Abstract
Background and Purpose. Paroxysmal Atrial fibrillation/Flutter (PAF) detection rates in cryptogenic strokes have been variable. We sought to determine the percentage of patients with cryptogenic stroke who had PAF on prolonged non-invasive cardiac monitoring. Methods and Results. Sixty-two consecutive patients with stroke and TIA in a single center with a mean age of 61 (+/− 14) years were analyzed. PAF was detected in 15 (24%) patients. Only one patient reported symptoms of shortness of breath during the episode of PAF while on monitoring, and 71 (97%) of these 73 episodes were asymptomatic. A regression analysis revealed that the presence of PVCs (ventricular premature beats) lasting more than 2 minutes (OR 6.3, 95% CI, 1.11–18.92; P = .042) and strokes (high signal on Diffusion Weighted Imaging) (OR 4.3, 95% CI, 5–36.3; P = .041) predicted PAF. Patients with multiple DWI signals were more likely than solitary signals to have PAF (OR 11.1, 95% CI, 2.5–48.5, P < .01). Conclusion. Occult PAF is common in cryptogenic strokes, and is often asymptomatic. Our data suggests that up to one in five patients with suspected cryptogenic strokes and TIAs have PAF, especially if they have PVCs and multiple high DWI signals on MRI.
1. Introduction
Atrial fibrillation/flutter both persistent and paroxysmal are significant risk factors for cardioembolic ischemic stroke and is often asymptomatic [1, 2]. Atrial Fibrillation (both persistent and paroxysmal) are significant predictors of first and recurrent cerebrovascular and cardiac events, with more than 75 000 cases of stroke per year ascribed to AF (atrial Fibrillation) in the United States [3]. Oral anticoagulant therapy is the keystone of management of such patients, which reduces recurrences of stroke and stroke severity [4, 5]. A baseline electrocardiogram and routine Holter monitoring detects AF in up to 5–7 percent of patients with ischemic strokes within the first 72 hours [6, 7]. A large proportion of strokes, up to 40% in some reports, has no apparent etiology on routine diagnostic testing and is often classified as cryptogenic strokes [8]. The etiology of some of these strokes, however, may be cardioembolic even though the initial diagnostic evaluation may be negative.
Cardiac monitors have improved our capabilities to investigate the presence of paroxysmal atrial fibrillation or flutter (PAF) in patients with stroke for longer time periods. Higher detection rates of PAF have been reported with prolonged cardiac monitoring in patients with cryptogenic cerebrovascular events [9]. We investigated our cohort of cryptogenic stroke patients and sought to determine the percentage of patients who presented to our institution with cryptogenic strokes who have PAF on long-term monitoring. We also sought predictors of PAF in patients with cryptogenic strokes.
2. Methods
Between July 2007 to March 2010, we studied and retrospectively analyzed 62 consecutive patients with cryptogenic stroke (n = 50) or TIA (n = 12) who underwent prolonged non-invasive cardiac monitoring up to 28 days after discharge. These represented 10 percent of the total ischemic stroke and TIA population (n = 629). We sought to determine the percentage of patients with cryptogenic stroke (high signal on DWI) or TIA (symptoms lasting <24 hours and DWI imaging negative) who had PAF on prolonged outpatient non-invasive cardiac monitoring. This retrospective study was approved by IRB.
2.1. Non-Invasive Cardiac Monitoring
CardioNet MCOT (Conshohocken, PA), is a pocket-sized device worn by patients for up to 28 days for cardiac rhythm monitoring. Patients wear three chest leads attached to a small sensor that detects heartbeats and wirelessly transmits this information to the monitor. The monitor analyzes each heartbeat, detects arrhythmias, and transmits each event to the CardioNet monitoring center via a built-in cellular modem. Patients keep a log of their symptoms. Episodes of PAF were defined by duration of more than or 30 seconds [2]. The duration and frequency of PAF episodes were recorded. Manual review was done by 2 physicians to confirm the presence or absence of PAF episodes was recorded. The cardiologist report which was considered gold standard.
2.2. Statistical Analysis
Data was analyzed retrospectively. We performed logistic regression analysis (P < .05 with 95% CI) to determine factors predicting presence or absence of PAF. All analyses were done using (SPSSinc, IBM).
3. Results
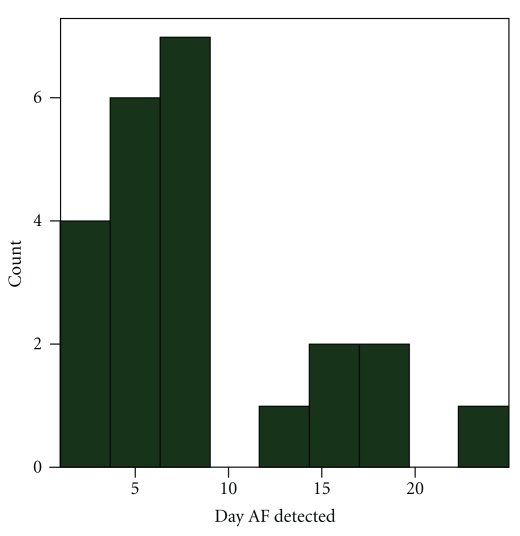
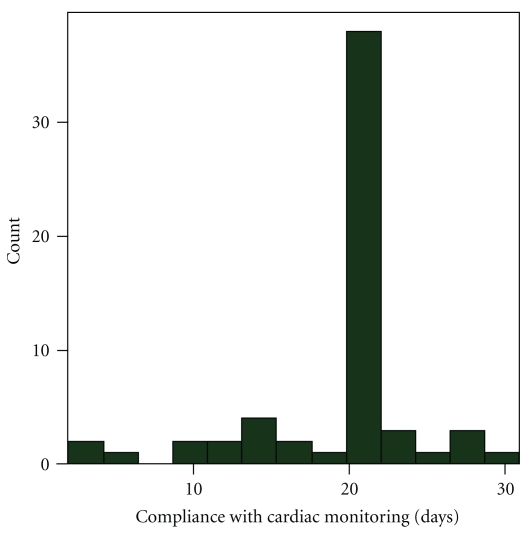
Sixty-two consecutive patients (Table 1) with stroke (n = 50) and TIA (n = 12), with mean age of 61 (± 14) years, were analyzed. All patients underwent an MRI scan with DWI imaging. Ninety-five percent of patients had undergone TEE (transesophageal echocardiogram),TTE (transthoracic echocardiogram) with bubbles (5%), and a CT angiogram (100%). All these tests did not reveal a cause for the stroke. All patients had EKG on admission and at least 24 hours of cardiac telemetry. PAF was detected in 15 (24%) patients. The majority, 14 (93%), of the PAF was detected within the first 21 days (Figure 1). Median duration of non-invasive monitoring was 21 days (range 2–28) (Figure 2), and median day when the first PAF episode was detected was day 7. There were 3 patients who underwent less than 7 days of monitoring. PAF was detected early in those patients, and monitoring was therefore terminated. A total of 73 episodes of paroxysmal PAF were detected among these 15 patients, with range (1–21) and median 5 episodes per patient. Only one patient reported symptoms of shortness of breath during the episode of PAF while monitoring, and 71 (97%) of these 73 episodes were apparently asymptomatic. In patients with PAF, mean maximum heart rate was 111 and mean minimum heart rate was 74. Median start date of non-invasive monitoring was day 29 (range 16–48) after discharge.
Table 1.
Patient characteristics.
| Characteristics | Total | Afib | No-Afib |
|---|---|---|---|
| N = 62 | N = 15 | N = 47 | |
| Mean age | 61 years | 64 | 59 |
| Female (n = 30) | 48% | 52% | 48% |
| HTN (n = 16) | 26% | 27% | 23% |
| CAD (n = 8) | 13% | 20% | 11% |
| DM (n = 3) | 5% | 6% | 4% |
| Dyslipidemia (n = 26) | 42% | 45% | 41% |
| Mean EF (ejection fraction) | 58% | 56% | 59% |
| Percentage getting TEE (n = 59) (transesophageal echocardiogram) | 95% | 93% | 96% |
| Percentage getting CTA (CTAngiogram) | 100% | 100% | 100% |
Figure 1.
Day PAF detected on prolonged non-invasive cardiac monitoring.
Figure 2.
Compliance in days by patients with non-invasive cardiac monitoring.
A logistic regression analysis revealed that the presence of PVCs lasting more than 2 minutes (n = 12, OR 6.32, 1.11–18.92, 95% CI, P = .042) significantly predicted PAF. In addition, strokes (high signal on diffusion weighted imaging) (OR 4.3, 95% CI, 5 to 36.3, P = .041) predicted PAF. There were 50 patients with high DWI signal (strokes) and 12 patients with TIA. Multiple DWI signals were observed in 20 out of 50 patients. There were 14 patients with high DWI signal with PAF and 1 patient with TIA with PAF. Out of the 14 patients with high DWI signals, 11 had multiple signals and 3 had solitary signals. Patients with multiple DWI signals were more likely than solitary signals to have PAF (OR 11.1, 95%CI, 2.5–48.5, P < .01). Other factors such as age, sex, diabetes, hypertension, and heart disease were not significantly associated with PAF detection.
4. Discussion
Our findings indicate that around 25% of patients with cryptogenic stroke had PAF, and most (97%) of these PAF episodes were silent (absence of symptoms suggestive of arrhythmia). If we used the 5-minute definition, 6 patients (9%) had PAF. Previous studies (Table 2) reported PAF detection rates ranging from 4% to 29 % on prolonged cardiac monitoring which ranged from 4 to 30 days in patients with strokes or TIAs. However, there was significant heterogeneity in the design and outcomes of those studies. In 4 studies, [9, 12, 14, 15] which specifically studied cryptogenic strokes, the PAF detection rate was 4–23%. Different types of cardiac monitors were used with variable duration of monitoring (4–30 days). In fact, two studies [13, 15] used implantable devices to evaluate for PAF, and found variable results (4% to 29% detection) on longer term monitoring (up to 1.4 years). A recent cost-effective analysis showed that non-invasive outpatient cardiac monitoring is a cost-effective option for detection of PAF in patients with stroke [16].
Table 2.
Previous studies evaluating prolonged cardiac monitoring.
| Study | Design | Patient selection | N | Device/Days | Percent with PAF/Outcome definition |
|---|---|---|---|---|---|
| Barthelemy et al, 2003 [10] | Observational | Ischemic stroke or TIA | 60 | Cardiac monitoring 4 days | 7.7% with PAF ≥30 secs |
| Jabaudon et al, 2004 [11] | Observational | Ischemic stroke or TIA | 88 | Cardiac monitoring 7 days | 5.7% with PAF |
| Tayal et al, 2008 [9] | Retrospective | Cryptogenic stroke or TIA | 56 | Cardiac monitoring 21 days | 23% with any PAF |
| Elijovich et al, 2009 [12] | Retrospective | Cryptogenic stroke or TIA | 20 | Cardiac monitor 30 | 20% with PAF ≥30 secs |
| Zeigler et al, 2010 [13] | Observational | Stroke or TIA | 183 | Implantable defib/pacer ≥21 days | 29% with PAF/AT ≥5 mins |
| Gaillard et al. 2010 [14] | Observational | Cryptogenic strokes or TIA | 98 | Cardiac monitor 30 days | 9.2% with PAF ≥32 secs |
| Dion et al. 2010 [15] | Observational | Cryptogenic strokes or TIA | 24 | Implantable defib/pacer ≥21 days | 4% with PAF ≥ 30 secs |
The use of the term “cryptogenic” has been variable among stroke physicians. A recent study [15] found very low incidence of PAF on long term monitoring (1/24) in a population of cryptogenic stroke patients. However, in that study, not all patients underwent evaluation of the intracranial and extracranial vasculature and it is possible that the etiology of stroke was due to atherosclerosis. In fact, some patients only had carotid Doppler imaging. All stroke or TIA patients in our study had a CT angiogram to visualize the aortic arch, extracranial, and intracranial blood vessels. In addition, 95% of patients had a TEE and 5% had surface echo with agitated saline injection for detection of right-to-left shunt. We believe the term “cryptogenic” stroke should be reserved for patients who have undergone a comprehensive cardiac and vascular work up.
The duration of monitoring may be important. In our study, although patients were prescribed monitoring for 21–28 days, not all patients were able to complete the full duration of prescribed monitoring (Figure 2). Monitoring was discontinued in three patients who underwent less than 7 days of monitoring, as PAF was detected early. Although previous studies have focused on monitoring for 30 days, existing data indicate that PAF detection is possible even beyond this time period. In a pooled estimate of stroke patients from the Virtual International Stroke Trials Archive data of more than 2504 patients [7], new PAF was detected in 6.9% patients, of which in 68% of patients, PAF was detected after 48 hours of presentation. Another study [17] evaluating long-term monitoring in stroke patients for more than 21 days showed that 62% of PAF cases were detected beyond the initial 21 days of monitoring.
Previous studies used different definitions for PAF (Table 2). We used the ACC/AHA definition of paroxysmal PAF [2] which is self-terminating PAF episodes lasting 30 seconds or longer. In fact, 85% of the episodes of PAF in the study by Tayal et al. [9] did not meet the current criteria of paroxysmal PAF. Zeigler et al. defined paroxysmal PAF as self-terminating episodes lasting 5 minutes or longer, but did not differentiate between PAF and other atrial tachycardias. In our study, all 15 patients satisfied the current ACC/AHA definition of PAF. In addition to the 15 patients, 8 patients had less than 30-second episodes of PAF, which did not satisfy criteria of PAF by AHA.
Multiple high signals on DWI MRI and runs of PVCs may help to predict PAF. In a recent study [14] using transtelephonic evaluation, where EKGs were obtained every day for 30 days, the detection rate was 9% (9/98). This study also showed that positive DWI signal on MRI scan (P = .042) and presence of >100 premature atrial contractions (P = .007) predicted the presence of PAF. Our study, however, showed for the first time that presence of PVCs for more than 2 minutes and multiple high signals on DWI were predictive of higher detection rates of paroxysmal PAF. A recent analysis of ARIC (atherosclerosis risk in communities) study showed that presence of frequent PVCs lasting more than 2 minutes were associated with increased risk of stroke in participants free of hypertension and diabetes [18]. In conclusion, although limited to a single center retrospective sample, our study suggests that PAF is common in cryptogenic stroke patients. In addition, it is always difficult to include cryptogenic TIA patients, as some of these patients may not represent true cerebrovascular events. However, we used traditional definition of TIA and we acknowledge that the low number of TIA patients may result in possibility of bias. Also, five percent (n = 3) of patients did not have TEE in our population. They were evaluated with TTE with bubbles (agitated saline) which is a relatively sensitive test to detect shunt. Patients with multiple DWI signals on MRI and PVCs lasting longer than 2 minutes may have higher PAF prevalence rates—this novel finding warrants further study. The majority of the PAF episodes are asymptomatic. Whether detecting PAF in these patients warrants change in therapy needs further study. Further studies using larger patient population are necessary. Sensitivity and specificity of these devices to detect PAF needs to be evaluated in a systematic fashion. In addition, role of implantable devices to detect PAF may facilitate longer duration of monitoring, which needs to be investigated.
Conflict of Interests
There is no conflict of interests to declare.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for practice guidelines (Writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation) Circulation. 2006;114(7):e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 3.Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke—co-sponsored by the Council on Cardiovascular Radiology and Intervention. The American Academy of Neurology affirms the value of this guideline. Circulation. 2006;113(10):e409–e449. [PubMed] [Google Scholar]
- 4.McBride R, Blackshear JL, Baker VS, et al. Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: stroke prevention in Atrial Fibrillation III Randomised Clinical Trial. The Lancet. 1996;348(9028):633–638. [PubMed] [Google Scholar]
- 5.Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. The New England Journal of Medicine. 2003;349(11):1019–1026. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- 6.Liao J, Khalid Z, Scallan C, Morillo C, O'Donnell M. Noninvasive cardiac monitoring for detecting paroxysmal atrial fibrillation or flutter after acute ischemic stroke: a systematic review. Stroke. 2007;38(11):2935–2940. doi: 10.1161/STROKEAHA.106.478685. [DOI] [PubMed] [Google Scholar]
- 7.Kamel H, Lees KR, Lyden PD, et al. Delayed detection of atrial fibrillation after ischemic stroke. Journal of Stroke and Cerebrovascular Diseases. 2009;18(6):453–457. doi: 10.1016/j.jstrokecerebrovasdis.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Annals of Neurology. 1989;25(4):382–390. doi: 10.1002/ana.410250410. [DOI] [PubMed] [Google Scholar]
- 9.Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology. 2008;71(21):1696–1701. doi: 10.1212/01.wnl.0000325059.86313.31. [DOI] [PubMed] [Google Scholar]
- 10.Barthélémy JC, Féasson-Gérard S, Garnier P, et al. Automatic cardiac event recorders reveal paroxysmal atrial fibrillation after unexplained strokes or transient ischemic attacks. Annals of Noninvasive Electrocardiology. 2003;8(3):194–199. doi: 10.1046/j.1542-474X.2003.08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jabaudon D, Sztajzel J, Sievert K, Landis T, Sztajzel R. Usefulness of ambulatory 7-day ECG monitoring for the detection of atrial fibrillation and flutter after acute stroke and transient ischemic attack. Stroke. 2004;35(7):1647–1651. doi: 10.1161/01.STR.0000131269.69502.d9. [DOI] [PubMed] [Google Scholar]
- 12.Elijovich L, Josephson SA, Fung GL, Smith WS. Intermittent atrial fibrillation may account for a large proportion of otherwise cryptogenic stroke: a study of 30-day cardiac cardiac monitors. Journal of Stroke and Cerebrovascular Diseases. 2009;18(3):185–189. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Ziegler PD, Glotzer TV, Daoud EG, et al. Incidence of newly detected atrial arrhythmias via implantable devices in patients with a history of thromboembolic events. Stroke. 2010;41(2):256–260. doi: 10.1161/STROKEAHA.109.571455. [DOI] [PubMed] [Google Scholar]
- 14.Gaillard N, Deltour S, Vilotijevic B, et al. Detection of paroxysmal atrial fibrillation with transtelephonic EKG in TIA or stroke patients. Neurology. 2010;74(21):1666–1670. doi: 10.1212/WNL.0b013e3181e0427e. [DOI] [PubMed] [Google Scholar]
- 15.Dion F, Saudeau D, Bonnaud I, et al. Unexpected low prevalence of atrial fibrillation in cryptogenic ischemic stroke: a prospective study. doi: 10.1007/s10840-010-9485-5. Journal of Interventional Cardiac Electrophysiology. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamel H, Hegde M, Johnson DR, Gage BF, Johnston SC. Cost-effectiveness of outpatient cardiac monitoring to detect atrial fibrillation after ischemic stroke. Stroke. 2010;41(7):1514–1520. doi: 10.1161/STROKEAHA.110.582437. [DOI] [PubMed] [Google Scholar]
- 17.Glotzer TV, Daoud EG, Wyse DG, et al. The Relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk the trends study. Circulation. 2009;2(5):474–480. doi: 10.1161/CIRCEP.109.849638. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal SK, Heiss G, Rautaharju PM, Shahar E, Massing MW, Simpson RJ., Jr. Premature ventricular complexes and the risk of incident stroke: the atherosclerosis risk in communities (ARIC) study. Stroke. 2010;41(4):588–593. doi: 10.1161/STROKEAHA.109.567800. [DOI] [PMC free article] [PubMed] [Google Scholar]