Abstract
Adult rats display taste avoidance and disgust reactions when stimulated with gustatory stimuli previously paired with aversive agents such as lithium chloride (LiCl). By the second postnatal week of life, preweanling rats also display specific behaviors in response to a tastant conditioned stimulus (CS) that predicts LiCl-induced malaise. The present study compared conditioned disgust reactions induced by LiCl or ethanol (EtOH) in preweanling rats. In Experiment 1 we determined doses of ethanol and LiCl that exert similar levels of conditioned taste avoidance. After having equated drug dosage in terms of conditioned taste avoidance, 13-Day old rats were given a single pairing of a novel taste (saccharin) and either LiCl or ethanol (2.5 g/kg; Experiment 2). Saccharin intake and emission of disgust reactions were assessed 24 and 48 hours after training. Pups given paired presentations of saccharin and the aversive agents (ethanol or LiCl) consumed less saccharin during the first testing Day than controls. These pups also showed more aversive behavioral reactions to the gustatory CS than controls. Specifically, increased amounts of grooming, general activity, head shaking and wall climbing as well as reduced mouthing were observed in response to the CS. Conditioned aversive reactions but not taste avoidance were still evident on the second testing Day. In conclusion, a taste CS paired with post-absorptive effects of EtOH and LiCl elicited a similar pattern of conditioned rejection reactions in preweanling rats. These results suggest that similar mechanisms may be underlying CTAs induced by LiCl and a relatively high EtOH dose.
Keywords: ethanol, LiCl, taste aversion, disgust reactions, infant rat
The ontogenetic analysis of ethanol (EtOH) intake and reinforcement has revealed strikingly high ethanol consumption (Sanders & Spear, 2007; Truxell & Spear, 2004; Truxell et al, 2007) and a predisposition to acquire ethanol-mediated appetitive reinforcement during the first two postnatal weeks of life (Arias & Chotro, 2006a; Chotro & Arias, 2007; Molina, Pautassi, Truxell, & Spear, 2007; Nizhnikov, Varlinskaya, Petrov, & Spear, 2006; Petrov, Varlinskaya, & Spear, 2003). These appetitive effects of ethanol have been found even when employing relatively high ethanol doses, between 2 and 3 g/kg (Arias & Chotro, 2006a; Chotro & Arias, 2007; Molina et al., 2007). In addition, during the second postnatal week of life, relatively high ethanol doses (between 1.25 and 2.5 g/kg) induce locomotor activating effects (Arias, Molina, Mlewski, Pautassi and Spar, 2008; Arias, Mlewski, Molina and Spear, 2009b; Arias, Mlewski, Miller, Molina, & Spear, 2009), an effect modulated by dopamine, GABA B and opioid receptors (Arias, Mlewski, Hansen, Molina and Spear, 2010; Arias, Molina and Spear, 2010; Arias, Mlewski, Molina and Spear, 2009a). Drug-induced locomotor stimulation is thought to be associated with the positive reinforcing properties of substances of abuse (Wise & Bozarth, 1987).
Ethanol also supports aversive learning in infant rats. For example, these young organisms can learn to avoid a novel tasting solution (i.e., saccharin, conditioned stimulus, CS) that signals the pharmacological effects of either low (0.4 g/kg) or high (2 or 3 g/kg) ethanol doses (Arias & Chotro, 2006a, 2006b; Hunt, Kraebel, Rabine, Spear, & Spear, 1993; Hunt, Molina, Spear, & Spear, 1990). In preweanling rats the magnitude of this aversive learning is known to be modulated by several factors such as the stage of development in which training takes place (Arias & Chotro, 2006a; Hunt, Spear, & Spear, 1991) or individual differences in response to a novel environment (Arias, Molina, & Spear, 2009). In summary, it seems clear that a given dose of ethanol may induce differential (i.e., appetitive or aversive) motivational effects, depending on a variety of variables.
Conditioned taste aversion training results not only in avoidance of the taste signaling the US. Subjects also display a distinctive set of behavioral reactions when intraorally stimulated with this CS (Parker, 1995, 2003). These reactions, collectively termed “conditioned disgust reactions” and measured in a so-called taste reactivity test (TRT) (Arias & Chotro, 2005a, 2005b; Berridge, 2000; Parker, 1995, 2003), are thought to reflect a change in the palatability of the gustatory CS. Following taste aversion training, the typical behavioral repertoire in terms of taste reactivity includes gaping, chin rubbing, paw treading, head shaking, increased locomotion, facial wiping and wall climbing (Arias & Chotro, 2005a, 2005b; Berridge, 2000; Parker, 1995, 2003). Mouthing and tongue protrusions are common reactions of the hedonic component of the TRT; a reduction of these responses after conditioned taste aversion training is considered a reduction in palatability (Berridge, 2000).
To our knowledge, there is a scarcity of work on the expression of conditioned disgust reactions elicited by a tastant CS previously paired with ethanol, particularly early in ontogeny. In adult rats, Davies and Parker (1990) found increased aversive reactions and supressed ingestive behaviors in response to a gustatory CS that was previously paired with ethanol (2 g/kg).
The study of ethanol-mediated motivational learning during early ontogeny is important for several reasons. As mentioned, this stage of development is characterized by heightened affinity for ethanol. Moreover, unlike the mature rat which rarely exhibits appetitive conditioned reinforcement by ethanol, the infant shows both appetitive and aversive ethanol-mediated learning. The second week of life in the rat is a critical window for the study of aversive learning. By this age the rat pup is capable of expressing differential aversive conditioning as a function of the US employed. (Hoffmann, Hunt, & Spear, 1991; Hoffmann, Hunt, & Spear, 1990). Hoffmann and co-workers found that 15-, but not 5-Day old rats displayed specific conditioned disgust reactions when stimulated with a LiCl-paired taste. Specifically, in 15-Day-old subjects conditioned disgust reactions elicited by the LiCl-paired taste were qualitatively different (increased wall-climbing and paw-treading, and decreased mouthing) from those mediated by a footshock.
The present study sought to analyze disgust reactions elicited by a relatively high ethanol dose (2.5 g/kg) in preweanling rats. The expression of these aversive reactions was analyzed with a taste reactivity test adapted to the infant rat (Arias & Chotro, 2005a, 2005b; Hall & Bryan, 1981; Vigorito & Sclafani, 1988). Thirteen-Day old rats were given a single pairing between a novel taste CS (saccharin) and post-absorptive effects of ethanol (2.5 g/kg, Experiment 2). Saccharin intake and emission of disgust reactions were assessed 24 and 48 hours after training. Previous studies have shown that taste avoidance is extinguished by the second testing Day under similar experimental conditions (Arias & Chotro, 2006b). In the present experiments measurement of responsiveness towards the taste CS was conducted to analyze potential divergence between taste avoidance and taste reactivity patterns during extinction. Although comparison between patterns of extinction of taste avoidance learning and conditioned disgust reaction has been assessed in adult rats (Cántora, Lopez, Aguado, Rana, & Parker, 2006), the expression of such phenomenon in infants has yet to be investigated. Cántora et al (2006) found that rats continued to avoid a lithium-paired flavour, even when conditioned disgust reactions were completely extinguished. In addition, we also included in the experimental design a group of subjects treated with LiCl. In Experiment 1 the LiCl dose selected as the US was designed to generate a level of conditioned intake suppression similar to that found when the US is 2.5 g/kg ethanol. Examination of whether ethanol-mediated taste avoidance in preweanling rats is accompanied by conditioned disgust reactions similar to those found when employing LiCl may help to understand the mechanisms underlying ethanol’s motivational effects.
2.Experiment 1
The goal of the present experiment was to determine a LiCl dose that exerts a level of conditioned taste avoidance similar to that observed with a relatively high ethanol dose (2.5 g/kg).
2.1 Material and Methods
2.1.1 Subjects
Forty-seven 13-Day-old Sprague-Dawley pups (23 females and 24 males), representative of 6 litters were employed. Animals were born and reared at the vivarium of the Center for Developmental Psychobiology (Binghamton University, NY) under conditions of constant room temperature (22 ± 1.0 °C), on a 12-hour light: 12-hour dark cycle. Births were examined daily and the Day of parturition was considered as postnatal Day 0 (PD0). All litters were culled to 10 pups (5 females and 5 males when possible) within 48 hours after birth. Animal maintenance and experimental procedures were in accordance with the Guide for Care and Use of Laboratory Animals (Institute-of-Laboratory-Animal-Resources, 1996) and the guidelines indicated by the Binghamton University handling review committee. In this, as well as in the following experiment, no more than one male and female from a given litter was assigned to the same treatment condition (Holson & Pearce, 1992). Male and female subjects across experimental groups was balanced.
2.2 Procedures
2.2.1 Conditioning phase (PD13)
Pups were separated from their mothers and randomly assigned to one of four independent groups as a function of the different Conditioning treatments: CS-only (n=13), LiCl 1% (n=13), LiCl 0.5% (n=13) or EtOH (n=16). Pups were placed in couples in holding cages (45 × 20 × 20 cm) lined with clean wood shavings and maintained at 30–32°C by means of a heating pad. An intraoral cannula (PE 10 polyethylene tubing, length: 5 cm, Clay Adams, Parsippany, NJ) was implanted in the right cheek of each pup, as previously described (Chotro & Alonso, 2003; Hall & Rosenblatt, 1979). Briefly, a flanged end of the cannula was shaped by exposure to a heat source (external diameter: 1.2 mm). A dental needle (30-gauge Monoject, Sherwood Medical, Munchen, Germany) was attached to the non-flanged end of the cannula and positioned in the middle portion of the intraoral mucosa. The needle was inserted through the cheek and the cannula was pulled through the tissue until the flange end rested on the mouth’s mucosa. This cannulation procedure requires no more than 20 s per subject and does not induce major stress in preweanling rats (Spear, Specht, Kirstein, & Kuhn, 1989). Animals were deprived from maternal care for 90 minutes prior to commencement of conditioning. Previous work (Hunt et al., 1991) indicates that this time interval is appropiate for acquisition of conditioned taste aversion in preweanling pups. Also, by using a relatively short deprivation we sought to avoid the severe stress associated with lengthier deprivation and maternal separation treatments. These procedures can exert aversive unconditional consequences (Smith, Kucharski, & Spear, 1985) likely to interact with the learning processes under analysis. Ninety minutes after cannulation, pup’s bladders were voided by gentle brushing of the anogenital area. Following this procedure body weights were recorded and subjects were placed into individual Plexiglas chambers (10 × 10 × 12 cm). Then pups received a saccharin intraoral infusion (CS, 0.05 % w/v, duration: 7.5 min). Total administration volume was equivalent to 2.75 % of the subject’s pre-infusion body weight. Saccharin was delivered at a constant rate by means of a 10-syringe infusion pump (KD Scientific, Holliston, MA) connected to the oral cannula of each pup by a polyethylene catheter (Clay Adams, PE 50 Parsippany, NJ). When employing these infusion parameters, pups are capable of either consuming or rejecting the infused solution (Arias & Chotro, 2006a; Chotro & Alonso, 2003). After the infusion procedure, subjects were weighed to estimate saccharin consumption scores. Percentage body weight gain (% BWG) was calculated as follows: [Post-infusion weight − Pre-infusion weight/Pre-infusion weight × 100].
Immediately following CS exposure, pups received LiCl [intraperitoneal, i.p., 0.5% or 1.0% of body weight of a 0.3 M LiCl solution (Sigma Aldrich, St Louis, MO, USA)], EtOH (2.5 g/kg, intragastric, i.g.) or remained untreated. Ethanol was administered in a volume equivalent to 0.015 ml per gram of body weight of a 21 % v/v ethanol solution. Pups were then returned to their holding cage, where they remained undisturbed for three hours until being reunited with their mother. The dose and route of administration of ethanol were selected on the basis of studies indicating that 2.5 g/kg i.g. ethanol is capable of inducing reliable one-trial conditioned taste avoidance in preweanling rats (Pautassi, Godoy, Spear & Molina, 2002; Pautassi, Ponce & Molina, 2005; Pautassi et al., unpublished). In administering LiCl via the intraperitoneal route we followed previous work that assessed mechanisms supporting LiCl-mediated conditioned taste aversion during the preweanling period (Hoffman et al., 1991; Vogt and Rudy, 1984).
2.2.2 Testing phase (PDs 14 – 15)
During each testing Day, pups were separated from their mothers, intraorally cannulated and placed in couples during ninety minutes in a heated holding cage (30–32°C). Then pups were tested in terms of saccharin intake for 7.5 min. Apparatus, parameters and behavioral recordings of this test replicate those described for the saccharin infusion procedure conducted on PD 13.
2.2.3 Data analysis
No significant effect of sex or interaction with the remaining factors was found in terms of saccharin acceptance. Hence, for the inferential analysis and descriptive presentation of the results, data were collapsed across sex.
Intake scores were analyzed by means of two-way mixed ANOVAs. Conditioning treatment (CS-only, LiCl-1 %, LiCl-0.5 % or EtOH 2.5 g/kg) served as a between-group factor, while Day (conditioning Day, testing Day 1 and testing Day 2) was considered the within-group variable. Significant main effects and/or interactions were further analyzed by means of follow-up ANOVAs and post-hoc analysis [Fisher’s least significant difference (LSD) test]. All inferential analyses conducted in the present study employed an α level equal to 0.05. When appropriate, we report effect size calculated using the algorithm provided by Myers and Well (Myers & Well, 2003).
2.3 Results
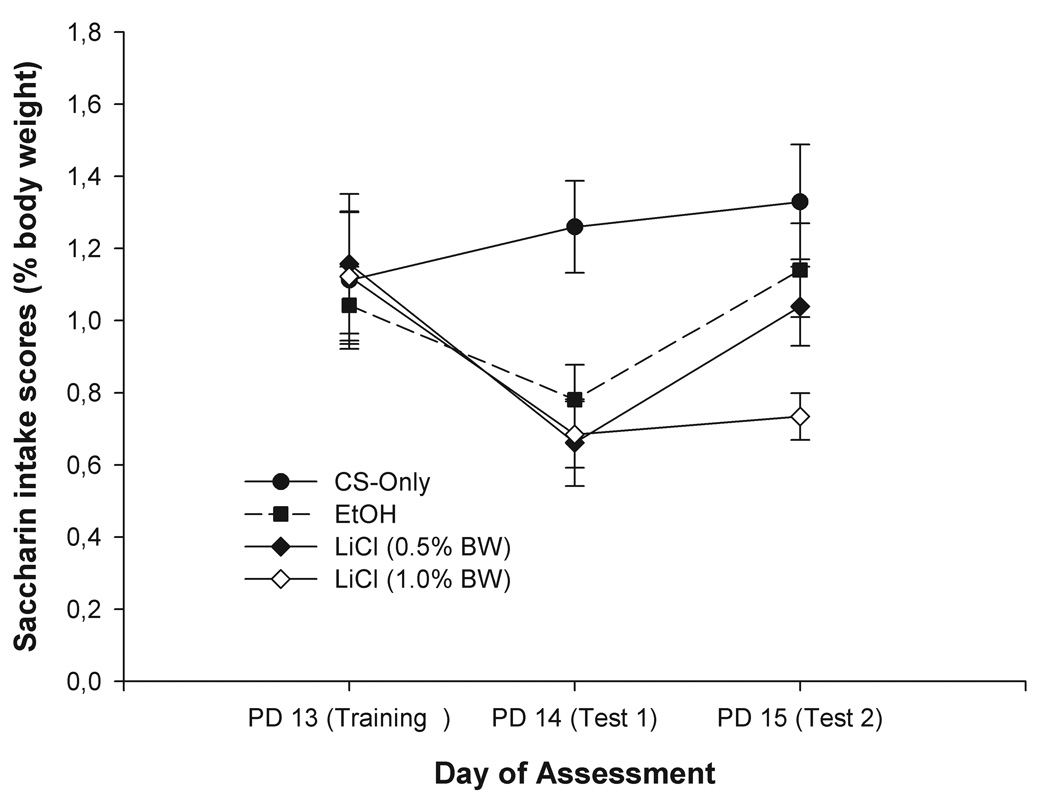
Figure 1 depicts intake scores during conditioning and extinction sessions. As can be observed, acceptance of the tastant CS was fairly similar across groups during initial training but changed dramatically after repeated testing. On the first testing Day (PD 14), pups from the CS-only condition ingested more saccharin than pups given 0.5, 1.0 % LiCl or EtOH. During the second testing Day (PD15) those pups that received 1.0 % LiCl ingested lower amounts of saccharin than the remaining groups. Interestingly, on PD15, saccharin acceptance in pups given EtOH or 0.5% LiCl did not differ from the CS-only control condition. These observations were confirmed by the corresponding ANOVA. This analysis revealed significant effects of Conditioning treatment [F(3, 43) = 3.79, p < 0.05] and Day [F(2, 86) = 59.76, p < 0.001]. The interaction between the latter factors also achieved significance, F(6, 86) = 2.87, p < 0.05. To determine the loci of this significant interaction, follow-up one-way ANOVAs (independent factor: Conditioning treatment) were performed. While no significant differences were detected during conditioning, the ANOVA revealed a significant effect of the main factor Conditioning treatment at testing Day 1 and 2 [F(3, 43) = 4.39, p < 0.05, Cohen’s f = 0.48, and F(3, 43) = 3.31, respectively, p < 0.05, Cohen’s f = 0.61]. On testing Day 1 (PD 14), post-hoc tests indicated lower CS intake in pups given EtOH or LiCl (0.5 or 1.0 %) than in controls. During the second testing Day only those pups treated with the highest LiCl dose (1.0 % body weight) ingested significantly lower amounts of saccharin than the CS-only group.
Figure 1.
Intake scores during conditioning (PD 13) and extinction sessions (PDs 14 and 15) as a function of the different Conditioning treatments (CS-only, LiCl-1 %, LiCl-0.5 % or EtOH 2.5 g/kg). Vertical lines illustrate standard errors of the means.
In summary, pups showed avoidance of a gustatory CS previously paired with postabsorptive consequences of EtOH or LiCl. Magnitude of the EtOH-mediated taste avoidance was equivalent to that induced by 0.5 % LiCl. Perhaps more important, time course of expression of the taste avoidance was highly similar in pups given 2.5 g/kg EtOH or 0.5 % LiCl. Specifically, amount of CS consumption in the latter drug-treated groups did not differ during the second testing and was equivalent to that found in CS-only controls, a phenomenon probably indicating extinction.
3. Experiment 2
In the previous experiment, EtOH and LiCl were assessed in terms of their capability to mediate conditoned taste avoidance. Similar effects of 0.5 % LiCl and 2.5 g/kg EtOH were found in terms of repeated CS intake measurements.. The present experiment compared taste avoidance in terms of CS intake and conditioned disgust reactions induced by the CS after pairing with EtOH or LiCl. That is, aversive reactivity towards the saccharin CS was assessed in terms of (a) saccharin intake and (b) patterns of behavioral reactivity elicited by the taste of saccharin. Doses of EtOH and LiCl corresponded to those shown to have equivalent effects on CTA expression in Experiment 1 (i.e., 2.5 g/kg EtOH and 0.5 % LiCl). In this experiment, a more stringent control condition was included to control for possible nonspecific toxic effects of LiCl or EtOH-- exposure to both the US and the CS but in an explicitly unpaired manner (i.e., unpaired controls).
3.1 Material and Methods
3.1.1 Subjects
Fifty-four 13-Day-old Sprague-Dawley pups (26 females and 28 males), representative of 8 litters were employed. Rearing conditions were similar to those described in Experiment 1.
3.2 Procedures
3.2.1.Conditioning phase (PD13)
The design of the present experiment was a 2 × 2 factorial defined by the following factors: Unconditioned stimulus (EtOH or LiCl) and Conditioning treatment (paired or unpaired). On PD 13 pups were separated from their mothers and randomly assigned to one of the four independent groups: LiCl-Paired (n = 16), LiCl-Unpaired (n = 11), EtOH-Paired (n = 15) or EtOH-Unpaired (n = 12). Pups were intraorally cannulated one hour before receiving intraoral saccharin infusion (CS, 0.05 % w/v; duration: 7.5 min). Immediately after CS exposure, paired pups were given LiCl (0.05 % of body weight of a 0.3 M LiCl solution, i.p.) or EtOH (2.5 g/kg of a 21 % v/v EtOH solution, i.g.). Unpaired animals received the corresponding US treatment (i.e., LiCl or EtOH) two hours after the CS presentation. This interval has been shown to be sufficient to prevent an association between the CS and the US in infant rats when employing either EtOH or LiCl (Hunt et al., 1991; Arias et al., 2006; Chotro and Alonso, 2003). After US administration, pups were kept in heated holding cages with a littermate from the opposite sex and from the same treatment condition. They returned to their homecage 180 minutes after US administration.
3.2.2 Testing phase (PDs 14 – 15)
Every testing Day, pups were separated from their mothers, intraorally cannulated and placed in couples during ninety minutes in a heated holding cage (30–32° C). Then pups were tested in a 12-min taste reactivity test, immediately followed by a 7.5 min saccharin intake test.
3.2.3 Taste reactivity test
Behavioral reaction to the gustatory CS was assessed as described in previous studies (Arias & Chotro, 2005a, 2005b). Briefly, the evaluation was conducted in a trapezoid-shaped chamber with a front wall (29 cm wide) made of clear glass while the remaining walls (back 18 cm, sides 11.5 cm) and floor were made of a mirror glass. This chamber allows analysis of orofacial and body movements regardless of the location of the pup. The chamber was 12.5 cm high and divided into two equal sections. Two pups were evaluated at a time, one per section of the chamber. Pups remained in the test chamber for 2 minutes of habituation to the environment before commencement of the intraoral infusions of saccharin (baseline phase). Then, pups received five intraoral pulses of saccharin (15-sec each), one every two minutes, for a total time of 10 min. Infusion rates employed during the taste reactivity test were 0.60 and 0.76 ml per minute on PDs 14 and 15, respectively, which were selected in order to use similar infusion rates as those employed during the intake test in Experiment 1. Pup’s behavior was videotaped for subsequent analysis.
Based on previous studies using taste reactivity tests with infant rats (Arias & Chotro, 2005a, 2005b, 2006a; Hall & Bryan, 1981; Hoffmann et al., 1990; Hoffmann, Molina, Kucharski, & Spear, 1987; Pautassi, Arias, Molina, & Spear, 2008; Vigorito & Sclafani, 1988) and adult rats (Berridge, 2000; Davies & Parker, 1990; Kiefer, Hill, Coonfield, & Ferraro, 2005; Limebeer & Parker, 2000; Parker, 1991, 1993, 1995, 2003), the following behavioral measures were selected: general activity, wall climbing, grooming, facial wiping, head shaking and mouthing movements.
The behavioral measures were analyzed from the videotapes and scored every 30 seconds. General activity was rated in seven categories as described by Hall and Bryan (Hall & Bryan, 1981): 0 = no movement, except for occasional twitches; 1 = slight movement, usually of the head or paw, sustained for five seconds; 2 = substantial movement of the head and paws, including grooming, but no locomotion; 3 = locomotion involving forelimbs and often including rooting and probing but with hind limbs motionless and usually serving as a pivot; 4 = clear and sustained locomotion about the test container; 5 = vigorous locomotion, often including rolling, kicking, and wall climbing; 6 = an extreme, but occasionally observed, form of rating five in which the pup tumbled about its container for most of the 30 sec interval, locomotion, rolling, probing, wall climbing and jumping. The range of the score was 1–6. When a given behavior was done for the most part of the 30 sec interval we considered that the pup was doing such behavior.
A pup was considered to be wall climbing when standing on its rear limbs with its forepaws against the wall of the testing chamber. Time in seconds for wall climbing was registered for each pup every 30 sec during the baseline and test intervals. Head shaking was the rapid side-to-side movement of the head frequently associated with fluid expulsion. Number of head-shakes was registered throughout each successive 30 sec interval. Grooming was defined as a sequence of movements lasting approximately 5-sec, beginning with rapid elliptical strokes by the paws over the nose. The amplitude of such movements progressively increases and is continued by large bilateral strokes. This sequence of movements concludes with a postural turn followed by a period of body licking directed to the flank (Berridge, Fentress, & Parr, 1987; Kalueff & Tuohimaa, 2005). Facial wiping was operationalized through the placement of the forepaw onto the face followed by a downward stroke of the paw across the face, generically from ear to nose (Brumley & Robinson, 2004). Mouthing was defined as any obvious movement of the mouth and jaws. Time in seconds for grooming, mouthing and facial wiping was registered every 30 sec. Three trained researchers blind to the experimental conditions performed all behavioral observations. In a preliminary phase, they rated all the data for a subset of the subjects. This phase allowed assessment of the reliability between raters through Pearson’s p (all values > .90). Afterwards, each rater scored some of the behaviors under analysis.
3.2.4 Intake test
On PDs 14 and 15, immediately after termination of the taste reactivity test, pups were tested in terms of saccharin intake. Animals were removed from the chamber in which the taste reactivity test was conducted and transferred to an adjacent room. Then, the pups’ bladders were voided by gentle brushing of the anogenital area. Body weights were immediately recorded and consumption was evaluated using a procedure similar to that described in Experiment 1. The dependent variable analyzed was intake expressed as a percentage of body weight gained during the 7.5 min test.
3.2.5 Data analysis
No significant effect of sex or interaction with the remaining factors was found in terms of saccharin acceptance or taste reactivity measures. This led to data being collapsed across sex.
Saccharin intake during conditioning and testing phases were analyzed using three-way mixed ANOVAs. These analyses included as independent variables Conditioning treatment (paired or unpaired) and Unconditioned stimulus (EtOH 2.5 g/kg or LiCl 0.5 %). Day (conditioning, testing Day 1 and testing Day 2) was considered the within-subject variable.
Behavioral scores collected during the taste reactivity test were collapsed into two values, one corresponding to baseline (duration: 2 min), and the other corresponding to the test section in which intraoral pulses were delivered (duration: 10 minutes). Behavioral responsiveness (mouthing, locomotion, wall climbing, facial wiping, grooming and head-shaking) collected during baseline and the intraoral infusion phase was scrutinized by separate three-way mixed ANOVAs. These analyses included Conditioning treatment (paired or unpaired) and Unconditioned stimulus (EtOH or LiCl) as independent variables and Day (testing Day 1 and testing Day 2) as a within factor.
Significant main effects and/or interactions were further analyzed by means of follow-up ANOVAs and post-hoc analysis [Fisher’s LSD test]. The effect size is reported, whenever appropriate, by the algorithm provided by Myers and Well (Myers & Well, 2003).
We further analyzed the behavioral measures recorded during the taste reactivity test and the intake data by means of a correlational approach. Hence, a matrix of correlations (Pearson’s product-moment correlation) was created for each testing Day. This analysis was meant to supplement and not to replace the analysis of mean differences in behavioral responsiveness by ANOVAs. While the latter approach assessed the expression of conditioned taste aversion across groups, the specific question pursued by the correlation analysis was to what extent the different behaviors were related to one another or to the intake scores. In other words, a correlational analysis is important towards understanding the internal structure of the taste reactivity components and their relationship with intake scores. There has been considerable interest in assessing the relationship between taste reactivity test indices. Previous literature has considered that increased wall climbing, facial wiping or head shaking may be reflecting negative hedonic value of a given taste, while increased mouthing movements often accompany positive hedonic reactions (Arias & Chotro, 2005a, 2005b; Hall & Bryan, 1981; Hoffmann et al., 1991; Hoffmann et al., 1990; Hoffmann et al., 1987). This information is important to validate the combination of taste reactivity responses in aversive and appetitive clusters, a procedure that has been widely employed (e.g.; Hill and Kiefer, 1997).
3.3 Results
3.3.1 Taste reactivity test
Baseline
No significant effects or significant interactions were found when behavioral responsiveness (mouthing, locomotion, wall climbing, facial wiping, grooming and head-shaking) during baseline was analyzed.
Mouthing
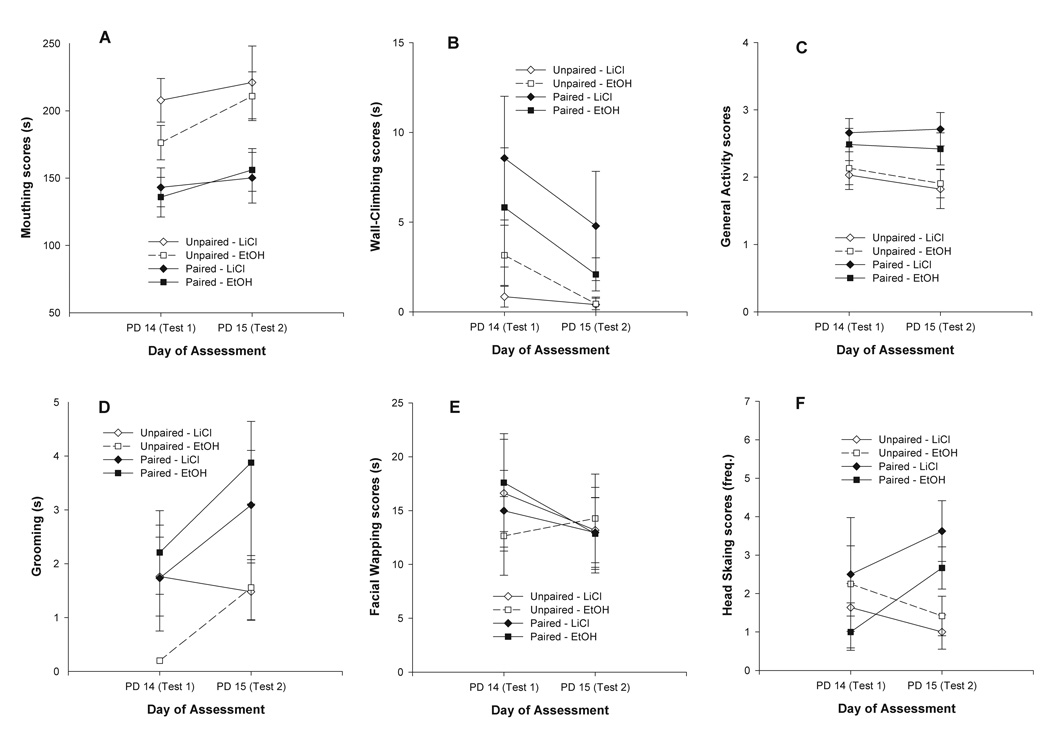
Figure 2a depicts mouthing scores during the taste reactivity tests as a function of Conditioning treatment (paired or unpaired) and Unconditioned stimulus (LiCl or EtOH). During the actual saccharin infusions, the corresponding ANOVA revealed significant effects of the main factors, Conditioning treatment [F(1, 50) = 14.29, p < 0.001, Cohen’s f = 0.53] and Day [F(1, 50) = 4.60, p < 0.05, Cohen’s f = 0.29]. As revealed by subsequent post-hoc tests, paired animals, regardless of the US (i.e., LiCl or EtOH), showed significantly less time giving mouthing movements than control counterparts. Across conditioning and for both USs, duration of this behavior was higher on the second testing Day.
Figure 2.
Behavior scores of the different reactions registered during the taste reactivity tests (PDs 14 and 15) as a function of Conditioning treatment (paired or unpaired) and Unconditioned stimulus (LiCl or EtOH). Vertical lines illustrate standard errors of the means.
Wall Climbing
During the taste reactivity test, pups given paired CS/US presentations spent more time wall climbing than pups from unpaired conditions (Figure 2b). The inferential analysis of the data confirmed this observation. The ANOVA revealed a significant effect of the main factor, Conditioning treatment, F(1, 50) = 4.31, p < 0.05, Cohen’s f = 0.29. No significant effects or interactions involving Unconditioned stimulus (LiCl vs. EtOH) were observed.
General activity
Conditioning treatment significantly affected general activity scores at test [F(1, 50) = 7.51, p < 0.05, Cohen’s f = 0.39]. As already observed in relation with wall climbing scores, pups that received paired CS/US presentations displayed higher levels of general activation than unpaired control pups (see Figure 2c).
Grooming
The ANOVA conducted with grooming scores at test revealed significant effects of Conditioning treatment [F(1, 50) = 4.67, p < 0.05, Cohen’s f = 0.49], and Day [F(1, 50) = 7.31, p < 0.01, Cohen’s f = 0.39]. Scores corresponding to this behavior were significantly higher during the second testing Day than the first one. More important for the aim of the present study is the fact that pups from groups LiCl-Paired and EtOH-Paired spent more time grooming than those from unpaired conditions (see Figure 2d).
Facial wiping
When facial wiping scores at test were analyzed, the ANOVA did not detect significant effects of Conditioning treatment or US. This analysis revealed only a significant effect of Day [F(1, 50) = 6.57, p < 0.05, Cohen’s f = 0.35] that interacted with the nature of the Unconditioned stimulus given to the pups [F(1, 50) = 11.79, p < 0.01, Cohen’s f = 0.48]. Follow-up within-factor ANOVAs for each Unconditioned stimulus revealed that facial wiping scores were lower at the second testing Day, but only when LiCl was employed as a US (see Figure 2e).
Head shaking
Figure 2f depicts head shaking scores registered during the taste reactivity test. On PD 15, amount of headshakes was significantly higher in pups from paired conditions than in those from the unpaired ones. The ANOVA revealed a significant interaction comprising Conditioning treatment and Day, F(1, 50) = 5.11, p < 0.05. Subsequent follow-up ANOVAs including Conditioning treatment as a between factor were conducted for each testing Day. These analyses revealed only a significant effect of Conditioning treatment during the second testing Day, F(1, 50) = 9.16, p < 0.01, Cohen’s f = 0.44, indicating that on PD 15, head shaking was significantly higher in paired relative to unpaired pups.
3.3.2 Intake test
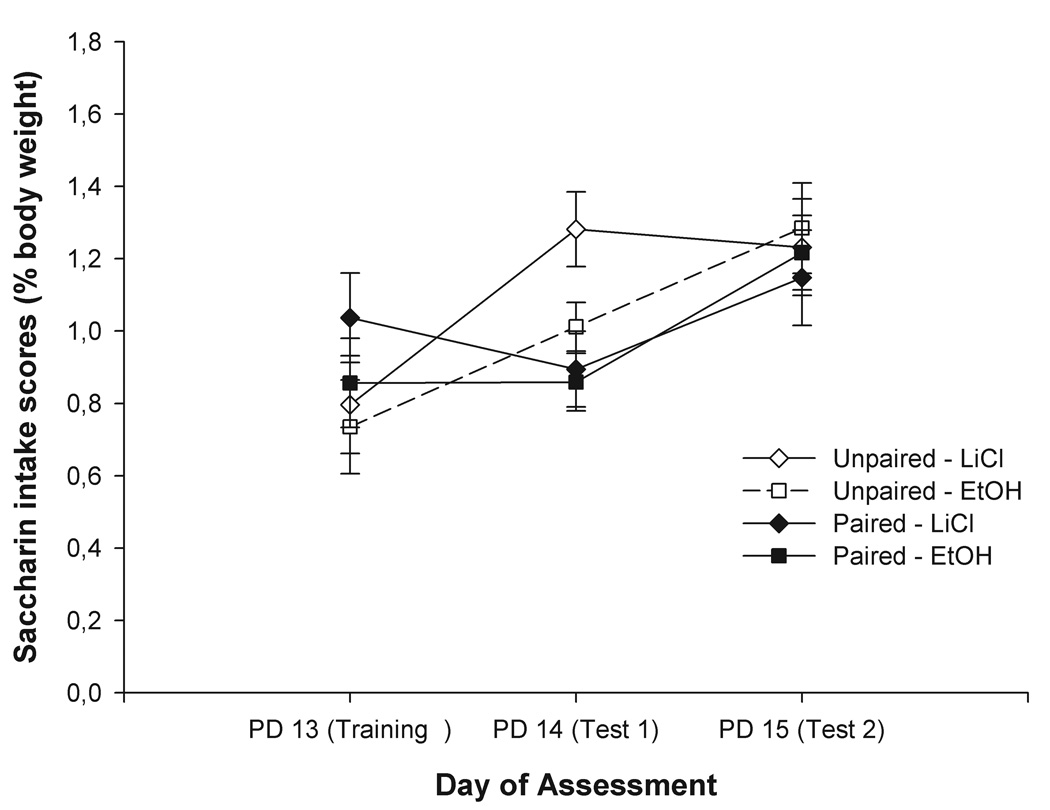
Figure 3 depicts intake of saccharin as a function of Conditioning treatment and nature of Unconditioned stimulus given to the pups. At testing Day 1, pups from Paired-LiCl and Paired-EtOH groups consumed less saccharin than controls, an effect that was no longer evident during the second testing Day. The ANOVA revealed a significant effect of the main factor, Day, F(2, 100) = 15.10, p < 0.0001. The Conditioning treatment-by-Day interaction was also significant, F(4, 100) = 5.79, p < 0.01. To determine the loci of this significant interaction, follow-up one-way ANOVAs were performed considering Conditioning treatment as the only independent factor, and analyzing separately intake data from conditioning or each testing Day. No significant differences between groups were detected at conditioning. The ANOVA for testing Day 1 revealed a significant effect of Conditioning treatment [F(1, 50) = 8.39, p < 0.01, Cohen’s f = 0.40], an effect that failed to interact with Unconditioned stimulus. Pups from paired conditions ingested less saccharin than those from unpaired conditions. The ANOVA for intake scores at testing Day 2 indicated no significant effects.
Figure 3.
Intake of saccharin as a function of Conditioning treatment (paired or unpaired) and nature of Unconditioned stimulus given to the pups (LiCl or EtOH). Vertical lines illustrate standard errors of the means.
3.3.3 Analysis of correlations
Correlations encompassing behavioral responsiveness and intake scores from testing Days 1 and 2 are presented in Tables 1 and 2, respectively. At both testing Days, mouthing negatively and significantly correlated with wall climbing and general activity scores. On the other hand, facial wiping, grooming and head shaking were significantly and positively correlated. Another interesting observation is that during the second testing Day, head shaking correlated significantly and positively with wall climbing and general activity. On the other hand, on PD 15 a significant negative association was found between head shaking and mouthing. It should be noted that, as previously stated, head shakes were more frequent on PD 15, particularly in paired pups. Finally, no significant correlations were observed when examining the association between intake data and the remaining behaviors.
Table 1. Testing Day 1.
Correlations encompassing behavioral responsiveness and intake scores from testing days 1 (Table 1) and 2 (Table 2). At both testing days, mouthing negatively and significantly correlated with wall climbing and general activity scores. Facial wiping, grooming and head shaking were significantly and positively correlated. No significant correlations were observed between intake data and the remaining behaviors.
| Saccharin Intake (% BW) |
Mouthing (s) |
Wall- Climbing (s) |
General Activity |
Grooming (s) |
Facial Wipng (s) |
Head Shaking (freq) |
|
|---|---|---|---|---|---|---|---|
| Saccharin Intake (% BW) | ----------- | 0.20 | −0.24 | −0.08 | 0.17 | −0.11 | 0.03 |
| Mouthing(s) | 0.20 | ----------- | −0.42* | −0.70* | 0.15 | −0.19 | 0.01 |
| Wall-Climbing(s) | −0.24 | −0.42* | ----------- | 0.49* | 0.17 | −0.22 | −0.09 |
| General Activity | −0.08 | −0.70* | 0.49* | ---------- | −0.03 | −0.07 | −0.12 |
| Grooming (s) | 0.17 | −0.15 | 0.17 | −0.03 | ----------- | 0.43* | 0.33* |
| Facial Wiping (s) | −0.11 | −0.19 | −0.22 | −0.07 | 0.43* | ----------- | 0.44* |
| Head Shaking(freq) | 0.03 | 0.01 | −0.09 | −0.12 | 0.33* | 0.44* | ---------- |
Correlations significant at p < 0.05 are marked with asterisks and in bold.
Table 2. Testing Day 2.
Correlations encompassing behavioral responsiveness and intake scores from testing days 1 (Table 1) and 2 (Table 2). At both testing days, mouthing negatively and significantly correlated with wall climbing and general activity scores. Facial wiping, grooming and head shaking were significantly and positively correlated. No significant correlations were observed between intake data and the remaining behaviors.
| Saccharin Intake (% BW) |
Mouthing (s) |
Wall- Climbing (s) |
General Activity |
Grooming (s) |
Facial Wiping (s) |
Head Shaking (freq) |
|
|---|---|---|---|---|---|---|---|
| Saccharin Intake (% BW) | ----------- | −0.22 | −0.09 | −0.19 | 0.08 | 0.17 | 0.15 |
| Mouthing(s) | −0.22 | ----------- | −0.36* | −0.70* | −0.02 | −0.22 | −0.40* |
| Wall-Climbing(s) | −0.09 | −0.36* | ----------- | 0.45* | −0.15 | −0.16 | 0.28* |
| General Activity | −0.19 | −0.70* | 0.45* | ---------- | 0.12 | 0.08 | 0.32* |
| Grooming (s) | 0.08 | −0.02 | −0.15 | 0.12 | ----------- | 0.31* | 0.41* |
| Facial Wiping (s) | 0.17 | −0.22 | −0.16 | 0.08 | 0.31* | ----------- | 0.12 |
| Head Shaking(freq) | 0.15 | −0.40* | 0.28* | 0.32* | 0.41* | 0.12 | ---------- |
Correlations significant at p < 0.05 are marked with asterisks and in bold.
In summary, these results confirm that EtOH and LiCl, at the doses employed in the present experiment, induced equivalent levels of taste avoidance in preweanling rats. It is important to note that, in the present experiment, the intake test was conducted after the taste reactivity test. This could have facilitated extinction of the taste avoidance, since pups received intermittent non-reinforced exposure to the CS. Nevertheless, significant differences were detected on testing Day 1 in terms of fluid rejection as a function of Conditioning treatment. Rapid extinction of conditioned taste avoidance was again observed on testing Day 2, as indexed by paired pups exhibiting CS consumption similar to that of their control counterparts. In other words, despite the procedural changes, expression of drug-mediated taste avoidance was highly similar to that found in Experiment 1 and equivalent whether the US was LiCl or EtOH.
4. Discussion
Preweanling rats rapidly learned to avoid a tastant CS previously paired with either LiCl (1 or 0.5 % of body weight, 0.3 M) or a relatively high ethanol dose (2.5 g/kg). Magnitude of the ethanol-induced taste avoidance was similar to that produced by the lower LiCl dose (Experiments 1 and 2). Specifically, in both experiments, a single conditioning trial (postnatal Day 13) resulted in equivalently suppressed CS consumption after pairing with LiCl or EtOH when assessed 24 hour later (first testing Day, PD 14) and rapid extinction in a later assessment (second testing Day, PD 15).
We found (Experiment 2) a similar quantitative and qualitative pattern of rejection reactions following conditioning with ethanol or LiCl. Regardless of the US employed at training, paired pups showed heightened head shaking and overall activity, and also spent more time wall climbing and grooming than unpaired controls. In addition, infants from paired conditions mouthed less than did controls. Head shaking, increased locomotion, facial wiping and wall climbing have been frequently considered as aversive reactions in preweanling and adult rats (Arias & Chotro, 2005a, 2005b, 2006a; Berridge, 2000; Hall & Bryan, 1981; Hoffmann et al., 1991; Parker, 1991, 1993, 1995, 2003; Vigorito & Sclafani, 1988). On the other hand, mouthing has been included under the category of ingestive or appetitive behaviors in rats (Arias & Chotro, 2005a, 2005b, 2006a; Berridge, 2000; Hall & Bryan, 1981; Hoffmann et al., 1991; Parker, 1991, 1993, 1995, 2003; Vigorito & Sclafani, 1988). The present results indicate that the pairing of post-absorptive effects of ethanol and a taste CS produces: (a) taste avoidance and, perhaps more important, (b) a shift in the motivational value (i.e., palatability) of the taste CS. In other words, the gustatory CS acquired negative hedonic value after being associated with ethanol.
It is of considerable interest to discuss these findings within the framework of the ontogeny of learning and behavior, as motivated by aversive reinforcers. Hoffmann and coworkers (Hoffmann et al., 1991) found that 15-Day-old pups exhibit less mouthing than controls when stimulated with a 15% w/v sucrose solution previously paired with LiCl, citric acid intraoral infusion or footshock. In other words, not only malaise-inducing agents (LiCl) but also exteroceptive aversive stimuli (footshock) resulted in reduced mouthing, suggesting that this variable is not a specific index of malaise-induced taste aversion at this age. Mouthing has been classified primarily as a positive or hedonic response (Hill & Kiefer, 1997; 2002; Higley & Kiefer, 2006) and in some situations can enter within the neutral reactions category (Berridge, 2000). In fact, mouthing, overall activity and grooming are all behaviors that can be classified as neutral for adult rats (Berridge, 2000). The term “neutral” refers to the fact that these behaviors are often under control of several other factors that are not directly linked to the hedonic value of the taste (Berridge, 2000). In contrast, wall climbing and paw treading are specifically observed when 15-Day-old rats are given paired presentations of a taste CS and LiCl (Hoffmann et al., 1991). These results indicate that conditioned aversions during the second postnatal week of life in the rat are differentially expressed as a function of the US employed. The present study allows the conclusion that, during this developmental stage, ethanol and LiCl induce similar patterns of conditioned disgust reactions. This pattern includes reduced mouthing and increased overall activity as well as heightened wall climbing. A reduction in mouthing together with an increase in the aversive components of the taste reactivity test indicates undoubtedly a reduction in palatability of the conditioned taste. As described, increases in wall climbing have been reported in 15-Day-old rats particularly when emetic agents such as LiCl are employed (Hoffmann et al., 1991).
Also relevant for the broader field of the ontogeny of learning is that, in the present study, disgust reactions and taste avoidance showed differential patterns of extinction. Behaviors such as head shaking showed a delayed pattern of emergence, being particularly evident during the second testing Day, a result congruent with recent observations of preweanling rats (Pautassi et al., 2008). In the present study, conditioned taste avoidance, but not disgust reactions, was extinguished by the end of testing. Employing adult rats, Cántora et al (Cántora et al., 2006) reported the opposite, persistence of lithium-mediated taste avoidance after complete extinction of conditioned disgust reactions, suggesting the existence of age-related differences in the expression of aversive learning. Besides age-related differences, there are procedural factors that may explain the apparent discrepancy between these studies. In the present work, the CS was intraorally delivered while in Cántora et al. (Cántora et al., 2006), a two bottle test was employed to assess taste avoidance. As these authors mentioned, conditioned taste avoidance extinguishes faster when using intraoral infusion procedures than when employing two-bottle test (Fouquet, Oberling, & Sandner, 2001; Wolgin & Wade, 1990; Yamamoto, Fresquet, & Sandner, 2002). This observation is congruent with the fact that preweanling rats treated with 3.0-g/kg ethanol also show rapid extinction of conditioned intake suppression when assessed by intraoral infusion (Arias & Chotro, 2006b). In addition, these authors also observed ethanol-induced conditioned disgust reactions after a 3-Day interval between conditioning and testing, but no differences were detected in terms of intake (Arias & Chotro, 2006a).
According to Parker (Parker, 1995, 2003, 2006), conditioned rejection reactions reflect “conditioned nausea” in rats. Conditioned nausea is a theoretical construct, inferred from the fact that, although rats cannot vomit, they display these rejection behaviors in response to drug treatments that induce vomit in human and in emetic species, including LiCl, cyclophosphamide or apomorphine (Andrews & Horn, 2006). These behaviors are not present when employing treatments that do not induce vomit, even when dosage of these drugs is titrated to produce equivalent taste avoidance to that found with LiCl (Parker, 1991, 1995, 2003). Also, acquisition of disgust reactions induced by emetic drugs such as LiCl is inhibited by treatments that inhibit nausea in humans and retching in ferrets, such as Ondansetron or Domperidone (Lau, Ngan, Rudd, & Yew, 2005; Limebeer & Parker, 2000). A similar finding has been recently observed in infant rats. The administration of Domperidone to 14-Day olds inhibited several LiCl-mediated disgust reactions, including wall climbing, without affecting the magnitude of the taste avoidance induced by the emetic agent (Pautassi et al., 2008). It should be noted that those behaviors (e.g. gaping, chin rubbing and paw pushing) more frequently interpreted as “conditioned nausea” in rats (Parker, 1995, 2003) were not observed in our study, regardless of the US employed. In adult rats, these aversive behaviors have been observed in response to a gustatory CS previously paired with a relatively high ethanol dose (2 g/kg) (Davies & Parker, 1990). In our study, we did observe conditioned suppression of mouthing, which seems to be a nonspecific index of conditioned taste aversion. That is, conditioned reduction in mouthing is observed when employing emetic (LiCl) as well as non-emetic USs, such as citric acid or footshock (Hoffmann et al., 1991). There are some behaviors specifically elicited in infants by emetic agents, notably wall climbing (Hoffmann et al., 1991; Pautassi et al., 2008). As already mentioned, in Experiment 2 paired pups exhibited longer durations of wall climbing than control counterparts across testing sessions, a result that was not affected by the nature of the US (i.e., LiCl or ethanol).
In conjunction with previous studies (Davies & Parker, 1990; Hoffmann et al., 1991; Hoffmann et al., 1990; Pautassi et al., 2008), the present results suggest that, in infant rats, CTAs mediated by LiCl and ethanol may share a similar mechanism. This hypothesis is supported by other empirical evidence. For example, CSs paired with high ethanol doses or LiCl induce similar patterns of c-Fos activity in the area postrema, the lateral parabrachial nucleus and the nucleus of the solitary tract, areas directly involved in nausea (Thiele, Roitman, & Bernstein, 1996). In addition, taste-aversion-prone rats consume lower ethanol amounts than taste-aversion-resistant rats (Orr, Walters, & Elkins, 1997; Orr, Whitford-Stoddard, & Elkins, 2004). These rat strains have been selected specifically according to the magnitude of taste avoidance acquired when employing cyclophosphamide as US, an emetic-class agent (Elkins, 1986).
A consistent pattern of correlations emerged on both testing Days. Wall climbing and overall activity were positively correlated, and both behaviors correlated negatively with mouthing. Furthermore, significant positive correlations were found between facial wiping, grooming and head shaking scores. In the second testing Day, head shaking also correlated positively with other disgust reactions (wall climbing and overall activity), and negatively with mouthing. Interestingly, increased head shakes were observed in paired groups only during the second testing Day. The multiple association comprising wall climbing, head shaking and overall activity is consistent with the notion that they reflect a shared negative hedonic component acquired by the tastant CS during training. It is not surprising that grooming, head shaking and facial wiping were significantly correlated. In the context of aversive learning, facial wiping and head shaking can occur in close temporal contiguity, at least in adult animals (Berridge, 2000). In addition, facial wiping in adult rats has also been linked to grooming (Berridge, 2000). Grooming is a complex behavior pattern associated with several actions, including alleviation of stress (Kalueff & Tuohimaa, 2005).
None of these conditioned disgust reactions were correlated significantly with intake scores. This lack of association seems to support the hypothesis that these indexes reflect different processes (Berridge, 2000). Specifically, taste reactivity patterns reflect whether or not a given taste is liked (i.e., palatability). Palatability is often -- but not always -- correlated with the willingness to ingest. There are many situations in which palatability and ingestion can be dissociated (Berridge, 2000). Animals can ingest a given taste without exhibiting positive hedonic taste reactivity components, or show a neutral hedonic pattern but still reject a taste. Parker (1995) found that although most drugs of abuse induce taste avoidance, only those inducing internal malaise also induce aversive taste reactivity. Extensive 6-OHDA lesions that disrupt dopamine transmission suppress intake but do not affect the normal hedonic pattern of response to tastants (Berridge, 2000). In summary, several studies have indicated the taste reactivity components can be independent of intake scores. Furthermore, the lack of association between taste reactivity and intake scores found in Experiment 2 is consistent with previous work. We recently assessed, in preweanling rats, taste reactivity and saccharin intake after saccharin-LiCl pairings (Pautassi et al., 2008). As in the present study, we found no significant correlation between saccharin consumption levels at test and taste reactivity scores.
In summary, preweanling rats exhibited similar patterns of conditioned disgust reactions when stimulated with a taste previously paired with either LiCl or ethanol, even when these drugs were titrated to induce similar levels of taste avoidance. These results suggest that, at least when high doses are employed, ethanol may share some aversive postabsorptive consequences with the emetic agent LiCl. The results have implications for the interpretation of ethanol-mediated CTA. It is still a matter of discussion if CS intake suppression (i.e., taste avoidance) following conditioning with stimulant drugs is driven by aversive or appetitive drug effects. The present results strongly suggest that, during infancy, when employing relatively high ethanol doses, CTA is mainly driven by the drug’s aversive effects. The present study also provides relevant information for the ontogeny of aversively motivated learning and illustrates the value of the taste reactivity technique to capture the hedonic components involved in the appetitive and consummatory processes during early ontogeny.
Acknowledgements
This work was supported by Supported by grants from the NIAAA (AA11960, AA013098, AA015992) and the NIMH (MH035219) to NES and the Agencia Nacional de Promocion Cientifica y Tecnologica (PICT 05-14024) to JCM, as well as a Postdoctoral fellowship from Ministerio de Educacion y Ciencia from Spain to CA. The authors wish to express their gratitude to Teri Tanehaus and Heather Murphy for their technical assistance.
References
- Andrews PL, Horn CC. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auton Neurosci. 2006;125(1–2):100–115. doi: 10.1016/j.autneu.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Increased palatability of ethanol after prenatal ethanol exposure is mediated by the opioid system. Pharmacol Biochem Behav. 2005a;82(3):434–442. doi: 10.1016/j.pbb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Increased preference for ethanol in the infant rat after prenatal ethanol exposure, expressed on intake and taste reactivity tests. Alcohol Clin Exp Res. 2005b;29(3):337–346. doi: 10.1097/01.alc.0000156115.35817.21. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Ethanol-induced preferences or aversions as a function of age in preweanling rats. Behav Neurosci. 2006a;120(3) doi: 10.1037/0735-7044.120.3.710. In press. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Interactions between prenatal ethanol exposure and postnatal learning about ethanol in rat pups. Alcohol. 2006b;40(1):51–59. doi: 10.1016/j.alcohol.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Arias C, Molina JC, Mlewski EC, Pautassi RM, Spear NE. Acute sensitivity and acute tolerance to ethanol in preweanling rats with or without prenatal experience with the drug. Pharmacol Biochem Behav. 2008;89(4):608–622. doi: 10.1016/j.pbb.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Miller S, Molina JC, Spear NE. Novelty modulates the stimulating motor effects of ethanol in preweanling rats. Pharmacol Biochem Behav. 2009;92(3):448–456. doi: 10.1016/j.pbb.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Molina JC, Spear NE. Ethanol-mediated aversive learning as a function of locomotor activity in a novel environment in infant Sprague-Dawley rats. Pharmacol Biochem Behav. 92(4):621–628. doi: 10.1016/j.pbb.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Molina JC, Spear NE. Ethanol induces locomotor activating effects in preweanling Sprague-Dawley rats. Alcohol. 2009a;43(1):13–23. doi: 10.1016/j.alcohol.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Molina JC, Spear NE. Naloxone and baclofen attenuate ethanol's locomotor-activating effects in preweanling Sprague-Dawley rats. Behav Neurosci. 2009b;123(1):172–180. doi: 10.1037/a0014049. [DOI] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Hansen C, Molina JC, Paglini G, Spear NE. Dopamine receptors modulate ethanol's locomotor-activating effects in preweanling rats. Dev Psychobiol. 2010;52(1):13–23. doi: 10.1002/dev.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Molina JC, Spear NE. Differential role of micro, delta and kappa opioid receptors in ethanol-mediated locomotor activation and ethanol intake in preweanling rats. Physiol Behav. 2010;99(3):348–354. doi: 10.1016/j.physbeh.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24(2):173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Fentress JC, Parr H. Natural syntax rules control action sequence of rats. Behav Brain Res. 1987;23(1):59–68. doi: 10.1016/0166-4328(87)90242-7. [DOI] [PubMed] [Google Scholar]
- Brumley MR, Robinson SR. Facial wiping in the rat fetus: variation of chemosensory stimulus parameters. Dev Psychobiol. 2004;44(4):219–229. doi: 10.1002/dev.20005. [DOI] [PubMed] [Google Scholar]
- Cántora R, Lopez M, Aguado L, Rana S, Parker LA. Extinction of a saccharin-lithium association: assessment by consumption and taste reactivity. Learn Behav. 2006;34(1):37–43. doi: 10.3758/bf03192869. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Alonso G. Stimulus preexposure reduces generalization of conditioned taste aversions between alcohol and non-alcohol flavors in infant rats. Behav Neurosci. 2003;117(1):113–122. [PubMed] [Google Scholar]
- Chotro MG, Arias C. Ontogenetic difference in ethanol reinforcing properties: the role of the opioid system. Behavioural Pharmachology. 2007;18(7):661–666. doi: 10.1097/FBP.0b013e3282f00754. [DOI] [PubMed] [Google Scholar]
- Davies BT, Parker LA. Novel versus familiar ethanol: a comparison of aversive and rewarding properties. Alcohol. 1990;7(6):523–529. doi: 10.1016/0741-8329(90)90043-c. [DOI] [PubMed] [Google Scholar]
- Elkins RL. Separation of taste-aversion-prone and taste-aversion-resistant rats through selective breeding: implications for individual differences in conditionability and aversion-therapy alcoholism treatment. Behav Neurosci. 1986;100(1):121–124. doi: 10.1037//0735-7044.100.1.121. [DOI] [PubMed] [Google Scholar]
- Fouquet N, Oberling P, Sandner G. Differential effect of free intake versus oral perfusion of sucrose in conditioned taste aversion in rats. Physiol Behav. 2001;74(4–5):465–474. doi: 10.1016/s0031-9384(01)00585-6. [DOI] [PubMed] [Google Scholar]
- Hall WG. Neural systems for early independent ingestion: regional metabolic changes during ingestive responding and dehydration. Behav Neurosci. 1989;103(2):386–411. doi: 10.1037//0735-7044.103.2.386. [DOI] [PubMed] [Google Scholar]
- Hall WG, Bryan TE. The ontogeny of feeding in rats: IV. Taste development as measured by intake and behavioral responses to oral infusions of sucrose and quinine. J Comp Physiol Psychol. 1981;95(2):240–251. doi: 10.1037/h0077771. [DOI] [PubMed] [Google Scholar]
- Higley A, Kiefer S. Delta receptor antagonism, ethanol taste reactivity, and ethanol consumption in outbred male rats. Alcohol. 2006;40(3):143–150. doi: 10.1016/j.alcohol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Hill K, Kiefer S. Naltrexone treatment increases the aversiveness of alcohol for outbred rats. Alcoholism-Clinical and Experimental Research. 1997;21(4):637–641. [PubMed] [Google Scholar]
- Hoffmann H, Hunt P, Spear N. Ontogenetic differences in Cs palatability following conditioned taste aversion training. Learning and Motivation. 1991;22:329–352. [Google Scholar]
- Hoffmann H, Hunt P, Spear NE. Ontogenetic differences in the association of gustatory and tactile cues with lithium chloride and footshock. Behav Neural Biol. 1990;53(3):441–450. doi: 10.1016/0163-1047(90)90324-y. [DOI] [PubMed] [Google Scholar]
- Hoffmann H, Molina JC, Kucharski D, Spear NE. Further examination of ontogenetic limitations on conditioned taste aversion. Dev Psychobiol. 1987;20(4):455–463. doi: 10.1002/dev.420200409. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14(3):221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Kraebel KS, Rabine H, Spear LP, Spear NE. Enhanced ethanol intake in preweanling rats following exposure to ethanol in a nursing context. Dev Psychobiol. 1993;26(3):133–153. doi: 10.1002/dev.420260302. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Molina JC, Spear LP, Spear NE. Ethanol-mediated taste aversions and state-dependency in preweanling (16-Day-old) rats. Behav Neural Biol. 1990;54(3):300–322. doi: 10.1016/0163-1047(90)90650-u. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Spear LP, Spear NE. An ontogenetic comparison of ethanol-mediated taste aversion learning and ethanol-induced hypothermia in preweanling rats. Behav Neurosci. 1991;105(6):971–983. doi: 10.1037//0735-7044.105.6.971. [DOI] [PubMed] [Google Scholar]
- Institute-of-Laboratory-Animal-Resources. Washington, DC: National Academic Press; National Research Council. Guide for the Care and Use of Laboratory Animals. 1996
- Kalueff AV, Tuohimaa P. Contrasting grooming phenotypes in three mouse strains markedly different in anxiety and activity (129S1, BALB/c and NMRI) Behav Brain Res. 2005;160(1):1–10. doi: 10.1016/j.bbr.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Hill KG, Coonfield DL, Ferraro FM., 3rd Ethanol familiarity and naltrexone treatment affect ethanol responses in rats. Alcohol. 2005;37(3):167–172. doi: 10.1016/j.alcohol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Lau AH, Ngan MP, Rudd JA, Yew DT. Differential action of domperidone to modify emesis and behaviour induced by apomorphine in the ferret. Eur J Pharmacol. 2005;516(3):247–252. doi: 10.1016/j.ejphar.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Parker LA. The antiemetic drug ondansetron interferes with lithium-induced conditioned rejection reactions, but not lithium-induced taste avoidance in rats. J Exp Psychol Anim Behav Process. 2000;26(4):371–384. doi: 10.1037//0097-7403.26.4.371. [DOI] [PubMed] [Google Scholar]
- Molina JC, Pautassi RM, Truxell E, Spear N. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007;41(1):41–55. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JL, Well AD. Research design and statistical analysis (2nd ed.) Mahwah NJ, USA: Lawrence Erlbaum Associates Publishers; 2003. [Google Scholar]
- Nizhnikov ME, Varlinskaya EI, Petrov ES, Spear NE. Reinforcing properties of ethanol in neonatal rats: involvement of the opioid system. Behav Neurosci. 2006;120(2):267–280. doi: 10.1037/0735-7044.120.2.267. [DOI] [PubMed] [Google Scholar]
- Orr TE, Walters PA, Elkins RL. Differences in free-choice ethanol acceptance between taste aversion-prone and taste aversion-resistant rats. Alcohol Clin Exp Res. 1997;21(8):1491–1496. [PubMed] [Google Scholar]
- Orr TE, Whitford-Stoddard JL, Elkins RL. Taste-aversion-prone (TAP) rats and taste-aversion-resistant (TAR) rats differ in ethanol self-administration, but not in ethanol clearance or general consumption. Alcohol. 2004;33(1):1–7. doi: 10.1016/j.alcohol.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Parker LA. Taste reactivity responses elicited by reinforcing drugs: a dose-response analysis. Behav Neurosci. 1991;105(6):955–964. doi: 10.1037//0735-7044.105.6.955. [DOI] [PubMed] [Google Scholar]
- Parker LA. Taste reactivity responses elicited by cocaine-, phencyclidine-, and methamphetamine-paired sucrose solutions. Behav Neurosci. 1993;107(1):118–129. doi: 10.1037//0735-7044.107.1.118. [DOI] [PubMed] [Google Scholar]
- Parker LA. Rewarding drugs produce taste avoidance, but not taste aversion. Neurosci Biobehav Rev. 1995;19(1):143–157. doi: 10.1016/0149-7634(94)00028-y. [DOI] [PubMed] [Google Scholar]
- Parker LA. Taste avoidance and taste aversion: evidence for two different processes. Learn Behav. 2003;31(2):165–172. doi: 10.3758/bf03195979. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Arias C, Molina JC, Spear N. Domperidone interferes with conditioned disgust reactions but not taste avoidance evoked by a LiCl-paired taste in infant rats. Dev Psychobiol. 2008;50(4):343–352. doi: 10.1002/dev.20288. [DOI] [PubMed] [Google Scholar]
- Pautassi R, Godoy J, Spear N, Molina J. Early responsiveness to stimuli paired with different stages within the state of alcohol intoxication. Alcohol Clin Exp Res. 2002;26(5):644–654. [PubMed] [Google Scholar]
- Pautassi R, Ponce L, Molina J. Effects of early exposure to ethanol on subsequent learning mediated by the unconditional attributes of the drug. Latinamerican Journal of Psychology. 2005;37(1):149–166. [Google Scholar]
- Petrov ES, Varlinskaya EI, Spear NE. Reinforcement from pharmacological effects of ethanol in newborn rats. Alcohol Clin Exp Res. 2003;27(10):1583–1591. doi: 10.1097/01.ALC.0000089960.62640.58. [DOI] [PubMed] [Google Scholar]
- Sanders S, Spear NE. Ethanol acceptance is high during early infancy and becomes still higher after previous ethanol ingestion. Alcohol Clin Exp Res. 2007;31(7):1148–1158. doi: 10.1111/j.1530-0277.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- Smith GJ, Kucharski D, Spear NE. Conditioning of an odor aversion in preweanlings with isolation from home nest as the Unconditioned stimulus. Dev Psychobiol. 1985;18(5):421–434. doi: 10.1002/dev.420180507. [DOI] [PubMed] [Google Scholar]
- Spear LP, Specht SM, Kirstein CL, Kuhn CM. Anterior and posterior, but not cheek, intraoral cannulation procedures elevate serum corticosterone levels in neonatal rat pups. Dev Psychobiol. 1989;22(4):401–411. doi: 10.1002/dev.420220407. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Roitman MF, Bernstein IL. c-Fos induction in rat brainstem in response to ethanol- and lithium chloride-induced conditioned taste aversions. Alcohol Clin Exp Res. 1996;20(6):1023–1028. doi: 10.1111/j.1530-0277.1996.tb01941.x. [DOI] [PubMed] [Google Scholar]
- Truxell E, Spear NE. Immediate acceptance of ethanol in infant rats: ontogenetic differences with moderate but not high ethanol concentration. Alcohol Clin Exp Res. 2004;28(8):1200–1211. doi: 10.1097/01.alc.0000134220.34842.18. [DOI] [PubMed] [Google Scholar]
- Vigorito M, Sclafani A. Ontogeny of polycose and sucrose appetite in neonatal rats. Dev Psychobiol. 1988;21(5):457–465. doi: 10.1002/dev.420210505. [DOI] [PubMed] [Google Scholar]
- Vogt MB, Rudy JW. Ontogenesis of learning: I. Variation in the rat's reflexive and learned responses to gustatory stimulation. Dev Psychobiol. 1984;17(1):11–33. doi: 10.1002/dev.420170103. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94(4):469–492. [PubMed] [Google Scholar]
- Wolgin DL, Wade JV. Effect of lithium chloride-induced aversion on appetitive and consummatory behavior. Behav Neurosci. 1990;104(3):438–440. doi: 10.1037//0735-7044.104.3.438. [DOI] [PubMed] [Google Scholar]
- Yamamoto J, Fresquet N, Sandner G. Conditioned taste aversion using four different means to deliver sucrose to rats. Physiol Behav. 2002;75(3):387–396. doi: 10.1016/s0031-9384(01)00671-0. [DOI] [PubMed] [Google Scholar]