Abstract
Background and Purpose
This study compared 6-MV IMRT and proton therapy in terms of organ specific second cancer lifetime attributable risks (LARs) caused by scattered and secondary out-of-field radiation.
Material and Methods
Based on simulated organ doses, excess relative and excess absolute risk models were applied to assess organ-specific LARs. Two treatment sites (cranium and central spine) were considered involving 6 treatment volumes and 6 patient ages (9-month, 4-year, 8-year, 11-year, 14-year, and adult).
Results
The LARs for thyroid cancer from a 6 cm diameter field treating a brain lesion in a 4-year old patient were estimated to be 1.1% and 0.3% in passive proton therapy and IMRT, respectively. However, estimated LARs for bladder cancer, more than 25 cm from the field edge for the same patient and treatment field, were estimated to be 0.2% and 0.02% from IMRT and proton therapy, respectively. Risks for proton beam scanning was found to be an order of magnitude smaller compared to passive proton therapy.
Conclusion
In terms of out-of-field risks, IMRT offers advantage close to the primary field and an increasing advantage for passive proton therapy is noticed with increasing distance to the field. Scanning proton beam therapy shows the lowest risks.
Keywords: Proton therapy, IMRT, Second cancer risk, Whole-body phantom, Pediatric radiation oncology
Introduction
The risk for developing a radiation-induced second cancer, particularly among pediatric patients, is of concern in radiation therapy as the number of cancer survivors with a long post treatment life is increasing. We have previously shown that young adults and pediatric patients tend to receive, on average, higher secondary organ doses than adults due to geometrical factors [1,2]. In addition to these dosimetric disadvantages for young patients, risk models show an increased risk per absorbed dose due to greater chance for the effects to manifest in his/her post treatment lifetime and more proliferating cells in such young patients [3,4]. The early childhood cancer survivor study [5,6] has shown that the risk of developing a second cancer in the first 25 years from initial treatment can be as high as 25% [3]. A recent review by Tubiana [7] has shown that second primary malignancies are observed for in-field organs receiving doses in excess of 2 Gy and cases of thyroid and breast cancers are observed in children after receiving doses as low as 100 mGy. Concern has been raised that there might be considerable risks for second cancers in highly conformal treatment delivery techniques, like IMRT or proton therapy [7–18]. For 6-MV IMRT, the scattered photon dose is primarily caused by leakage through the accelerator head and internal scatter within the patient. On the other hand, passive scattered proton therapy produces secondary neutrons that form the primary source of out-of-field radiation. Neutrons are generated both in the treatment head (external neutrons) and inside the patient (internal neutrons) through proton-nuclear interactions [19]. In the case of ideal scanned proton therapy delivery (without beam shaping devices in the treatment head) neutrons are only generated inside patient.
The purpose of this study was to show the adverse effects of out-of-field radiation doses in IMRT and proton therapy. Therefore, this study deals only with the second cancer incidences for organs located away from the treatment site.
Methods and Materials
Whole body voxelized phantoms and equivalent dose simulations
The dosimetric information used in this analysis is based on results from Monte Carlo simulations to determined energy deposition events in patients receiving radiation therapy with protons or IMRT [20]. We used six gender-specific whole-body computational phantoms [21–23]. The implementation of the phantoms in our computational framework was previously described [1,2]. The patient characteristics considered were: adult 39-year old (male), 14-year old (male), 11-year old (male), 8-year old (female), 4-year old (female), and 9-month old (male). The neutron equivalent doses from passive scattered proton therapy and the scattered photon doses from IMRT were simulated as previously described [2,20].
Table 1 summarizes the beam and field parameters considered. For proton therapy fields, the beam energies at the treatment head entrance were 196.2 MeV and 178.3 MeV for range/modulation widths of 10 cm/5 cm and 15 cm/10 cm, respectively [2]. The IMRT fields used in this study had a circular shape to match as closely as possible the proton therapy fields. The jaws settings were matched to circumscribe the circular apertures [19]. Circular aperture fields are used as representative field shapes of various sizes for brain and spine tumors. To model for scanning proton beam therapy we simply ignored the secondary neutrons produced in the treatment head and only considered neutron doses produced inside the patient. Secondary neutron equivalent doses from proton therapy were analyzed as mSv per treatment Gy applying an RBE of 1.1 [mSv/Gy(RBE)] whereas scattered photon doses from IMRT were analyzed as mSv/Gy. Energy dependent radiation weighting factors for neutrons were assigned according to the recommendation by the ICRP [24] as described elsewhere [20].
Table 1.
Diameter, range and modulation widths for each treatment field considered. Proton beam energies refer to the treatment head entrance. The normalization point for the target dose ensured that there is comparable dose in the center of the target for proton therapy and IMRT simulations.
| Field | Aperture diameter [cm] | Protons | IMRT | |||
|---|---|---|---|---|---|---|
| Beam Energy [MeV] | SOBP range [cm] | SOBP Modulation width [cm] | Beam Energy [MV] | Normalization depth [cm] | ||
| 1 | 3 | 169.2 | 10 | 5 | 6 | 7.5 |
| 2 | 6 | 169.2 | 10 | 5 | 6 | 7.5 |
| 3 | 9 | 169.2 | 10 | 5 | 6 | 7.5 |
| 4 | 3 | 178.3 | 15 | 10 | 6 | 10 |
| 5 | 6 | 178.3 | 15 | 10 | 6 | 10 |
| 6 | 9 | 178.3 | 15 | 10 | 6 | 10 |
To simulate typical scenarios for the brain fields, the beams were positioned lateral to the head (i.e. gantry angle at 90 degrees). In the spine field setup, the beams were positioned posteriorly pointing towards a location between vertebrae-t and vertebrae-l (centered around vertebrae T12) with the gantry angle at 180 degrees. We considered all 6 patient groups on both treatment sites (cranium and spine) with all six fields as described in table 1.
Low-dose Cancer Induction Risk
Risk assessments were based on two risk models presented in the Biological Effects of Ionizing Radiation (BEIR VII) report [4], namely, the excess relative risk (ERR) and the excess absolute risk (EAR). Risk transport models discussed in BEIR VII were derived from the atomic bomb survivors and based on the assumption that acute doses were delivered in one fraction. The report assumes a latency for solid tumors to be ~5 years and for leukemia to be ~2 years.
For lifetime attributable risk (LAR) calculations we integrated risks for all attainable ages from the age at exposure (i.e. treatment time) to 100 years (conservative number). Furthermore, according to BEIR VII, for sites other than lung, LAR values should be calculated by weighting ERR with 0.7 and EAR with 0.3. We re-evaluated baseline incidences for 2001–2005 from age and gender specific baseline risks compiled by Anderson and DeTurk [25] and in the SEER report [26]. Our study took into account fractionation effects. To account for low doses and low dose rates in therapeutic irradiation, a dose and dose-rate effective factor (DDREF) has been introduced [24,27]. Accordingly, we assumed DDREF to be unity for high-LET radiation such as neutrons [24]. For scattered photons from IMRT the risk values were reduced by a DDREF factor of 1.5 as recommended in BIER VII report.
Although doses for more than 50 organs were determined in the previous study [20], we did not estimate the risk for all organs because of a lack of baseline cancer incidence data [26]. For the scenario of cranial fields, organs considered for female patients were larynx, thyroid, bronchi, breasts, lungs, esophagus, liver, pancreas, uterus, ovaries, and bladder wall. For the spinal fields, female organs considered for the risk estimates were brain, pharynx, larynx, thyroid, bronchi, breasts, esophagus, lung, rectosigmoid wall, uterus, ovaries, and bladder wall. For male patients breast tissue as well as uterus and ovaries were replaced by testes and prostate.
Results
Lifetime Attributable Risk: Cranial Case
In the interest of space we only present the results for 2 out of the 6 considered patient age groups. For one of the considered treatment fields (field 6 having the largest treatment volume), table 2 shows LAR values for solid cancer incidences assuming a 8-year old female and a 14-year old male patient. We considered a prescription dose of 54-Gy. For breast tissue, we quote the estimated LAR values for ERR and EAR separately following a suggestion in the BEIR report [4]. For comparison, the unexposed lifetime baseline risks are shown in the last column of table 2. As expected, younger patients show higher second cancer incidence risk compared to older patients.
Table 2.
Lifetime attributable risks (LAR) for second solid cancer incidences assuming an 8/14-year old female/male treated with 54-Gy (Gy[RBE]) target dose (cranial lesion; field 6). For breast, instead of combining the two transport models we quote the estimates separately.
| Organs | LAR (%) |
Baseline(%) | ||
|---|---|---|---|---|
| Passive scattered proton therapy | IMRT | Proton beam scanning | ||
| Larynx | 0.08/0.14 | 0.04/0.12 | 0.02/<0.01 | 0.15/0.61 |
| Thyroid | 1.39/0.15 | 0.64/0.06 | 0.18/0.01 | 1.10/0.38 |
| Bronchi | 0.68/0.23 | 0.17/0.13 | 0.03/0.03 | 6.67/7.76 |
| Breasts EAR | 0.78/- | 0.33/- | 0.04/- | 13/- |
| Breasts ERR | 0.45/- | 0.19/- | 0.02/- | 13/- |
| Lungs | 0.39/0.14 | 0.16/0.14 | 0.04/0.02 | 6.67/7.76 |
| Esophagus | 0.04/0.07 | 0.01/0.05 | 0.01/ | 0.24/0.75 |
| Liver | 0.01/0.01 | 0.01/0.03 | <0.01/<0.01 | 0.41/0.90 |
| Pancreas | 0.04/0.01 | 0.09/0.18 | 0.01/<0.01 | 1.24/1.25 |
| Kidneys | 0.01/0.01 | 0.04/0.08 | <0.01/<0.01 | 1.02/1.69 |
| Uterus/Prostate | <0.01/<0.01 | 0.01/0.01 | <0.01/<0.01 | 2.5/16.01 |
| Ovaries/Testes | <0.01/<0.01 | 0.02/0.02 | <0.01/<0.01 | 1.42/0.37 |
| Bladder Wall | <0.01/0.01 | 0.04/0.02 | <0.01/<0.01 | 1.25/3.98 |
Apparently, in passive scattered proton therapy, organs near the field edge receive higher secondary neutron equivalent doses compared to photon doses in similar IMRT fields. Proton beam scanning offers a distinct advantage compared to the other two modalities. The reason for the slight out-of-field advantage of IMRT compared to passive scattered proton therapy close to the field is two-fold: the radiation weighting factor for neutrons and the DDREF value of 1.5 for photons. Further away from the target though, in both patients (8 and 14-year old), organs located below the kidneys show higher second cancer risks from IMRT relative to the risks from similar proton therapy fields. When analyzing all fields (Fig. 1), it is apparent that for IMRT, there is a clear increase in risk as the treatment volume increases. The situation is not as clear in proton therapy as there is the effect of increase secondary dose from patient generated neutrons and decrease secondary dose from treatment head generated neutrons as the treatment volume increases [1,2].
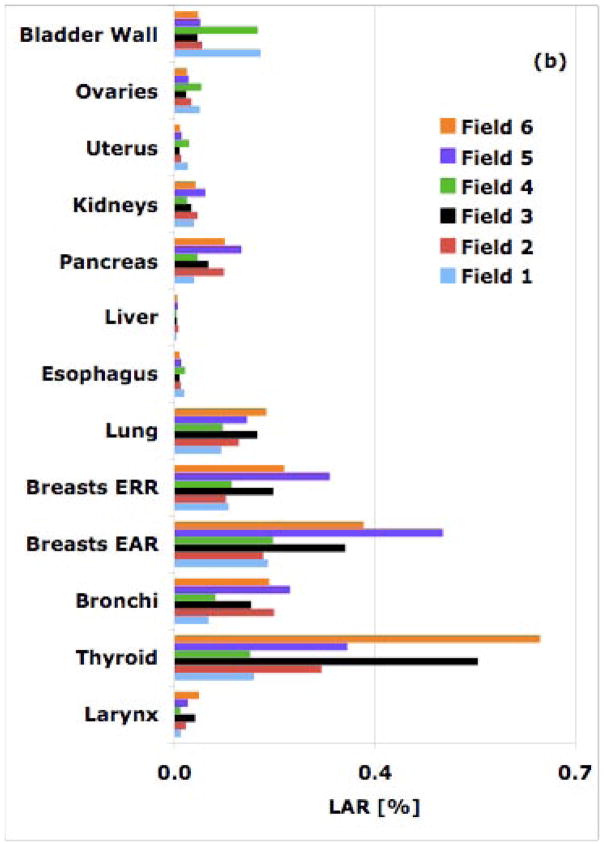
Figure 1.
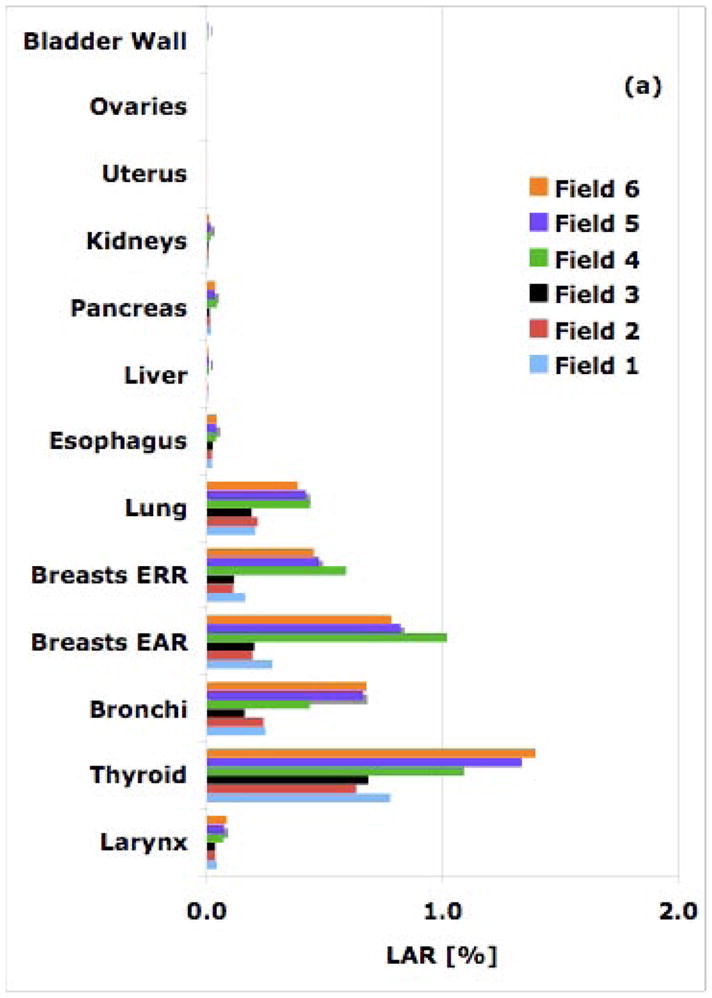
Simulated LAR values from all six cranial fields for an 8-year old patient for a 54-Gy treatment: (a) proton therapy; (b) 6-MV IMRT.
Breast and thyroid cancer are of concern for female patients as the baseline cancer incidences for breasts and thyroid in women are 13% and 1.1%, respectively. Our estimation for a cranial field (field 6) shows a LAR for developing thyroid cancer from a 54-Gy[RBE] proton treatment of an 8-year old female patient of 1.4%, i.e. higher than the baseline risk. The corresponding LAR for a similar IMRT field was estimated to be 0.6%, i.e. half the baseline risk.
Lifetime Attributable Risk: Spine Case
As in the cranial case, the second cancer risks for out-of-field organs close to the field edge are higher from proton therapy compared to similar IMRT fields. However, with increasing distance to the field (beyond ~25cm from the field edge) the risks from IMRT becomes higher compared to similar proton therapy fields. LAR values for various out-of-field organs are summarized in table 3.
Table 3.
Lifetime attributable risks (LAR) for second cancer incidences assuming an 8/14-year old female/male treated with 54 Gy (Gy[RBE]) target dose (spine lesion; field 6). For breast, instead of combining the two transport models we quote the estimates separately.
| Organs | LAR (%) |
Baseline(%) | ||
|---|---|---|---|---|
| Passive scattered proton therapy | IMRT | Proton beam scanning | ||
| Brain | 0.01/<0.01 | 0.08/0.04 | <0.01/<0.01 | 0.56/0.65 |
| Pharynx | 0.02/0.01 | 0.02/0.03 | 0.01/<0.01 | 0.67/1.41 |
| Larynx | 0.01/0.01 | 0.01/0.01 | <0.01/<0.01 | 0.15/0.61 |
| Thyroid | 0.18/0.02 | 0.21/0.01 | 0.04/<0.01 | 1.1/0.38 |
| Bronchi | 0.59/0.28 | 0.19/0.16 | 0.06/0.04 | 6.67/7.76 |
| Breast EAR | 0.68/- | 0.55/- | 0.13/- | 13/- |
| Breast ERR | 0.40/- | 0.32/- | 0.07/- | 13/- |
| Esophagus | 0.05/0.06 | 0.02/0.06 | 0.01/0.01 | 0.24/0.75 |
| Lung | 1.16/0.33 | 0.59/0.23 | 0.16/0.05 | 6.67/7.75 |
| Rectosigmoid Wall | 0.62/0.40 | 0.36/0.38 | 0.10/0.01 | 5.38/5.67 |
| Uterus/Prostate | 0.06/0.06 | 0.036/0.05 | 0.01/0.01 | 2.5/16.01 |
| Ovaries/Testes | 0.13/0/04 | 0.074/0.04 | 0.02/0.01 | 1.42/0.37 |
| Bladder Wall | 0.29/0.21 | 0.14/0.13 | 0.04/0.03 | 1.25/3.98 |
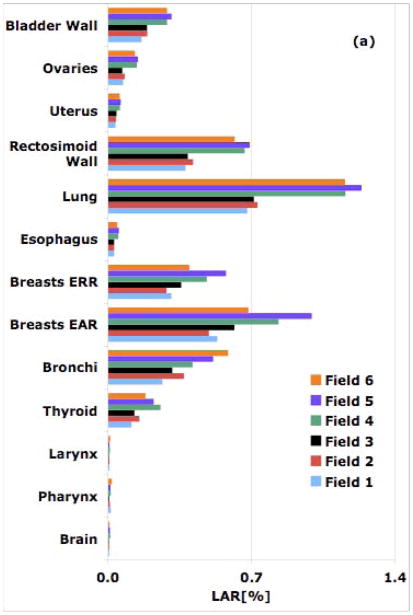
The rectosigmoid wall is found to have the highest risk in the 11 and the 14-year old male patients owing to small lateral distances from the field edge. In proton therapy, LARs for the rectosigmoid wall in an 11 and a 14-year old patient were found to be 0.5% and 0.4%, respectively. In IMRT, the corresponding risks are estimated to be 0.1% and 0.4%, respectively. On the other hand brain and pharynx, farther away from the field edge, receive higher doses from IMRT compared to those from proton therapy fields. Again, as in the case of cranial fields, proton therapy offers an advantage for organs distant to the target while closer to the field the risk due to scattered dose in IMRT seems to be lower. Consequently, the relative (protons versus IMRT) out-of-field risk shifts more in favor of protons as the patient age increases. Close to the target, the difference between the two modalities becomes smaller as the field size increases (Fig. 2). In passive scattered proton therapy, the second cancer incidence risk decreases as the field size increases [1,2]. In contrast, for IMRT, the largest field (9-cm diameter) for both shallow and deep-seated tumors results in the highest LAR values.
Figure 2.
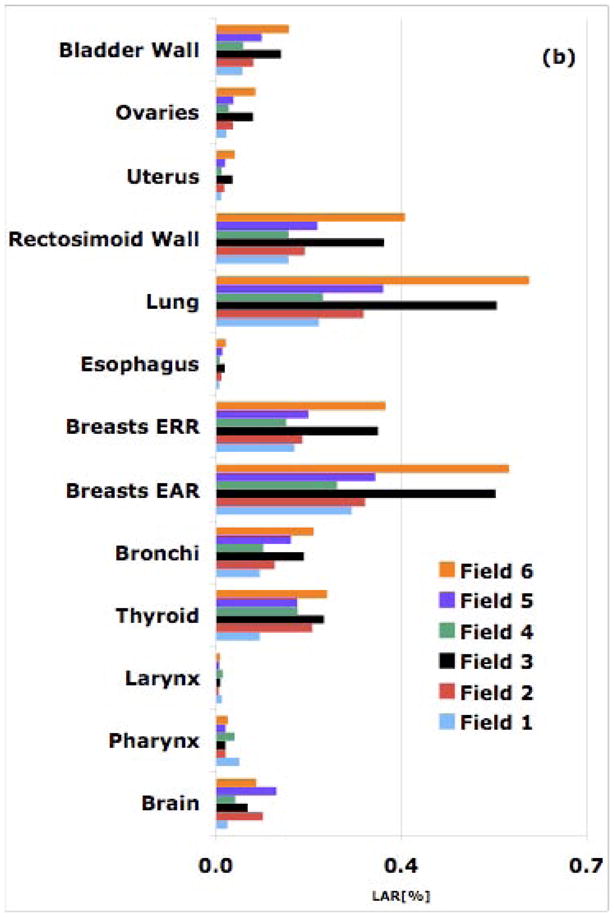
Simulated LAR [%] values from all 6 spine fields for an 8-year old patient for a 54-Gy treatment: (a) proton therapy; (b) IMRT.
Organs of particular concern are the lungs with large baseline risks for both males (6.7%) and females (7.8%). For spine fields lungs are close to the field edge. In passive scattered proton therapy the LAR value for lungs from field 6 varies from 2.0% to 0.3% as age progresses from 4 to 14 years. Similarly, for IMRT the LAR varies from 1.2% to 0.2%. For comparison, for a scanned beam the LAR for lungs varies from 0.41% to 0.05% for all pediatric scenarios.
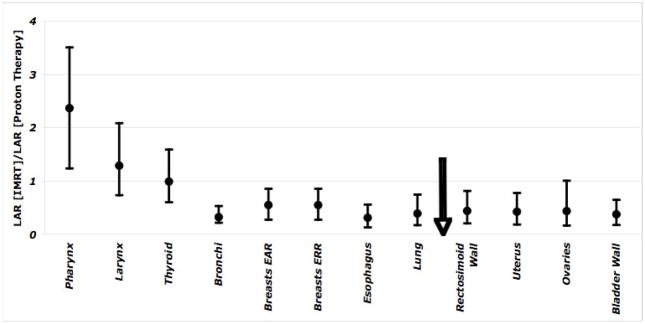
Fig. 3 shows the ratio of second cancer induction risks (averaged over six spinal fields) from IMRT and proton therapy averaged over six spinal fields for an 8-year old female patient. The arrow indicates the approximate location of the isocenter. Error bars show the variation of LARs for six spinal fields. Organs located closer to the field-edge, for example bladder wall to bronchi, show a higher cancer risk from proton therapy, relative to IMRT. But, organs located at large distances from the field-edge (e.g., thyroid, larynx, pharynx, and brain) show a considerably higher risk from IMRT relative to proton therapy.
Figure 3.
The ratio of the LAR values from IMRT and proton therapy assuming a 54-Gy (Gy[RBE]) treatment of a 8-year old female patient. Error bars show the range of ratios for six fields. The arrow shows the approximate location of the treatment field.
Uncertainties
There are considerable uncertainties in risk models and model parameters we adopted for this study. These uncertainties account for an error of ~50% according to a previous study [4]. Further, in proton therapy, the uncertainty in neutron weighting factors is at least 50% [3]. There are also statistical uncertainties in dose simulated for different organs, depending on the distance to the field. Organs located laterally away from the field-edge or have small volumes are associated with larger statistical errors. The estimated statistical errors in the secondary neutron equivalent doses are as much as 30% [2] and the uncertainties in the scattered doses from IMRT fields are up to 40% [20]. Most importantly, our comparison of IMRT and proton therapy is valid only for those fields considered in our analysis, which may not be representative for some treatment scenarios.
Conclusions
We have compared passive scattered proton therapy, scanned proton therapy, and 6-MV IMRT fields in terms of lifetime attributable risks for organs outside of the main radiation field for different patient ages and field sizes. We conclude that young patients show significantly higher risks than adults or patients of a large stature. Deep-seated tumors are associated with elevated risks relative to shallow treatment fields. In proton therapy we noticed a decrease in the second cancer risk as the field aperture is increased due to less material in the primary beam [1,2], whereas in IMRT fields risks increase with aperture size. Our results suggest that, in ideal proton scanning beam therapy (without significant amount of absorbers in the treatment head) the out-of-field second cancer risks will be much lower than those from IMRT or passive scattered proton therapy.
Protons offer a distinct advantage over IMRT within the main radiation field due to a much lower integral dose to the patient when compared with IMRT (roughly a factor of 2–3 [28]). In other words, much lower in-field doses are delivered to patients when using proton therapy. This is one of the reasons for the great success of proton therapy in pediatric radiation oncology. A lower integral dose should translate into a lower risk for a second malignancy although for intermediate to high dose levels the dose response relationship will no longer be linear.
In this study we have only considered out-of-field risks, which are most likely much lower than the in-field risks since most second malignancies are seen within the main irradiated area. Nevertheless, an analysis was warranted based on the continuing discussion in the radiation oncology field about the potential danger of scattered and secondary doses. It turns out that organs near the field-edge (but out of field) show higher LAR values from passive scattered proton therapy relative to similar IMRT fields. At roughly more than 25 cm laterally from the target, the risk from IMRT becomes higher than the risk from proton therapy. In both cranial and spine cases, the majority of second cancer risks estimated for various organs except for the thyroid, are found to be well below the baseline risks.
Other groups [14–16] have recently published second cancer risk comparisons between IMRT and passive scattered proton therapy. Due to differences in field sizes, treatment sites, and risk models we cannot directly compare our results. Fontenot et al [16] considered the colon and bladder inside the main treatment fields constituting 90% of the total risk. Neutron production in the treatment head is also facility dependent.
Acknowledgments
This work was supported by institutional funds (ECOR award by Partners Health Care Inc., Ira Spiro award for translational research by the Department of Radiation Oncology at Massachusetts General Hospital (MGH)) and a NIH/NCI grant (CO6-CA059267).
Footnotes
Conflicts of Interest Notification
There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Basit S. Athar, Email: Basit.Athar@hamptonproton.org, Department of Radiation Oncology, Massachusetts General Hospital & Harvard Medical School, Francis H Burr Proton Therapy Center, 55 Fruit Street, Boston MA 02114, Phone: 617-726-4490, Fax: 617-724-0368
Harald Paganetti, Email: hpaganetti@parteners.org, Department of Radiation Oncology, Massachusetts General Hospital & Harvard Medical School, Francis H Burr Proton Therapy Center, 55 Fruit Street, Boston MA 02114, Phone: 617-726-5847, Fax: 617-724-0368
References
- 1.Zacharatou Jarlskog C, et al. Assessment of organ specific neutron doses in proton therapy using whole-body age-dependent voxel phantoms. Phys Med Biol. 2008;53:693–717. doi: 10.1088/0031-9155/53/3/012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Athar SB, Paganetti H. Neutron equivalent doses and associated lifetime cancer incidence risks for head & neck and spinal proton therapy. Phys Med Biol. 2009;54:4907–4026. doi: 10.1088/0031-9155/54/16/005. [DOI] [PubMed] [Google Scholar]
- 3.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65:1–7. doi: 10.1016/j.ijrobp.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 4.BEIR VII. Health risks from exposure to low level of ionizing radiation. National Research Council, National Academy of Science; 2006. Biological Effects of Ionizing Radiation Committee. Report 2006. [Google Scholar]
- 5.Bassal M, Mertens AC, Taylor, et al. Risk of selected subsequent carcinomas in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:476–483. doi: 10.1200/JCO.2005.02.7235. [DOI] [PubMed] [Google Scholar]
- 6.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancers: Childhood Cancer Survivor Study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 7.Tubiana M. Can we reduce the incidence of second primary malignancies occurring afer radiotherapy? A critical review. Radiother Oncol. 2009;91:4–15. doi: 10.1016/j.radonc.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Hall EJ, Wuu CS. Radiation-induced second cancers: The impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56:83–88. doi: 10.1016/s0360-3016(03)00073-7. [DOI] [PubMed] [Google Scholar]
- 9.Hall EJ. Henry S Kaplan Distinguished Scientist Award 2003 the crooked shall be made straight; dose response relationships for carcinogenesis. Int J Radiat Biol. 2004;80:327–37. doi: 10.1080/09553000410001695895. [DOI] [PubMed] [Google Scholar]
- 10.Kry SF, et al. The calculated risk of the fatal secondary malignancies from the intentsity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2005;62:1105–1208. doi: 10.1016/j.ijrobp.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 11.Paganetti H, Bortfeld T, Delaney TF. Neutron dose in proton radiation therapy: In regard to Eric J.Hall (Int J Radiat Oncol Biol Phys 65 1–7) Int J Radiat Oncol Biol Phys. 2006;66:1594–1595. doi: 10.1016/j.ijrobp.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Zacharatou Jarlskog C, Paganetti H. Risk of Developing Second Cancer from Neutron Dose in Proton Therapy as a Function of Field Characteristics, Organ, and Pateint Age. Int J Radiat Oncol Biol Phys. 2008;72:228–235. doi: 10.1016/j.ijrobp.2008.04.069. [DOI] [PubMed] [Google Scholar]
- 13.Brenner DJ, Hall EJ. Secondary neutrons in clinical proton radiotherapy: A charged issue. Radiother Oncl. 2008;86:165–170. doi: 10.1016/j.radonc.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Newhauser WD, Fontenot JD, Mahajan A, Kornguth D, et al. The risk of developing a second cancer after receiving craniospinal proton irradiation. Phys Med Biol. 2009;54:2277–2291. doi: 10.1088/0031-9155/54/8/002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taddei PJ, Mirkovic D, Fontenot JD, Giebeler A, Zheng Y, et al. Stray radiation dose and second patient receiving craniospinal irradiation with proton beam. Phys Med Biol. 2009;54:2259–2275. doi: 10.1088/0031-9155/54/8/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontenot JD, Lee AK, Newhauser WD. Risk of secondary malignant neoplams from proton therapy and intensità-modulated X-ray therapy for early-stage prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:616–622. doi: 10.1016/j.ijrobp.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson VC, Mcdonough J, Tochner Z. Proton beam irradiation in pediatric oncology: an overview. J Ped Hemat Oncol. 2005;27:444–8. doi: 10.1097/01.mph.0000174030.55485.54. [DOI] [PubMed] [Google Scholar]
- 18.Kry SF, Salepour M, Titt U, White RA, Stovall M, Followill D. Monte Carlo study shows no significant difference in second cancer risk between 6- and 18-MV intensity-modulated radiation therapy. Radiother Oncol. 2009;91:132–7. doi: 10.1016/j.radonc.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Medin J, Andrea P. Monte Carlo calculated stopping-power ratios, water/air, for clinical proton dosimetry (50–250MeV) Phys Med Biol. 1997;42:89–105. doi: 10.1088/0031-9155/42/1/006. [DOI] [PubMed] [Google Scholar]
- 20.Athar SB, Bednarz B, Seco J, Hancox C, Paganetti H. Comparison of out-of-field photon doses in 6-MV IMRT and neutron doses in proton therapy for adult and pediatric patients. Phys Med Biol. 2009;55:2879–2891. doi: 10.1088/0031-9155/55/10/006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu XG, Chao TC, Bozkurt A. VIP-MAN: an image-based whole-body adult male model constructed from color photographs of the Visible Human Project for multi-particle Monte Carlo calculations. Health Phys. 2000;78:476–85. doi: 10.1097/00004032-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Lee C, Bolch W. Construction of a tomographic computational model of a 9-mo-old and its Monte Carlo calculation time comparison between the MCNP4C and MCNPX codes. Health Phys. 2003;84:S259. [Google Scholar]
- 23.Lee C, Williams JL, Lee C, Bolch WE. The UF series of tomographic computational phantoms of pediatric patients. Med Phys. 2005;32:3537–48. doi: 10.1118/1.2107067. [DOI] [PubMed] [Google Scholar]
- 24.ICRP. International Commission on Radiological Protection. Oxford; Pergamon: ICRP Publication; 2003. Relative Biological Effectiveness (RBE), QualityFactor (Q), and Radiation Weighting Factor (wR) p. 92. [DOI] [PubMed] [Google Scholar]
- 25.Anderson RN, DeTurk PB. United States Life Tables. National Vital Statistics Reports. 2002;50:1–12. [PubMed] [Google Scholar]
- 26.SEER. Cancer Statistics Review 1975–2005. http://seer.cancer.gov/csr/1975-2005.
- 27.Kocher DC, Apostoei AI, Hoffman FO. Radiation effectiveness factors for use in calculating probability of causation of radiogenic cancers. Health Phys. 2005:3–32. doi: 10.1097/01.hp.0000154172.48895.45. [DOI] [PubMed] [Google Scholar]
- 28.ICRU, Prescribing, Recording, and Reporting Proton-Beam Therapy. J of the ICRU, Report. 2007:78. [Google Scholar]