Abstract
Despite the growing research investigating the sex-specific organization of courtship behavior in Drosophila melanogaster, much remains to be understood about the sex-specific organization of the motor circuit that drives this behavior. To investigate the sex-specification of a tightly patterned component of courtship behavior, courtship song, we used the GAL4/UAS targeted gene expression system to feminize the ventral ganglia in male Drosophila and analyzed the acoustic properties of courtship song. More specifically, we used the thoracic-specifying teashirt promoter (tshGAL4) to express feminizing transgenes specifically in the ventral ganglia. When tshGAL4 drove expression of transformer (tra), males were unable to produce prolonged wing extensions. Transgenic expression of an RNAi construct directed against male-specific fruitless (fruM) transcripts resulted in normal wing extension, but highly defective courtship song, with 58% of males failing to generate detectable courtship song. Of those that did sing, widths of individual pulses were significantly broader than controls, suggesting thoracic fruM function serves to mediate proprioceptive-dependent wing vibration damping during pulse song. However, the most critical signal in the song, the interpulse interval, remained intact. The inability to phenocopy this effect by reducing fruM expression in motor neurons and proprioceptive neurons suggests thoracic interneurons require fruM for proper pulse song execution and patterning of pulse structure, but not for pulse timing. This provides evidence that genes establishing sex-specific activation of complex behaviors may also be used in establishing pattern-generating motor networks underlying these sex-specific behaviors.
Keywords: Drosophila behavior, Acoustic, fruitless, Sexual dimorphism, Motor control
Introduction
The behaviorally reproducible and stereotyped behavior of male Drosophila melanogaster courtship and its genetic amenability provide hope for a detailed and multilevel understanding of a complex and adaptive behavior. (Dickson, 2008; Villella and Hall, 2008). Completely understanding courtship behavior requires understanding its sensory transduction and integration, coordination of sub-behaviors, and its motor pattern generation. While a history of ongoing research focuses on the initiation and organization of courtship (Manoli and Baker, 2004; Datta et al., 2008; Dickson, 2008; Kimura et al., 2008), progress in understanding the motor networks themselves lags behind. Our study aims to elucidate organizational properties of one courtship motor network, the song circuit.
D. melanogaster courtship is composed of a stereotyped sequence of motor acts including female-directed following, abdomen tapping, singing, and attempting copulation (Hall, 1994). During courtship song, the male extends and then vibrates one wing. It is believed that descending brain neurons activate a thoracic circuit producing the song motor pattern (von Schilcher and Hall, 1979; Huber et al., 1989; Konopka et al., 1996; Clyne and Miesenböck, 2008). Courtship song is controlled by direct and indirect muscles (Bennet-Clark and Ewing, 1968), both of which receive phase-locked inputs from motor neurons during song (Ewing, 1977, 1979b). This phase-locking is due to proprioceptive wing feedback upon direct flight muscle motor neurons (Ewing, 1979a; Tauber and Eberl, 2001) and reciprocal inhibition among indirect flight muscle motor neurons (Levine, 1973; Ewing, 1977; Harcombe and Wyman, 1977).
Gynandromorph studies revealed a male requirement of the dorsal brain to initiate song through wing extension (Hall, 1977), and the thoracic ganglia to generate the song motor pattern (von Schilcher and Hall, 1979). Thus, considered with the abnormal song produced by females artificially induced to sing (Clyne and Miesenböck, 2008), some thoracic song components are apparently sexually dimorphic.
Female-specific transformer (tra) controls sexually dimorphic splicing of doublesex (dsx) and fruitless (fru), and the splice isoform, fruM, is only found in males. fruM function has been shown to be both necessary and sufficient to initiate many of the male courtship behaviors (Baker et al., 2001; Demir and Dickson, 2005; Manoli et al., 2005), but sex-specific dsx function is required to fully establish the network properly (Villella and Hall, 1996; Rideout et al., 2007; Kimura et al., 2008). Mutant fruM males exhibit disrupted courtship song production (Villella et al., 1997), but fruM expression in females is not sufficient for song initiation (Rideout et al., 2007). Further, artificially induced song in decapitated females resulted in pulse structure like that of wild type males upon ectopic fruM expression (Clyne and Miesenböck, 2008).
It has been proposed that fruM function is important at all levels of the nervous system related to courtship, as fruM is expressed in widespread neuronal populations from sensory neurons to motor neurons (Baker et al., 2001). Yet, the use of classic fruM mutants to study song has precluded direct analysis of motor networks. We investigated the putative thoracic courtship song patterning circuit selectively by using the homeotic teashirt (tsh) gene, a transcription factor specifying thoracic and abdominal segments (Röder et al., 1992), whose expression is selectively thoracic. These genetic manipulations allowed us to investigate whether the thoracic song circuit of male Drosophila is sex-specifically organized, and if the sex determination genes tra and fruM play a role in this organization.
Materials and Methods
Flies
Flies were maintained at 25° C in 12:12 LD conditions. All transgenes used in tshGAL4 (tshGAL4-md621; Calleja et al., 1996) experiments were outcrossed into a common isogenic Canton-S (CS) w1118 background for six generations. We feminized the nervous system in the tsh pattern by expressing tra with a UAS-tra.F20J7 (UAS-tra) construct (Ferveur et al., 1995) or by reducing fruM expression with a fruM-RNAi construct, UAS-fruMIR (Manoli and Baker, 2004). All flies carrying UAS-fruMIR contain a UAS insert on the second and third chromosome (Manoli and Baker, 2004), although the genotype is subsequently abbreviated to only reflect the second chromosomal insertion. The n-sybGAL80 line drives expression of GAL80, a GAL4 inhibitor, under the control of the neuronal-synaptobrevin (n-syb) pan-neuronal promoter (DiAntonio et al., 1993) and was used heterozygously, although it is represented homozygously for clarity. The UAS-fruMIR stocks were generously provided by Bruce Baker (Manoli and Baker, 2004), and the n-sybGAL80 stock was a generous gift from Julie Simpson (Janelia Farm Research Campus). All stocks other than n-sybGAL80 and UAS-fruMIR were obtained from the Bloomington Drosophila Stock Center (Bloomington, IN, USA). The motor neuron driver, D42 (Gustafson and Boulianne, 1996), the chordotonal driver, atoGAL4.3.6.10 (Hassan et al., 2000), the proximal wing base driver, 30A (Brand and Perrimon, 1993), and the myosin heavy chain mutant, Mhc5, previously known as bashed (Homyk and Emerson, 1988), have been previously described. In addition to thoracic and abdominal specification, tsh also functions in delineation of the thoracic-labial border (Dezulueta et al., 1994), establishment of domains along the proximo-distal axis of the developing wing and leg via wingless and nubbin (Zirin and Mann, 2007), as well as specification of the eye (Singh et al., 2002). All transgenic lines were in a w1118 background, while the wildtype control used here (+ / +) was Canton-S (all in the same isogenic background) to control for eye color.
Behavioral assays and song analysis
Courtship observations were made on a focal 5–6 day old male and a 3–5 day old CS virgin subject female, both isolated as virgins within 6 hours of eclosion. Individual flies were aspirated into a 10 × 6 mm cylindrical chamber with an acrylic ceiling and a fine mesh copper flooring mounted over a heat block held at 25° C. Acoustic recordings were made using a calibrated velocity sensitive microphone (Tauber and Eberl, 2001; Cator et al., 2009) beneath the chamber. Courtship and songs were recorded using digitally sampled audio (48 kHz) and video (standard NTSC). Recordings were made for five minutes or until successful copulation, whichever occurred first. The frequency response of the recording apparatus was flat within 2 dB up to 2 kHz. Recordings were high-pass filtered at 50 Hz, anti-alias filtered, and digitally resampled at 4 kHz, and passed through an integrating filter to compensate for the frequency-amplitude relationship of particle velocity measurements.
Courtship index (CI) and wing extension index (WEI), the proportion of time spent courting and extending a wing, respectively, were calculated from video. A wing extension (Figure 1) began with the promotion of the wing and ended with the retraction of the wing to resting position or initiation of another wing promotion. Pulses were initially detected through thresholding using Matlab software written for courtship song analysis and confirmed through inspection of audio and video (unilateral wing extension) records, and pulse times were logged as time of midpoint of total energy within the pulse. Pulse trains were defined as a sequence of at least 3 pulses separated by no more than a specified window (Wheeler et al., 1988) of 60 ms. A longer window of 100 ms affected the measured IPI of tshGAL4/UAS-fruMIR males by less than 1.0% (data not shown). Pulse width was defined as the smallest window necessary to encompass 90% of the pulse energy. Sine song was detected as sinusoidal hums coinciding with unilateral wing extension (only hums longer than 100 ms were scored). Also, some tshGAL4/UAS-fruMIR males display wing generated output coinciding with wing extension that was not classifiable as either pulse or sine song (data not shown). Although these wing generated outputs coincided with unilateral wing extension and courtship behavior, they did not share the tonal properties of sine song or the rapid amplitude modulation of pulse song. These were taken to be failed courtship song attempts, and were classified as neither pulse nor sine song. Calculation of sound particle velocity levels (SPVL) used a standard reference of 50 nm s−1. Each datum measuring the song in general (Fig. 2) represents the mean within a fly, while each datum measuring pulse characteristics (Fig. 3) represents the median within each fly due to the larger sample size.
Figure 1.
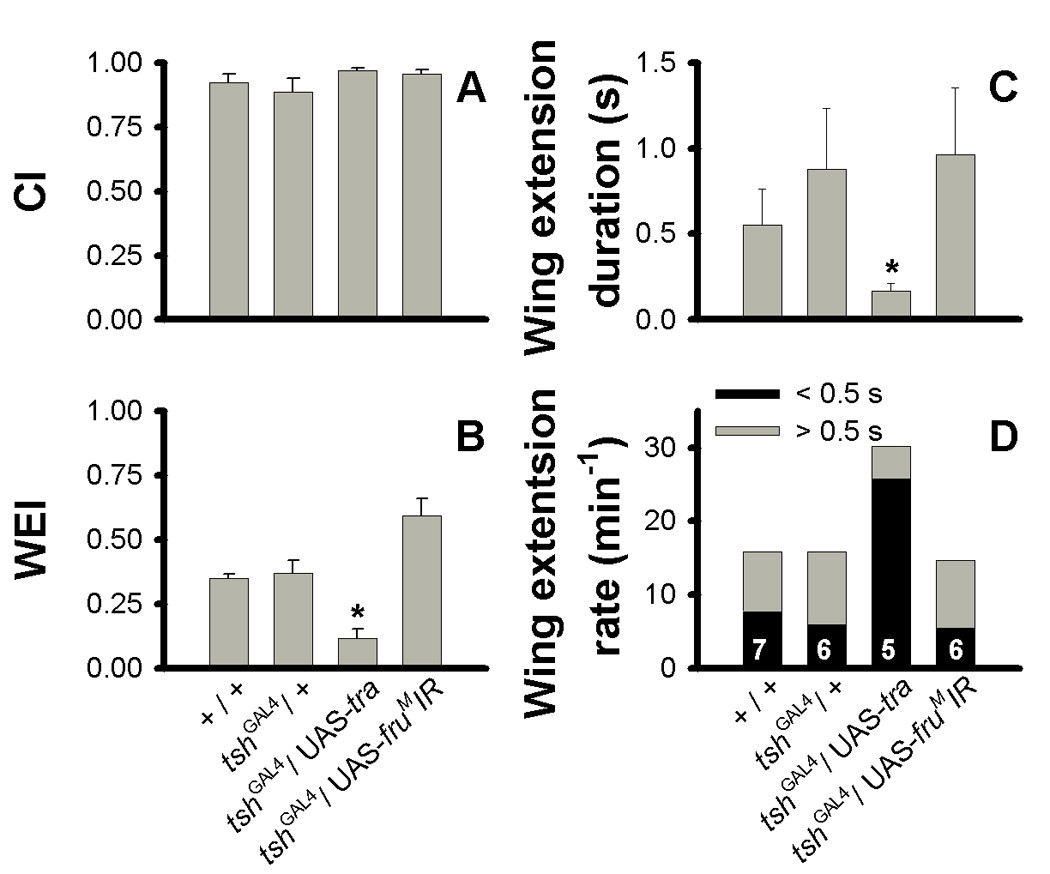
Measurements of wing extension behavior. (A) No differences in courtship index (CI) were observed among genotypes (mean ± s.e.m.). (B) tshGAL4/UAS-tra flies had a significantly decreased median wing extension duration compared to tshGAL4/+ controls, while tshGAL4/UAS-fruMIR males were no different than controls. (C) Mean of a fly’s median wing extension duration. tshGAL4/UAS-tra males display significantly shorter wing extensions than tshGAL4/+ controls. (D) Wing extension frequency, separating extensions shorter and longer than 0.5 s. tshGAL4/UAS-tra flies are not different in frequency of total wing extensions, but exhibit more frequent wing extensions shorter than 0.5 s and less frequent wing extensions longer than 0.5 s compared to tshGAL4/+ controls. There is no difference between tshGAL4/UAS-fruMIR males and tshGAL4/+ control males. Sample size indicated within bars in (D). *: p < 0.05.
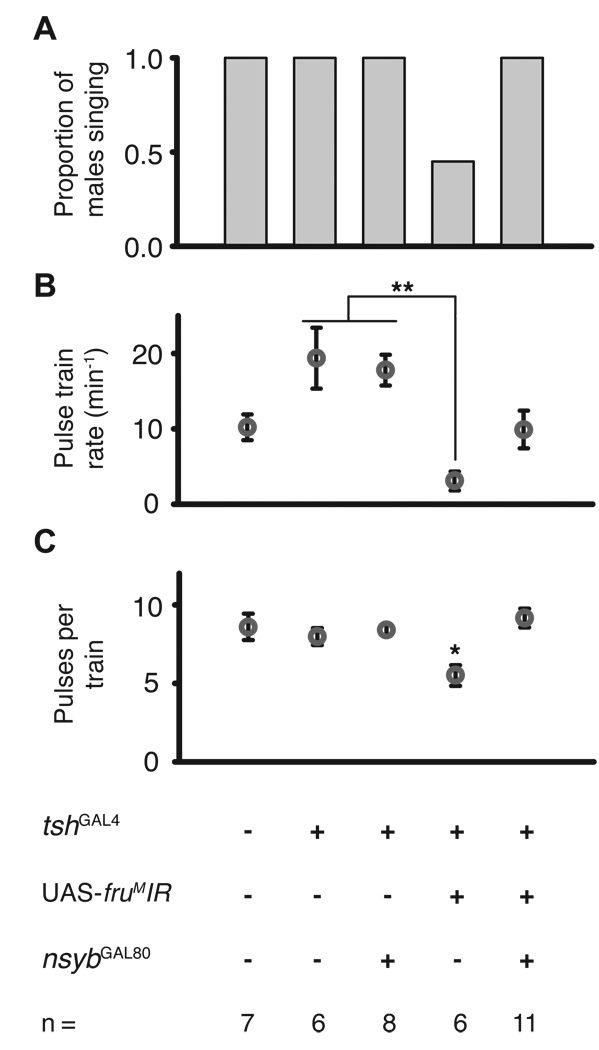
Figure 2.
Expression of fruMIR in tsh-specific pattern reduces amount of courtship song. Proportion of flies that produced audible output classified as pulse song (A). Only tshGAL4/UAS-fruMIR males failed to produce courtship song. tshGAL4/UAS-fruMIR also males exhibited fewer pulse trains per minute (B) and pulses per train (C) than tshGAL4/+ controls, while tshGAL4/UAS-fruMIR; n-sybGAL80 males were no different than controls. tshGAL4/UAS-fruMIR; n-sybGAL80 males rescued the decreased pulses per train in tshGAL4/UAS-fruMIR males. *: p < 0.05, **: p < 0.01.
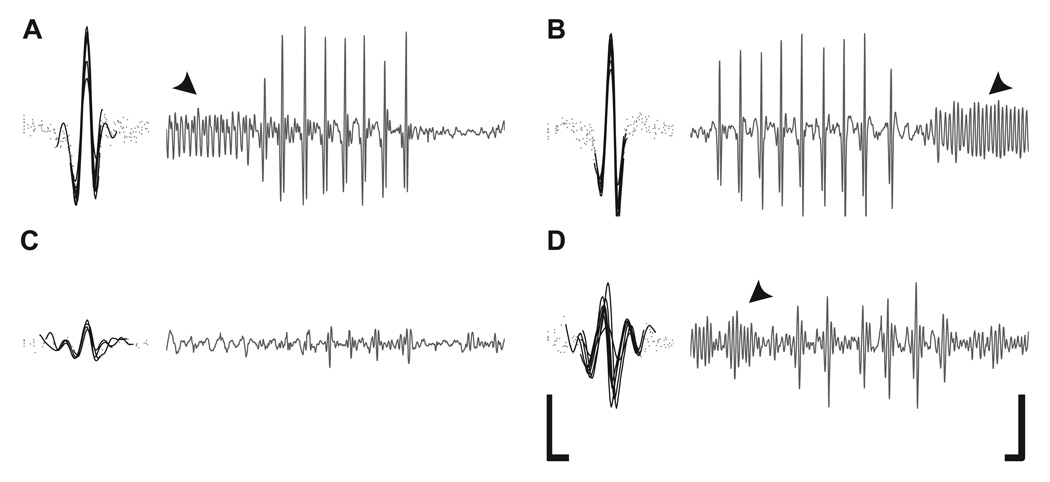
Figure 3.
Representative traces of courtship song output. The left panel displays individual pulses within a pulse train aligned by midpoint of energy. Solid lines indicate amount of trace required to include 90% of the signal’s energy (pulse width). Right panel displays the whole trace. Arrowheads indicate sine song. (A) tshGAL4/+ controls, (B) tshGAL4/UAS-fruMIR; n-sybGAL80 rescue flies, (C) representative small amplitude and (D) polycyclic nature of tshGAL4/UAS-fruMIR courtship song. Scale bars: Left panel, horizontal 5 ms, vertical 5 mm s−1. Right panel, horizontal 25 ms, vertical 5 mm s−1.
Immunocytochemistry
The rat α-FruM antibody, kindly provided by Bruce Baker (Janelia Farm Research Campus), targets the male-specific 101-amino acid sequence at the N-terminus of the peptide (Lee et al., 2000). Within 6 hours of eclosion, adult w; tshGAL4, UAS-mCD8-GFP/ CyO male central nervous systems were dissected out and fixed in 3.5% paraformaldehyde, incubated in 1:300 α-FruM overnight, and incubated in 1:1000 TRITC-conjugated goat anti-rat (Jackson ImmunoResearch, West Grove, PA, USA) for two hours. Preparations were viewed with a TCS SP2 Leica confocal microscope system.
Flight assays
Flight ability was measured in an assay adapted from Drummond et al. (1991). Three- to five-day-old males were released on a platform in the center of an open-topped cylinder 45 cm wide and 54 cm high with a light source at the top. Flies were recorded as having landed on the bottom, landed on the side, or flown above the top of the cylinder. If no flight was initiated within 30 seconds, the fly was reapplied to the platform. Flies that never left platform after at least five trials were eliminated from the study.
Results
Ectopic tra expression reduces wing extension
We utilized the trunk-specific expression of teashirt (tsh) to specifically manipulate gene expression in this area, allowing us to investigate the putative thoracic song patterning circuit. We used the tshGAL4 allele to express GAL4 in a tsh-expressing cell-specific pattern (Brand and Perrimon, 1993; Duffy, 2002) to cell-specifically activate a UAS promoter to invert male-specific gene expression. We first investigated wing extension, a prerequisite step to producing courtship song. Video analysis of courtship trials showed that the feminizing construct UAS-tra driven by tshGAL4 had no detectable effect on courtship intensity measured by proportion of time spent courting (CI), compared to wild-type control males (+) or control males carrying tshGAL4 alone (tshGAL4/+) (Fig. 1A; Kruskal-Wallis, p = 0.76). During courtship, however, tshGAL4/UAS-tra males showed a significant decrement in (1) proportion of time spent extending a wing toward the female (wing extension index, WEI) as compared to tshGAL4/+ controls (Fig. 1B; Kruskal-Wallis: p < 0.005, Tukey-Kramer: p < 0.05) and (2) median wing extension duration (Fig. 1C; Kruskal-Wallis, p < 0.005, Tukey-Kramer, p < 0.05). Furthermore, these tshGAL4/UAS-tra males display an unusual wing extension profile consisting of many rapid wing extensions, too fast to be measured with standard video. tshGAL4/UAS-tra males showed more rapid wing extensions (Kruskal-Wallis: p < 0.01, Tukey-Kramer: p < 0.05) and fewer sustained wing extensions (Kruskal-Wallis: p < 0.05, Tukey-Kramer: p < 0.05) (defined as being shorter or longer than 0.5 s, respectively) than tshGAL4/+ controls (Fig. 1D). However, there was no significant effect of genotype across all wing extension events (Kruskal-Wallis: p = 0.0517).
Since the lack of wing extension exhibited by tshGAL4/UAS-tra males precluded courtship song production, we asked if elimination of fruM, a downstream target of tra in the sex-determination hierarchy, would more selectively produce defective song that could be analyzed for its defects. We utilized an RNAi construct directed at fruM transcripts (UAS-fruMIR) to reduce fruM expression (Manoli and Baker, 2004) in a tsh-specific pattern. As observed in tshGAL4/UAS-tra males, males carrying both tshGAL4 and UAS-fruMIR showed no defect in CI (Fig. 1A). However, unlike tshGAL4/UAS-tra males, measurements of wing extension revealed no differences between tshGAL4/UAS-fruMIR males and controls (Fig. 1B–D). We therefore continued our study of courtship song utilizing the UAS-fruMIR transgene.
fruM RNAi reduces amount of courtship song
Courtship song is comprised of a pulse component (“pulse song”), consisting of a train of discrete, single pulses composed of one to several cycles, and a sinusoidal (125–200 Hz) component (“sine song”) as seen in flight, but slower (Bennet-Clark and Ewing, 1968).
Utilization of the UAS-fruMIR transgene to express RNAi directed at fruM in tsh-expressing neurons produced a strong phenotype of reduced courtship song. All control flies included in this analysis exhibited pulse song, but 42% (5/12) of tshGAL4/UAS-fruMIR males exhibited no detectable pulse song (Fig. 2A) despite vigorous courtship (Fig 1A), a statistically significant effect (Pearson’s χ2, experiment-wide: p < 0.0005, pair-wise with Bonferonni: p < 0.05). The 58% of tshGAL4/UAS-fruMIR males exhibiting song sang at a significantly lower rate measured by pulse trains per minute (Fig 2B), as compared to tshGAL4/+ control males (Kruskal-Wallis: p < 0.005, Tukey-Kramer: p < 0.01). They also exhibited fewer pulses per pulse train than control flies (Fig 2C; Kruskal-Wallis: p < 0.001, Tukey-Kramer: p < 0.05). The high proportion of tshGAL4/UAS-fruMIR males that did not produce song or sang at a decreased rate indicates that expression of fruMIR in a tsh-specific pattern disrupts execution of pulse song.
To ensure that the reduction in courtship song had a neural basis rather than being due to broad tsh expression across thoracic tissues, we included another transgene expressing the GAL4 inhibitor GAL80 in a pan-neuronal, n-synaptobrevin (n-syb) pattern to block the effects of fruMIR in the nervous system. The pulses per train deficit was fully rescued in tshGAL4/UAS-fruMIR; n-sybGAL80 (abbreviated from w; tshGAL4/UAS-fruMIR; n-sybGAL80/UAS-fruMIR, see Materials and Methods) rescue males (Fig. 2), and trended towards a rescue in pulse train rate, arguing that the phenotype is indeed neuronal.
fruM RNAi disrupts song structure
Representative data show that pulse song typically consists of a train of many pulses, each consisting of one or two cycles as seen in tshGAL4/+ controls (Fig. 3A) and tshGAL4/UAS-fruMIR; n-sybGAL80 rescue flies (Fig. 3B). Those tshGAL4/UAS-fruMIR males that sang show several defects, such as decreased amplitudes (Fig. 3C) and polycyclic pulses (Fig 3D), in which extra cycles are present before and after the peak particle velocity.
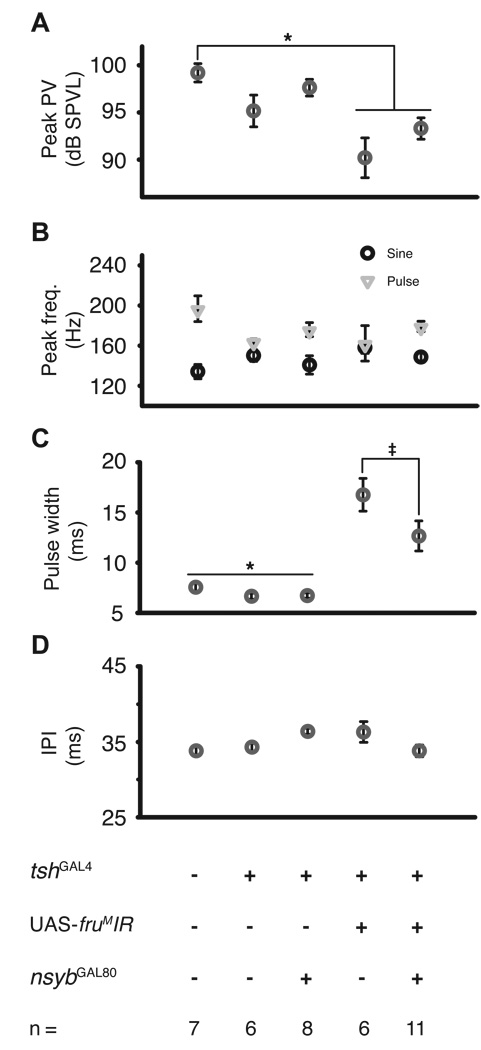
Wild-type control males exhibited a peak pulse particle velocity of 99.2 ± 1.0 db SPVL (re: 50 nm s−1, mean ± SE). Pulse amplitudes from tshGAL4/UASfruMIR males showed a non-significant trend towards reduction of amplitude compared to tshGAL4/+ controls and a significant decrease compared to wildtype controls (Fig. 4A; Kruskal-Wallis: p < 0.01, Tukey-Kramer: p < 0.01). Although tshGAL4/UAS-fruMIR; n-sybGAL80 rescue males showed a trend of increased amplitude compared to tshGAL4/UAS-fruMIR males, these males also sang at a decreased amplitude compared to wild-type controls (p < 0.05), but not compared to tshGAL4/+ controls. Peak intrapulse frequencies and peak sine song frequencies were not found to differ among genotypes (Fig. 4B; Kruskal-Wallis: p = 0.43 and p = 0.80, respectively). The similarity of mean pulse and sine peak frequencies in tshGAL4/UAS-fruMIR males should not be interpreted as a single underlying frequency for both song modes, as pulse and sine song frequency did not correlate in each fly examined, and intermale variation was quite high. Pulse widths were broader (Kruskal-Wallis: p < 0.001, Tukey-Kramer: p < 0.05) in tshGAL4/UAS-fruMIR males as compared to tshGAL4 and wildtype controls (Fig. 4C). Pulse widths of tshGAL4/UAS-fruMIR and tshGAL4/UAS-fruMIR; n-sybGAL80 males were not significantly different (Tukey-Kramer). However, because tshGAL4/UASfruMIR; n-sybGAL80 males serve as rescue males, an a priori prediction of narrower pulse width in rescue versus tshGAL4/UAS-fruMIR males could be made. Using a one-tailed test, the pulse width phenotype was rescued in tshGAL4/UASfruMIR; n-sybGAL80 vis-à-vis tshGAL4/UAS-fruMIR males (Tukey-Kramer: p < 0.05).
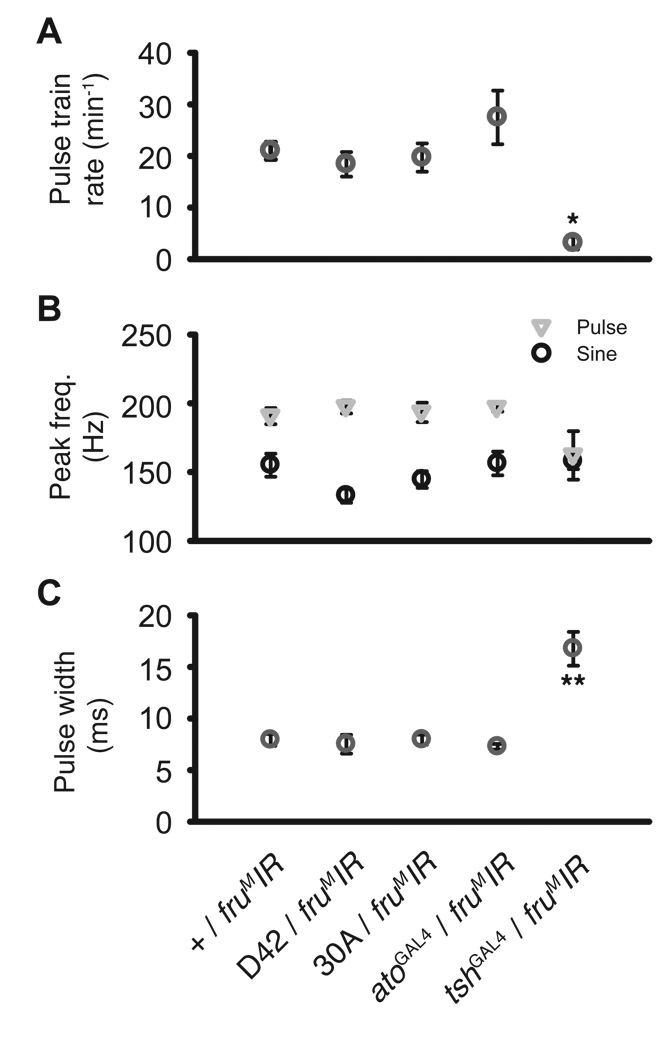
Figure 4.
Intrapulse data. (A) Peak particle velocity within a pulse is reported here as a mean of medians in dB SPVL, using 50 nm s−1 as a reference. There no significant differences were detected in dB SPVL, but there was a trend for a reduction in pulse amplitude for tshGAL4/UAS-fruMIR males. (B) Peak frequency of sine songs (circle) and individual pulses (triangle). No significant differences were observed. (C) Pulse widths from tshGAL4/UAS-fruMIR males were significantly broader compared to tshGAL4/+ controls. This broadened pulse width is rescued in tshGAL4/UAS-fruMIR; n-sybGAL80 males. (D) Mean interpusle interval (IPI) was unaffected by genotype. *: p < 0.05, **: p < 0.01, ‡: one-tailed, p < 0.05.
Pulses within a pulse train are separated by a species-specific interpulse interval (IPI) (Bennet-Clark, 1971) which itself oscillates in a species-specific manner (Kyriacou and Hall, 1980). In many species IPI is critical to maximizing a male’s chance of copulation (Bennet-Clark and Ewing, 1969; Kyriacou and Hall, 1982, 1986). Interestingly, males of tshGAL4/UAS-fruMIR did not show a disrupted IPI (Fig. 4D, Kruskal-Wallis: p < 0.05, no effects from Tukey-Kramer), the most important feature of the courtship song.
tshGAL4 is expressed in FruM domains
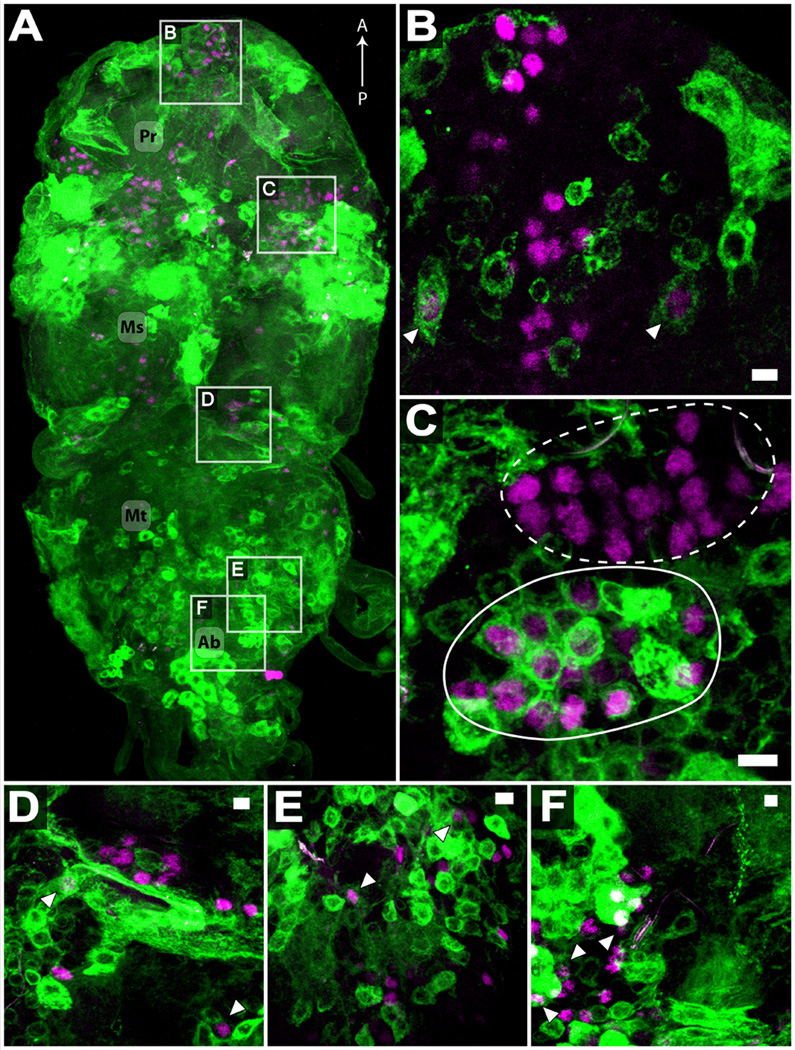
To identify the specific regions of the nervous system affected by the tshGAL4/UAS-fruMIR genotype, we analyzed the tshGAL4 expression pattern in the CNS of recently emerged males using a membrane-bound GFP reporter gene (mCD8-GFP) and determined its corresponding overlap with FruM immunoreactivity. Expression of tshGAL4-driven mCD8-GFP in the brain was very limited (occasionally absent) and inconsistent with no observed overlap with FruM immunoreactivity (Fig. S1). In contrast, tshGAL4 driven mCD8-GFP was widely expressed throughout the somata and neuropils of the ventral ganglia, including all neuromeres (Fig. 5A). No obvious sexual dimorphisms were detected in GFP labeled neurons (data not shown), but the extensive labeling by tshGAL4 makes definitive analysis difficult. Colocalization of GFP and FruM immunoreactivity was observed in all five previously described (Lee et al., 2000) groups of FruM ventral ganglia neurons (Fig. 5B–F). The most extensive colocalization of FruM and tshGAL4-driven GFP expression was observed at the anterior margin of the ventral mesothoracic neuromere (Fig. 5C). Although tshGAL4 expression was observed in somata of widely varying size, FruM immunoreactivity was only detected in those tshGAL4-expressing neurons with small somata (~ 5 µm in diameter), consistent with previous reports of limited motor neuron FruM expression (Ryner et al., 1996; Manoli et al., 2005). The somata of direct flight muscle motor neurons (DFMns) are known to be located in the anterior mesothoracic region of the ventral ganglion (Trimarchi and Schneiderman, 1994), raising the possibility that DFMns require fruM for proper courtship song functioning. However, we did not observe colocalization of GFP and FruM immunoreactivity in neurons with larger somata (≥ 10 µm) characteristic of DFM motor neurons (Trimarchi and Schneiderman, 1994).
Figure 5.
Immunocytochemistry of adult w; tshGAL4, UAS-mCD8-GFP/CyO CNS, visualizing endogenous, membrane bound GFP (green) and FruM immunoreactivity (magenta). (A) Dorsal-ventral view of adult ventral ganglia. Extensive labeling is visible in prothoracic (Pr), mesothoracic (Ms), metathoracic (Mt), and abdominal (Ab) segments. Anterior-posterior axis is indicated. (B–F) 3 – 5 µm representative sections of the five groups of fruM neurons in the ventral ganglia, according to (Lee et al., 2000). FruM neural cluster 16 (B), 17 (C), 18 (D), 19 (E), and 20 (F). Arrowheads indicate examples of neurons coexpressing FruM and mCD8-GFP. In (C), a FruM-expressing cluster clearly coexpresses GFP (solid line), while an adjacent FruM cluster does not (dashed line). Scale bars (B–F) represent 5 µm.
Elimination of fruM in motor neurons and wing sensory neurons does not affect song
Motor neurons and proprioceptive sensory neurons are known to be critical members of pattern generating networks (Levine, 1973; Harcombe and Wyman, 1977; Ewing, 1979a), and fruM is expressed in subsets of motorneurons and wing sensory neurons (Manoli et al., 2005; Rideout et al., 2007). Feminizing the entire pool of thoracic neurons resulted in a strong courtship song deficit (Fig. 2–4), so we used additional GAL4 tools to eliminate fruM in a subset of these neurons. We first looked at motor neurons by driving fruMIR with the GAL4 driver D42 (Gustafson and Boulianne, 1996) that expresses in all motor neurons. Since power and timing of sound pulses is provided by the direct and indirect flight muscles (Ewing, 1977, 1979b), we hypothesized that fruM activity in the motor neurons that innervate these muscles may be critical for proper song production. We eliminated expression of fruM in motor neurons by driving fruMIR with D42GAL4, which is expressed in direct and indirect flight muscle motor neurons (Gustafson and Boulianne, 1996; Usui-Aoki et al., 2000). No differences between D42GAL4/UAS-fruMIR and UAS-fruMIR controls were observed. This is consistent with another motor neuron GAL4 driver, P103.3 (Consoulas et al., 2002), in that P103.3GAL4/UAS-fruMIR males also exhibited no detectable song defects (n = 4, data not shown). As motor neurons receive wing proprioceptive input entraining the song pattern and some sensory organs at base of the wing are known to express fruM (Manoli et al., 2005), we hypothesized that fruM function in the sensory organs themselves may be necessary for proper courtship song production. The atoGAL4 construct drives expression in proprioceptive organs (Hassan et al., 2000), such as those expressing fruM at the wing base, but exhibited no detectable effect on courtship song when driving UAS-fruMIR (Fig. 6). Similarly, driving expression of UAS-fruMIR with 30A, a GAL4 driver that is expressed at the presumptive wing base of the imaginal disc, including precursors for the wing proprioceptive organs (Brand and Perrimon, 1993), does not produce a detectable courtship song phenotype.
Figure 6.
Courtship song is unaffected by driving fruMIR in motor neurons and sensory neurons. No significant effects of genotype were found on (A) pulse rate, (B) pulse and sine song peak frequency, or (C) pulse width compared to UAS-fruMIR controls. The tshGAL4/UAS-fruMIR mutant phenotype is replotted from Fig. 2 and Fig. 4 for comparison. n = 6 – 8. *: p < 0.005, **: p < 0.001.
fruMIR does not affect flight
Because flight and courtship song use overlapping motor components but only courtship song behavior is sexually dimorphic, we tested the flight ability of males exhibiting courtship song defects. In an assay adapted from Drummond et al. (1991), males were released in the center of a large cylinder, allowed to fly freely, and their landing site was recorded. We compared tshGAL4/UAS-fruMIR males with wild-type controls and a known flight mutant, Mhc5, which has been previously reported as a “poor flier”, rather than “flightless” (Homyk and Emerson, 1988). Flies that never left the platform after four 30-second trials were excluded from analysis (1 of 24 wild-type controls, 4 of 41 tshGAL4/UAS-fruMIR males, and 1 of 20 MHC5 males). As expected, Mhc5 mutants exhibited a significant flight defect compared to wild-type and tshGAL4/UAS-fruMIR males (Pearson’s χ2, experiment-wide: p < 0.0005, pair-wise with Bonferonni: p < 0.0005). Interestingly, no difference in flight ability was detected between wild-type males and tshGAL4/UAS-fruMIR males (Fig. 7), suggesting the abnormalities in wing coordination induced by reducing fruM expression were specific to song production.
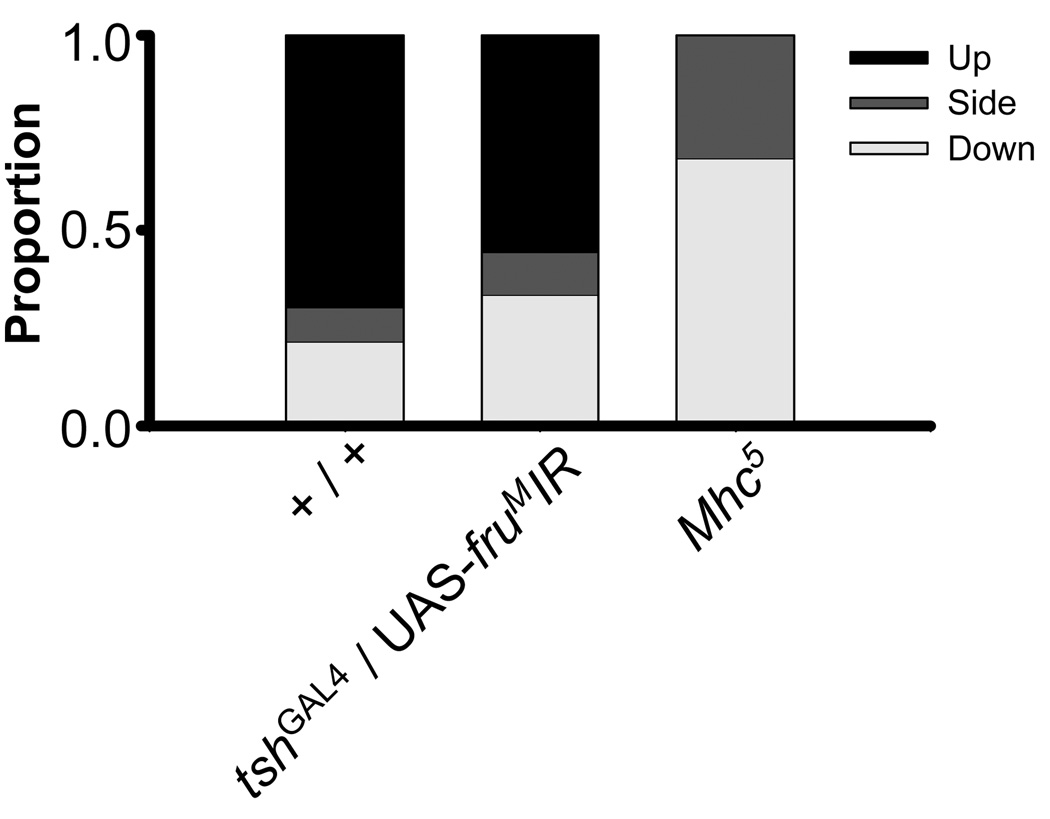
Figure 7.
Flight ability of tshGAL4/UAS-fruMIR males. Males were placed in the center of a cylinder and allowed to freely fly. Landing sites were recorded as the bottom (white), side (gray), or out of the cylinder (black). There was a significant effect of genotype on flight performance (p < 0.001). Flight performance of control + / + males (n = 23) was not significantly different from tshGAL4 / UAS-fruMIR males (n = 27), but both performed better than the known flight mutant, Mhc5 (n = 19) (p < 0.0005).
Discussion
Although the role of sex-determination genes in the initiation of courtship behaviors is well studied, very little is known about what role these genes play in the neuronal circuits directly controlling these behaviors. Previous studies of fruM mutants exhibiting aberrant courtship song (Ryner et al., 1996; Villella et al., 1997) were ambiguous as to whether fruM is needed during the initiation of courtship song (i.e., the brain) or the execution of courtship song (i.e., the thoracic ganglia). Other experiments providing data in support of a role for fruM in the song circuit rely on artificial activation of networks in a decapitated preparation (Clyne and Miesenböck, 2008). Our results indicate fruM function is necessary in the thoracic song circuit itself for normal courtship song execution. To our knowledge, this perturbation of the song circuit leaving the descending song control intact is the first in an intact, freely behaving animal. Reducing fruM expression in motor neurons and sensory neurons resulted in normal song, suggesting that the fruM requirement is restricted to interneurons.
Our data provide evidence that the courtship song pattern generator is sex-specifically organized. It has been previously argued that the song circuit in females is intact but remains latent and is simply not activated (Clyne and Miesenböck, 2008), as photoexcitation of fruM neurons in decapitated females was sufficient to induce a motor output recognizably resembling courtship song, but this motor output was non-functional (i.e., it did not increase mating when played back during courtship). Considering our evidence that disruption of fruM expression in the song circuit strongly disrupts song in males, that expression of fruM in the song circuit functionally improves artificially-induced song in females (Clyne and Miesenböck, 2008), and a sex-specific thoracic focus critical for courtship song was identified through gynandromorphic analysis (von Schilcher and Hall, 1979), we argue that an essential component of the song circuit vital for its proper function is indeed male-specific and fruM is responsible for establishing the most important aspects of the circuit’s function. Further, these fruM-dependant idiosyncrasies appear to selectively affect wing coordination with respect to courtship song, as males unable to sing normally exhibited no detectable flight deficit. These unidentified fruM-dependant circuit modifications allow coordination of wing control mechanisms specific to song production over those required for flight.
fruM and IPI
The interpulse interval (IPI), and the rate at which it oscillates during singing, encodes the male’s species-identity and provides the most salient feature of courtship song throughout Drosophila (Bennet-Clark et al., 1976; Talyn and Dowse, 2004; Markow and O'Grady, 2005). Despite the strong disruption of courtship song by elimination of fruM in tsh+ neurons, IPI, the most critical song parameter, is unaffected, reflecting its important courtship role. Villella et al. (1997) have shown that fruM mutant males have an extended mean IPI. Our data suggests that this dependence of IPI on fruM is not found in the thorax but in the brain, or that this dependence is conferred by other fruM, non-tsh+ thoracic neurons. The finding that fruM expression has no bearing on IPI in a decapitated fly (Clyne and Miesenböck, 2008) favors the former. An expansive enhancer trap study demonstrates that song features (e.g., IPI) are likely shaped by the function of a distributed set of neuropil regions (Moran and Kyriacou, 2009). The role of the circadian rhythm gene, period, on the cycling of IPIs is conferred in the thoracic ganglia (Konopka et al., 1996), so the mean IPI might be regulated by the brain while the thoracic ganglia might be responsible for instilling the oscillation of IPI. The descending IPI information might be encoded by tonic drive, whereby stronger song circuit excitation results in shorter IPIs (Bentley, 1977), such that the long IPIs of flies photoinduced to sing (Clyne and Miesenböck, 2008) may result from photoactivation providing less circuit activation than endogenous excitation.
fruM and wing extension
Males expressing tra in a tshGAL4 pattern were unable to produce prolonged wing extensions, suggesting tra-dependent sex-specification of tsh-expressing neurons is critical for extending a wing long enough to sing. This thoracic execution requirement is in addition to the previously identified initiation requirement of male tissue in the dorsal brain (Hall, 1977). Recently, Koganezawa et al. (2009) showed that blocked activity of G32a-expressing neurons, like those found in tarsi, increases abnormal bilateral wing extension. Interestingly, feminizing tsh+ neurons suppresses wing extension, while blocking G32a+ neurons reduces the suppression of wing extension, suggesting these distinct neural populations are antagonistic or manipulations of the overlapping populations resulted in opposite effects. Alternatively, tra expression may result in a subtle wing cuticle or muscle phenotype in males, as tsh is important for determining the proximal wing domain (Zirin and Mann, 2007). In either case, the normal wing extensions observed in tshGAL4/UAS-fruMIR males suggest thoracic fruM, downstream of tra, is not responsible for the wing extension phenotype, emphasizing the emerging role that other downstream genes (e.g. doublesex) play in the organization and function of the courtship network (Rideout et al., 2007).
Potential substrates for fruM’s role in song
The fruM song phenotype may be produced by affecting any of four song circuit components: (1) descending inputs, (2) motor neurons, (3) proprioceptive neurons, and (4) local interneurons. First, the selective expression of tsh in the thorax and abdomen eliminates the role of descending brain interneurons in this fruM-dependent song phenotype. Second, elimination of fruM in motor neurons using D42GAL4 did not result in a detectable phenotype, despite the fact that invertebrate flight motor neurons integrate sensory afferents and related motor neuron efferents, thus are critical for flight pattern generation (Levine, 1973; Harcombe and Wyman, 1977). This is consistent with fruM’s lack of an effect on innervation patterns of direct flight muscles, although it is expressed in one of them (Rideout et al., 2007). Third, eliminating fruM in proprioceptive organs using atoGAL4 and at the developing wing base using 30AGAL4 had no detectable effect on courtship song, including pulse duration. Wing sensory neurons limit the number of cycles per pulse and ensure short pulse durations, as sensory information entrains elements damping the wing vibration (Ewing, 1979a; Tauber and Eberl, 2001), and fruM is expressed in sensory sensilla at the wing base (Manoli et al., 2005). Taken together, our results argue against a motor neuron, sensory neuron, or descending interneuron courtship song requirement for fruM, suggesting local interneurons have a sex-specific fruM requirement to properly assemble the song patterning circuit.
Although fruM interneurons are widely distributed throughout the thoracic ganglia, a large cluster of tsh and fruM co-expressing neurons is located ventrally along the anterior margin of the mesothoracic segment. This region has been speculated to be important in courtship song production (von Schilcher and Hall, 1979; Rideout et al., 2007), particularly since dsxM and fruM are highly co-expressed in this region, both of these factors are important in proper song production (Rideout et al., 2007), and this region is responsible for control of wing movement (Trimarchi and Schneiderman, 1994). These fruM-dependent song circuit neurons may overlap with the previously identified pool of sexually dimorphic neurons (Rideout et al., 2007) and neurons putatively expressing the tsh paralog, tiptop, which affect pulse width and IPI (Datta et al., 2009; Moran and Kyriacou, 2009).
Mechanisms of fruM function in the song circuit
The fruM requirement for courtship song demonstrated here may control wiring of the song circuit by controlling the song circuit’s connection to the descending command system or by sculpting the connections among song circuit components themselves. In the former case, a fruM “identity” signal in the song circuit may be required to receive projections from the fruM-expressing song command system in the brain, perhaps through a thoracic fruM-specific projection that is absent when fruM is eliminated (Datta et al., 2008; Kimura et al., 2008). Thus, song circuits not expressing fruM may not receive descending excitation (or insufficient descending excitation), resulting in aberrant courtship song. On the other hand, fruM may be required for the song patterning circuit itself to be wired properly. This is consistent with the observation of Clyne and Miesenböck (2008) that fruM expression in females improves song circuit function. Although we have focused on fruM’s developmental ability to mediate sex-specific differences in neuronal projection patterns (Kimura et al., 2005; Datta et al., 2008; Kimura et al., 2008), we cannot rule out a neurophysiological role for fruM, despite a limited set of studies addressing this issue (Datta et al., 2008).
Interpretation of the abnormal courtship song parameters in males with disrupted thoracic fruM expression may provide insights into how fruM affects neuromuscular control of song. The neuromuscular mechanism producing pulse song is still unclear, but one hypothesis is that pulse timing is achieved by direct, timed inputs onto an unidentified direct flight muscle that moves the wing and distorts the thorax to trigger a contraction of a power-delivering, stretch-activated fibrillar muscle (Ewing, 1977). Lagging sensory input via proprioceptive feedback (Ewing, 1979a; Reddy et al., 1997; Tauber and Eberl, 2001) damps the ensuing antagonistic indirect flight muscle contraction directly by activating opposing direct flight muscles or indirectly by releasing tension in the thoracic box to prevent its stretch-activation and the subsequent polycylic pulse. In this model, the decreased amplitude of tshGAL4/UAS-fruMIR pulse song suggests that indirect flight muscles may not be sufficiently recruited in the absence of fruM. The broadened, polycyclic pulse width suggests that damping may not occur properly.
Courtship song intensity
To our knowledge, our recordings are the first calibrated measure of acoustic output of a courting male fly reported in the literature. The mean sound particle velocity level (SPVL) of a wild-type male sound pulse as measured here is 99.2 ± 1.0 db SPVL (re: 50 nm s−1, mean ± s.e.m.). As males were allowed to freely move around the chamber while singing at distances from 1 – 6 mm from -- and unrestricted angles to -- the microphone, a standardized output intensity is confounded by distance and directional effects, as well as complex near-field propagation physics. Nonetheless, this figure corresponds well to the predictive figure calculated by Bennet-Clark (1971) of 95 dB SPVL at 5 mm in front of the courting male. The near-field dipteran auditory organ, the arista, attenuates the vibration amplitude at 166 Hz by approximately only 2.5 dB (Gopfert and Robert, 2002), thus it is obviously sensitive enough to detect sounds we measured.
This report provides evidence that sex-specification via fruM function is required specifically in the thoracic ganglia to perform courtship song, and suggests other courtship-specific motor patterns (e.g., genitalia licking, copulation attempts) may be controlled by circuits requiring fruM. However, fruM does not affect the most important feature of that pattern, IPI. Identification of neurons critical to patterning the song in a fruM-dependent matter will further our understanding of how complex behaviors are produced by the nervous system.
Supplementary Material
Figure S1. Adult male w; tshGAL4, UAS-mCD8-GFP/CyO (green) stained with an anti-fruM antibody (magenta). Little or no GFP expression is visible in the brain (br), while strong expression is visible in the ventral ganglia (vg). Anterior-posterior axis is indicated.
Acknowledgements
We would like to thank Bruce Baker for generously providing the UAS-fruMIR stocks and α-FruM antibody, Julie Simpson for providing the n-sybGAL80 stock and tsh suggestions, Ben Arthur, Bruce Land, and Gus Lott for helpful acoustics suggestions, and Ron Booker, David Deitcher and Cole Gilbert for thoughtful comments on this manuscript. Support was provided by HHMI and NIH (NIDCD) to RRH and an NIGMS predoctoral Training Grant to CDR.
References
- Baker BS, Taylor BJ, Hall JC. Are complex behaviors specified by dedicated regulatory genes? Reasoning from Drosophila. Cell. 2001;105:13–24. doi: 10.1016/s0092-8674(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Bennet-Clark HC. Acoustics of insect song. Nature. 1971;234:255–259. [Google Scholar]
- Bennet-Clark HC, Ewing AW. The wing mechanism involved in the courtship of Drosophila. Journal of Experimental Biology. 1968;49:117–128. [Google Scholar]
- Bennet-Clark HC, Ewing AW. Pulse interval as a critical parameter in courtship song of Drosophila melanogaster. Animal Behaviour. 1969;17:755–759. [Google Scholar]
- Bennet-Clark HC, Dow M, Ewing AW, Manning A, Schilcher FV. Courtship stimuli in Drosophila melanogaster. Behavior Genetics. 1976;6:93–95. doi: 10.1007/BF01065681. [DOI] [PubMed] [Google Scholar]
- Bentley D. Control of cricket song patterns by descending interneurons. Journal of Comparative Physiology. 1977;116:19–38. [Google Scholar]
- Brand AH, Perrimon N. Targeted gene-expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Calleja M, Moreno E, Pelaz S, Morata G. Visualization of gene expression in living adult Drosophila. Science. 1996;274:252–255. doi: 10.1126/science.274.5285.252. [DOI] [PubMed] [Google Scholar]
- Cator LJ, Arthur BJ, Harrington LC, Hoy RR. Harmonic convergence in the love songs of the dengue vector mosquito. Science. 2009:1166541. doi: 10.1126/science.1166541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne JD, Miesenböck G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell. 2008;133:354–363. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Consoulas C, Restifo LL, Levine RB. Dendritic remodeling and growth of motoneurons during metamorphosis of Drosophila melanogaster. Journal of Neuroscience. 2002;22:4906–4917. doi: 10.1523/JNEUROSCI.22-12-04906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta RR, Lurye JM, Kumar JP. Restriction of ectopic eye formation by Drosophila teashirt and tiptop to the developing antenna. Developmental Dynamics. 2009;238:2202–2210. doi: 10.1002/dvdy.21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Vasconcelos ML, Ruta V, Luo S, Wong A, Demir E, Flores J, Balonze K, Dickson BJ, Axel R. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature. 2008;452:473–477. doi: 10.1038/nature06808. [DOI] [PubMed] [Google Scholar]
- Demir E, Dickson BJ. fruitless splicing specifies male courtship behavior in Drosophila. Cell. 2005;121:785–794. doi: 10.1016/j.cell.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Dezulueta P, Alexandre E, Jacq B, Kerridge S. Homeotic complex and teashirt genes cooperate to establish trunk segmental identities in Drosophila. Development. 1994;120:2287–2296. doi: 10.1242/dev.120.8.2287. [DOI] [PubMed] [Google Scholar]
- DiAntonio A, Burgess RW, Chin AC, Deitcher DL, Scheller RH, Schwarz TL. Identification and characterization of Drosophila genes for synaptic vesicle proteins. J Neurosci. 1993;13:4924–4935. doi: 10.1523/JNEUROSCI.13-11-04924.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BJ. Wired for sex: the neurobiology of Drosophila mating decisions. Science. 2008;322:904–909. doi: 10.1126/science.1159276. [DOI] [PubMed] [Google Scholar]
- Drummond DR, Hennessey ES, Sparrow JC. Characterization of missense mutations in the Act88f gene of Drosophila melanogaster. Molecular & General Genetics. 1991;226:70–80. doi: 10.1007/BF00273589. [DOI] [PubMed] [Google Scholar]
- Duffy JB. GAL4 system in Drosophila: A fly geneticist's Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- Ewing AW. Neuromuscular basis of courtship song in drosophila: the role of indirect flight muscles. Journal of Comparative Physiology. 1977;119:249–265. [Google Scholar]
- Ewing AW. Role of feedback during singing and flight in Drosophila melanogaster. Physiological Entomology. 1979a;4:329–337. [Google Scholar]
- Ewing AW. Neuromuscular basis of courtship song in Drosophila: the role of the direct and axillary wing muscles. Journal of Comparative Physiology. 1979b;130:87–93. [Google Scholar]
- Ferveur JF, Stortkuhl KF, Stocker RF, Greenspan RJ. Genetic feminization of brain structures and changed sexual orientation in male Drosophila. Science. 1995;267:902–905. doi: 10.1126/science.7846534. [DOI] [PubMed] [Google Scholar]
- Gopfert MC, Robert D. The mechanical basis of Drosophila audition. Journal of Experimental Biology. 2002;205:1199–1208. doi: 10.1242/jeb.205.9.1199. [DOI] [PubMed] [Google Scholar]
- Gustafson K, Boulianne GL. Distinct expression patterns detected within individual tissues by the GAL4 enhancer trap technique. Genome. 1996;39:174–182. doi: 10.1139/g96-023. [DOI] [PubMed] [Google Scholar]
- Hall JC. Portions of central nervous system controlling reproductive behavior in Drosophila melanogaster. Behavior Genetics. 1977;7:291–312. doi: 10.1007/BF01066800. [DOI] [PubMed] [Google Scholar]
- Hall JC. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- Harcombe ES, Wyman RJ. Output pattern generation by Drosophila flight motoneurons. Journal of Neurophysiology. 1977;40:1066–1077. doi: 10.1152/jn.1977.40.5.1066. [DOI] [PubMed] [Google Scholar]
- Hassan BA, Bermingham NA, He Y, Sun Y, Jan Y-N, Zoghbi HY, Bellen HJ. atonal regulates neurite arborization but does not act as a proneural gene in the Drosophila brain. Neuron. 2000;25:549–561. doi: 10.1016/s0896-6273(00)81059-4. [DOI] [PubMed] [Google Scholar]
- Homyk T, Emerson CP. Functional Interactions between Unlinked Muscle Genes within Haploinsufficient Regions of the Drosophila Genome. Genetics. 1988;119:105–121. doi: 10.1093/genetics/119.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber F, Moore TE, Loher W, editors. Cricket Behavior and Neurobiology. Ithaca, NY, US: Cornell University Press; 1989. [Google Scholar]
- Kimura K, Hachiya T, Koganezawa M, Tazawa T, Yamamoto D. Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron. 2008;59:759–769. doi: 10.1016/j.neuron.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Kimura KI, Ote M, Tazawa T, Yamamoto D. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature. 2005;438:229–233. doi: 10.1038/nature04229. [DOI] [PubMed] [Google Scholar]
- Koganezawa M, Matsuo T, Kimura KI, Yamamoto D. Shaping of Drosophila Male Courtship Posture by a Gustatory Pheromone. In: Finger TE, editor. International Symposium on Olfaction and Taste; Oxford: Blackwell Publishing; 2009. pp. 497–501. [DOI] [PubMed] [Google Scholar]
- Konopka RJ, Kyriacou CP, Hall JC. Mosaic analysis in the Drosophila CNS of circadian and courtship song rhythms affected by a period clock mutation. Journal of Neurogenetics. 1996;11 doi: 10.3109/01677069609107066. 117-&. [DOI] [PubMed] [Google Scholar]
- Kyriacou CP, Hall JC. Circadian rhythm mutations in Drosophila melanogaster affect short-term fluctuations in the males courtship song. Proceedings of the National Academy of Sciences of the United States of America-Biological Sciences. 1980;77:6729–6733. doi: 10.1073/pnas.77.11.6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriacou CP, Hall JC. The function of courtship song rhythms in Drosophila. Animal Behaviour. 1982;30:794–801. doi: 10.1006/anbe.1998.0976. [DOI] [PubMed] [Google Scholar]
- Kyriacou CP, Hall JC. Interspecific genetic control of courtship song production and reception in Drosophila. Science. 1986;232:494–497. doi: 10.1126/science.3083506. [DOI] [PubMed] [Google Scholar]
- Lee G, Foss M, Goodwin SF, Carlo T, Taylor BJ, Hall JC. Spatial, temporal, and sexually dimorphic expression patterns of the fruitless gene in the Drosophila central nervous system. Journal of Neurobiology. 2000;43:404–426. doi: 10.1002/1097-4695(20000615)43:4<404::aid-neu8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Levine J. Properties of nervous system controlling flight in Drosophila melanogaster. Journal of Comparative Physiology. 1973;84:129–166. [Google Scholar]
- Manoli DS, Baker BS. Median bundle neurons coordinate behaviours during Drosophila male courtship. Nature. 2004;430:564–569. doi: 10.1038/nature02713. [DOI] [PubMed] [Google Scholar]
- Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- Markow TA, O'Grady PM. Evolutionary genetics of reproductive behavior in Drosophila: Connecting the dots. Annual Review of Genetics. 2005;39:263–291. doi: 10.1146/annurev.genet.39.073003.112454. [DOI] [PubMed] [Google Scholar]
- Moran CN, Kyriacou CP. Functional neurogenomics of the courtship song of male Drosophila melanogaster. Cortex. 2009;45:18–34. doi: 10.1016/j.cortex.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Reddy S, Jin P, Trimarchi J, Caruccio P, Phillis R, Murphey RK. Mutant molecular motors disrupt neural circuits in Drosophila. J Neurobiol. 1997;33:711–723. doi: 10.1002/(sici)1097-4695(19971120)33:6<711::aid-neu1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Rideout EJ, Billeter JC, Goodwin SF. The sex-determination genes fruitless and doublesex specify a neural substrate required for courtship song. Current Biology. 2007;17:1473–1478. doi: 10.1016/j.cub.2007.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röder L, Vola C, Kerridge S. The role of the teashirt gene in trunk segmental identity in Drosophila. Development. 1992;115 doi: 10.1242/dev.115.4.1017. 1017-&. [DOI] [PubMed] [Google Scholar]
- Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, Hall JC, Taylor BJ, Wasserman SA. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell. 1996;87:1079–1089. doi: 10.1016/s0092-8674(00)81802-4. [DOI] [PubMed] [Google Scholar]
- Singh A, Kango-Singh M, Sun YH. Eye suppression, a novel function of teashirt, requires Wingless signaling. Development. 2002;129:4271–4280. doi: 10.1242/dev.129.18.4271. [DOI] [PubMed] [Google Scholar]
- Talyn BC, Dowse HB. The role of courtship song in sexual selection and species recognition by female Drosophila melanogaster. Animal Behaviour. 2004;68:1165–1180. [Google Scholar]
- Tauber E, Eberl DF. Song production in auditory mutants of Drosophila: the role of sensory feedback. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology. 2001;187:341–348. doi: 10.1007/s003590100206. [DOI] [PubMed] [Google Scholar]
- Trimarchi JR, Schneiderman AM. The motor neurons innervating the direct flight muscles of Drosophila melanogaster are morphologically specialized. Journal of Comparative Neurology. 1994;340:427–443. doi: 10.1002/cne.903400311. [DOI] [PubMed] [Google Scholar]
- Usui-Aoki K, Ito H, Ui-Tei K, Takahashi K, Lukacsovich T, Awano W, Nakata H, Piao ZF, Nilsson EE, Tomida J, Yamamoto D. Formation of the male-specific muscle in female Drosophila by ectopic fruitless expression. Nature Cell Biology. 2000;2:500–506. doi: 10.1038/35019537. [DOI] [PubMed] [Google Scholar]
- Villella A, Hall JC. Courtship anomalies caused by doublesex mutations in Drosophila melanogaster. Genetics. 1996;143:331–344. doi: 10.1093/genetics/143.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villella A, Hall JC. Neurogenetics of courtship and mating in Drosophila. In: Hall JC, Friedmann T, Dunlap JC, editors. Advances in Genetics. Academic Press; 2008. pp. 67–184. [DOI] [PubMed] [Google Scholar]
- Villella A, Gailey DA, Berwald B, Ohshima S, Barnes PT, Hall JC. Extended reproductive roles of the fruitless gene in Drosophila melanogaster revealed by behavioral analysis of new fru mutants. Genetics. 1997;147:1107–1130. doi: 10.1093/genetics/147.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schilcher F, Hall JC. Neural topography of courtship song in sex mosaics of Drosophila melanogaster. Journal of Comparative Physiology. 1979;129:85–95. [Google Scholar]
- Wheeler DA, Fields WL, Hall JC. Spectral analysis of Drosophila courtship songs: Drosophila melanogaster, Drosophila simulans, and their interspecific hybrid. Behavior Genetics. 1988;18:675–703. doi: 10.1007/BF01066850. [DOI] [PubMed] [Google Scholar]
- Zirin JD, Mann RS. Nubbin and Teashirt mark barriers to clonal growth along the proximal-distal axis of the Drosophila wing. Developmental Biology. 2007;304:745–758. doi: 10.1016/j.ydbio.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Adult male w; tshGAL4, UAS-mCD8-GFP/CyO (green) stained with an anti-fruM antibody (magenta). Little or no GFP expression is visible in the brain (br), while strong expression is visible in the ventral ganglia (vg). Anterior-posterior axis is indicated.