Abstract
Context
Although ZAP-70 is required for T-cell development, it’s unclear how this kinase controls both positive and negative selection.
Objective and Methods
Using OT-I pre-selection thymocytes and a panel of pMHC ligands of defined affinity, the recruitment, phosphorylation and activity of ZAP-70 was determined at the interface with antigen-presenting cells.
Results
Peptide-MHC ligands promoting negative selection induce a discrete elevation of ZAP-70 recruitment, phosphorylation and enzymatic activity in the thymocyte:APC interface.
Discussion
The quantity of ZAP-70 kinase activity per cell is a key parameter controlling the fate of a developing thymocyte since partial inhibition of ZAP-70 kinase activity converted negative into positive selection. Surprisingly, the amount of ZAP-70 enzymatic activity observed during negative selection is not controlled by differential phosphorylation of the ZAP-70 protein but rather by the total amount of TCR and co-associated ZAP-70 recruited to the thymocyte:APC interface.
Conclusions
These data provide evidence that a burst of ZAP-70 activity initiates the signaling pathways for negative selection.
Keywords: negative selection, thymic development, TCR signaling
Introduction
Following αβ T-cell receptor (TCR) engagement by pMHC ligands, the Syk family protein tyrosine kinase (PTK) ZAP-70 is activated and phosphorylates several downstream target molecules (1–3). ZAP-70 initially binds to phosphorylated immunoreceptor tyrosine-based activation motifs (ITAMs) of the ζ and CD3ε subunits of the TCR complex through its tandem Src-homology region 2 (SH2) domain (4, 5). Subsequently, ζ-bound ZAP-70 is tyrosine phosphorylated (6–8) ZAP-70 is a key signaling component in thymocytes since ZAP-70 deficient mice are blocked in both positive and negative selection (9).
Studies have shown that ZAP-70 dysfunction can lead to autoimmunity. A spontaneous occurring single amino acid substitution in the second SH2-domain (W163C) alters TCR-mediated signaling and thereby the outcome of thymic selection, causes joint inflammation and infiltration of CD4+ T-cells, a pathology reminiscent of rheumatoid arthritis in humans (10). Apparently, the efficiency of negative selection is altered in these mutant mice due to the reduction of ZAP-70 activity. A similar result was obtained by Siggs et al. who studied point mutations within the zap-70 gene (11). In patients with selective T-cell deficiency (STD), who suffer from persistent infections reminiscent of severe combined immunodeficiency, a point mutation leads to alternative splicing of the zap-70 gene (12). This mutation results in a three amino acid insertion in the kinase domain, abolishing its enzymatic activity. T-cells from patients, homozygous for this point mutation exhibit markedly reduced tyrosine phosphorylation; nevertheless some Ca2+ mobilization remains in activated peripheral T-cells. The authors suggest that the Src kinase Fyn takes over some ZAP-70 functions in human peripheral T-cells. On the other hand, ZAP-70 deficiency in mice blocks both positive and negative selection of DP thymocytes (9). Another study demonstrates that a spontaneously occurring point mutation in the DLAARN motif (R464C) of ZAP-70’s kinase domain results in defective TCR signaling and a complete arrest of thymocyte development at the DP stage (13). These mice express a catalytically inactive form of ZAP-70, again demonstrating the requirement for ZAP-70 activity in thymocyte development.
The number of CD3 ITAMs and by the extension the quantity of ZAP-70 kinase activity seems to play a central role in the establishment of central tolerance. In transgenic mice expressing either class I or class II MHC restricted TCRs, the percentage of positively selected CD8+ or CD4+ SP cells, respectively, decreases by reducing the number of ζ chain ITAMs (14). This change of ITAM multiplicity alters the efficiency of thymic selection by reducing ZAP-70 binding and downstream signaling. Another study shows that reducing the number of CD3 and ζ-chain ITAMs, thereby lowering the TCR signal strength, results in autoimmunity due to a failure in deleting self-reactive T-cells, which are instead positively selected in the thymus (15). The authors suggest that the main reason for the TCR/CD3 complex to have a total of 10 ITAMs is rather quantitative than qualitative in order to guarantee scalable signaling and efficient negative selection. On the other hand, CD8+ T cells expressing the P14 transgenic TCR and normal CD3-γδε but non-functional ζ-ITAMs were positively selected and did not exhibit defective effector functions, suggesting more specific roles for the individual ITAMs of the TCR/CD3 complex (16). However, the P14 TCR may function somewhat independent of differential ITAM phosphorylation since low concentrations of antigen can mediate positive selection of P14 transgenic thymocytes (17).
In vitro, cell-free binding assays have revealed the parameters for ZAP-70 binding to the CD3 subunits (5). ZAP-70 binding to ζ or CD3ε through its tandem SH2-domain is highly cooperative, which ensures a high affinity for the doubly phosphorylated ζ or CD3ε ITAM. The binding affinities of the ZAP-70 tandem SH2-domains to peptides containing individual phosphorylated ζ or CD3ε ITAMs was measured and the hierarchy described as ITAM(ζ1) ≥ ITAM(ζ2) > ITAM (CD3ε) ≥ ITAM (ζ3) (18). Affinity differences of as much as 30-fold between the individual phosphor-ITAMs suggests that ZAP-70 may bind to ITAMs sequentially, provided that they are phosphorylated. Indeed, a study shows that ζ ITAM phosphorylation is sequential and appears to be ordered (19). The authors detected a basal level of partially phosphorylated ζ in resting T-cells whereas fully phosphorylated ζ is induced only upon stimulation with agonist pMHC ligands. On the other hand, antagonist pMHC ligands promote intermediate ζ phosphorylation in mature T-cells, which did not detectably activate ZAP-70. Similarly, T-cell clones stimulated with altered peptide ligands (APLs) induce only partial ζ ITAM phosphorylation, which greatly reduces ZAP-70 activation and PLC-γ1 mediated phospholipid hydrolysis (20).
Fully phosphorylated ζ dimers are sufficient to promote ZAP-70 trans-phosphorylation and kinase activity in vitro, whereas monomeric ζ fails to activate ZAP-70 (21). Interestingly, murine thymocytes exhibit a constitutive association of ZAP-70 with partially phosphorylated ζ, yet ZAP-70 is not activated (22, 23). The difference may originate from a negative regulation pathway, which is not active in cell-free assays. Indeed, the phosphatase SHP-1 increases its dephosphorylation activity by directly binding to ZAP-70, which negatively regulates ZAP-70 kinase activity (24, 25).
Several phosphorylation sites on ZAP-70 are important for increasing its kinase activity. Tyrosine 319 is a substrate for the Src kinase Lck and phosphorylation of this residue is a critical step in activating ZAP-70 kinase activity (26). Accordingly, a ZAP-70 mutant carrying an optimized binding site for Lck (Y319EEI instead of wildtype Y319SDP) displays a gain-of function phenotype, encompassing increased tyrosine phosphorylation and catalytic activity. On the other hand, substitution of tyrosine 319 with phenylalanine (Y319F) in the interdomain B region of ZAP-70 leads to dominant negative behavior (8). Y319F prevents phosphorylation of further ZAP-70 tyrosine residues such as Y493 in the activation loop, which ultimately prevents antigen-induced IL-2 expression. Another study suggests that Y315 and Y319 function as an autoinhibitory switch, thereby regulating ZAP-70’s kinase activity (27). By phosphorylating Y319 (and possibly Y315), Lck or other kinases activate ZAP-70, similar to the juxtamembrane region of several RTKs. These findings are supported by the crystallographic structure of ZAP-70 where phosphorylation at Y315 and Y319 in the interdomain B stabilizes a conformational change required to activate the kinase (28). In contrast to the Y319F mutant, a Y315A/Y319A mutant leads to Y493 phosphorylation, presumably via intermolecular trans-phosphorylation. The Y315A/Y319A mutant protein may be in an open conformation and is therefore released from autoinhibition, similar to wildtype Y319-phosphorylated ZAP-70 (27).
Tyrosine phosphorylation at residue Y493 is another positive regulatory step (6, 29). Substitution of Y493 with phenylalanine (Y493F) prevents ZAP-70 activation even if Y493F is co-expressed with constitutively active Lck. Furthermore, Lck is not very efficient in phosphorylating Y493 in a kinase-inactive ZAP-70 mutant (K369A), even when Y319 is phosphorylated and available for Lck-binding. This suggests that Y493 in wildtype ZAP-70 is poorly phosphorylated by Lck, if at all. Interestingly, the K369A kinase-inactive mutant is phosphorylated at both Y319 and Y493 in Cos or 293 cells. Therefore, it seems that Lck can inefficiently phosphorylate Y493 under certain conditions (26). Hypothetically, Lck may be responsible for the initial but rather inefficient phosphorylation of Y493 on several ZAP-70 molecules that trans-autophosphorylate other ZAP-70 molecules, which are present as dimers on paired ITAMs of the CD3 subunits.
The current study shows how the level of ZAP-70 activity controls the outcome of thymic selection. ZAP-70 is differentially recruited and phosphorylated in the interface of DP OT-I transgenic thymocytes with antigen presenting cells (APCs) loaded with positive or negative selecting peptides. pMHC ligands with a TCR affinity just over the negative selection threshold induce an abrupt increase in TCR and ZAP-70 recruitment at the interface formed between a DP thymocyte and a peptide-loaded APC. This discrete elevation of TCR and ZAP-70 recruitment occurring at the negative selection threshold is associated with a discontinuous increase in ZAP-70 enzymatic activity. This tightly regulated increase in ZAP-70 activity accounts for the transition from positive to negative selection. This is supported by experiments, which artificially reduce ZAP-70 enzymatic activity using a low molecular weight ZAP-70 kinase inhibitor. Use of this inhibitor also demonstrates that virtually all Y493 phosphorylation is dependent on ZAP-70 kinase activity and results from transphosphorylation. These data are consistent with the idea that the density of TCR/ZAP-70 at the thymocyte/APC interface determines the extent of Y493 phosphorylation, the amount of ZAP-70 activity and the outcome of thymic selection.
Methods
Animals
All animal experiments have been carried out in accordance with the cantonal and federal laws in Switzerland, regulating the humane treatment of laboratory animals.
Peptides and antibodies
OVA variant peptides were synthesized and purified as described {Alam, 1996 #55;Santori, 2002 #56} and had the following affinity hierarchy for the OT-I TCR: OVA > Q4 > Q4R7 > Q4H7 > G4 > VSV {Mallaun, 2008 #41;Daniels, 2006 #51}. Anti-CD4 (RM4-5), anti-CD8β (53-5.8), anti-Vα2 (B20.1), anti Vβ5 (MR9-4) and capturing anti-ZAP-70 (cl. 29) are from BD Biosciences. Probing antibodies anti-ZAP-70 (99F2), anti-p-Y319 (65E4), anti-p-Y493 (2704) are from Cell Signaling. For flow cytometry, fluorochrome-conjugated anti-ZAP-70 (1E7.2) is from Santa Cruz Biotechnology. Anti-phosphotyrosine (4G10) and anti-LAT (rabbit polyclonal) are from Upstate. Capturing and probing anti-CD3ε (145-2C11) and anti-ζ (H146-968) respectively, were from purified hybridoma supernatants.
Microscopy
CD19+ positive B-cells from C57 Bl/6 mice were used as APCs for microscopy. Spleen cell suspensions were subjected to red blood cell lysis and depletion of CD4, CD8α and NK1.1 positive cells by monoclonal antibodies and binding to magnetic beads (AutoMACS system; Miltenyi Biotec). Non-depleted cells (85–90% CD19 positive) were stained with Cy5 monoreactive dye (GE Healthcare) and loaded with 2μM peptide. Thymocytes from OT-I Rag−/−β2m−/− transgenic mice were stimulated with peptide-loaded APCs (1:1 ratio) by brief centrifugation and incubation at 37°C. Fixation with formaldehyde, permeabilization with Triton X-100 and staining with monoclonal antibodies was carried out on (3-aminopropyl)triethoxysilane (Sigma) coated cover slips. Microscopy was performed on an Olympus IX81 fluorescence microscope. The recruitment or phosphorylation of fluorescently labeled proteins to the contact area with APCs was calculated as background-corrected ratio of the mean fluorescence intensity (MFI) at the thymocyte:APC interface divided by the MFI of a membrane region outside the thymocyte:APC interface: ‘Fold recruitment/phosphorylation’ = (MFIInterface − MFIbkgd)/( MFIMembrane − MFIbkgd). Values were plotted as mean values ± SEM using the Prism software (GraphPad Software Inc.). Statistical analysis was performed using Student’s two-sample t test assuming two-tailed distribution and unequal variance.
Stimulation of DP thymocytes and immunoprecipitation
APCs (3LBM 13.1 B-cell hybridomas) expressing H-2Kb were loaded with 2∝M peptide, followed by fixation with 0.1% glutaraldehyde. DP thymocytes from OT-I Rag−/−β2m−/− transgenic mice were stimulated with peptide-loaded APCs by brief centrifugation and incubation at 37°C. For immunoprecipitations, cells were lysed with 1% nonionic detergent (Brij58 for TCR-IPs, NP-40 for LAT-IPs and digitonin for ZAP-70 IPs) and isotonic lysis buffer to generate post-nuclear lysates. Immunoprecipitations were performed with 1μg anti-CD3ε (145-2C11) monoclonal antibody and protein G sepharose (GE Healthcare). SDS-PAGE under reducing conditions and Western blotting was performed according to standard techniques. Nitrocellulose membranes were probed with primary antibodies and subsequently with horseradish peroxidase (HRP)-conjugated secondary antibodies. HRP mediated conversion of the ECL-reagent (GE Healthcare) was detected on ECL hyperfilms (GE Healthcare). Films were developed on a Curix80 processor (Agfa) and analyzed using the Gel Doc 2000 densitometer and the Quantity One software (BioRad). If required, membranes were stripped with Restore Western blot stripping buffer (Thermo Scientific) and reprobed. Mean grey values of ZAP-70 and phospho-specific bands were background corrected and normalized to the loading control (ζ) and the values measured at 0min.
In vitro kinase assay
ZAP-70 was co-immunoprecipitated from activated TCR complexes using the monoclonal anti-CD3ε (145-2C11) antibody. Immunoprecipitated complexes were incubated with 0.5μg recombinant LAT (Upstate), 5mM MgCl2 and 10μM ATP for 10min at 37°C. Reducing SDSPAGE, Western blotting and membrane probing was performed as described above.
Fetal thymic organ culture (FTOC)
FTOC was performed as described (30). In brief, thymic lobes were excised from OT-I Rag−/−β2m−/− transgenic mice at a gestational age of day 15.5. Exogenous β2m (5μg/ml), peptide(2μM for negative-selecting and 20μM for positive selecting peptides) and varying concentrations of the ZAP-70 kinase inhibitor were added to the FTOCs. After 7 days of culture, thymocytes were analyzed by flow cytometry.
Inhibition of ZAP-70 kinase activity by NIBR001
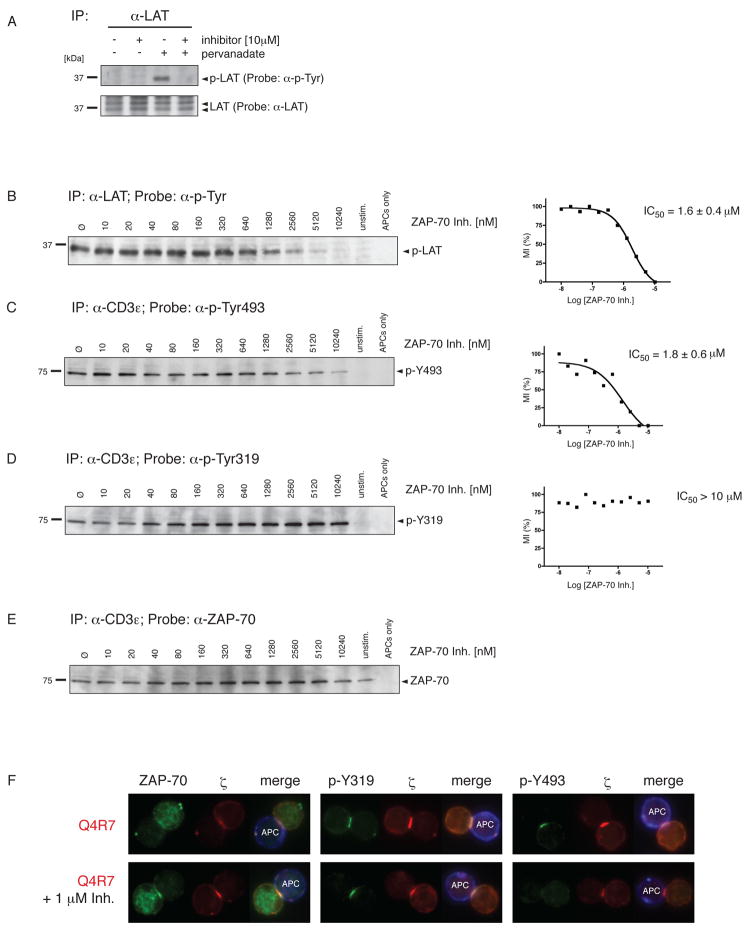
The specific ZAP-70 kinase inhibitor NIBR001 was produced at the Novartis Institutes for Biomedical Research (Basel, Switzerland). DP thymocytes from OT-I Rag−/−β2m−/− transgenic mice were pre-incubated with corresponding concentrations of NIBR001 for 30min. Stimulation with peptide-loaded APCs, immunoprecipitation of LAT or the TCR complex and Western blotting was performed as described above. The specificity of NIBR001 for ZAP-70 was confirmed by cell-free in vitro kinase assays described in supplementary figure 1.
Results
ZAP-70 recruitment and phosphorylation with positive and negative selecting ligands
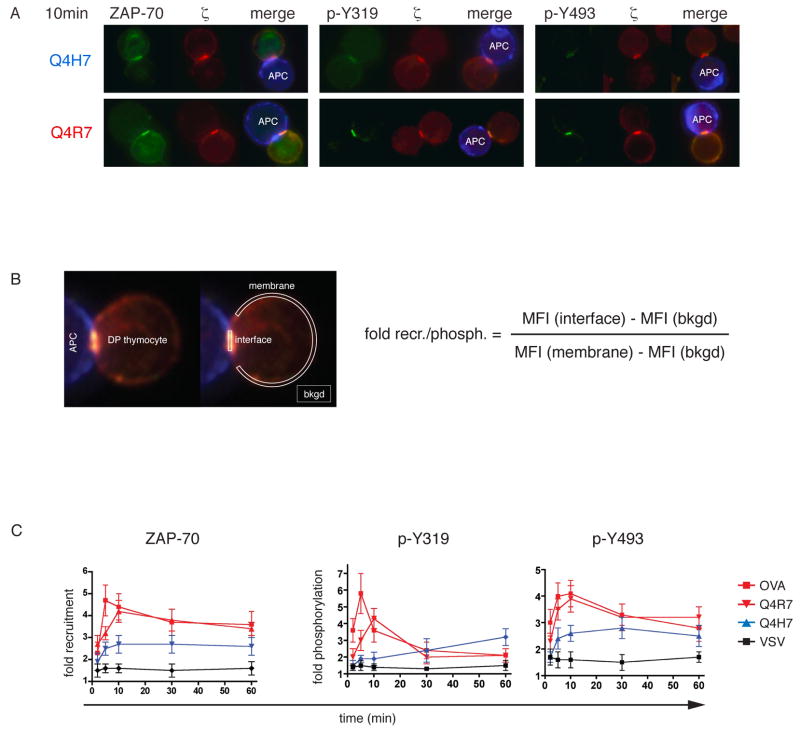
We used a well-defined set of peptides, inducing positive or negative selection of thymocytes expressing the OT-I TCR (31, 32). Transgenic mice of the OT-I Rag−/−β2m−/− mice are blocked in T-cell development and accumulate large numbers of pre-selection CD8+ CD4+ double-positive (33) thymocytes. These DP OT-I thymocytes were stimulated with peptide-loaded APCs and subsequently analyzed by fluorescence microscopy. OVA, Q4R7, Q4H7 or the null ligand, VSV were used in these experiments. OVA is a high affinity negative selector for the OT-I receptor. Q4R7 is the lowest affinity negative selector, whereas Q4H7 has the highest affinity ligand, which still induces positive selection of OT-I thymocytes (31). Specific staining for phosphorylated tyrosine at position 319 of ZAP-70 (p- Y319) revealed an increase in response to the negative-selecting peptide Q4R7 compared to the positive-selecting peptide Q4H7 (Fig 1A; middle panels). Similar results were obtained for phosphorylation of the tyrosine at position 493 (p-Y493) located in the kinase domain of ZAP-70 (Fig 1A; right panels). Increased ZAP-70 tyrosine phosphorylation was accompanied by increased ZAP-70 and ζ recruitment to the thymocyte:APC interface (Fig 1A; left panels). To quantify ZAP-70 recruitment and phosphorylation, fluorescence intensity was measured at the thymocyte:APC interface and normalized to the value measured on the thymocyte plasma membrane outside the interface (Fig 1B, legend and experimental procedures). For time course experiments, microscopy images were analyzed with this method and quantified at indicated time points (Fig 1C). OVA and Q4R7, the strongest and the weakest negative selecting peptides induced increased phosphorylation of Y319 and Y493 at the thymocyte:APC interface, as well as increased ZAP-70 recruitment. However, the peak of Q4R7-induced ZAP-70 phosphorylation and recruitment appeared to be only slightly reduced or delayed compared to OVA. In contrast, Q4H7, the positive selector, recruited significantly less ZAP-70 to the APC interface in spite of the fact that Q4H7/Kb only has a marginally lower affinity for the OT-I TCR compared to Q4R7/Kb. The difference in ZAP-70 recruitment between positive and negative selectors was already evident at 2 minutes, the earliest time point measured. Non-cognate VSV, a non-selecting peptide for the OT-I TCR failed to induce ZAP-70 recruitment or phosphorylation.
Fig 1. ZAP-70 recruitment and phosphorylation at the thymocyte:APC interface.
A, OT-I Rag−/−β2m−/− DP thymocytes were stimulated with peptide-loaded APCs for 10min. Cells were stained with monoclonal antibodies against ζ (33), ZAP-70 (1) or phosphorylated ZAP-70 species (1) and subjected to fluorescence microscopy analysis. Q4H7 (positive selector) stimulations are shown in the upper panel and Q4R7 (negative selector) stimulations in the lower panel. Enriched B-cells from C57 Bl/6 spleens (blue) were used as APCs.
B, Numerical evaluation of ZAP-70 recruitment and phosphorylation at the thymocyte:APC interface. The mean fluorescence intensity (MFI) of the ZAP-70 or the phospho-ZAP-70 signal was measured from user-defined regions encompassing the contact area or the membrane, indicated by white regions. The ‘fold recruitment’ or ‘fold phosphorylation’ value was then calculated according to the indicated algorithm.
C, Time-course of ZAP-70 recruitment and phosphorylation at the thymocyte:APC interface. APC-stimulated thymocytes were imaged by fluorescence microscopy (as in A) at indicated time points. ‘Fold recruitment’ of ZAP-70 or ‘fold phosphorylation’ of Y319 and Y493 in the contact area with APCs was measured as described in Experimental Procedures. For OVA, Q4R7 and Q4H7, n ≥ 20 cell conjugates were analyzed and for VSV n ≥ 10 and plotted as mean values ± SEM. The differences in ZAP-70 recruitment and phosphorylation induced by the weakest negative selector, Q4R7, or the strongest positive selector, Q4H7, were significant (p < 0.05) at 5 and 10 min.
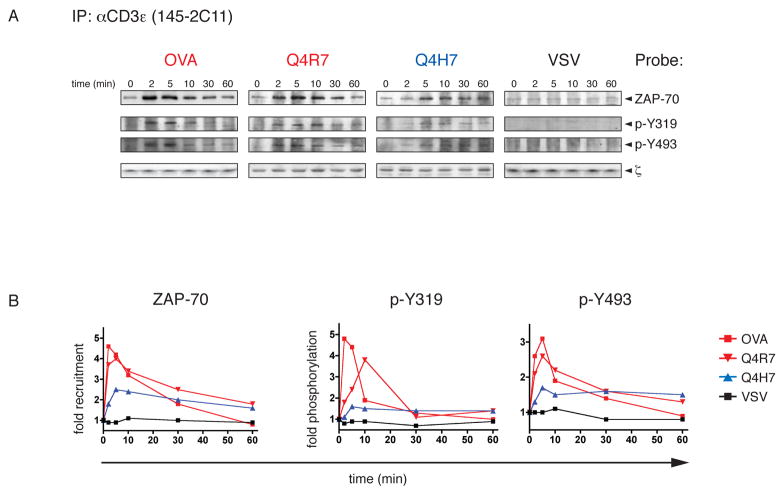
We also investigated ZAP-70 phosphorylation and recruitment to activated TCR complexes by Western analysis in order to confirm the results obtained by microscopy. Immunoprecipitated TCR complexes from OT-I Rag−/−β2m−/− thymocytes stimulated with peptide-loaded APCs were probed for the presence of tyrosine phosphorylation of ZAP-70 (Fig 2A). Similar to what we observed by microscopy, ZAP-70 recruitment to the TCR was enhanced with negative-selecting ligands. Relative to the positive selector Q4H7, Q4R7 increases ZAP-70 association to the TCR/CD3 complex as well as Y319 and Y493 phosphorylation by 2-fold. Stimulation with non-cognate VSV shows a background level of ZAP-70 association to the TCR complex but fails to induce any detectable Y319 or Y493 phosphorylation. This is in accordance with previous observations that ZAP-70 is to some degree associated with the TCR ζ-chain, even in resting cells (3). The amount of TCR captured in these immunoprecipitations was similar as shown by probing for ζ. Densitometric analysis (see experimental procedures) confirmed the patterns of ZAP-70 association to the TCR observed by microscopy (Fig 2B). pMHC ligands above the negative selection threshold induce a discrete elevation of TCR and ZAP-70 recruitment as well as ZAP-70 phosphorylation at the thymocyte:APC interface.
Fig 2. Biochemical analysis of ZAP-70 recruitment and phosphorylation.
A, Immunoprecipitation of TCR complexes from OT-I Rag−/−β2m−/− DP thymocytes stimulated with peptide-loaded APCs. At indicated time points, TCR complexes were immunoprecipitated a monoclonal CD3ε antibody and subjected to Western blot analysis. Co-immunoprecipitated ZAP-70 was probed with monoclonal antibodies against ZAP-70 (first row), p-Y319 (second row) or p-Y493 (third row). Membranes were stripped and reprobed for ζ (third row) as loading control. Representative Western blots are shown from several experiments performed for each peptide (n=3 for OVA, Q4R7 and Q4H7; n=2 for VSV).
B, Graphical evaluation of ZAP-70 recruitment and phosphorylation. Western blots as described in A were quantified by densitometry. Mean grey values of the corresponding bands were background-corrected and normalized to the loading control (ζ) and the values observed at 0min. Graphs show the mean values of several (2–3) experiments performed.
ZAP-70 kinase activity in positive and negative selection
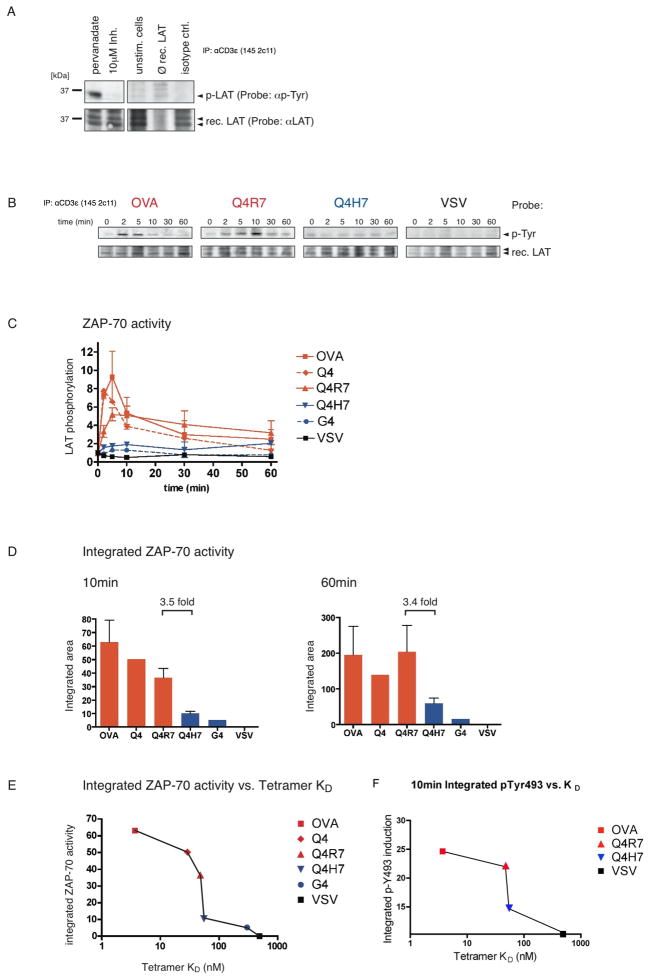
We developed an in vitro kinase assay to assess ZAP-70 kinase activity under positive or negative selecting conditions. ZAP-70 kinase activity was measured using a recombinant LAT substrate in presence of ATP (Fig 3). LAT is the main ZAP-70 substrate, which orchestrates TCR downstream signaling (34, 35). The specificity of the assay was confirmed by using the ZAP-70 kinase inhibitor NIBR001, which abrogated pervanadate-induced ZAP-70 kinase activity in this in vitro assay (Fig 3A; lanes 1 and 2). Control reactions showed that no ZAP-70 kinase activity was detected in unstimulated thymocytes (Fig 3A; lane 3). Furthermore, endogenous LAT is not co-immunoprecipitated with the TCR under these conditions as shown in the absence of recombinant LAT (Fig 3A; lane 4). These controls suggest that LAT phosphorylation in this assay specifically comes from TCR-associated, activated ZAP-70.
Fig 3. ZAP-70 activity with positive and negative selecting ligands.
A, In vitro kinase assay to assess ZAP-70 activity. Thymocytes from C57 Bl/6 mice were tested for ZAP-70-mediated phosphorylation of an exogenously added kinase substrate (recombinant LAT). Pervanadate-induced ZAP-70 kinase activity was abolished by 10 μM of the ZAP-70 kinase inhibitor NIBR001 (lanes 1&2). Unstimulated cells fail to induce phospho-LAT (lane 3). Experimental conditions do not co-immunoprecipitate endogenous LAT (lane 4). Immunoprecipitation with isotype control antibody (lane 5).
B, ZAP-70 kinase activity in stimulated DP thymocytes. OT-I Rag−/−β2m−/− DP thymocytes were stimulated with peptide-loaded APCs for indicated time points and TCR/CD3/ZAP-70 complexes were co-immunoprecipitated with anti-CD3ε. Phosphorylation of a recombinant LAT substrate was probed after the in vitro kinase assay. Representative Western blots are shown from several experiments performed for each peptide (n=3 for OVA, Q4R7 and Q4H7; n=2 for VSV).
C, Graphical evaluation of ZAP-70 kinase activity. Western blots as described in B were quantified by densitometry. All values were normalized to ZAP-70 kinase activity at 0 min.
D, Integrated ZAP-70 activity. ZAP-70 activity as plotted in C was assessed over the commencement of the experiment (10min, left panel) or the whole time-course (60min, right panel). Areas under the curves were integrated and normalized to VSV.
E, Integrated ZAP-70 activity vs. pMHC tetramer binding affinity. The integrated ZAP-70 activity during the commencement of the experiment (as shown in D; 10min) was plotted against the pMHC tetramer binding affinity (KD values from {Daniels, 2006 #51}).
F, Integrated p-Y493 phosphorylation vs. pMHC tetramer binding affinity. Tyrosine 493 phosphorylation was integrated over the initiation phase of thymocyte stimulation (taken from Figure 2B; first 10min) and plotted against pMHC tetramer binding affinity (similar to E).
OT-I DP thymocytes were stimulated with peptide-loaded APCs and ZAP-70 kinase activity was determined from co-immunoprecipitated TCR/CD3/ZAP-70 complexes using the in vitro kinase assay. The negative-selecting peptides OVA and Q4R7 induced increased ZAP-70 activity at early time points compared to the positive-selecting peptide Q4H7 (Fig 3B). The decline of ZAP-70 activity at later time points may be due to reduced ZAP-70 association with the ζ ITAMs or the result of negative feedback pathways (36). ZAP-70 dissociation from activated TCR/CD3 complexes has been previously described (37). The non-cognate peptide, VSV failed to induce any detectable ZAP-70 activity (Fig 3B). Phospho-LAT signals from these Western blots were quantified by densitometry for 3 negative selectors (OVA, Q4, Q4R7), 2 positive selectors (Q4H7, G4) and non-cognate VSV (Fig 3C, see experimental procedures for affinity hierarchy). To estimate the total ZAP-70 activity over the initiation phase of TCR signaling or the whole time-course of the experiment, we integrated the area under the curves (Fig 3D). In both cases, the integrated ZAP-70 activity was at least 3-fold higher for the weakest negative selector Q4R7 compared to the strongest positive selector Q4H7. During the initial phase of stimulation (10min), the strongest negative selector, OVA induced somewhat more ZAP-70 kinase activity than the weak negative selector, Q4R7 (Fig 3D, left panel); however, over the entire time course of the experiment (60 min) both negative selecting peptides induced a similar amount of ZAP-70 activity (Fig 3D, right panel). We also plotted the pMHC tetramer binding affinity (KD values taken from (31) for each peptide ligand used versus the integrated ZAP-70 kinase activity during the initial phase of stimulation (Fig 3E). This emphasizes that at the affinity threshold of thymic selection, negative-selecting ligands abruptly induce a 2- to 3 fold increase in ZAP-70 recruitment and phosphorylation. The steepest slope of this curve occurs at the selection threshold (between Q4H7 and Q4R7), which was confirmed by comparing Y493 phosphorylation obtained from stimulated TCR complexes (values taken from Figure 2B) versus pMHC tetramer binding affinity (Fig 3F).
Partial ZAP-70 inhibition converts negative into positive selection
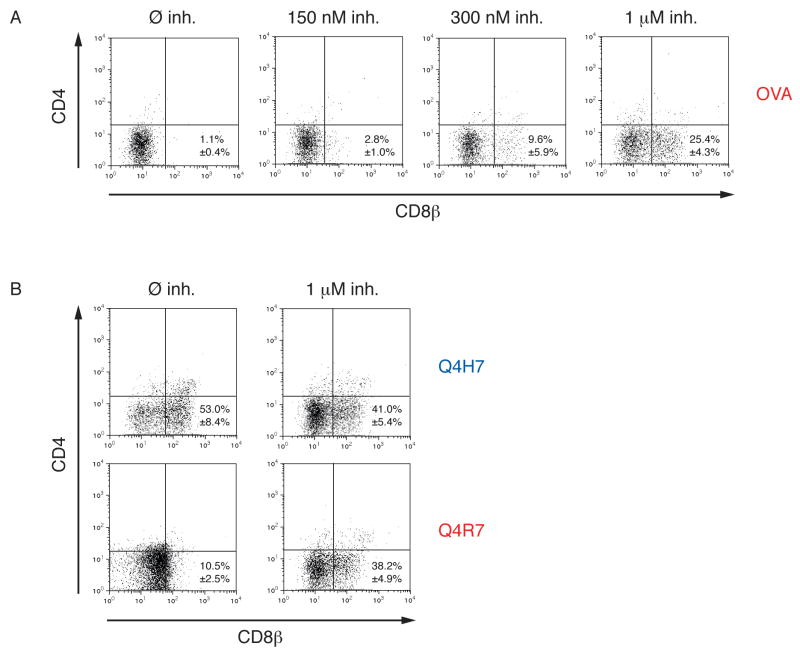
The above results raised the question whether the amount of ZAP-70 activity is a dose-dependent determinant of thymocyte selection outcome. We wondered whether lowering ZAP-70 activity using the ZAP-70 inhibitor NIBR001 could actually convert negative selection of DP thymocytes into positive selection. To assess this question we analyzed selection of OT-I DP thymocytes in fetal thymic organ cultures (FTOC) under negative or positive selecting conditions in either presence or absence of the ZAP-70 inhibitor. The ability of the ZAP-70 kinase inhibitor NIBR001 to influence thymic selection outcome was tested using FTOC (30) OVA induced negative selection by deleting virtually all DP thymocytes (Fig 4A; left panel). In contrast, non-cognate VSV induced neither positive nor negative selection (not shown; see reference 32). In presence of increasing concentrations of the ZAP-70 kinase inhibitor NIBR001, OVA stimulated FTOCs generated increased numbers of positively selected CD8αβ+ SP cells (Fig 4A; right panels). This demonstrates that positive selection depends on a limited amount of ZAP-70 kinase activity. In FTOCs stimulated with the weakest negative selector Q4R7, partial inhibition of ZAP-70 kinase activity led to positive selection of OT-I DP thymocytes (Fig 4B, lower panels). Interestingly, partial inhibition of ZAP-70 activity produced only a mild reduction of positive selection induced by Q4H7 (Fig 4B, upper panels). Although NIBR001 cross-inhibits a few kinases, the effects we observed in FTOC are best explained by inhibition of ZAP-70 activity (see Supplementary Figure 1 for a discussion of this point).
Fig 4. ZAP-70 inhibition converts negative into positive selection.
A, Dose dependent effect of ZAP-70 kinase activity in thymic selection. Thymic lobes from OT-I Rag−/−β2m−/− mice at gestational day 15.5 were incubated with 0.2 μM OVA in presence or absence of 150 nM, 300nM or 1 μM of the ZAP-70 kinase inhibitor NIBR001. After 7 days of culture, thymocytes were stained with fluorescently labeled antibodies against TCRβ, CD4 and CD8β and subjected to flow cytometry analysis. The percentage of TCRβ-positive, CD8αβ SP cells were determined using the FlowJo software.
B, ZAP-70 kinase inhibition at the negative selection threshold. FTOCs were incubated with 2 μM negative-selecting Q4R7 or 20 μM positive selecting Q4H7 in presence or absence of 1 μM ZAP-70 kinase inhibitor NIBR001. Thymocytes were processed and analyzed as in A.
We further investigated the specificity of NIBR001 by utilizing the structurally related but inactive compound, NIBR002. This did not affect negative selection induced by OVA (Supplementary Figure 2A).
Control of Y493 phosphorylation and ZAP-70 activity
The phosphorylation of Y493 enhances ZAP-70 activity, but it has been controversial whether p-Y493 is generated by a separate kinase or by trans-phosphorylation involving two ZAP-70 molecules. We addressed this using the ZAP-70 kinase inhibitor (NIBR001). NIBR001 prevented pervanadate-induced phosphorylation of endogenous LAT in DP thymocytes (Fig 5A; lanes 3&4). Untreated samples or the inhibitor alone had no effect on LAT phosphorylation (Fig 5A; lanes 1&2). The inhibitor had no effect on ζ phosphorylation (data not shown).
Fig 5. ZAP-70 activity correlates with Y493 phosphorylation.
A, The ZAP-70 kinase inhibitor NIBR001 abrogates endogenous LAT phosphorylation. LAT was immunoprecipitated from unstimulated or pervandate-induced thymocytes from C57 Bl/6 mice and analyzed by Western blot. Pervanadate-induced ZAP-70 activity could be inhibited by 10 μM NIBR001. LAT phosphorylation was detected by probing with an antiphosphotyrosine mAb (upper panel) and verified by probing with an anti-LAT mAb (lower panel).
B, Inhibition of endogenous LAT phosphorylation. OT-I Rag−/−β2m−/− DP thymocytes were stimulated for 5min with OVA-loaded APCs in presence of titrating ZAP-70 inhibitor concentrations. LAT immunoprecipitation was performed as in A. Phospho-LAT bands were quantified by densitometry and normalized to non-inhibited phospho-LAT. Half-maximal inhibition concentration (IC50) was calculated by non-linear regression analysis.
C, D, E ZAP-70 phosphorylation in presence of increasing inhibitor concentrations. Cells were stimulated as in B. TCR/CD3/ZAP-70 complexes were co-immunoprecipitated and probed with either anti-p-Y493 mAb (C), anti-p-Y319 mAb (D) or anti-ZAP-70 mAb (E). IC50 values were calculated as in B. All values were normalized to ZAP-70 recruitment obtained from E. Statistical analysis was based on at least 3 independent experiments and revealed no significant difference between the IC50 of LAT phosphorylation and the IC50 of ZAP-70 Y493 phosphorylation (p=0.78; Student’s two-sample t-test assuming unequal variances).
F, Inhibition of Y493 phosphorylation in the thymocyte:APC interface. OT-I Rag−/−β2m−/− DP thymocytes were stimulated for 10min with Q4R7-loaded APCs either in presence or absence of 1 μM ZAP-70 kinase inhibitor. Cells were stained and analyzed as in figure 1A. 38.3 ± 8.8 % of the DP thymocytes showed ≤ 2-fold reduction of p-Y493 phosphorylation compared to non-inhibited cells (n = 47; 3 independent experiments).
Using OVA-loaded APCs to stimulate OT-I DP thymocytes, we found that increasing concentrations of the ZAP-70 inhibitor abrogated endogenous LAT phosphorylation with an IC50 = 1.6 ± 0.4 e−6 M (Fig 5B). We also calculated the IC50 of the same inhibitor to prevent Y493 phosphorylation (IC50 = 1.8 ± 0.6 e−6 M) (Fig 5C). The two IC50 values are almost identical (p = 0.78) indicating that phosphorylation of Y493 is dependent on ZAP-70 kinase activity. The data are also consistent with the idea that enhanced ZAP-70 kinase activity in DP thymocytes is dependent on Y493 phosphorylation (6). However, even at the highest concentrations of the ZAP-70 kinase inhibitor there is a residual background of Y493 phosphorylation. It has been suggested that other kinases might initiate Y493 phosphorylation on a few molecules and that ZAP-70 trans-phosphorylation propagates and sustains Y493 phosphorylation (26).
As a specificity control we tested whether the ZAP-70 kinase inhibitor would prevent Y319 phosphorylation or ZAP-70 recruitment to the TCR (Fig 5D and 5E). Even in the presence of high inhibitor concentrations (up to 10μM), ZAP-70 was recruited to the TCR and its regulatory Y319 was phosphorylated. These results suggest that ZAP-70 association to activated TCR complexes and its phosphorylation at Y319 does not require ZAP-70 kinase activity. Moreover these data confirm previous results that Y319 phosphorylation is not mediated by ZAP-70 trans-phosphorylation but by another kinase(s), most likely Lck (26).
To further investigate the inhibition of Y493 phosphorylation at the thymocyte:APC interface, we analyzed Q4R7-stimulated DP thymocytes by microscopy (Fig 5F). As described in figure 1, Q4R7 induces strong ZAP-70 recruitment and Y319/Y493 phosphorylation. In the presence of 1 μM ZAP-70 kinase inhibitor, a concentration slightly less than the IC50 of ZAP-70 kinase inhibition (IC50 = 1.6 ± 0.4 e−6 M), Y493 phosphorylation was markedly reduced Fig 5F, right panels). 38.3 ± 8.8 % of all cells stimulated by Q4R7-loaded APCs showed a ≥ 2-fold reduction of Y493 phosphorylation (3 independent experiments; n = 47 cell conjugates analyzed). In contrast, ZAP-70 recruitment to the thymocyte:APC interface and its phosphorylation at Y319 remained intact in virtually all thymocyte-APC conjugates examined. These results confirm the co-immunoprecipitation experiments in figures 5B–E.
TCR/ZAP-70 ratio in positive and negative selection
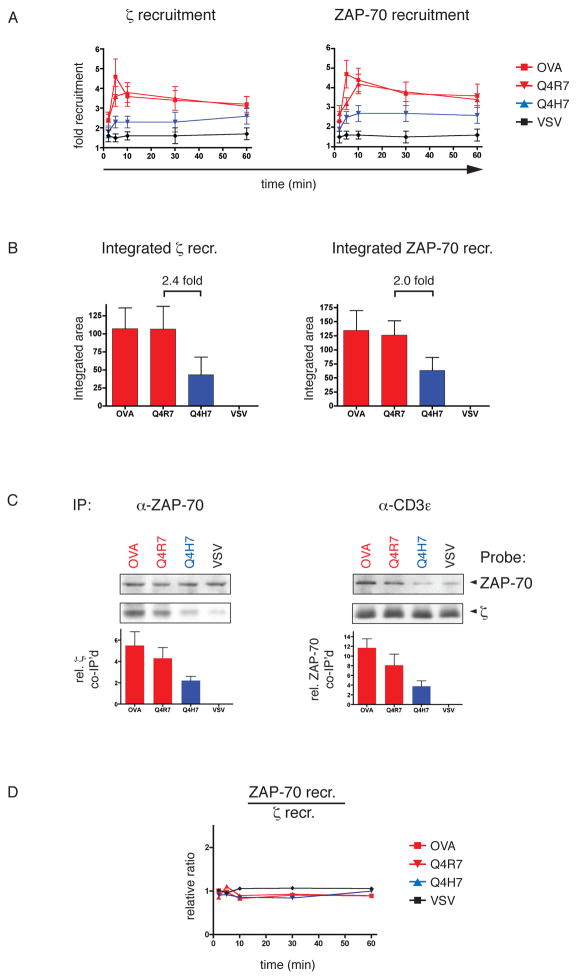
Following TCR engagement, CD3ε and ζ contain various phospho-ITAMs that promote SH2-mediated ZAP-70 binding with different affinities (18). We wondered whether this multiplicity of potential ZAP-70 binding sites is reflected in the ratio of ZAP-70 molecules per TCR complex under different selection conditions. Using microscopy, we examined the recruitment of the TCR and ZAP-70 into the contact zone with APCs in response to positive and negative selecting ligands (Fig 6A). Surprisingly, the relative recruitment of TCR and ZAP-70 into the thymocyte:APC interface was similar with both kinds of ligands. This was further confirmed by the integrated (total) recruitment of TCR and ZAP-70 over the whole time-course of the experiment (Fig 6B). This result points towards a selection mechanism where the increased number of ZAP-70 molecules in the interface with an APC is the consequence of increased TCR recruitment. However, since microscopy cannot distinguish between a scenario where TCR and ZAP-70 are simply co-localized in the thymocyte:APC interface or whether there is a (SH2-mediated) molecular association, we assessed the ratio between the two molecules by co-immunoprecipitation.
Fig 6. Relative ZAP-70/TCR ratio in positive and negative selection.
A, ζ and ZAP-70 recruitment to the thymocyte:APC interface. OT-I Rag−/−β2m−/− DP thymocytes were stimulated, stained and analyzed by microscopy as in figure 1.
B, Integrated ζ and ZAP-70 recruitment to the thymocyte:APC interface. ζ and ZAP-70 recruitment as plotted in A was assessed over the entire time-course. Areas under the curves were calculated manually.
C, ZAP-70 to TCR association by co-immunoprecipitation. OT-I Rag−/−β2m−/− DP thymocytes were stimulated for 10min with APCs loaded with OVA, Q4R7, Q4H7 or VSV. Coimmunoprecipitations performed with either CD3ε (left panel) or ZAP-70 (right panel) were probed with anti-ZAP-70 mAb (upper row) or anti-ζ mAb (lower row). Signals were quantified by densitometry, normalized to the corresponding loading control and VSV. Values are indicated below the blots. One representative experiment is shown of several performed.
D, Relative ZAP-70/ζ ratio in the thymocyte:APC interface. Values obtained from the relative recruitment of ZAP-70 to the APC interface (A) were normalized to ζ recruitment.
Immunoprecipitation of all TCR complexes from OT-I DP thymocytes stimulated with peptide-loaded APCs revealed that the relative amount of TCR associated ZAP-70 correlated with the thymic selection potential of the presented peptide. The data in figure 6C (right panels) show that the negative selectors OVA and Q4R7 induced higher levels of TCR-associated ZAP-70 than the positive selector Q4H7 (Fig 6C; right panel). This raised the question whether the increased ZAP-70 association to the TCR under negative selection conditions is due to an increased ZAP-70/TCR ratio or due to increased levels of activated TCRs that are each associated with the same number of ZAP-70 molecules. To address this, we immunoprecipitated ZAP-70 from stimulated OT-I thymocytes and probed for the TCR/CD3 component, ζ (Fig 6C; left panel). The level of TCR associated to ZAP-70 was clearly higher in response to the negative-selecting ligands OVA and Q4R7 compared to the positive selector Q4H7 (Fig 6C; left panel). These data argue against the idea that negative selecting ligands increase the number of ZAP-70 molecules recruited to each triggered TCR; instead these data support the hypothesis that negative selecting ligands actually increase the number of actively signaling TCRs. We also calculated the relative ZAP-70/TCR ratio in the thymocyte:APC interface from the values obtained in figure 6A. Indeed, the ratio remained constant for all peptides at all time points, independent of their selection properties (Fig 6D).
Taken together, these experiments suggest, that in the contact area of DP thymocytes with APCs, elevated ZAP-70 levels induced by negative selection are not due to an increased number of ZAP-70 molecules recruited to each triggered TCR, but rather to an increase in the number of TCRs that recruit ZAP-70. For both positive and negative selecting ligands, the relative ZAP-70/TCR ratio remains constant.
Discussion
To understand how ligand affinity is translated into a thymic selection signal, we studied the activation and localization of ZAP-70 in thymocytes stimulated with ligands of varying affinity. In OT-I DP thymocytes stimulated with negative-selecting peptides, we observed a strong and transient ZAP-70 recruitment to the thymocyte:APC interface (Fig 1). In response to a positive-selecting ligand whose affinity is only slightly below the selection threshold, ZAP-70 recruitment to the thymocyte:APC interface was significantly reduced and delayed. The positive selector, Q4H7 fails to induce an early peak of ZAP-70 recruitment, arguing for differential ZAP-70 regulation over the negative selection threshold. ZAP-70 activation involves phosphorylation of the regulatory tyrosine, Y319 in the interdomain B (8, 26) and Y493 in the activation loop of the kinase domain (6, 28). In our experiments, phosphorylation at both tyrosine residues was quickly induced by negative-selecting ligands, but poorly induced by a positive-selector. No ZAP-70 phosphorylation was detected when DP thymocytes were stimulated with a non-cognate ligand. The poor phosphorylation at Y319 is likely due to the weak TCR/CD8 interaction that is characteristic of positive selecting ligands (32, 38). A weaker TCR/CD8 interaction would limit lck’s availability around the CD3 complex and therefore limit Y319 phosphorylation. These microscopy results were confirmed by co-immunoprecipitation experiments, examining ZAP-70’s association to the CD3 complex and its phosphorylation state (Fig 2). The increased recruitment and phosphorylation of ZAP-70 by ligands above the selection threshold suggests that efficient ZAP-70 phosphorylation is a hallmark of negative selection.
ZAP-70 kinase activity was determined using in an in vitro kinase assay to correlate ZAP-70’s enzymatic activity with its phosphorylation state (Fig 3). In response to the negative selecting ligands OVA, Q4 and Q4R7, ZAP-70 kinase activity was quickly induced and diminished over the time course of the experiment (Fig 3). The positive selectors Q4H7 and G4 induced a ZAP-70 kinase activity that was only slightly above that induced by a noncognate ligand, VSV. Integrated over the initial phase (10min) or the whole time-course of the experiment (60min), negative selectors (compared to positive selectors) increased ZAP-70 kinase activity by 3.5- and 3.4-fold, respectively (Fig 3D).
Interestingly, all negative selecting ligands studied induce a similar amount of ZAP-70 kinase activity (Fig 3E). Despite the fact that OVA displays a > 10-fold higher affinity for the OT-I TCR than the weak negative selector Q4R7, ZAP-70 kinase activity was not proportionally increased. Positive selectors, over a wide range of affinities below the selection threshold induce a similar low level of ZAP-70 kinase activity (Fig 3E; compare G4 with Q4H7). Strikingly, the steepest increase in ZAP-70 kinase activity occurs precisely at the selection threshold (Fig 3E; see slope of curve between Q4H7 and Q4R7). ZAP-70 represents one of the most proximal steps in the signaling pathway where an analogue input (varying pMHC affinity) is converted into a quasi-digital output (low versus high ZAP-70 kinase activity). This observation was confirmed by Y493 phosphorylation, which presumably reflects ZAP-70 activity (6) and also exhibits a discontinuous increase at the negative selection threshold (Fig 3F).
ZAP-70 mediated phosphorylation of endogenous LAT was investigated using the specific ZAP-70 kinase inhibitor, NIBR001 (Supplementary Figure 1). Our experiments confirm that LAT is primarily phosphorylated by ZAP-70 and not by other PTKs (34) since endogenous LAT phosphorylation was almost entirely prevented at high inhibitor concentrations (Fig 5B). Additionally, NIBR001 substantially inhibits Y493 phosphorylation, implying that phosphorylation of this tyrosine residue is mediated by ZAP-70 itself (i.e. by transphosphorylation). The IC50 values for the inhibition of LAT phosphorylation and the generation of ZAP-70 phospho-Y493 are similar (p = 0.78), indicating that ZAP-70 kinase activity mediates both reactions (Fig 5B,C). The importance of tyrosine 493 phosphorylation for maximal ZAP-70 kinase activation has been demonstrated previously by point mutations, which reduce ZAP-70 kinase activity (6, 29).
In contrast, phosphorylation at Y319 in the regulatory domain of ZAP-70 was not affected by inhibiting ZAP-70 kinase activity, which argues that this residue is a phosphorylation target of other PTKs, such as Lck (Fig 5D). Indeed, overexpression studies have shown that phosphorylation at Y319 was only achieved upon cotransfection with Lck (27); furthermore, Y319 was also identified as binding site for Lck (26). Microscopy studies revealed that phosphorylated Y493 accumulates at the thymocyte:APC interface; this was inhibited by the ZAP-70 inhibitor, NIBR001. NIBR001 affected neither ZAP-70 recruitment to the APC interface nor phosphorylation of ZAP-70 at Y319. Taken together, these data argue that Y493 phosphorylation is the result of trans-phosphorylation between nearby ZAP-70 molecules.
In ZAP-70 knockout mice, thymocytes are arrested in their development at the DP stage and fail to become positively selected (9). Negative selection is also impaired in these animals. Given that ZAP-70 is a likely branch point for thymic selection signaling, we wondered whether we could influence the selection outcome by modulating the amount of ZAP-70 kinase activity. In FTOC, partial inhibition of ZAP-70 kinase activity converted negative selection into positive selection. Our results are supported by the study of Siggs et al., which describes two mutant mouse strains with amino acid substitutions in the ZAP-70 kinase domain that affect thymic selection and generate subsequent immunodeficiency to varying degrees (11). When the two zap-70 gene variants were intercrossed, intermediate TCR signaling resulted in defective thymic negative selection, but only mildly affected positive selection.
Maintaining control over the induction of ZAP-70 activity in thymocytes and in peripheral T cells is critical for selecting a self-tolerant T-cell repertoire. At the level of ZAP-70, the “strength of signal” seems to determine the outcome of thymic selection. Our experiments make an additional and important point: To change the selection outcome, the increase in signal strength, i.e. pMHC affinity, must cross the selection threshold. Other studies of TEC kinases (39) and the adaptor LAT (40) are also consistent with the “strength of signal” hypothesis. However, at more distal points in the signaling pathways, the strength of signal hypothesis does not seem to be operating. Inhibition of MEK1 selectively impairs positive selection while p38 inhibition selectively impairs negative selection (41). At these distal points in the signaling cascade, the positive and negative selection pathways are independent.
The increase in ZAP-70 kinase activity in negative selection might be related to a different molecular ratio of ZAP-70/TCR in the thymocyte:APC interface. Previous studies have identified partially phosphorylated ζ-chains in response to antagonist ligands (19), which may provide a mechanism to alter the number of associated ZAP-70 molecules per TCR complex. ZAP-70 binds to doubly phosphorylated ITAMs using both of its SH2-domains (5) and has varying binding affinities for the individual ζ and CD3ε ITAMs (18). It’s conceivable that this contributes to an elevated ZAP-70/TCR ratio during negative selection. However, our microscopy experiments indicate that TCR and ZAP-70 are proportionally recruited to the thymocyte:APC contact area by both positive and negative selecting ligands. Negative selection does not increase the relative ratio of ZAP-70/TCR but rather increases the total amount of TCR and ZAP-70 at the APC interface (Fig 6). This finding was further supported by co-immunoprecipitations of the TCR complex and ZAP-70 from APC-stimulated DP thymocytes (Fig 6). As expected, TCR-associated ZAP-70 was increased in response to the negative-selecting ligands. However, a pulldown of ZAP-70 and subsequent probing for TCR revealed that an increased amount of TCR is associated with ZAP-70 in lysates of DP thymocytes that had been stimulated with negative selecting ligands. Therefore, we postulate a model of negative selection signaling where increased TCR recruitment into the interface with an APC drives a proportional increase in ZAP-70 recruitment. Although this is our preferred interpretation, we cannot rule out the possibility that a small percentage of recruited TCRs in thymocytes stimulated with negative selecting peptides have an increased ZAP-70/TCR stoichiometry.
Conclusions
We and others have previously shown that negative-selecting ligands quickly induce close TCR-coreceptor approximation and exhibit strong ζ phosphorylation whereas positive selecting ligands promote less TCR-coreceptor association and reduced ζ phosphorylation (32, 38). We speculate that coreceptor-associated Lck phosphorylates the regulatory Y319 of TCR-associated ZAP-70. Subsequently, ZAP-70 is released from autoinhibition and transphosphorylates other nearby ZAP-70 molecules at Y493. Following the recognition of a high affinity self-antigen, the higher density of ZAP-70 molecules in the APC contact area could facilitate ZAP-70 trans-phosphorylation. During positive selection, the concentration of ZAP-70 molecules in the APC contact area is low and consequently the limited Y493 transphosphorylation fails to promote sufficient ZAP-70 kinase activity to induce negative selection signaling. Therefore, the 3-fold elevation of ZAP-70 kinase activity, which occurs above the affinity threshold is a proximal critical parameter of negative selection signaling.
Supplementary Material
Acknowledgments
The authors would like to thank B. Hausmann for FTOC experiments; E. Wagner for animal husbandry; and D. Fabbro, P. Drueckes and J. Trappe, (Novartis Institutes for Biomedical Research, Basel) for profiling the ZAP-70 inhibitor in various kinase assays. The authors thank D. Naeher for comments on the manuscript and T. Hoefer for fruitful discussions. The work is supported by grants from the Swiss National Science Foundation, Sybilla (EU FP7) and National Institutes of Health (AI074074).
Footnotes
Conflict of Interest:
Gerhard Zenke is an employee of Novartis Pharma AG, Basel.
References
- 1.Hatada MH, Lu X, Laird ER, Green J, Morgenstern JP, Lou M, et al. Molecular basis for interaction of the protein tyrosine kinase ZAP-70 with the T-cell receptor. Nature. 1995 Sep 7;377(6544):32–8. doi: 10.1038/377032a0. [DOI] [PubMed] [Google Scholar]
- 2.Wange RL, Kong AN, Samelson LE. A tyrosine-phosphorylated 70-kDa protein binds a photoaffinity analogue of ATP and associates with both the zeta chain and CD3 components of the activated T cell antigen receptor. J Biol Chem. 1992 Jun 15;267(17):11685–8. [PubMed] [Google Scholar]
- 3.Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992 Nov 13;71(4):649–62. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 4.Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994 Feb 25;263(5150):1136–9. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 5.Wange RL, Malek SN, Desiderio S, Samelson LE. Tandem SH2 domains of ZAP-70 bind to T cell antigen receptor zeta and CD3 epsilon from activated Jurkat T cells. J Biol Chem. 1993 Sep 15;268(26):19797–801. [PubMed] [Google Scholar]
- 6.Chan AC, Dalton M, Johnson R, Kong GH, Wang T, Thoma R, et al. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 1995 Jun 1;14(11):2499–508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong G, Dalton M, Bubeck Wardenburg J, Straus D, Kurosaki T, Chan AC. Distinct tyrosine phosphorylation sites in ZAP-70 mediate activation and negative regulation of antigen receptor function. Mol Cell Biol. 1996 Sep;16(9):5026–35. doi: 10.1128/mcb.16.9.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Bartolo V, Mege D, Germain V, Pelosi M, Dufour E, Michel F, et al. Tyrosine 319, a newly identified phosphorylation site of ZAP-70, plays a critical role in T cell antigen receptor signaling. J Biol Chem. 1999 Mar 5;274(10):6285–94. doi: 10.1074/jbc.274.10.6285. [DOI] [PubMed] [Google Scholar]
- 9.Negishi I, Motoyama N, Nakayama K, Senju S, Hatakeyama S, Zhang Q, et al. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995 Aug 3;376(6539):435–8. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003 Nov 27;426(6965):454–60. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 11.Siggs OM, Miosge LA, Yates AL, Kucharska EM, Sheahan D, Brdicka T, et al. Opposing functions of the T cell receptor kinase ZAP-70 in immunity and tolerance differentially titrate in response to nucleotide substitutions. Immunity. 2007 Dec;27(6):912–26. doi: 10.1016/j.immuni.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arpaia E, Shahar M, Dadi H, Cohen A, Roifman CM. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking zap-70 kinase. Cell. 1994 Mar 11;76(5):947–58. doi: 10.1016/0092-8674(94)90368-9. [DOI] [PubMed] [Google Scholar]
- 13.Wiest DL, Ashe JM, Howcroft TK, Lee HM, Kemper DM, Negishi I, et al. A spontaneously arising mutation in the DLAARN motif of murine ZAP-70 abrogates kinase activity and arrests thymocyte development. Immunity. 1997 Jun;6(6):663–71. doi: 10.1016/s1074-7613(00)80442-2. [DOI] [PubMed] [Google Scholar]
- 14.Shores EW, Tran T, Grinberg A, Sommers CL, Shen H, Love PE. Role of the multiple T cell receptor (TCR)-zeta chain signaling motifs in selection of the T cell repertoire. J Exp Med. 1997 Mar 3;185(5):893–900. doi: 10.1084/jem.185.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holst J, Wang H, Eder KD, Workman CJ, Boyd KL, Baquet Z, et al. Scalable signalling mediated by T cell antigen receptor-CD3 ITAMs ensures effective negative selection and prevents autoimmunity. Nat Immunol. 2008 Jun;9(6):658–66. doi: 10.1038/ni.1611. [DOI] [PubMed] [Google Scholar]
- 16.Ardouin L, Boyer C, Gillet A, Trucy J, Bernard AM, Nunes J, et al. Crippling of CD3-zeta ITAMs does not impair T cell receptor signaling. Immunity. 1999 Apr;10(4):409–20. doi: 10.1016/s1074-7613(00)80041-2. [DOI] [PubMed] [Google Scholar]
- 17.Ashton-Rickardt PG, Bandeira A, Delaney JR, Van Kaer L, Pircher HP, Zinkernagel RM, et al. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994 Feb 25;76(4):651–63. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 18.Isakov N, Wange RL, Burgess WH, Watts JD, Aebersold R, Samelson LE. ZAP-70 binding specificity to T cell receptor tyrosine-based activation motifs: the tandem SH2 domains of ZAP-70 bind distinct tyrosine-based activation motifs with varying affinity. J Exp Med. 1995 Jan 1;181(1):375–80. doi: 10.1084/jem.181.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kersh EN, Shaw AS, Allen PM. Fidelity of T cell activation through multistep T cell receptor zeta phosphorylation. Science. 1998 Jul 24;281(5376):572–5. doi: 10.1126/science.281.5376.572. [DOI] [PubMed] [Google Scholar]
- 20.Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-zeta and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994 Dec 2;79(5):913–22. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 21.LoGrasso PV, Hawkins J, Frank LJ, Wisniewski D, Marcy A. Mechanism of activation for Zap-70 catalytic activity. Proc Natl Acad Sci U S A. 1996 Oct 29;93(22):12165–70. doi: 10.1073/pnas.93.22.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Oers NS, Killeen N, Weiss A. ZAP-70 is constitutively associated with tyrosinephosphorylated TCR zeta in murine thymocytes and lymph node T cells. Immunity. 1994 Nov;1(8):675–85. doi: 10.1016/1074-7613(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 23.Madrenas J, Wange RL, Wang JL, Isakov N, Samelson LE, Germain RN. Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science. 1995 Jan 27;267(5197):515–8. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- 24.Plas DR, Johnson R, Pingel JT, Matthews RJ, Dalton M, Roy G, et al. Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science. 1996 May 24;272(5265):1173–6. doi: 10.1126/science.272.5265.1173. [DOI] [PubMed] [Google Scholar]
- 25.Brockdorff J, Williams S, Couture C, Mustelin T. Dephosphorylation of ZAP-70 and inhibition of T cell activation by activated SHP1. Eur J Immunol. 1999 Aug;29(8):2539–50. doi: 10.1002/(SICI)1521-4141(199908)29:08<2539::AID-IMMU2539>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 26.Pelosi M, Di Bartolo V, Mounier V, Mege D, Pascussi JM, Dufour E, et al. Tyrosine 319 in the interdomain B of ZAP-70 is a binding site for the Src homology 2 domain of Lck. J Biol Chem. 1999 May 14;274(20):14229–37. doi: 10.1074/jbc.274.20.14229. [DOI] [PubMed] [Google Scholar]
- 27.Brdicka T, Kadlecek TA, Roose JP, Pastuszak AW, Weiss A. Intramolecular regulatory switch in ZAP-70: analogy with receptor tyrosine kinases. Mol Cell Biol. 2005 Jun;25(12):4924–33. doi: 10.1128/MCB.25.12.4924-4933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deindl S, Kadlecek TA, Brdicka T, Cao X, Weiss A, Kuriyan J. Structural basis for the inhibition of tyrosine kinase activity of ZAP-70. Cell. 2007 May 18;129(4):735–46. doi: 10.1016/j.cell.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 29.Wange RL, Guitian R, Isakov N, Watts JD, Aebersold R, Samelson LE. Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of ZAP-70. J Biol Chem. 1995 Aug 11;270(32):18730–3. doi: 10.1074/jbc.270.32.18730. [DOI] [PubMed] [Google Scholar]
- 30.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994 Jan 14;76(1):17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 31.Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006 Dec 7;444(7120):724–9. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 32.Mallaun M, Naeher D, Daniels MA, Yachi PP, Hausmann B, Luescher IF, et al. The T cell receptor’s alpha-chain connecting peptide motif promotes close approximation of the CD8 coreceptor allowing efficient signal initiation. J Immunol. 2008 Jun 15;180(12):8211–21. doi: 10.4049/jimmunol.180.12.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, et al. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996 Jun 13;381(6583):616–20. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998 Jan 9;92(1):83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, Trible RP, et al. Essential role of LAT in T cell development. Immunity. 1999 Mar;10(3):323–32. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 36.Acuto O, Di Bartolo V, Michel F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat Rev Immunol. 2008 Sep;8(9):699–712. doi: 10.1038/nri2397. [DOI] [PubMed] [Google Scholar]
- 37.Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005 Dec;6(12):1253–62. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 38.Yachi PP, Ampudia J, Zal T, Gascoigne NR. Altered peptide ligands induce delayed CD8-T cell receptor interaction--a role for CD8 in distinguishing antigen quality. Immunity. 2006 Aug;25(2):203–11. doi: 10.1016/j.immuni.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Schaeffer EM, Broussard C, Debnath J, Anderson S, McVicar DW, Schwartzberg PL. Tec family kinases modulate thresholds for thymocyte development and selection. J Exp Med. 2000 Oct 2;192(7):987–1000. doi: 10.1084/jem.192.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sommers CL, Lee J, Steiner KL, Gurson JM, Depersis CL, El-Khoury D, et al. Mutation of the phospholipase C-gamma1-binding site of LAT affects both positive and negative thymocyte selection. J Exp Med. 2005 Apr 4;201(7):1125–34. doi: 10.1084/jem.20041869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugawara T, Moriguchi T, Nishida E, Takahama Y. Differential roles of ERK and p38 MAP kinase pathways in positive and negative selection of T lymphocytes. Immunity. 1998 Oct;9(4):565–74. doi: 10.1016/s1074-7613(00)80639-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.