Abstract
AMP-activated protein kinase (AMPK) is a highly conserved cellular energy sensor that plays a central role in metabolic homeostasis. A recent study in Science (Egan et al., 2011) identifies ULK1 as a substrate for AMPK phosphorylation, a modification required for selective autophagy of mitochondria and cell survival during starvation.
Keywords: lysosome, membrane biogenesis, mitophagy, stress, vacuole
Macroautophagy (hereafter autophagy) is an evolutionarily conserved catabolic process in which portions of the cytoplasm, including superfluous or damaged organelles and misfolded or aggregated proteins, are engulfed in double-membrane vesicles called autophagosomes for degradation and recycling. Autophagy helps cells, organs and entire organisms endure various stress conditions, such as limited nutrients, decreased energy supply or low oxygen levels. Due to its pivotal role in survival, homeostasis and development, autophagy has attracted intensive interest, and it has been implicated it in a wide range of processes and diseases including cancer, neurodegeneration, immune response, development and aging. Pioneering work in yeast followed by studies in a range of higher eukaryotes during the past two decades has significantly improved our knowledge of the molecular mechanism, with 35 proteins now identified at different steps of autophagy (Yang and Klionsky, 2010). However, many questions still remain unanswered. For example, how different stimuli trigger the sensing system and then relay their signals to induce autophagy, and how different signaling pathways in the context of a network coordinate its regulation. In their recent study, Egan and colleagues further connect AMPK with autophagy regulation, showing that it phosphorylates and activates ULK1.
AMPK is a conserved metabolic switch that senses cellular energy status and governs energy homeostasis through its regulation of glucose and lipid metabolism. This kinase couples cell growth with environmental nutrient availability, and dysregulation of this pathway underlies pathophysiologies such as cancer, cardiovascular disease, diabetes and other metabolic syndromes (Shackelford and Shaw, 2009). Several lines of evidence suggest a role for AMPK in autophagy induction (Herrero-Martin et al., 2009; Liang et al., 2007); however, the molecular mechanism remains largely unexplored. A recent paper by Lee et al. (2010) addressed this issue by showing that AMPK binds to ULK1 (the mammalian homolog of yeast Atg1), and this interaction is required for ULK1-mediated autophagy. The authors suggest that autophagy induction is mediated through AMPK-dependent phosphorylation of raptor, leading to inactivation of mTORC1. Now, research by Egan et al. (2011) adds another piece to the picture, suggesting that ULK1 is directly phosphorylated by AMPK, and this phosphorylation is essential for mitochondria homeostasis and cell survival. The ULK1 kinase is a central component of the core machinery involved in autophagosome formation. This study therefore expands our knowledge on the upstream regulation of ULK1 and sheds light on the connection between cellular energy metabolism and autophagy.
In their study, Egan et al. (2011) carried out a two-part screen to identify AMPK substrates that function in cell growth and metabolism. Using a bioinformatics approach, the authors first identified proteins that contain a conserved AMPK substrate motif. Candidates were then analyzed for the ability to interact with the 14-3-3 phospho-binding protein during energy stress conditions and only in an AMPK-dependent manner. One putative substrate they identified was ULK1, and three of the four predicted phosphorylation sites were detected by tandem mass spectrometry. Both in vivo and in vitro assays confirmed that ULK1 is a bona-fide substrate for AMPK. Phenotypic characterization of AMPK- or ULK1-deficient murine liver or primary hepatocytes unveiled defects in autophagy. For example, an established marker for autophagy, p62, accumulates in AMPK-deficient livers. Since p62 is involved in mitochondria clearance, the authors, through a series of assays, identified defects in selective degradation of mitochondria by autophagy (mitophagy), and a corresponding mitochondria accumulation and abnormality in AMPK- or ULK1-deficient hepatocytes. A non-phosphorylatable (4SA) ULK1 mutant is unable to complement the morphological and functional mitochondria defects, or the loss of cell survival after starvation in ULK1- and ULK2-deficient mouse embryonic fibroblasts compared to wild-type ULK1, suggesting that AMPK phosphorylation of ULK1 is important for its function. The authors also extended their analysis to test whether AMPK and ULK1 have conserved roles in C. elegans. They show that AMPK activity is both necessary and sufficient for the induction of autophagy, and ULK1 is essential for this induction.
In this study, AMPK activation was achieved through pharmacological activation with the AMPK agonists metformin, a widely used type 2 diabetes drug, and phenformin, a more potent analogue. Whether physiological conditions that can activate AMPK, such as glucose starvation, oxidative stress, hypoxia and exercise also exert the same effect merits consideration. Furthermore, mitophagy is a specific type of autophagy that selectively degrades mitochondria (Kanki and Klionsky, 2010). It has not been determined whether this AMPK-dependent phosphorylation of ULK1 is also involved in nonspecific bulk autophagy; the latter may need different sites of phosphorylation on ULK1 or a different mode of phosphorylation events. Pursuit of this question may ultimately shed light on the regulatory pathways and mechanisms that specify different types of autophagy in response to different stress conditions.
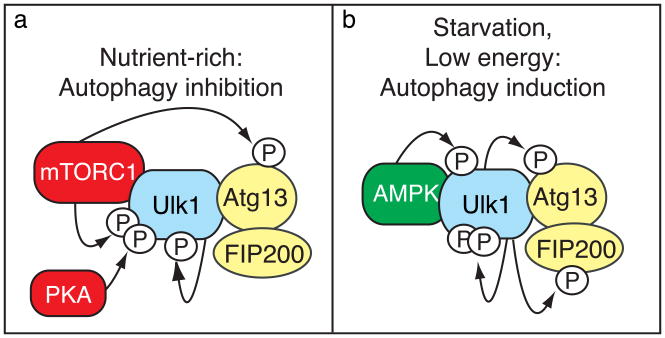
The results from Egan et al. (2011) reveal a direct connection between energy sensing and core autophagy proteins, yet, as usual in science, the information they reveal leads to further questions. For example, autophagy is a tightly regulated process, and excessive autophagy can be as deleterious as insufficient autophagy. Therefore, feedback regulation is important for the fine-tuning and proper termination of autophagy. It will be important to determine if ULK1 or some other component phosphorylates and inhibits AMPK as an opposing mechanism. Similarly, determination of the complementary phosphatases involved in this process will provide valuable insights into autophagy downregulation. Another issue is that ULK1 forms a complex with mAtg13 and FIP200 (the mammalian counterparts of yeast Atg13 and Atg17). Under autophagy-inducing conditions when mTORC1 is inactivated, mTORC1 dissociates from the ULK1-mAtg13-FIP200 complex, ULK1 becomes active, increases its autophosphorylation, and phosphorylates mAtg13 and FIP200 (Mizushima, 2010; Fig. 1). Will AMPK also interact with (and possibly phosphorylate) mAtg13 and/or FIP200? If so, what is the function and impact of this interaction? It will also be interesting to determine whether this pathway is conserved in all eukaryotes going back to yeast. Due to its facile genetics, studies in yeast may rapidly uncover additional players in this pathway. Nonetheless, we may expect to see differences between mammals and yeast. The greater complexity in mammals may reflect the fact that they have adopted a greater diversity of regulatory mechanisms for development- or tissue-specific functions. Finally, AMPK inhibits mTORC1 by phosphorylating TSC2 (Inoki et al., 2003) and raptor (Gwinn et al., 2008), whereas mTORC1 inhibits the ULK1 complex by phosphorylating ULK1 and mAtg13. The elucidation of the dynamic interplay among AMPK, mTORC1 and the ULK1 complex and possibly other signaling pathways involving sensors such as protein kinase A will provide further information about the interconnected network of autophagy regulation.
Figure 1.
Phosphorylation-dependent regulation of the Ulk1 kinase complex. (a) Under nutrient-rich conditions, mTORC1 binds the Ulk1 kinase complex and inhibits autophagy by phosphorylating Ulk1 and Atg13. Ulk1 is also inhibited by protein kinase A-dependent phosphorylation, but is stabilized in part through autophosphorylation. (b) When cells are starved or depleted of energy, mTORC1 dissociates from the complex, Ulk1 autophosphorylation increases and the kinase phosphorylates Atg13 and FIP200, and Ulk1 now binds and is phosphorylated by AMPK, resulting in autophagy induction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Selected Reading
- Egan DF, Shackelford DB, Mihaylova MM, Gelino SR, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011 doi: 10.1126/science.1196371. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero-Martin G, Hoyer-Hansen M, Garcia-Garcia C, Fumarola C, Farkas T, Lopez-Rivas A, Jaattela M. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 2009;28:677–685. doi: 10.1038/emboj.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Kanki T, Klionsky DJ. The molecular mechanism of mitochondria autophagy in yeast. Mol Microbiol. 2010;75:795–800. doi: 10.1111/j.1365-2958.2009.07035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Park S, Takahashi Y, Wang HG. The association of AMPK with ULK1 regulates autophagy. PLoS One. 2010;5:e15394. doi: 10.1371/journal.pone.0015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22:132–139. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]