Abstract
Objectives
To examine the effects of aerobic exercise on cognition and other biomarkers associated with Alzheimer disease pathology for older adults with mild cognitive impairment, and assess the role of sex as a predictor of response.
Design
Six-month, randomized, controlled, clinical trial.
Setting
Veterans Affairs Puget Sound Health Care System clinical research unit.
Participants
Thirty-three adults (17 women) with amnestic mild cognitive impairment ranging in age from 55 to 85 years (mean age,70 years).
Intervention
Participants were randomized either to a high-intensity aerobic exercise or stretching control group. The aerobic group exercised under the supervision of a fitness trainer at 75% to 85% of heart rate reserve for 45 to 60 min/d, 4 d/wk for 6 months. The control group carried out supervised stretching activities according to the same schedule but maintained their heart rate at or below 50% of their heart rate reserve. Before and after the study, glucometabolic and treadmill tests were performed and fat distribution was assessed using dual-energy x-ray absorptiometry. At baseline, month 3, and month 6, blood was collected for assay and cognitive tests were administered.
Main Outcome Measures
Performance measures on Symbol-Digit Modalities, Verbal Fluency, Stroop, Trails B, Task Switching, Story Recall, and List Learning. Fasting plasma levels of insulin, cortisol, brain-derived neurotrophic factor, insulinlike growth factor-I, and β-amyloids 40 and 42.
Results
Six months of high-intensity aerobic exercise had sex-specific effects on cognition, glucose metabolism, and hypothalamic-pituitary-adrenal axis and trophic activity despite comparable gains in cardiorespiratory fitness and body fat reduction. For women, aerobic exercise improved performance on multiple tests of executive function, increased glucose disposal during the metabolic clamp, and reduced fasting plasma levels of insulin, cortisol, and brain-derived neurotrophic factor. For men, aerobic exercise increased plasma levels of insulinlike growth factor I and had a favorable effect only on Trails B performance.
Conclusions
This study provides support, using rigorous controlled methodology, for a potent nonpharma-cologic intervention that improves executive control processes for older women at high risk of cognitive decline. Moreover, our results suggest that a sex bias in cognitive response may relate to sex-based differences in glucometabolic and hypothalamic-pituitary-adrenal axis responses to aerobic exercise.
Salutary effects of exercise on cognitive function have been demonstrated in animal models and in a growing number of clinical studies with older adults.1-3 Potential mechanisms to account for the cognition-enhancing effects of exercise, identified primarily through animal research, include favorable effects on neuronal survivability and function, neuroinflammation, vascularization, neuroendocrine response to stress, and brain amyloid burden.4-14 Exercise also has positive effects on physiological processes such as glucoregulation and cardiovascular health that, when compromised, increase the risk of developing cognitive impairment and Alzheimer disease (AD).15-18 Exercise benefits executive control processes of cognition including selective attention, planning, organizing, multitasking, inhibition, and working memory, and these effects may be more pronounced for older women than older men.19 Favorable effects of exercise on memory have been reported in a few clinical trials,20,21 although most support for a memory benefit comes from animal research.8,11,22,23 In humans, results of cross-sectional24 and prospective25 brain imaging studies suggest that increased aerobic fitness in cognitively healthy older adults is associated with reduced age-related atrophy and increased perfusion in regions that support executive control and memory processes, yet are most vulnerable to aging.19,26
Physical conditioning has positive effects not only on normal aging but also age-related neurodegenerative disease. In the Canadian Study of Health and Aging, physical activity reduced the risks of cognitive impairment and AD.27 For adults with AD, increased cardiorespiratory fitness is associated with reduced whole-brain atrophy and increased white matter volume.28 Studies examining the beneficial effect of aerobic exercise on memory-impaired older adults are limited and primarily rely on retrospective measures of physical activity, unstructured (eg, home-based) programs, or general measures of cognitive function, but nonetheless suggest promising effects.19,29
In this study, we tested the feasibility of an exercise intervention for older adults with mild cognitive impairment (MCI), and hypothesized that a 6-month program of aerobic exercise relative to a stretching control would benefit cognitive function, particularly executive control processes. We also examined intervention effects on AD-related biomarkers in blood including insulin, cortisol, brain-derived neurotrophic factor (BDNF), insulinlike growth factor I (IGF-I), and β-amyloids 40 and 42 (Aβ40 and Aβ42) to explore putative mechanisms linking exercise with improved cognitive function.
Methods
Subjects
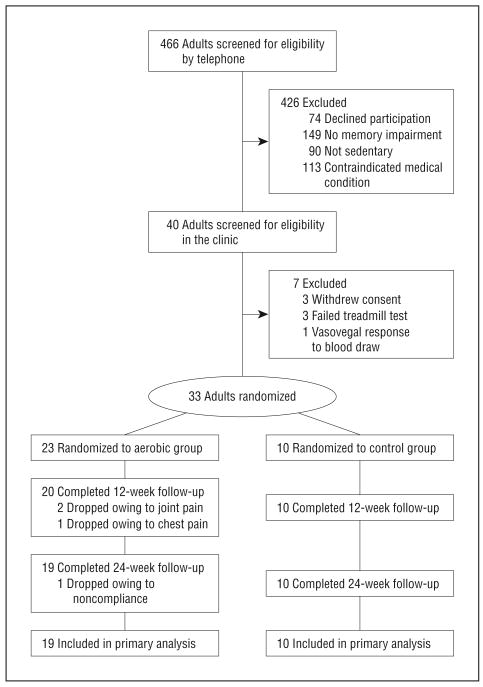
The study was approved by the University of Washington institutional review board. Thirty-three subjects diagnosed with amnestic MCI (single or multiple domain) using Petersen criteria30 in a memory disorders clinic provided written informed consent and were enrolled in the study. Exclusion criteria included unstable cardiac disease, significant cerebrovascular disease, musculoskeletal impairment, or presence of other medical conditions with significant psychiatric, neurologic, or metabolic sequela. Only sedentary adults (self-report of <30 minutes of structured physical activity <3 times per week in the last 6 months, confirmed with peak oxygen consumption during graded exercise treadmill test) were enrolled. Use of statins or antihypertensives was permitted, while use of diabetes medications was not. At enrollment, the aerobic and control groups were comparable with respect to age, general cognitive status, cardiorespiratory fitness, adiposity, insulin sensitivity, and fasting plasma levels of insulin, cholesterol, IGF-I, and cortisol (P > .17 for all). Overall, women at baseline had higher Mini-Mental State Examination scores, body fat, cholesterol (total and high-density lipoprotein), and cortisol levels, and lower IGF-I levels than men. Sample characteristics at study entry are provided in the Table, and the CONSORT (Consolidated Standards of Reporting Trials) diagram outlining subject flow from first contact to study completion is provided in Figure 1. Four subjects (2 men) randomized to the aerobic group dropped out prior to the 6-month assessment. Fasting baseline plasma glucose levels were higher for dropouts compared with completers (P<.001; 112 vs 92 mg/dL for completers [to convert to millimoles per liter, multiply by 0.0555]). Dropouts and completers were comparable for other baseline measurements. Imputations for missing data resulting from spoiled samples or testing error were not performed given the limited number of occurrences (<5%).
Table. Subject Characteristics at Enrollment.
| Mean (SD) | ||||
|---|---|---|---|---|
| Aerobic | Stretching | |||
| Women | Men | Women | Men | |
| Sample, No. | 10 | 9 | 5 | 5 |
| Receiving a β-blocker, No. | 2 | 4 | 0 | 3 |
| Receiving a statin, No. | 4 | 3 | 1 | 2 |
| Age, y | 65.3 (9.4) | 70.9 (6.7) | 74.6 (11.1) | 70.6 (6.1) |
| MMSE scorea | 28.4 (1.7) | 25.6 (2.4) | 28.6 (1.7) | 27.2 (1.8) |
| DRS score | 137 (3.3) | 131 (9.8) | 134 (7.6) | 135 (12.9) |
| V̇o2peak, mL/kg | 22.6 (4.2) | 25.2 (5.4) | 20.4 (1.9) | 23.6 (5.9) |
| BMI | 28.0 (5.5) | 29.1 (4.1) | 29.8 (5.3) | 28.6 (2.1) |
| DEXA, %a | 40.0 (6.4) | 27.0 (4.8) | 40.6 (3.5) | 27.8 (2.8) |
| IMGDc | 16.5 (9.1) | 11.7 (5.6) | 12.6 (4.0) | 13.7 (4.5) |
| FPI, μIU/mL | 7.1 (5.2) | 6.3 (4.4) | 7.5 (2.2) | 5.7 (1.4) |
| Cholesterol, mg/dLa | 204 (39) | 184 (29) | 197 (44) | 150 (25) |
| HDL, mg/dLa | 71 (17) | 51 (20) | 63 (18) | 49 (13) |
| IGF-I, ng/mLa | 86.0 (32.3) | 96.7 (45.0) | 58.8 (36.1) | 107.5 (24.5) |
| Cortisol, μg/dLb | 11.84 (3.76) | 9.99 (2.52) | 12.30 (3.99) | 9.98 (3.50) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DEXA dual-energy x-ray absorptiometry-measured percentage of body fat, excluding head; DRS, Dementia Rating Scale; FPI, fasting plasma insulin; HDL, fasting high-density lipoprotein cholesterol; IGF-I, fasting plasma insulinlike growth factor I; IMGD, insulin-mediated glucose disposal; MMSE, Mini-mental State Examination; V̇o2peak, peak oxygen consumption.
SI conversion factors: To convert total and HDL cholesterol to millimoles per liter, multiply by 0.0259; insulinlike growth factor to nanomoles per liter, 0.131; insulin to picomoles per liter, 6.945; cortisol to nanomoles per liter, 27.588.
P<.05; baseline differences by sex.
P=.06; baseline differences by sex.
Dextrose (mL) and insulin (mU/kg) infused during 30-minute metabolic clamp.
Figure 1.
Subject flow diagram from initial contact through study completion.
Procedure
Participants were randomized using a 2:1 ratio to aerobic exercise or stretching control groups. Cognitive testing and 12-hour fasting blood collection occurred between 8 am and 10 am at baseline and at months 3 and 6. Before and after the 6-month intervention, insulin sensitivity (via hyperinsulinemic-euglycemic clamp), peak cardiorespiratory capacity (via graded exercise treadmill test), and percentage of body fat (via dual-energy x-ray absorptiometry) were assessed for all subjects. Study personnel involved in collection of outcome measures were blinded to randomization assignment.
Intervention Protocols
Participants in both the aerobic and stretching groups carried out their activity routines 4 d/wk for 45 to 60 minutes per session for 6 months. Participants maintained a constant diet and extracurricular activity level for the duration of the study. Most exercise sessions (90%) were conducted at local Young Mens Christian Associations (YMCA). The first 8 sessions were supervised by the trainer. Thereafter, the trainer supervised 1 session per week per participant. Subjects also received a weekly call from the study coordinator to ensure compliance and completed daily logs tracking exercise duration and heart rate (HR) monitor measurements. Compliance data were reviewed weekly by an exercise physiologist. Exercise duration and intensity were gradually increased over the first 6 weeks of the program until participants in the aerobic group were exercising at 75% to 85% of HR reserve31 using a treadmill, stationary bicycle, or elliptical trainer. This intensity was then maintained for the study's duration. The treadmill was the most commonly selected machine, regardless of sex. Participants in the control group carried out a prescribed routine of stretching and balance exercises, maintaining HR at or below 50% of HR reserve. Only 1 participant was dropped from the study owing to noncompliance (completed <3 sessions per week for a 4-week period). For 2 participants (n=1, stretching group), the study period was extended by 1 month to ensure that target goals were attained for a minimum of 22 weeks. Participants in both groups completed an average of 3.75 sessions per week.
Cardiorespiratory Fitness Assessment
At baseline and following the 6-month intervention, participants completed a modified Balke32 maximal-graded exercise treadmill test, with HR and oxygen uptake continuously monitored by an automated metabolic cart (MedGraphics, St Paul, Minnesota). Peak oxygen consumption (V̇o2peak) was measured at test termination, triggered by the onset of symptoms or participant report of exhaustion.
Hyperinsulinemic-Euglycemic Clamp
At baseline and following the 6-month intervention, fasting participants received a hyperinsulinemic-euglycemic clamp,33 as previously described.34 The quantity of dextrose infusate (mL) required to maintain euglycemia under the condition of steady-state hyperinsulinemia for 30 minutes was recorded. Values were adjusted for the volume of insulin infused in 30 minutes (mU/kg); greater values reflected increased glucose disposal and, consequently, increased insulin sensitivity.
Cognitive Assessment
Three comparable versions of the protocol were constructed and randomly assigned in counterbalanced order. An additional version was given prior to baseline to familiarize participants with procedures. The protocol included tests of executive function and short-term memory with documented sensitivity to aging or early neurodegenerative disease, as described below.
Tests of Executive Function
For the Trail Making Test,35 subjects drew lines to connect alphanumeric stimuli in ascending order that were randomly placed on a page. In the more difficult condition (Trails B), subjects alternately tracked 2 sets of stimuli (letters, numbers) while performing the task. The Stroop Color and Word Test,36,37 a test of selective attention and response inhibition, was given using a computer equipped with a voice key. Color names were presented on a computer screen 1 at a time in concordant or discordant font colors (eg, the word red presented in red or green font). For each of 4 alternating trial blocks, subjects either read the word or named the color as quickly as possible, and response latency and content were recorded. Each trial was preceded by a displayed reminder regarding task instruction to minimize memory load. Task Switching38,39 measures the cost of switching between tasks. Pairs of stimuli including a letter and a number were presented clockwise around a 2 × 2 matrix displayed on a computer screen. Every 2 trials, the participant made an odd-even decision or a consonant-vowel decision. Each trial was triggered by the previous response. Verbal Fluency40,41 was measured by the number of words generated across four 60-second trials. Subjects listed words that began with a letter of the alphabet for the first 2 trials and that belonged to a semantic category for the remaining 2 trials. For Symbol Digit Modalities,42 a test of processing speed, subjects saw a legend that paired numbers with abstract symbols and drew the matching symbols for a series of numbers as quickly as possible. The number of correct responses in 120 seconds was recorded.
Tests of Memory
For Story Recall,43,44 a test of declarative memory, subjects heard a narrative that contained 44 informational bits and recalled as much as possible both immediately and after a 30-minute delay. Credit was awarded for verbatim recall and accurate paraphrases. For List Learning,45 subjects heard a list of 12 words and recalled as many items as possible across 3 learning trials, and then again after a 20-minute delay. For Delayed-Match-To-Sample,46 a test of visual memory, 20 abstract geometric designs were presented in series for 10 seconds on a touch-screen monitor. Following a 30-minute delay, 20 design triplets were displayed in series, and subjects touched the single design per set that was previously studied.
Assays
Plasma insulin, IGF-I, and cortisol levels were quantified using radioimmunoassay. Plasma BDNF and platelet factor 4 were measured by enzyme-linked immunosorbent assay (ELISA) (Promega, Madison, Wisconsin; and Aniara, Mason, Ohio). Although BDNF is highly concentrated in the central nervous system, it is also stored and released by activated blood platelets.47 Thus, we assayed platelet factor 4 to estimate platelet activity and adjusted total BDNF levels by these values. Plasma Aβ40 and Aβ42 levels were determined using ELISA, as previously described.48
Statistical Analysis
Multiple regression and correlation procedures were used to residualize the 6-month data from baseline to create change scores. Residualized cognitive measures were subjected to separate multivariate analyses of variance (MANOVA) by domain (ie, executive function, memory), with treatment group as the independent variable. Covariates statistically considered for inclusion in the model included age, education, baseline insulin sensitivity (glucose disposal), V̇o2peak, and cognitive status (Mini-Mental State Examination). In light of reports suggesting a sex bias in cognitive response,2,19 sex was included as a predictor variable. Cardiorespiratory outcomes (V̇o2peak, treadmill grade, treadmill time to exhaustion) and measures of glucose homeostasis (glucose disposal and an estimate of insulin sensitivity using the homeostasis model assessment49) were subjected to similarly structured MANOVAs. For significant MANOVAs, separate univariate ANOVAs were conducted. Pairwise comparisons were performed using t tests when appropriate. Secondary analyses examined aerobic exercise effects on adiposity (dual-energy x-ray absorptiometry–determined percentage of body fat), cardiovascular outcomes (lipids, blood pressures), hypothalamic-pituitary-adrenal (HPA) axis (plasma cortisol) and trophic (plasma IGF-I, BDNF) activity, and plasma β-amyloid levels using similarly structured ANOVAs. Exercise-related associations were examined using multiple regression and correlation for measures of cognition, cardiorespiratory fitness, insulin sensitivity, adiposity, cortisol, BDNF, IGF-I, and β-amyloid. Positively skewed distributions were log-transformed prior to analysis.
Results
Cardiorespiratory Fitness
Six months of controlled aerobic exercise vs stretching improved cardiorespiratory fitness indexed by exercise treadmill test measures of V̇o2peak (F1,26=17.93; P=.003; aerobic, +11%; stretching, −7%), treadmill grade (F1,26=12.79; P =.001; aerobic, +49%; stretching, −9%), and treadmill time to exhaustion (F1,26=6.63; P=.02; aerobic, +38%; stretching, +4%). Neither sex nor β-blocker use were contributory in these analyses.
Cognitive Function
Six months of controlled aerobic exercise improved executive control processes of multitasking, cognitive flexibility, information processing efficiency, and selective attention (Symbol-Digit Modalities, Verbal Fluency, Stroop, Trails B, and Task Switching; MANOVA, F5,19=3.05; P=.04). When sex was included in the model as a predictor variable, a significant interaction indicated that this treatment effect differed for men and women (group × sex F5,17=2.98; P =.04). For women, increasing V̇o2peak was associated with improved executive function (P=.05). The results of univariate analyses for the constituent cognitive tests are provided below. A similarly structured MANOVA performed on measures collected 3 months into the study failed to reach significance, likely because participants had only completed 6 weeks of the program at the targeted intensity.
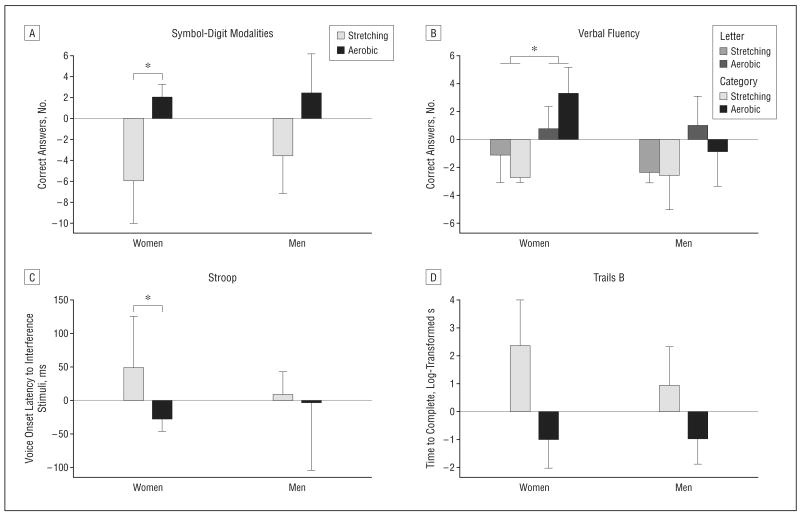
Favorable effects of aerobic exercise were apparent for Symbol-Digit Modalities (F1,26=4.18; P=.05) (Figure 2A) and Verbal Fluency (F1,25=4.87; P=.04). When Verbal Fluency was dissected into letter or category components, group effects were more apparent for category (letter, P=.20; category, P=.03) (Figure 2B). Category fluency involves search of the semantic network and is often impaired at the earliest stages of AD.41,50 Separate analyses by sex indicated that effect size magnitude (f) was larger for women than men on both tasks (symbol-digit: fwomen=0.67, P =.04; fmen=0.29, P=.33; category fluency: fwomen=0.88, P =.01; fmen=0.28, P=.39).
Figure 2.
Mean (standard error of the mean) values representing the change from baseline for cognitive measures, expressed as residual scores. A, For the Symbol-Digit Modalities test, the number of correct responses (in 120 seconds) increased for those in the aerobic group relative to the stretching group (P=.05); this effect was more pronounced for women (P=.04) than men (P=.33). B, For the Verbal Fluency test, word generation was increased for those in the aerobic group relative to the stretching group (P=.04). For women only, aerobic exercise increased category fluency (P=.01). C, For the Stroop test, voice onset latencies to interference stimuli were reduced for women in the aerobic exercise vs stretching group (P=.02). D, For the Trails B test, aerobic exercise reduced the time to complete the task (P=.04), and this effect was comparable for women (P=.09) and men (P=.05). *P<.05.
Sex differences were also observed on the Stroop test (Figure 2C). For this test, voice-onset latencies to interference stimuli (naming font color of discordant color words, eg, the word blue in red font) were analyzed. Baseline voice-onset latencies in a control condition (reading blue printed in blue font) were included in the model to adjust for baseline differences in reading time. For the women, Stroop performance improved for those in the aerobic group but not the stretching group (fwomen=0.76; F1,12=6.93; P=.02), while for men, aerobic exercise had no effect (fmen=0.05; P=.86).
Trails B performance differed by treatment group (F1,25=4.58; P =.04) (Figure 2D). The aerobic group was faster to complete the task relative to baseline (P=.05), whereas the stretching control group tended to be slower (P=.12). This effect was similar for women and men (fwomen=0.56, P =.09; fmen=0.70, P=.05). Performance in the less demanding condition of this task, Trails A, was unaffected by the exercise manipulation. For Task Switching, a task with similar set-shifting demands, accuracy in trials in which the task was switched (eg, from a consonant-vowel discrimination to an odd-even discrimination), controlling for age, tended to benefit from aerobic exercise for both men and women (P=.09).
Tests of verbal declarative memory including List Learning and Story Recall were unaffected by the exercise manipulation. For Delayed-Match-To-Sample, performance was at or near chance level for all subjects, so no analyses were conducted. No effects were observed after 3 months of exercise (MANOVA, P=.33).
Glucose Metabolism, Lipids, and Adiposity
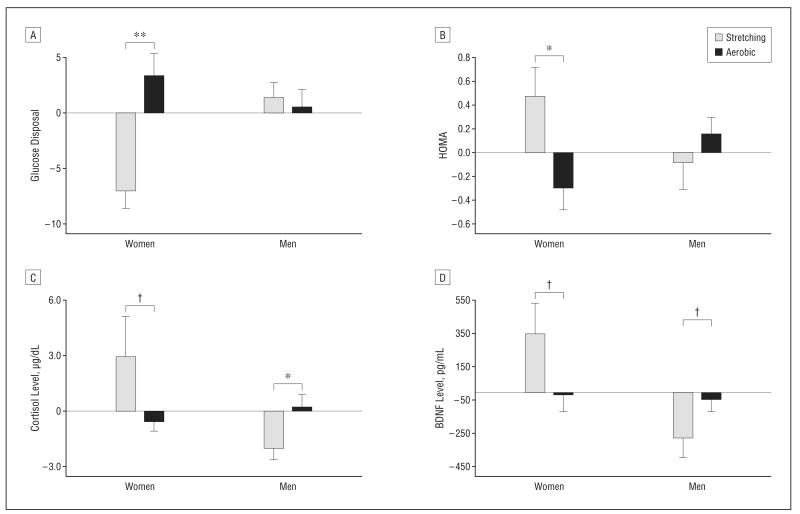
Aerobic exercise was associated with sex-specific improvements in glucoregulation and insulin sensitivity (group × sex MANOVA, F3,19=3.84; P=.03). For women, glucose disposal during the hyperinsulinemic-euglycemic clamp increased for aerobic exercisers relative to controls, an effect that was not apparent for men (group × sex ANOVA, F1,22=7.49; P=.01) (Figure 3A). Similar sex-specific effects were noted for other measures of glucoregulation including fasting plasma insulin (F1,24=4.10; P=.05) and homeostasis model assessment (F1,24=5.73; P=.02) (Figure 3B). For women, 6-month changes in insulin sensitivity predicted V̇o2peak (P=.003) and executive function (P=.01). Total body fat decreased for women and men in the aerobic relative to the stretching group (F1,26=4.16; P =.05), primarily owing to reduced truncal adiposity (P=.08). A similar pattern was observed for body mass index (P=.15). Total cholesterol levels increased for the stretching group and were reduced for the aerobic exercise group (F1,27=4.79; P=.04). An analogous pattern of results described low-density lipoprotein (P =.08) but not high-density lipoprotein (P=.87) or triglyceride levels (P=.64). Statin use did not affect these results.
Figure 3.
Mean (standard error of the mean) values representing the change from baseline for physiological measures, expressed as residual scores. Insulin sensitivity, estimated by glucose disposal (glucose [mL]/insulin [mU/kg]) during the 30-minute steady-state period of hyperinsulinemic-euglycemic clamp (A) and by homeostasis model assessment (HOMA) (B) improved for women in the aerobic group (glucose disposal P=.005; HOMA P=.04). Aerobic exercise had different effects on plasma levels of cortisol (C) and brain-derived neurotrophic factor (BDNF) (D) for women and men (group × sex: cortisol, P=.02; BDNF, P=.04). Relative to controls, aerobic exercise reduced cortisol and BDNF levels for women (cortisol, P=.05; BDNF, P=.06) and increased levels for men (cortisol, P=.04; BDNF, P=.10). Levels of BDNF were adjusted for platelet factor 4 levels and baseline insulin sensitivity. *P<.05; **P<.01; †P<.1.
Cortisol, Bdnf, Igf, and β-Amyloid
A sex-specific effect of aerobic exercise vs stretching was observed for plasma cortisol levels (Figure 3C) (group × sex ANOVA, F1,25=6.00; P=.02). Cortisol levels increased for women in the control group during the 6-month study period, but not for women in the aerobic group. For men, cortisol decreased over time for those in the stretching group while remaining stable for the aerobic group. At baseline, cortisol levels trended higher for women than for men (P=.06) and predicted treatment-related change in fasting plasma insulin levels. A higher basal cortisol level was associated with a greater exercise-induced drop in insulin for men and women in the aerobic exercise group (r=−0.60; P=.007). Consistent with the literature,51 total plasma BDNF levels, adjusted for the contribution of activated platelets, tended to be higher for women than men at baseline (P=.09). A sex-specific effect of aerobic exercise vs stretching was observed for plasma BDNF, adjusted for platelet reactivity and baseline insulin sensitivity (glucose disposal) (Figure 3D) (group × sex ANOVA, F1,23=4.68; P=.04). For the aerobic group, plasma BDNF and cortisol were positively correlated (r=0.51; P=.04). Plasma IGF-I was higher at baseline and increased in response to aerobic exercise for the men (P=.02). Finally, although mean plasma levels of Aβ42 decreased for aerobic exercisers (−6%) and increased for the control group (+24%) during the 6-month period, the difference failed to reach statistical significance (P=.13).
Comment
Six months of aerobic exercise relative to a stretching control improved cognitive function in older adults with MCI. These effects were more pronounced for women than men despite comparable gains in cardiorespiratory fitness. In particular, positive effects were observed for executive control abilities such as selective attention, search efficiency, processing speed, and cognitive flexibility, benefits that have been previously described for cognitively intact adults.2 In a recent exercise trial for older memory-impaired adults, Lautenschlager et al found benefits for memory and language but not executive function.20 However, in their study subjects were not sedentary at study entry and sex-specific effects were not examined. In our study, sex differences in cognitive response may relate to metabolic effects of exercise. Indices of glucoregulation and insulin sensitivity improved with aerobic exercise for women but not for men. In addition, the cortisol response to exercise manipulation was qualitatively different by sex. Relative to controls, aerobic exercise reduced cortisol levels for women and increased levels for men.
Six months of aerobic training had a beneficial effect on executive control processes for women with MCI, processes that are compromised by aging26 but disproportionately so in the earliest, preclinical stages of AD.52 The treatment effects in our study reflect both improvement for women in the aerobic group paired with worsening performance for women in the stretching control group. This finding suggests that aerobic exercise plays a protective role by attenuating progression of cognitive symptoms in MCI. Epidemiologic evidence for a similar sex-specific protective effect was recently reported for the Canadian Study of Health and Aging53 such that women had a reduced risk of developing cognitive impairment if they participated in heavy to moderate exercise, while for men, risk and physical activity were not associated.
In contrast to consistent beneficial effects of exercise on executive function for women, an exercise-associated cognitive benefit for men was observed only on a single test (Trails B). On average, effect size for women was more than twice that for men (f=0.72 vs f=0.33, respectively). This pattern is consistent with a meta-analysis conducted by Colcombe and Kramer19 that described greater cognitive benefits of aerobic exercise for studies including proportionately more women. Similarly, in a recent 12-month randomized controlled trial of moderate-intensity walking for adults with MCI, attention and memory improved for women in the study, whereas for men, only the most compliant showed improved memory, and attention was unchanged.54 Conceivably, sex differences may have been overlooked in other studies for which sex was not included as a predictor variable or was used as a covariate.
At baseline, the women tended to be less fit, carried more body fat, and had better lipid profiles (more high-density and less low-density lipoprotein), lower IGF-I levels, and higher cortisol levels. Insulin sensitivity improved for women in the aerobic group, and greater gain was associated with increased cardiorespiratory fitness. For men, although V̇o2peak increased and body fat and total cholesterol decreased with aerobic exercise, insulin sensitivity did not change. The sex-specific effect of aerobic exercise on insulin sensitivity that we observed has not been previously reported.
Hypothalamic-pituitary-adrenal axis response to aerobic exercise, as measured by circulating levels of plasma cortisol, also differed for men and women with MCI. Relative to controls, aerobic exercise reduced cortisol for women and increased cortisol for men. Although cortisol typically rises following an acute bout of exercise, chronic exposure to exercise and consequent improvement in aerobic fitness reduces acute HPA axis reactivity.55,56 In older adults, cortisol levels are elevated relative to younger adults,57,58 and this age effect is almost 3 times greater for women than men.59 With age, response efficiency of the HPA axis to stress is reduced owing to sluggish inhibition of adrenocorticotropic hormone secretion,60,61 and older women are at a greater disadvantage than older men given their increased physiological reactivity to stress.62-64
Elevated cortisol has deleterious cognitive consequences for women and memory-impaired adults. In a community-based longitudinal study of 200 older adults, women with the highest cortisol levels had the lowest test scores, and risk of cognitive decline increased when levels continued to climb during a 2.5-year period, while for men, cognitive performance was unrelated to fluctuations in cortisol.65 For patients in the early stages of AD, cortisol levels are markedly elevated relative to age-matched nondemented adults,66-70 and higher levels among patients predict more rapid disease progression.71 Hypothalamic-pituitary-adrenal axis overactivity may ultimately compromise brain resilience to stress, increasing vulnerability to neurodegeneration.57,66,72-74
Neurodegenerative disease has been linked to changes in growth factors such as BDNF. In our study, treatment effects on plasma BDNF paralleled those of cortisol, an association that has also been observed by others.75 Brain-derived neurotrophic factor is linked to glucoregulation and insulin sensitivity76-79 and is highly regulated by HPA axis activity.80 Interventions such as aerobic exercise that can markedly alter HPA axis activity61,81 may have the potential to confer clinically meaningful cognitive benefits, particularly for older women at elevated risk of AD.
Limitations of the present study include a small sample size, so replication with a larger group of adults with MCI is essential. It is conceivable that a cognitive benefit for men might be demonstrated in larger trials powered to detect smaller effect sizes (using our data, a minimum sample of 78 men will be needed to detect f ≥ 0.28 with 80% power and α=.05). Of the 466 older adults screened for eligibility in our study, 113 were excluded for medical reasons (Figure 1). This selection bias will likely affect the generalizability of our findings to population-based samples. We chose to exercise adults at a high level of intensity to maximize our ability to detect a true effect. Consequently, we were conservative regarding inclusion criteria to ensure patient safety and minimize liability. Studies that examine the effects of lower-intensity exercise have fewer exclusionary criteria. The demands of the aerobic intervention are suited for a controlled trial, but may not be well-tolerated in less structured, less supervised population-based studies. Other limitations include unequal representation by apolipoprotein E ε4 genotype (no ε4+ women) precluding examination of exercise effects on this AD risk factor and the possible misinterpretation of treatment effects in the context of a stretching control group. Arguably, a stretching program may not serve as an adequate control but rather a low-intensity exercise group with potential effects on the outcome measures of interest. The use of a stretching control provided subjects with comparable opportunities for social interaction (with staff and exercise facility personnel) and increased physical mobility, factors that could conceivably affect mood and HPA axis activity with implications for cognition.82,83
Aerobic exercise is a cost-effective practice that is associated with numerous physical benefits. The results of this study suggest that exercise also provides a cognitive benefit for some adults with MCI. Cognition-enhancing effects of aerobic exercise were most pronounced for executive control tasks in women, an effect that was paired with increased insulin sensitivity and reduced circulating levels of cortisol and BDNF. Six months of a behavioral intervention involving regular intervals of increased HR was sufficient to improve cognitive performance for an at-risk group without the cost and adverse effects associated with most pharmaceutical therapies. Further examination of associations between aerobic exercise–induced change in glucoregulation, HPA axis activity, and cognition may uncover mechanisms that could account for the sex bias in cognitive response.
Acknowledgments
Funding/Support: This study was supported by the Department of Veterans Affairs and Alzheimer's Association grant NIRG-04-1010.
Additional Contributions: We are grateful to the members of our laboratory who contributed many hours to this project including Karen Enstrom, RN, Darla Chapman, RN, Donna Davis, RN, Laura Fisher, Lauren Smith, Jaime Tidwell, Tracia Clark, Amy Morgan, Brenna Renn, and Meg Wojtowicz, and to Elizabeth Colasurdo for her assistance with the cortisol assays. Throughout the study, the Young Mens Christian Association (YMCA) of Greater Seattle, the YMCA of Tacoma-Pierce County, and the South Sound YMCA worked closely with us to provide exercise facilities for participants.
Footnotes
Financial Disclosure: None reported.
Author Contributions: Dr Baker had full access to all of the data and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Baker, Frank, Duncan, and Craft. Acquisition of data: Baker, Frank, Foster-Schubert, McTiernan, Watson, Cholerton, and Craft. Analysis and interpretation of data: Baker, Green, Wilkinson, Plymate, Fishel, and Mehta. Drafting of the manuscript: Baker, Green, Wilkinson, and Craft. Critical revision of the manuscript for important intellectual content: Baker, Frank, Foster-Schubert, Green, Wilkinson, McTiernan, Plymate, Fishel, Watson, Cholerton, Duncan, Mehta, and Craft. Statistical analysis: Baker. Obtained funding: Baker, Duncan, and Craft. Administrative, technical, and material support: Frank, Foster-Schubert, Green, Wilkinson, Plymate, Fishel, Watson, Cholerton, and Mehta. Study supervision: Baker, Frank, and McTiernan.
Disclaimer: Neither funding source provided scientific input for the study.
References
- 1.Fillit HM, Butler RN, O'Connell AW, et al. Achieving and maintaining cognitive vitality with aging. Mayo Clin Proc. 2002;77(7):681–696. doi: 10.4065/77.7.681. [DOI] [PubMed] [Google Scholar]
- 2.Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol. 2006;101(4):1237–1242. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- 3.Erickson KI, Kramer AF. Aerobic exercise effects on cognitive and neural plasticity in older adults. Br J Sports Med. 2009;43(1):22–24. doi: 10.1136/bjsm.2008.052498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fordyce DE, Farrar RP. Enhancement of spatial learning in F344 rats by physical activity and related learning-associated alterations in hippocampal and cortical cholinergic functioning. Behav Brain Res. 1991;46(2):123–133. doi: 10.1016/s0166-4328(05)80105-6. [DOI] [PubMed] [Google Scholar]
- 5.Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab. 1992;12(1):110–119. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- 6.Carro E, Trejo J, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21(15):5678–5684. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25(17):4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG, Cotman CW. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J Neuroinflammation. 2008;5:13. doi: 10.1186/1742-2094-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parachikova A, Nichol KE, Cotman CW. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol Dis. 2008;30(1):121–129. doi: 10.1016/j.nbd.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasse SK, Greenwood BN, Masini CV, et al. Chronic voluntary wheel running facilitates corticosterone response habituation to repeated audiogenic stress exposure in male rats. Stress. 2008;11(6):425–437. doi: 10.1080/10253890801887453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Um HS, Kang EB, Leem YH, et al. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer's disease in an NSE/APPsw-transgenic model. Int J Mol Med. 2008;22(4):529–539. [PubMed] [Google Scholar]
- 14.Nagahara AH, Merrill DA, Coppola G, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15(3):331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuusisto J, Koivisto K, Mykkanen L, et al. Association between features of the insulin resistance syndrome and Alzheimer's disease independently of apolipoprotein E4 phenotype: cross sectional population based study. BMJ. 1997;315(7115):1045–1049. doi: 10.1136/bmj.315.7115.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasparini L, Xu H. Potential roles of insulin and IGF-1 in Alzheimer's disease. Trends Neurosci. 2003;26(8):404–406. doi: 10.1016/S0166-2236(03)00163-2. [DOI] [PubMed] [Google Scholar]
- 17.Craft S. Insulin resistance syndrome and Alzheimer's disease: age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiol Aging. 2005;26(suppl 1):65–69. doi: 10.1016/j.neurobiolaging.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Helzner EP, Luchsinger JA, Scarmeas N, et al. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 2009;66(3):343–348. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colcombe S, Kramer A. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 20.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 21.Erickson KI, Prakash RS, Voss MW, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19(10):1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radák Z, Kaneko T, Tahara S, et al. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochem Int. 2001;38(1):17–23. doi: 10.1016/s0197-0186(00)00063-2. [DOI] [PubMed] [Google Scholar]
- 23.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 24.Colcombe S, Erickson K, Raz N, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58(2):176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 25.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 26.Daniels K, Toth J, Jacoby L. The aging of executive functions. In: Bialystok E, Craik F, editors. Lifespan Cognition: Mechanisms of Change. New York, NY: Oxford University Press; 2006. pp. 96–111. [Google Scholar]
- 27.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 28.Burns JM, Cronk BB, Anderson HS, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71(3):210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85(10):1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 31.Pate R, Blair S, Durstine J, et al. Guidelines for Exercise Testing and Prescription: American College of Sports Medicine. Philadelphia, PA: Lea & Febiger; 1991. [Google Scholar]
- 32.Hagberg JM. Exercise assessment of arthritic and elderly individuals. Baillieres Clin Rheumatol. 1994;8(1):29–52. doi: 10.1016/s0950-3579(05)80223-7. [DOI] [PubMed] [Google Scholar]
- 33.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 34.Watson GS, Peskind ER, Asthana S, et al. Insulin increases CSF Abeta42 levels in normal older adults. Neurology. 2003;60(12):1899–1903. doi: 10.1212/01.wnl.0000065916.25128.25. [DOI] [PubMed] [Google Scholar]
- 35.Shibuya-Tayoshi S, Sumitani S, Kikuchi K, et al. Activation of the prefrontal cortex during the Trail-Making Test detected with multichannel near-infrared spectroscopy. Psychiatry Clin Neurosci. 2007;61(6):616–621. doi: 10.1111/j.1440-1819.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- 36.Golden CJ. Stroop Color and Word Test. Chicago, IL: Stoelting; 1978. [Google Scholar]
- 37.Spieler DH, Balota DA, Faust ME. Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer's type. J Exp Psychol Hum Percept Perform. 1996;22(2):461–479. doi: 10.1037//0096-1523.22.2.461. [DOI] [PubMed] [Google Scholar]
- 38.Rogers RD, Sahakian B, Hodges J, Polkey C, Kennard C, Robbins T. Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson's disease. Brain. 1998;121(pt 5):815–842. doi: 10.1093/brain/121.5.815. [DOI] [PubMed] [Google Scholar]
- 39.Kramer AF, Hahn S, McAuley E, et al. Exercise, aging and cognition: healthy body, healthy mind? In: Fisk AD, Rogers W, editors. Human Factors Interventions for the Health Care of Older Adults. Hillsdale, NJ: Erlbaum; 2001. [Google Scholar]
- 40.Nutter-Upham KE, Saykin AJ, Rabin LA, et al. Verbal fluency performance in amnestic MCI and older adults with cognitive complaints. Arch Clin Neuropsychol. 2008;23(3):229–241. doi: 10.1016/j.acn.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lonie JA, Herrmann LL, Tierney KM, et al. Lexical and semantic fluency discrepancy scores in aMCI and early Alzheimer's disease. J Neuropsychol. 2009;3(pt 1):79–92. doi: 10.1348/174866408X289935. [DOI] [PubMed] [Google Scholar]
- 42.Wechsler D. Wechsler Adult Intelligence Scale. 3rd. San Antonio, TX: Harcourt Assessment; 2003. [Google Scholar]
- 43.Chodosh J, Reuben D, Albert M, Seeman T. Predicting cognitive impairment in high-functioning community-dwelling older persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc. 2002;50(6):1051–1060. doi: 10.1046/j.1532-5415.2002.50260.x. [DOI] [PubMed] [Google Scholar]
- 44.Craft S, Asthana S, Cook DG, et al. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer's disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28(6):809–822. doi: 10.1016/s0306-4530(02)00087-2. [DOI] [PubMed] [Google Scholar]
- 45.Blacker D, Lee H, Muzikansky A, et al. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol. 2007;64(6):862–871. doi: 10.1001/archneur.64.6.862. [DOI] [PubMed] [Google Scholar]
- 46.Buccafusco JJ, Terry A, Jr, Murdoch P. A computer-assisted cognitive test battery for aged monkeys. J Mol Neurosci. 2002;19(1-2):179–185. doi: 10.1007/s12031-002-0030-6. [DOI] [PubMed] [Google Scholar]
- 47.Fujimura H, Altar CA, Chen R, et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002;87(4):728–734. [PubMed] [Google Scholar]
- 48.Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol. 2000;57(1):100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 49.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 50.Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer's type: a meta-analysis. Neuropsychologia. 2004;42(9):1212–1222. doi: 10.1016/j.neuropsychologia.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Lommatzsch M, Zingler D, Schuhbaeck K, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26(1):115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Traykov L, Raoux N, Latour F, et al. Executive functions deficit in mild cognitive impairment. Cogn Behav Neurol. 2007;20(4):219–224. doi: 10.1097/WNN.0b013e31815e6254. [DOI] [PubMed] [Google Scholar]
- 53.Middleton L, Kirkland S, Rockwood K. Prevention of CIND by physical activity: different impact on VCI-ND compared with MCI. J Neurol Sci. 2008;269(1-2):80–84. doi: 10.1016/j.jns.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 54.van Uffelen JG, Chinapaw MJ, van Mechelen W, Hopman-Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? a randomised controlled trial. Br J Sports Med. 2008;42(5):344–351. doi: 10.1136/bjsm.2007.044735. [DOI] [PubMed] [Google Scholar]
- 55.Deuster PA, Petrides JS, Singh A, Lucci EB, Chrousos GP, Gold PW. High intensity exercise promotes escape of adrenocorticotropin and cortisol from suppression by dexamethasone: sexually dimorphic responses. J Clin Endocrinol Metab. 1998;83(9):3332–3338. doi: 10.1210/jcem.83.9.5110. [DOI] [PubMed] [Google Scholar]
- 56.Mastorakos G, Pavlatou M. Exercise as a stress model and the interplay between the hypothalamus-pituitary-adrenal and the hypothalamus-pituitary-thyroid axes. Horm Metab Res. 2005;37(9):577–584. doi: 10.1055/s-2005-870426. [DOI] [PubMed] [Google Scholar]
- 57.Peskind ER, Raskind MA, Wingerson D, et al. Hypothalamic-pituitary-adrenocortical axis responses to physostigmine: effects of Alzheimer's disease and gender. Biol Psychiatry. 1996;40(1):61–68. doi: 10.1016/0006-3223(95)00318-5. [DOI] [PubMed] [Google Scholar]
- 58.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 59.Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology. 2005;30(1):80–91. doi: 10.1016/j.psyneuen.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 60.Wilkinson CW, Petrie EC, Murray SR, Colasurdo EA, Raskind MA, Peskind ER. Human glucocorticoid feedback inhibition is reduced in older individuals: evening study. J Clin Endocrinol Metab. 2001;86(2):545–550. doi: 10.1210/jcem.86.2.7232. [DOI] [PubMed] [Google Scholar]
- 61.Traustado′ttir T, Bosch PR, Cantu T, Matt KS. Hypothalamic-pituitary-adrenal axis response and recovery from high-intensity exercise in women: effects of aging and fitness. J Clin Endocrinol Metab. 2004;89(7):3248–3254. doi: 10.1210/jc.2003-031713. [DOI] [PubMed] [Google Scholar]
- 62.Petrie EC, Wilkinson CW, Murray S, Jensen C, Peskind ER, Raskind MA. Effects of Alzheimer's disease and gender on the hypothalamic-pituitary-adrenal axis response to lumbar puncture stress. Psychoneuroendocrinology. 1999;24(4):385–395. doi: 10.1016/s0306-4530(98)00088-2. [DOI] [PubMed] [Google Scholar]
- 63.Seeman TE, Singer B, Wilkinson CW, McEwen B. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology. 2001;26(3):225–240. doi: 10.1016/s0306-4530(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 64.Traustado′ttir T, Bosch PR, Matt KS. Gender differences in cardiovascular and hypothalamic-pituitary-adrenal axis responses to psychological stress in healthy older adult men and women. Stress. 2003;6(2):133–140. doi: 10.1080/1025389031000111302. [DOI] [PubMed] [Google Scholar]
- 65.Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW. Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. J Clin Endocrinol Metab. 1997;82(8):2458–2465. doi: 10.1210/jcem.82.8.4173. [DOI] [PubMed] [Google Scholar]
- 66.Davis KL, Davis BM, Greenwald BS, et al. Cortisol and Alzheimer's disease I: basal studies. Am J Psychiatry. 1986;143(3):300–305. doi: 10.1176/ajp.143.3.300. [DOI] [PubMed] [Google Scholar]
- 67.Masugi F, Ogihara T, Sakaguchi K, et al. High plasma levels of cortisol in patients with senile dementia of the Alzheimer's type. Methods Find Exp Clin Pharmacol. 1989;11(11):707–710. [PubMed] [Google Scholar]
- 68.Swanwick GR, Kirby M, Bruce I, et al. Hypothalamic-pituitary-adrenal axis dysfunction in Alzheimer's disease: lack of association between longitudinal and cross-sectional findings. Am J Psychiatry. 1998;155(2):286–289. doi: 10.1176/ajp.155.2.286. [DOI] [PubMed] [Google Scholar]
- 69.Peskind ER, Wilkinson CW, Petrie EC, Schellenberg GD, Raskind MA. Increased CSF cortisol in AD is a function of APOE genotype. Neurology. 2001;56(8):1094–1098. doi: 10.1212/wnl.56.8.1094. [DOI] [PubMed] [Google Scholar]
- 70.Rasmuson S, Nasman B, Carlstrom K, Olsson T. Increased levels of adrenocortical and gonadal hormones in mild to moderate Alzheimer's disease. Dement Geriatr Cogn Disord. 2002;13(2):74–79. doi: 10.1159/000048637. [DOI] [PubMed] [Google Scholar]
- 71.Csernansky JG, Dong H, Fagan AM, et al. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiatry. 2006;163(12):2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maeda K, Tanimoto K, Terada T, Shintani T, Kakigi T. Elevated urinary free cortisol in patients with dementia. Neurobiol Aging. 1991;12(2):161–163. doi: 10.1016/0197-4580(91)90055-o. [DOI] [PubMed] [Google Scholar]
- 73.Hatzinger M, Z'Brun A, Hemmeter U, et al. Hypothalamic-pituitary-adrenal system function in patients with Alzheimer's disease. Neurobiol Aging. 1995;16(2):205–209. doi: 10.1016/0197-4580(94)00159-6. [DOI] [PubMed] [Google Scholar]
- 74.Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer's disease. J Neurosci. 2006;26(35):9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Begliuomini S, Lenzi E, Ninni F, et al. Plasma brain-derived neurotrophic factor daily variations in men: correlation with cortisol circadian rhythm. J Endocrinol. 2008;197(2):429–435. doi: 10.1677/JOE-07-0376. [DOI] [PubMed] [Google Scholar]
- 76.Mattson MP. Brain evolution and lifespan regulation: conservation of signal transduction pathways that regulate energy metabolism. Mech Ageing Dev. 2002;123(8):947–953. doi: 10.1016/s0047-6374(02)00032-5. [DOI] [PubMed] [Google Scholar]
- 77.Krabbe KS, Nielsen AR, Krogh-Madsen R, et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50(2):431–438. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- 78.Fujinami A, Ohta K, Obayashi H, et al. Serum brain-derived neurotrophic factor in patients with type 2 diabetes mellitus: relationship to glucose metabolism and biomarkers of insulin resistance. Clin Biochem. 2008;41(10-11):812–817. doi: 10.1016/j.clinbiochem.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 79.Stranahan AM, Norman ED, Lee K, et al. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18(11):1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith MA, Makino S, Kvetnansky R, Post RM. Effects of stress on neurotrophic factor expression in the rat brain. Ann N Y Acad Sci. 1995;771:234–239. doi: 10.1111/j.1749-6632.1995.tb44684.x. [DOI] [PubMed] [Google Scholar]
- 81.Traustado′ttir T, Bosch PR, Matt KS. The HPA axis response to stress in women: effects of aging and fitness. Psychoneuroendocrinology. 2005;30(4):392–402. doi: 10.1016/j.psyneuen.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 82.Unger JB, Johnson CA, Marks G. Functional decline in the elderly: evidence for direct and stress-buffering protective effects of social interactions and physical activity. Ann Behav Med. 1997;19(2):152–160. doi: 10.1007/BF02883332. [DOI] [PubMed] [Google Scholar]
- 83.Teri L, Logsdon RG, McCurry SM. Exercise interventions for dementia and cognitive impairment: the Seattle Protocols. J Nutr Health Aging. 2008;12(6):391–394. doi: 10.1007/BF02982672. [DOI] [PMC free article] [PubMed] [Google Scholar]