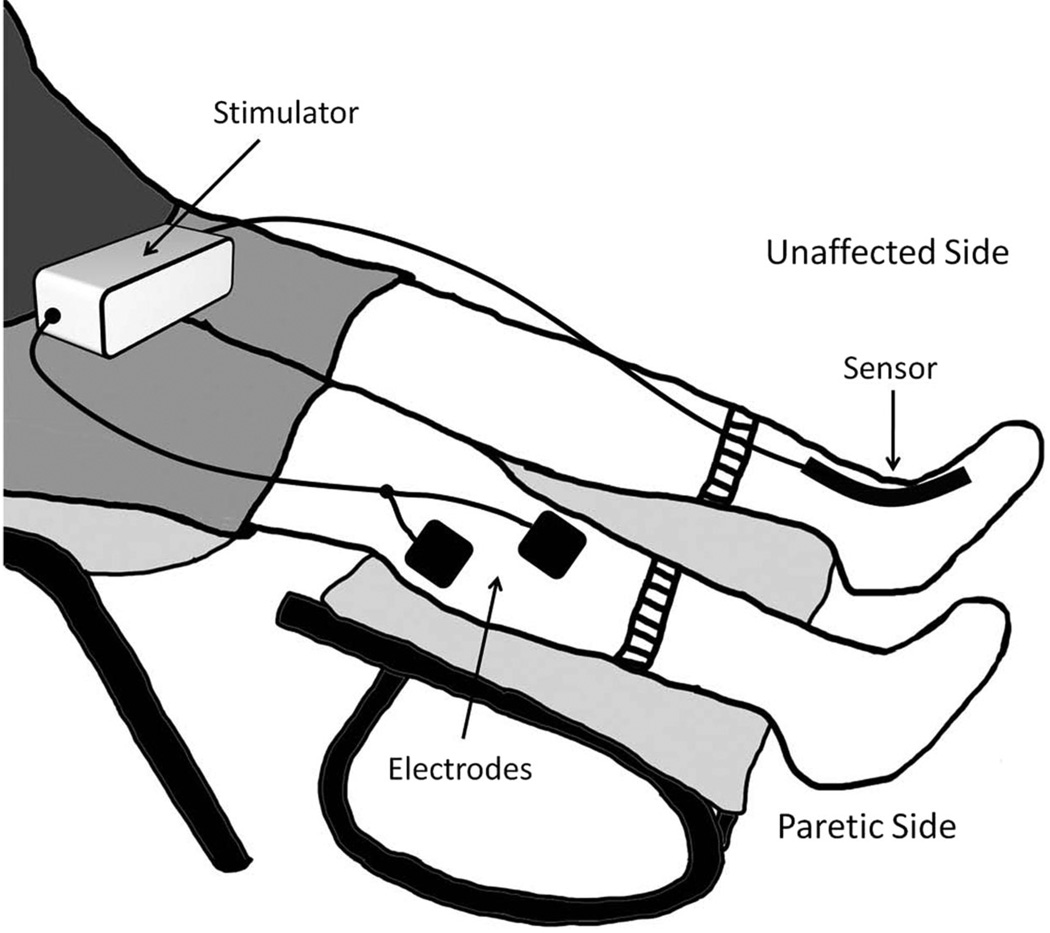
Contralaterally controlled neuromuscular electrical stimulation (CCNMES) for footdrop is a technique we have developed that aims to promote recovery of voluntary ankle dorsiflexion. CCNMES activates the paretic ankle dorsiflexors through surface electrodes positioned over the peroneal nerve and tibialis anterior muscle. The stimulation intensity is directly proportional to the degree of volitional dorsiflexion of the unimpaired contralateral ankle, which is detected by a sensor attached to a sock worn on the unaffected foot (Fig. 1). Thus, volitional dorsiflexion of the unaffected ankle (cognitive investment) produces stimulated dorsiflexion of the affected ankle, thereby enabling the individual to control their ankle movement. CCNMES treatment consists of using the stimulation system for a prescribed period of time to (1) perform active, repetitive ankle dorsiflexion exercises and (2) practice a goal-oriented motor control task that requires careful control of ankle dorsiflexion. Unlike FES footdrop systems, CCNMES is not used for walking. Rather, CCNMES is a motor control therapy that is designed to restore volitional ankle dorsiflexion so that an FES device or AFO is no longer needed to prevent footdrop during gait.
FIGURE 1.
Contralaterally controlled neuromuscular electrical stimulation (CCNMES) system. Volitional dorsiflexion of the unaffected ankle produces a proportional intensity of stimulation to the paretic ankle dorsiflexors.
This article reports on the first three stroke survivors with chronic (>6-mo poststroke) ankle dorsiflexor paresis to complete a 6-wk regimen of CCNMES and 3 mos of follow-up. The purpose of this pilot study was to evaluate the feasibility of applying CCNMES treatment to chronic stroke survivors and to investigate whether CCNMES might reduce ankle motor impairment.
METHODS
Participants
Participants were recruited from a stroke rehabilitation outpatient clinic of an academic medical center and assessed for eligibility using the following inclusion criteria: (1) ≥6 mos from hemorrhagic or nonhemorrhagic stroke, (2) ankle dorsiflexion strength while seated of ≤2/5 (Medical Research Council scale), (3) passive range of motion of paretic ankle to neutral, (4) surface electrical stimulation of peroneal nerve and tibialis anterior while seated with lower legs supported dorsiflexes the ankle to neutral without pain, (5) if ambulatory, demonstrates footdrop during ambulation such that gait instability or inefficient gait patterns are exhibited, and (6) demonstrates sufficient cognition to use the CCNMES system as instructed with the assistance of a caregiver if necessary. Individuals were excluded if they had intramuscular botulinum toxin injections in any lower limb muscle within the 3-mo period preceding study entry, uncompensated hemineglect, acute signs or symptoms of deep vein thrombosis or thromboembolism, edema of the affected lower leg, or absent sensation of the affected lower leg. The study protocol was approved by the institutional review board of the medical center, and written informed consent was obtained from each subject.
Electrical Stimulation System and Stimulation Parameters
The CCNMES system consists of a stimulator, a bend sensor attached to a sock, and surface electrodes (Fig. 1). The stimulator is a multipurpose custom-built multichannel programmable unit that can deliver up to seven independent monopolar channels (using a common anode) of biphasic current with pulse parameter ranges that are suitable for surface stimulation (0–250 µsecs, 0–100 mA, and up to 100 Hz). Only one channel (two electrodes) was needed for this study. One electrode was positioned just below the head of the fibula over the common peroneal nerve, and the second electrode was positioned over the motor point of the tibialis anterior muscle of the paretic leg to achieve balanced ankle dorsiflexion. The electrode positions were adjusted until full balanced dorsiflexion was achieved without discomfort. Additional channels of stimulation could have been used to recruit additional muscles to achieve balanced dorsiflexion, but we did not find this to be necessary. Square 2 × 2 in self-adhering pregelled electrodes were used (PALS, Axelgaard Manufacturing Co., Fallbrook, CA).
A single 4.5 in-long × 0.25 in-wide bend sensor (Images SI Inc., Staten Island, NY) enclosed in a velcro sheath was attached to a sock worn on the unaffected foot. The sensor was positioned so that it crossed the dorsal aspect of the ankle and was connected by cable to the stimulator. When the sensor bends with dorsiflexion or plantarflexion of the unimpaired ankle, impedance changes that are proportional to the amount of bend modulate the analog voltage input to the stimulator. This voltage input proportionally modulates the stimulation intensity (pulse duration) delivered through the electrodes on the paretic leg.
Stimulation parameters that produced balanced maximum ankle dorsiflexion were determined empirically for each subject and programmed into the stimulator. The strength of muscle contraction (and consequent degree of dorsiflexion) was modulated by changing the stimulus pulse duration in accordance with the voltage input from the bend sensor on the unaffected foot. As the participant moved the unaffected foot from its resting posture to full dorsiflexion, the corresponding voltage input mapped to a range of stimulus pulse durations. The minimum pulse duration was just below that which produced a faint sensation (sensory threshold) and corresponded to when the unaffected foot was at its resting posture. The maximum pulse duration was that which produced full ankle dorsiflexion without pain or discomfort and corresponded to when the unaffected foot was fully dorsiflexed. A fixed pulse frequency of 35 Hz and pulse amplitude of 40 mA were chosen as default values based on previous experience. If the longest pulse duration available (250 µsecs) did not produce full ankle dorsiflexion and then the current amplitude was increased.
To assist the participants in properly positioning the electrodes and performing the CCNMES ankle dorsiflexion exercises at home, a picture of the electrodes in their proper positions on the participant’s leg was taken and given to them to take home. Also, a user’s manual detailing how to put on and use the CCNMES system at home was reviewed with each subject and, if needed, their caregiver before they were sent home with it.
Intervention
The 6-wk intervention period consisted of two components: (1) self-administered active repetitive ankle dorsiflexion exercise daily at home and (2) ankle dorsiflexion tracking task practice performed twice a week in the research laboratory. Both of these components of the intervention involved the use of the CCNMES system.
The home exercise program consisted of two 55-min sessions per day, each session consisting of three 15-min sets separated by 5 mins of rest. During each 15-min set, the subjects were prompted by light and sound cues produced by the stimulator to repeatedly attempt to dorsiflex both ankles simultaneously for several seconds and then relax both ankles for several seconds. The ankle dorsiflex/relax cue durations were adjusted approximately every 2 wks during the 6-wk treatment period from 5/20 secs dorsiflex/relax to 6/16–8/14 secs. The purpose of the graded duty cycle was to prevent fatigue early in the intervention and to build greater strength with more repetitions and longer duration muscle contractions as the treatment progressed. The subjects were instructed to sit upright or slightly reclined in a comfortable chair with both lower legs extended and supported at the calves so that the feet did not touch the floor and ankle movement was not blocked by the chair or footrest. The subjects were instructed to separate the two exercise sessions by at least 2 hrs to avoid fatigue and to write in a diary (provided) indicating when they did their sessions. The diaries were collected weekly. Along with the diaries, compliance was monitored by the data logging capability of the stimulator, which logged the date and time the unit was turned on and off and the date and time of the start and completion of the cues corresponding to each set in an exercise session. On days that the subjects came to the laboratory for ankle motor control task practice (see below), they were to perform only one exercise session at home for at least 2 hrs after the laboratory session. Thus, each participant was prescribed 12 sessions of self-administered CCNMES exercise per week for 6 wks, for a total of 72 sessions.
The laboratory sessions twice a week focused on 15 mins of ankle dorsiflexion tracking task practice. After donning the CCNMES system, the participant was seated facing a computer screen with two parallel traces scrolling horizontally right to left across the screen, creating a “path.” The path was characterized by stretches of gradually rising and falling slopes and interspersed periods of abrupt jumps up and down during the course of 15 mins. An electrogoniometer that was taped across the paretic ankle displayed a cursor on the screen, its vertical position corresponding to the degree of dorsiflexion of the paretic ankle. The subjects’ lower limbs were extended and supported at the calves so that the feet did not touch the floor and were free to move at the ankles. The vertical range of the scrolling traces was set to correspond to the paretic ankle dorsiflexion/plantarflexion range achievable with the CCNMES stimulator. The subjects’ task was to keep the cursor between the parallel scrolling traces (i.e., on the path) by using the CCNMES system to carefully dorsiflex and plantarflex the paretic ankle. The subjects were instructed to exert effort to dorsiflex and plantarflex both ankles simultaneously during this task. The magnitude of deviation from the path was registered as an error, which was displayed on the screen and accumulated as the task progressed. At the end of the 15-min task, a final error score was recorded and used as a goal during the next session. If the subject did not feel too fatigued, he or she repeated the 15-min tracking task after resting 5 mins.
Assessment
Assessments of lower limb motor impairment were made four times before the 6-wk treatment, at the end of the treatment period, and 1 and 3 mos thereafter. The four pretreatment assessment sessions (baseline assessments) were made within the 2-wk period preceding the 6-wk treatment phase. The assessments included (1) maximum voluntary ankle dorsiflexion angle, (2) ankle movement tracking error, and (3) the lower limb portion of the Fugl-Meyer assessment of motor impairment. For the end-of-treatment assessment, the participants were instructed to refrain from using the stimulator for at least 24 hrs before the assessment to prevent muscle fatigue or eliminate any possible short-term carryover effect from affecting the outcomes.
Maximum voluntary ankle dorsiflexion angle was measured using an electrogoniometer (Biometrics Ltd., Gwent, United Kingdom) taped to the anterior aspect of the foot, so that the sensor spanned the ankle without impeding foot movement. The subjects were seated in a chair that was raised, so that the feet did not touch the floor. The thigh and lower leg were restrained with straps to prevent hip flexion and knee extension. From a resting posture, subjects were prompted by an audio tone to maximally dorsiflex the ankle for 4 secs. The subjects were instructed to respond as quickly and completely as possible to the audio tone. After one practice trial, three ankle dorsiflexion trials were run, separated by 1 min of rest to reduce the likelihood of fatigue affecting the results. The average dorsiflexion angle attained during the last second of the audio tone was calculated for each trial, and these magnitudes were averaged for the three trials.
Ankle movement tracking is an assessment of ankle motor control.13 The tracking assessment, using the same setup as in the maximum voluntary ankle dorsiflexion assessment, was similar to the tracking task described earlier, except that the track in the assessment was a 30-sec long single sine-wave trace with a frequency of 0.1 Hz (1 cycle in 10 secs), which scrolled right to left across the screen. This is a different trace than the path used in the dorsiflexion tracking task that was part of the treatment. The subject’s voluntary range of motion achieved the day of the assessment, as determined from the preceding test, was used to set the vertical range of the sine-wave track. The resting ankle angle was defined as 0% of the subject’s ankle range of motion, and the maximum dorsiflexion angle was defined as 100%. The peaks of the sine-wave track were then set at 85% of this range and the troughs of the sine-wave track were set at 15%; thus, the track was scaled to the middle 70% of the subject’s voluntary range of motion achieved the day of the assessment.13 Scaling the track to the subject’s voluntary range focuses the assessment on motor control and eliminates lack of adequate active range of motion as a reason for poor performance. The subject’s task was to try to keep the cursor on or as close to the scrolling sine-wave trace as possible by voluntarily dorsiflexing and plantarflexing the ankle with no stimulation. Each trial lasted 30 secs. A practice trial preceded three trials, which were separated by 1 min of rest to reduce the likelihood of fatigue affecting the results. The vertical distance from the cursor to the target trace was calculated for every time point of data collected. The final error for each trial was the average vertical distance (percentage of the subject’s range of motion) from the cursor to the target trace, and these magnitudes were averaged for the three trials. Errors less than ~3 (i.e., off the track by <3% of the subject’s range of motion) are expected to be in the normal range.
The Fugl-Meyer assessment is a valid and reliable measure of poststroke motor impairment.14 For the purpose of this study, only the lower limb motor impairment component of the Fugl-Meyer assessment was used. The subjects were asked to attempt to make various isolated and simultaneous movements of the hip, knee, and ankle. The measure takes into account synergy patterns as well as isolated strength, coordination, and hypertonia. Each requested movement was graded on a three-point ordinal scale (0, cannot perform; 1, perform partially; and 2, perform fully) by a trained and experienced physical therapist and summed to provide a final score. The maximum score of the lower limb Fugl-Meyer assessment is 34.
RESULTS
Subject 1
Subject 1 was a 57-yr-old man who sustained a right hemisphere cortical infarct complicated by a hemorrhagic conversion 6 yrs before treatment entry (Table 1). He presented with left upper and lower limb hemiparesis and was a community ambulator but required a molded AFO to help clear his toes during the swing phase and used a straight cane for balance. He walked with a stiff-legged gait with reduced hip and knee flexion and circumduction of the hemiparetic limb. Before treatment, he exhibited both flexion and extension synergy patterns of the lower limb while supine, mixed dynamic flexor and extensor synergy while seated, and no evidence of isolated knee or ankle movements while standing. This is consistent with a lower limb Fugl-Meyer score of 16 (average of four baseline assessments). He exhibited voluntary left ankle dorsiflexion to 59 degrees while seated, a modified Ashworth score of 1+ of the left ankle plantarflexors, and reduced sensation of the left lower leg. His comorbidities included diabetes mellitus, hypertension, and history of seizure disorder. He took antiepileptic medication (levetiracetam) for poststroke seizures, which he maintained throughout the study. He had no seizure for >3 yrs before starting the study. He reported occasional mild depression; he exhibited no cognitive deficits. Full stimulated ankle dorsiflexion was achieved with 35 Hz, 80 mA, and 200 µsecs current pulses. Subject 1 was able to put on the electrodes, sock, and sensor independently. He completed 49 (68%) of the prescribed 72 prescribed home exercise sessions, according to the stimulator’s datalogger, and he kept all 12 of the laboratory visits. During the laboratory visits, he performed the 15-min tracking task once.
TABLE 1.
Baseline characteristics of three stroke survivors receiving 6 wks of CCNMES treatment for ankle dorsiflexor paresis
| Subject | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| Age, yrs | 57 | 43 | 44 |
| Sex | M | F | F |
| Time since stroke, yrs | 6.0 | 6.7 | 6.9 |
| Type of stroke | Thrombotic, cortical | Intracerebral hemorrhage | Thrombotic, cortical |
| Paretic side | L | L | R |
| Sensation | Reduced | Intact | Intact |
| LE Fugl-Meyer scorea | 16 | 18 | 23 |
Average of four baseline assessments.
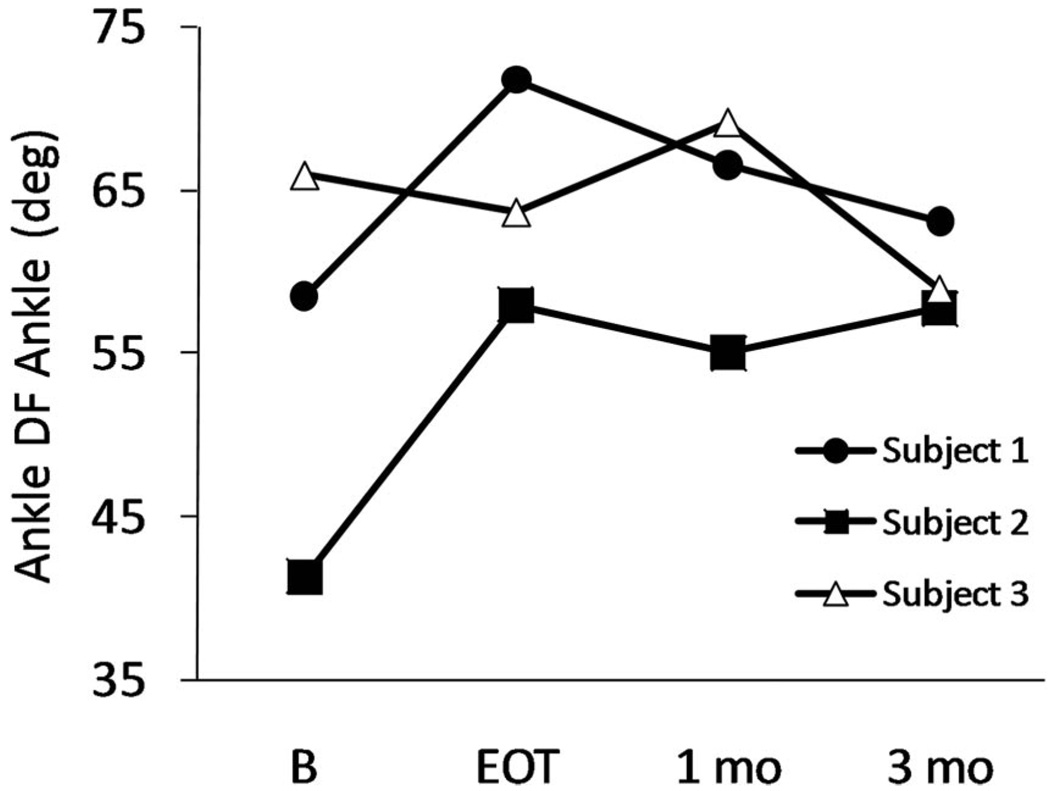
His maximum voluntary ankle dorsiflexion angle increased from an average of 59 degrees at baseline to 72 degrees by the end of treatment, for a gain of 13 degrees (Fig. 2 and Table 2). This gain progressively diminished at the 1- and 3-mo follow-up assessments. His ankle movement tracking error decreased from an average of 28 at baseline to 12 at the end of treatment, a 57% decrease (Table 2). He maintained this tracking performance during the 3-mo follow-up period. His lower limb Fugl-Meyer score increased four points by the end of treatment, but the gain did not persist at the follow-up assessments (Table 2).
FIGURE 2.
Maximum voluntary ankle dorsiflexion angle. Data points at baseline are the average values from four baseline assessment sessions. DF, dorsiflexion; B, baseline; EOT, end of treatment.
TABLE 2.
Lower limb motor impairment values of three stroke survivors receiving 6 wks of CCNMES treatment for ankle dorsiflexor paresis through 3 mos follow-up
| Subject | B1 | B2 | B3 | B4 | Bavg | EOT | 1 mo | 3 mos |
|---|---|---|---|---|---|---|---|---|
| Maximum voluntary ankle dorsiflexion angle, degrees | ||||||||
| 1 | 58 | 62 | 59 | 55 | 59 | 72 | 67 | 63 |
| 2 | 43 | 31 | 38 | 53 | 41 | 58 | 55 | 58 |
| 3 | 71 | 63 | 64 | 66 | 66 | 64 | 69 | 59 |
| Ankle movement tracking error | ||||||||
| 1 | 20 | 34 | 32 | 25 | 28 | 12 | 11 | 13 |
| 2 | 40 | 30 | 25 | 26 | 30 | 13 | 15 | 7 |
| 3 | 19 | 15 | 15 | 14 | 16 | 14 | 16 | 17 |
| Lower limb Fugl-Meyer score (maximum = 34) | ||||||||
| 1 | 13 | 17 | 17 | 18 | 16 | 20 | 16 | 17 |
| 2 | 16 | 19 | 19 | 19 | 18 | 23 | 23 | 23 |
| 3 | 23 | 24 | 24 | 22 | 23 | 22 | 23 | 23 |
B, baseline; Bavg, average of four baseline assessments; EOT, end of treatment.
Subject 2
Subject 2 was a 43-yr-old woman who sustained an intraventricular hemorrhage in the right frontal lobe measuring 6 cm in diameter with mild extension into the anterior aspect of the right lateral ventricle 6.7 yrs before treatment entry (Table 1). She presented with left upper and lower limb hemiparesis and was a community ambulator but required a molded AFO to help clear her toes during the swing phase and used a straight cane for balance. She walked with reduced hip and knee flexion during the swing phase and compensated with left hip external rotation with a forward flexed pelvis and hip retraction. Similar to subject 1, before treatment, she exhibited both flexion and extension synergy patterns while supine, mixed dynamic flexor and extensor synergy while seated, and no evidence of isolated knee or ankle movements while standing. This is consistent with a lower limb Fugl-Meyer score of 18 (average of four baseline assessments). She exhibited voluntary left ankle dorsiflexion to 41 degrees while seated, a modified Ashworth score of 1 of the left ankle plantarflexors, and no appreciable sensory deficit of the left lower leg. She took citalopram hydrobromide for history of depression and gabapentin for cervical radicular pain, medications that she maintained throughout the study. She exhibited no evidence of cognitive deficits. Full stimulated ankle dorsiflexion was achieved with 35 Hz, 40 mA, 160 and µsecs current pulses. Subject 2 was able to put on the electrodes, sock, and sensor independently. The stimulator’s datalogger indicated that she completed 54 (75%) of the 72 prescribed home exercise sessions, and she kept 10 of the 12 laboratory visits. At nearly all of the visits, she performed the 15-min tracking task twice, resting 5 mins between them.
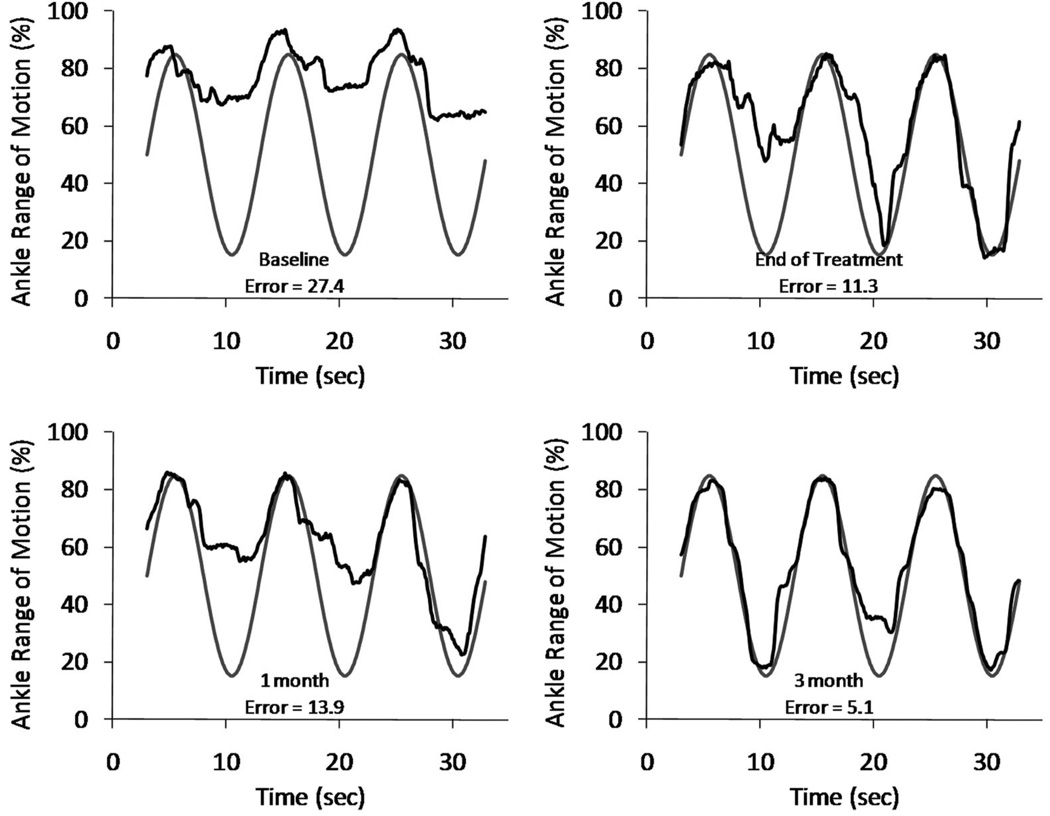
Her maximum voluntary ankle dorsiflexion angle increased from an average of 41 degrees at baseline to 58 degrees by the end of treatment for a gain of 17 degrees (Fig. 2 and Table 2). This gain persisted for 3 mos. Her ankle movement tracking error decreased from an average of 30 at baseline to 13 at the end of treatment, a 57% decrease (Table 2). She maintained this improvement in tracking during the follow-up period and in fact had further reduction in error at the 3-mo assessment (Fig. 3). Her Fugl-Meyer score increased by five points, and this gain persisted throughout the follow-up period (Table 2).
FIGURE 3.
Sample trials of ankle movement tracking assessment for subject 2. Vertical axis represents the subject’s ankle range of motion achieved that day, such that 100% corresponds to maximum voluntary dorsiflexion and 0% corresponds to ankle resting angle. Error indicated is for that particular trial.
Subject 3
Subject 3 was a 44-yr-old woman who sustained a left middle cerebral artery infarct 6.9 yrs before treatment entry (Table 1). She presented with right upper and lower limb hemiparesis. She ambulated in the community with an AFO independently. She ambulated independently in the home without the AFO. She exhibited a stiff-legged gait with reduced hip and knee flexion and no ankle dorsiflexion. Before treatment, she exhibited flexor and extensor synergy patterns of the paretic lower limb while supine, mixed dynamic flexor and extensor synergy while seated and isolated knee flexion, but not ankle dorsiflexion while standing. This consistent with a lower limb Fugl-Meyer score of 23 (average of four baseline assessments). She exhibited voluntary right ankle dorsiflexion to 66 degrees while seated, a modified Ashworth score of 2 of the right plantarflexors, and no sensory deficit of the right lower leg. She received medication for hypertension, which she maintained throughout the study protocol. There were no signs of depression. According to her caregiver, she had a premorbid learning disability, which prevented her from maintaining any gainful employment. The subject scored a 24 of 30 on a mini-mental state examination performed 1 yr before study enrolment; she was not able to demonstrate appropriate attention and calculation and exhibited difficulty with short-term memory. Full stimulated ankle dorsiflexion was achieved with 35 Hz, 40 mA, and 138 µsecs current pulses. Subject 3 relied on a caregiver to help her position the electrodes and put on the sock and sensor. She completed 39 (54%) of the 72 prescribed home exercise sessions according to the datalogger of the stimulator and kept 11 of the 12 laboratory visits. During the laboratory visits, she performed the 15-min tracking task once per visit.
For subject 3, the maximum voluntary dorsiflexion angle was 66 degrees at baseline and 64 degrees at the end of treatment; it increased to 69 degrees at 1 mo and decreased to 59 degrees at 3 mos (Fig. 2 and Table 2). Her ankle movement tracking error at baseline was 16 and remained fairly constant throughout the treatment and follow-up periods (Table 2). Similarly, her Fugl-Meyer score remained fairly constant throughout the study (Table 2).
DISCUSSION
This is the first study to apply the concept of contralaterally controlled electrical stimulation for the purpose of restoring voluntary ankle dorsiflexion. Our first application of the CCNMES paradigm was in the upper limb, where we stimulated the paretic hand of stroke survivors to open in response to volitional opening of the contralateral unaffected hand wearing an instrumented glove.15,16 This scheme puts the patient back in control of their hand and enables them to use it to practice functional tasks. The subjects in our pilot studies experienced reductions in hand impairment and were very enthusiastic about the treatment. Therefore, in addition to continuing our investigation of CCNMES for upper limb motor recovery in acute and chronic stroke survivors, we initiated this study to investigate the feasibility of CCNMES to reduce lower limb impairment.
There are several possible advantages of the CCNMES motor rehabilitation paradigm over other existing rehabilitation techniques. First, CCNMES capitalizes on the principle of synchronizing central and peripheral neural activity. Previous animal and clinical studies support the hypothesis that therapies that temporally link motor intention (central) to motor output (peripheral) and sensory feedback from the affected limb may facilitate neuroplastic changes leading to motor recovery.3,5 The Hebbian mechanisms believed to underlie activity-dependent cortical reorganization suggest that rehabilitation therapies that repeatedly generate synchronous pre- and postsynaptic neural activity along motor and sensory pathways might facilitate synaptic remodelling leading to neural reorganization and possibly motor recovery.17–20 More than other electrical stimulation paradigms (e.g., cyclic neuromuscular electrical stimulation, electromyography-triggered neuromuscular electrical stimulation, or FES footdrop systems), CCNMES maximizes the degree of synchronization between motor intention (central or presynaptic activity) and stimulated motor response (peripheral or postsynaptic activity) by making the stimulation intensity proportional to the amplitude of the control signal that the user produces volitionally (volitional dorsiflexion of the unimpaired contralateral ankle). Thus, the user not only controls the onset and duration of the stimulation (as in heelswitch- or electromyography-triggered stimulation) but also controls the intensity of the stimulation and resultant ankle movement, potentially creating a stronger perception of restored motor control. In summary, CCNMES provides motor output (peripheral efferent activation) and corresponding muscle and joint proprioceptive feedback (afferent activation) that are tightly coupled to the participants’ cognitive motor intention (bilateral central efferent activity) to dorsiflex both ankles.
Second, the CCNMES paradigm incorporates bilateral symmetric movement. Bilateral symmetric movement of the paretic and nonparetic upper limbs has been shown to reduce upper limb impairment in chronic stroke survivors,21 although it is not always efficacious.22 Other studies have shown that voluntary contraction of muscles in the nonparetic hand increases the excitability of contralateral motor pathways,23 and that bilateral gripping tasks enhance activation in the primary motor cortex of the affected hemisphere compared with unilateral paretic hand movement.24 Similar facilitatory effects of bilateral symmetric movement may be possible in the lower limb, but this has not been studied. A theoretical model proposes that simultaneous bilateral movement promotes interhemispheric disinhibition, which may allow reorganization by sharing of normal movement commands from the undamaged hemisphere.25 Disinhibition may also encourage recruitment of undamaged neurons to construct new task-relevant neural networks. In addition, it may be possible for ipsilateral, corticospinal neural pathways to take over motor control.26
Third, with CCNMES, the active motor intention that the patient must give to dorsiflexing the ankle during exercise and motor control task practice is much greater than with FES footdrop systems for ambulation. The CCNMES paradigm allows the individual to practice a goal-oriented motor control task (i.e., tracking) that requires carefully controlled ankle dorsiflexion. Although the ankle dorsiflexion tracking task may not be directly functional such as walking, it is a goal-oriented task that requires cognitive investment by having the subject concentrate on controlling the degree of dorsiflexion. This task requires a similar, perhaps even greater, degree of ankle motor control as operating a standard automobile foot pedal accelerator. It is a task that develops motor control skill and potentially creates a strong perception that motor intention is producing the desired modulated motor output. Creating a perception of restored motor control is important in motor rehabilitation and forms part of the basis of interventions such as motor imagery,27 mirror therapy,28 and virtual reality.29
Fourth, with CCNMES a higher dosage of stimulation can be delivered than with FES footdrop systems, which require the individual to walk to receive stimulation. With CCNMES, the subject performs the active repetitive movement exercise portion of the treatment while sitting comfortably at home. Teasell et al’s30 analysis of the role of therapy intensity concludes that, in general, the greater the time exposure to stroke rehabilitation therapies the better the outcomes.
Finally, CCNMES provides a method for mediating active repetitive movement even in severely hemiparetic individuals who have no residual ankle movement. Severe hemiparesis prevents a large segment of stroke survivors from participating in activity-dependent therapies that require the patient to generate active movements, but with CCNMES, the individual can provide the active motor intention that produces the subsequent motor response because the control signal is derived from movement of the unimpaired side, not the affected side. Thus, CCNMES may be more widely applicable than other treatments, and it may be also less expensive and require less therapist time because it can be self-administered, in part, at home.
Three stroke survivors with chronic footdrop participated in this first study of CCNMES. Two of the three participants showed improvements on three lower limb motor impairment measures, suggesting that some stroke survivors with chronic footdrop might benefit from CCNMES.
Subject 1 experienced improvements in maximum voluntary ankle dorsiflexion, ankle movement motor control (reduction in tracking error), and lower limb Fugl-Meyer score. His motor control gains persisted for 3 mos, but his maximum ankle dorsiflexion and Fugl-Meyer score diminished during the follow-up period. This is similar to what was seen in the upper limb, where active finger extension declined during the follow-up, but tracking performance was maintained.16 Why some outcomes persisted and others did not may be because neuromuscular electrical stimulation strengthened the target muscles during the treatment period, and once the treatment ended the muscles became weak again causing losses in active ankle dorsiflexion and ability to perform the movements requested during the Fugl-Meyer assessment. Loss of strength, however, would not necessarily cause a decrease in tracking performance, because the tracking task relies more on motor coordination than strength. The problem of nonenduring improvements could possibly be addressed by redosing with CCNMES after certain periods of time.
Subject 2 also experienced improvements on all three impairment measures. Figure 3 reveals how her ankle motor control improved over time. At baseline, she had difficulty relaxing her dorsiflexors and letting her foot plantarflex; by the end of treatment, she was better able to relax her dorsiflexors on demand. She experienced the most improvement of the three subjects and had the most persistent gains during the 3-mo follow-up period. In addition, she was the most compliant with the CCNMES home exercise regimen, she was the only participant who was able to consistently practice the 15-min tracking task twice per session in the laboratory, and she seemed to have a busier lifestyle that required her to be out of ambulating in the community more than the other two subjects. These factors may have contributed to her slightly better and more persistent results.
Subject 3 did not improve appreciably on any of our lower limb motor impairment measures. All of her scores remained relatively constant throughout the study. It is unknown why subjects 1 and 2 experienced measureable reductions in ankle impairment but subject 3 did not. Several stroke rehabilitation studies have shown that response to treatment is related to baseline impairment severity. With respect to the measures used in this study, subject 3 was less severely impaired at baseline than subjects 1 and 2. On the basis of other studies, we would have expected that subjects with less impairment would improve more than subjects with severe impairment.31–33 However, none of the subjects in this study could have been classified as having severe lower limb impairment; they all had some ankle movement while seated and were able to ambulate (with an AFO) independently. The lack of improvement in subject 3 does not seem to be related to her relative degree of baseline motor impairment.
Different lesion sizes and locations may account for the different responses among subjects to the motor relearning treatment. Unfortunately, greater detail about the subjects’ lesions was not available for this study. Another explanation for different responses might be differences in treatment dose received due to differences in adherence to the treatment protocol.30 Subject 3 completed fewer of the prescribed home exercise sessions than the other two subjects (54% vs. 68% and 75%). However, another possibility is that differences in cognitive deficits or depressive states could affect the capacity to cognitively invest in the CCNMES training and, therefore, might contribute to differences in the outcome among subjects. Subject 3 was the only participant with clear cognitive deficits manifested by impaired calculation and concentration and impaired short-term memory on the mini-mental state examination administered 1 yr before study enrolment.
The participants’ subjective response to the intervention was positive. In a questionnaire given at the end of treatment, all three subjects strongly agreed with the statement, “I wish this intervention was part of my original therapy.” Subjects 1 and 2 agreed with the statement, “The exercises associated with this study improved my ankle movement.” Subject 1 stated at the end-of-treatment assessment that when he walked without his AFO he noticed that his foot did not drag and that he can “walk without shoes with no problem.” At the 3-mo assessment, subject 1 said that in comparison with baseline, “I’m walking much better, but if I walk too fast, it [my foot] still catches.” All three subjects indicated that the 2 hrs of exercise per day done at home was not too much, that the 6-wk treatment period was not too long, that it was not difficult to put on the electrodes correctly, and that the stimulation was not uncomfortable. All three subjects agreed that the exercises at home and the tracking task done in the laboratory required a lot of mental concentration. Subjects 1 and 2 strongly agreed with the statement, “I think I would get more benefit if I continued to use the stimulation system.”
This pilot study did not examine the effect of CCNMES on gait; therefore, it is unknown whether the reductions in lower limb and ankle impairment measured in this study translate to more stable gait and less footdrop during walking. Even though none of the subjects were able to achieve 90 degrees of active ankle dorsiflexion, the gains that subjects 1 and 2 did achieve could make a significant difference if they carry over to the condition of walking, in which control of ankle dorsiflexion is normally subconscious. The comment from subject 1 that he had less footdrop after the treatment phase suggests that there might be a positive effect on walking, even though all three subjects continued to use their AFOs after the treatment period. Future studies will add CCNMES to gait training and will include measures of kinematic and spatiotemporal parameters during gait and a gait functional measure. Dose-response characteristics will also be investigated.
CONCLUSIONS
This pilot study demonstrated the feasibility of using CCNMES at the paretic ankle to reduce lower limb impairment in chronic hemiplegia. The results of this study suggest that CCNMES may have positive effects in some stroke survivors. The small sample size of this study does not allow a conclusion to be made regarding the strength of association between CCNMES treatment and impairment reductions or the probability of causation. Nevertheless, further investigations are warranted and will focus on evaluating the effect of CCNMES on ambulation in both chronic and acute lower limb hemiplegia.
ACKNOWLEDGMENTS
We gratefully acknowledge Jennifer Nagy, PT, and Maureen T. Hennessey, PT, for performing the lower limb Fugl-Meyer assessments in this study.
This work was supported by Grant Numbers K12RR023264 and KL2RR024990 from the National Institutes of Health (NIH) National Center for Research Resources (NCRR) and by Grant Number K24HD054600 from the National Institute of Child Health and Human Development (NICHD). The neuromuscular electrical stimulators used in this study were developed by the Technical Development Laboratory of the Cleveland FES Center.
Footnotes
Disclosures:
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
REFERENCES
- 1.Wade DT, Hewer RL. Functional abilities after stroke: Measurement, natural history and prognosis. J Neurol Neurosurg Psychiatry. 1987;50:177–182. doi: 10.1136/jnnp.50.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jorgensen HS, Nakayama H, Raaschou HO, et al. Recovery of walking function in stroke patients: The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:27–32. doi: 10.1016/s0003-9993(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 3.Nudo RJ, Wise BM, SiFuentes F, et al. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 4.Butefisch C, Hummelsheim H, Denzler P, et al. Repetitive training of isolated movements improves the outcome of motor rehabilitation of the centrally paretic hand. J Neurol Sci. 1995;130:59–68. doi: 10.1016/0022-510x(95)00003-k. [DOI] [PubMed] [Google Scholar]
- 5.Luft AR, Macko RF, Forrester LW, et al. Treadmill exercise activates subcortical neural networks and improves walking after stroke: A randomized controlled trial. Stroke. 2008;39:3341–3350. doi: 10.1161/STROKEAHA.108.527531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hesse S. Treadmill training with partial body weight support after stroke: A review. NeuroRehabilitation. 2008;23:55–65. [PubMed] [Google Scholar]
- 7.Winchester P, Querry R. Robotic orthoses for body weight-supported treadmill training. Phys Med Rehabil Clin N Am. 2006;17:159–172. doi: 10.1016/j.pmr.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Hidler J, Nichols D, Pelliccio M, et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil Neural Repair. 2009;23:5–13. doi: 10.1177/1545968308326632. [DOI] [PubMed] [Google Scholar]
- 9.Husemann B, Muller F, Krewer C, et al. Effects of locomotion training with assistance of a robotdriven gait orthosis in hemiparetic patients after stroke: A randomized controlled pilot study. Stroke. 2007;38:349–354. doi: 10.1161/01.STR.0000254607.48765.cb. [DOI] [PubMed] [Google Scholar]
- 10.Burridge JH, Taylor PN, Hagan SA, et al. The effects of common peroneal stimulation on the effort and speed of walking: A randomized controlled trial with chronic hemiplegic patients. Clin Rehabil. 1997;11:201–210. doi: 10.1177/026921559701100303. [DOI] [PubMed] [Google Scholar]
- 11.Stein RB, Chong S, Everaert DG, et al. A multicenter trial of a footdrop stimulator controlled by a tilt sensor. Neurorehabil Neural Repair. 2006;20:371–379. doi: 10.1177/1545968306289292. [DOI] [PubMed] [Google Scholar]
- 12.Kottink AI, Oostendorp LJ, Buurke JH, et al. The orthotic effect of functional electrical stimulation on the improvement of walking in stroke patients with a dropped foot: A systematic review. Artif Organs. 2004;28:577–586. doi: 10.1111/j.1525-1594.2004.07310.x. [DOI] [PubMed] [Google Scholar]
- 13.Carey JR, Anderson KM, Kimberley TJ, et al. fMRI analysis of ankle movement tracking training in subject with stroke. Exp Brain Res. 2004;154:281–290. doi: 10.1007/s00221-003-1662-7. [DOI] [PubMed] [Google Scholar]
- 14.Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63:1606–1610. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- 15.Knutson JS, Harley MY, Hisel TZ, et al. Improving hand function in stroke survivors: A pilot study of contralaterally controlled functional electric stimulation in chronic hemiplegia. Arch Phys Med Rehabil. 2007;88:513–520. doi: 10.1016/j.apmr.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knutson JS, Hisel TZ, Harley MY, et al. A novel functional electrical stimulation treatment for recovery of hand function in hemiplegia: 12-week pilot study. Neurorehabil Neural Repair. 2009;23:17–25. doi: 10.1177/1545968308317577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson BB. Brain plasticity and stroke rehabilitation. The Willis lecture. Stroke. 2000;31:223–230. doi: 10.1161/01.str.31.1.223. [DOI] [PubMed] [Google Scholar]
- 18.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 19.Malenka RC, Nicoll RA. Long-term potentiation—A decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 20.Rushton DN. Functional electrical stimulation and rehabilitation—An hypothesis. Med Eng Phys. 2003;25:75–78. doi: 10.1016/s1350-4533(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 21.Luft AR, McCombe-Waller S, Whitall J, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: A randomized controlled trial. JAMA. 2004;292:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards LG, Senesac CR, Davis SB, et al. Bilateral arm training with rhythmic auditory cueing in chronic stroke: Not always efficacious. Neurorehabil Neural Repair. 2008;22:180–184. doi: 10.1177/1545968307305355. [DOI] [PubMed] [Google Scholar]
- 23.Woldag H, Lukhaup S, Renner C, et al. Enhanced motor cortex excitability during ipsilateral voluntary hand activation in healthy subjects and stroke patients. Stroke. 2004;35:2556–2559. doi: 10.1161/01.STR.0000144651.07122.da. [DOI] [PubMed] [Google Scholar]
- 24.Staines WR, McIlroy WE, Graham SJ, et al. Bilateral movement enhances ipsilesional cortical activity in acute stroke: A pilot functional MRI study. Neurology. 2001;56:401–404. doi: 10.1212/wnl.56.3.401. [DOI] [PubMed] [Google Scholar]
- 25.Mudie MH, Matyas TA. Can simultaneous bilateral movement involve the undamaged hemisphere in reconstruction of neural networks damaged by stroke? Disabil Rehabil. 2000;22:23–37. doi: 10.1080/096382800297097. [DOI] [PubMed] [Google Scholar]
- 26.Fisher CM. Concerning the mechanism of recovery in stroke hemiplegia. Can J Neurol Sci. 1992;19:57–63. [PubMed] [Google Scholar]
- 27.Page SJ, Levine P, Leonard AC. Effects of mental practice on affected limb use and function in chronic stroke. Arch Phys Med Rehabil. 2005;86:399–402. doi: 10.1016/j.apmr.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Sutbeyaz S, Yavuzer G, Sezer N, et al. Mirror therapy enhances lower-extremity motor recovery and motor functioning after stroke: A randomized controlled trial. Arch Phys Med Rehabil. 2007;88:555–559. doi: 10.1016/j.apmr.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 29.Yang YR, Tsai MP, Chuang TY, et al. Virtual reality-based training improves community ambulation in individuals with stroke: A randomized controlled trial. Gait Posture. 2008;28:201–206. doi: 10.1016/j.gaitpost.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Teasell R, Bitensky J, Salter K, et al. The role of timing and intensity of rehabilitation therapies. Top Stroke Rehabil. 2005;12:46–57. doi: 10.1310/ETDP-6DR4-D617-VMVF. [DOI] [PubMed] [Google Scholar]
- 31.Dobkin BH. Strategies for stroke rehabilitation. Lancet Neurol. 2004;3:528–536. doi: 10.1016/S1474-4422(04)00851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyd LA, Quaney BM, Pohl PS, et al. Learning implicitly: Effects of task and severity after stroke. Neurorehabil Neural Repair. 2007;21:444–454. doi: 10.1177/1545968307300438. [DOI] [PubMed] [Google Scholar]
- 33.Chae J, Yu D. A critical review of neuromuscular electrical stimulation for treatment of motor dysfunction in hemiplegia. Assist Technol. 2000;12:33–49. doi: 10.1080/10400435.2000.10132008. [DOI] [PubMed] [Google Scholar]