Abstract
Poly(A) tail removal is often the initial and rate-limiting step in mRNA decay and is also responsible for translational silencing of maternal mRNAs during oocyte maturation and early development. Here we report that deadenylation in HeLa cell extracts and by a purified mammalian poly(A)-specific exoribonuclease, PARN (previously designated deadenylating nuclease, DAN), is stimulated by the presence of an m7-guanosine cap on substrate RNAs. Known cap-binding proteins, such as eIF4E and the nuclear cap-binding complex, are not detectable in the enzyme preparation, and PARN itself binds to m7GTP–Sepharose and is eluted specifically with the cap analog m7GTP. Xenopus PARN is known to catalyze mRNA deadenylation during oocyte maturation. The enzyme is depleted from oocyte extract with m7GTP–Sepharose, can be photocross-linked to the m7GpppG cap and deadenylates m7GpppG-capped RNAs more efficiently than ApppG-capped RNAs both in vitro and in vivo. These data provide additional evidence that PARN is responsible for deadenylation during oocyte maturation and suggest that interactions between 5′ cap and 3′ poly(A) tail may integrate translational efficiency with mRNA stability.
Keywords: cap structure/deadenylation/mRNA stability/oocyte maturation/poly(A) tails
Introduction
Deadenylation, i.e. the exonucleolytic degradation of the 3′ poly(A) tracts of eukaryotic mRNAs, plays an important role in both mRNA degradation and translational silencing. In Saccharomyces cerevisiae, two general pathways of mRNA decay have been characterized, both of which are initiated by poly(A) removal. In the deadenylation-dependent decapping pathway, removal of the 5′ cap occurs rapidly after the poly(A) tail has been shortened to ∼10 nucleotides. Decapping is then followed by 5′ exonucleolytic degradation of the remaining mRNA ‘body’ (Muhlrad et al., 1994; Beelman and Parker, 1995; Caponigro and Parker, 1996). In a second pathway, the 5′ cap is not removed, and 3′-exonucleolytic decay continues after poly(A) tail removal (Jacobs Anderson and Parker, 1998). In higher eukaryotes, the decay of many mRNAs is also initiated by deadenylation (Wilson and Treisman, 1988; Shyu et al., 1991; Chen and Shyu, 1995; Ross, 1995), but the subsequent degradation events remain undefined. The conservation of several genes encoding RNA turnover components (Bashkirov et al., 1997; Mitchell et al., 1997; Tharun and Parker, 1999) and additional indirect evidence (Couttet et al., 1997) suggest that the two pathways analyzed in yeast also exist in mammalian cells, but direct evidence is lacking, and their relative contributions to the degradation of specific mRNAs are unknown. In addition to the deadenylation-dependent pathways, mRNA decay can also be initiated by endonucleolytic cleavage within the 3′-untranslated region (3′-UTR) (Ross, 1995; Chernokalskaya et al., 1998).
Poly(A) removal not only initiates mRNA turnover in yeast and in somatic metazoan cells, but is also used to silence maternal mRNAs translationally during oocyte maturation and embryogenesis in diverse species (Richter, 1996). During oocyte maturation, deadenylation does not require specific cis-elements and is a default pathway for mRNAs that do not undergo compensatory poly(A) elongation (Fox and Wickens, 1990; Varnum and Wormington, 1990). In contrast, certain mRNAs which are polyadenylated during meiotic maturation contain 3′-UTR elements that promote their subsequent deadenylation after fertilization. Thus, the translation of these mRNAs is restricted to mature oocytes (Bouvet et al., 1994; Legagneux et al., 1995). In both cases, deadenylation does not destabilize mRNAs immediately, but is a pre- requisite for their degradation at later stages of development (Audic et al., 1997; Gillian-Daniel et al., 1998; Voeltz and Steitz, 1998). The uncoupling of deadenylation from mRNA decay in gametes and embryos contrasts with both yeast and metazoan cells in which poly(A) removal rapidly promotes mRNA degradation.
Both the dependence of decapping on prior deadenylation and the inhibition of translation initiation by poly(A) removal reflect interactions between the 5′ cap and the 3′ poly(A) tail of the mRNA. An increasing number of such interactions have been described recently (Wickens et al., 1997). The deadenylation dependence of RNA decapping is due to the ability of the poly(A)-binding protein 1 (Pab1p in yeast or PABP1 in metazoans) to inhibit decapping by an unknown mechanism (Muhlrad et al., 1994; Caponigro and Parker, 1995; Coller et al., 1998). Deadenylation removes the PABP1-binding sites and thus relieves the inhibition of decapping. Deadenylation concomitantly inhibits translation initiation presumably due to the involvement of Pab1p in ribosome loading at the 5′ end. The central player in translation initiation is the eIF4G subunit of the multimeric eIF4F complex. 40S ribosomal subunit recruitment is facilitated by the synergistic binding of eIF4G to both the cap–eIF4E complex and the poly(A)–Pab1p complex, leading to juxtaposition of both ends of the mRNA into a ‘closed loop’ (Jacobson, 1996; Tarun and Sachs, 1996; Tarun et al., 1997; Imataka et al., 1998; Piron et al., 1998; Preiss and Hentze, 1998; Wells et al., 1998; De Gregorio et al., 1999; Otero et al., 1999).
As cytoplasmic, translationally active mRNA is the substrate for degradation, and the two main targets of the degradation pathways, the 5′ cap and 3′ poly(A) tail, are also determinants of efficient translation initiation, translation and mRNA turnover would be expected to be integrated. Indeed, the most compelling example illustrating this linkage is the nonsense-mediated decay pathway, which leads to premature degradation of mRNAs containing inappropriate nonsense codons within their coding sequences (Jacobson and Peltz, 1996; Ruiz-Echevarria et al., 1996). In yeast, these RNAs undergo deadenylation-independent decapping (Muhlrad and Parker, 1994).
The 3′ exonucleases responsible for mRNA deadenylation in vivo remain largely unidentified. In S.cerevisiae, one deadenylase, PAN, has been characterized. Poly(A) shortening by PAN is involved in specifying nuclear poly(A) tail length (Brown and Sachs, 1998). The phenotype of PAN mutants indicates that a role for PAN in cytoplasmic deadenylation, if any, cannot be exclusive; additional enzymes must contribute to cytoplasmic poly(A) removal (Boeck et al., 1996; Brown et al., 1996). In vertebrates, one poly(A)-specific 3′ exonuclease has been purified and cloned. This enzyme, initially described as the deadenylating nuclease, DAN (Körner and Wahle, 1997; Körner et al., 1998), and subsequently renamed poly(A)-specific ribonuclease, PARN (Körner et al., 1998; Buiting et al., 1999), catalyzes deadenylation during the meiotic maturation of Xenopus oocytes (Körner et al., 1998). It is not known whether PARN is involved in mRNA deadenylation in somatic cells. The identity of another poly(A)-specific 3′ exonuclease (Astrom et al., 1991, 1992) and its relationship to PARN remain to be determined.
Here we report that deadenylation by PARN is stimulated by the presence of an N7-methyl guanosine cap. This cap dependence was found in cytoplasmic extracts from both HeLa cells and Xenopus oocytes, with purified bovine PARN and in Xenopus oocytes in vivo. Recognition of the cap appears to be an intrinsic property of PARN itself.
Results
The 7-methyl guanosine cap stimulates deadenylation in vitro and in vivo
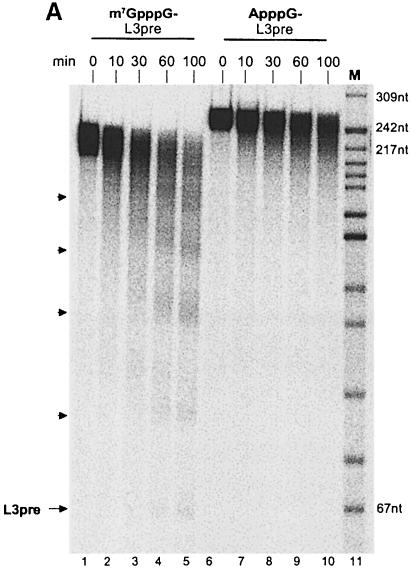
In agreement with previous results, a polyadenylated RNA with a 7-methyl guanosine cap (m7GpppG) at its 5′ end was deadenylated upon incubation in a HeLa cell cytoplasmic extract. The characteristic pattern of decay intermediates differing in length by ∼30 nucleotides has been described previously and is caused by partial protection of the poly(A) tail through the binding of PABP1 (Körner and Wahle, 1997). A small amount of completely deadenylated RNA accumulated upon longer incubation times (Figure 1A). In contrast, ApppG-capped substrate RNA generated neither the periodic pattern of decay intermediates nor an accumulation of fully deadenylated RNA (Figure 1A). Also, the full-length polyadenylated substrate was lost at a 2-fold lower rate (Figure 1A; quantitation not shown). These results suggested not only that RNA decay was retarded in the absence of a bona fide cap structure, but that the majority of ApppG-capped RNA was degraded by a different pathway. RNA lacking any cap was degraded at a rate similar to m7GpppG-capped substrate RNA, but again the partially deadenylated intermediates characteristic for deadenylation by PARN were not detected (data not shown).
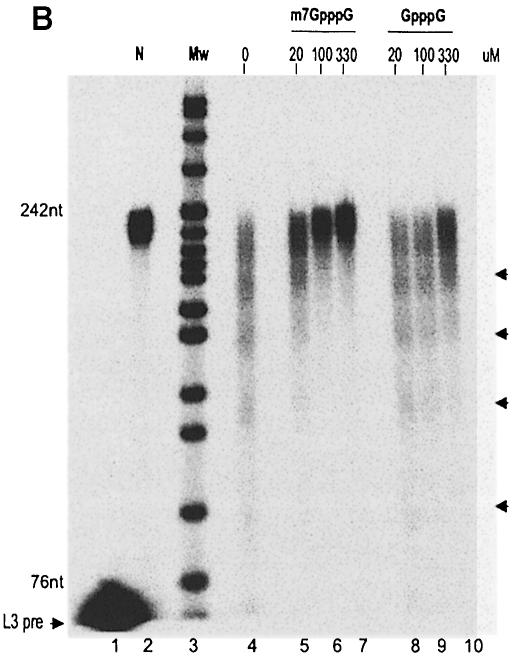
Fig. 1. (A) Cap-dependent deadenylation in a HeLa cytoplasmic extract. Two reaction mixtures were assembled on ice with 36 fmol of either m7GpppG-capped or ApppG-capped, polyadenylated L3 pre-RNA in 70 μl of reaction buffer. The reaction was started by the addition of 1.7 μl of a HeLa cell cytoplasmic extract and incubation at 37°C. Aliquots were taken at the times indicated and RNAs analyzed on an 8% denaturing polyacrylamide gel. Substrate, decay products and completely deadenylated L3 pre-RNA are indicated by arrowheads. Sizes (in nucleotides) of the DNA marker are shown on the right. (B) Competition with free cap in HeLa cell cytoplasmic extract. The 20 μl reaction mixtures were assembled on ice with 10 fmol of m7G-capped and polyadenylated (A160) L3 pre-RNA, 0.5 μl of HeLa cell cytoplasmic extract and 0, 20, 100 or 330 μM m7GpppG or GpppG. The deadenylation reaction was carried out at 37°C for 80 min. Products were separated on an 8% denaturing polyacrylamide gel and analyzed on a PhosphorImager. Lane 1, substrate RNA lacking a poly(A) tail; lane 2, polyadenylated substrate RNA without incubation; lane 3, DNA size marker; the sizes of two fragments are indicated on the left. Deadenylation intermediates are indicated by arrowheads on the right.
No difference in deadenylation efficiencies was observed between RNA substrates synthesized with either m7GpppG or non-methylated GpppG caps. As this lack of distinction may reflect cap methylation in the extract, a requirement for the 7-methyl group was tested further by inhibition experiments: free m7GpppG inhibited deadenylation at the lowest concentration tested, 20 μM, whereas a 5- to 10-fold higher concentration of unmethylated GpppG was required to achieve a similar effect (Figure 1B).
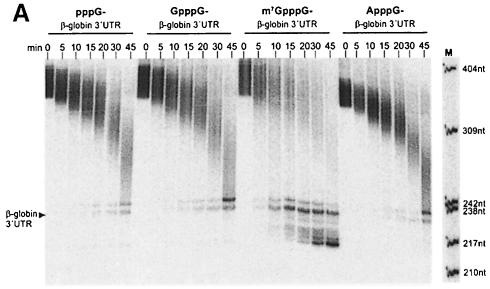
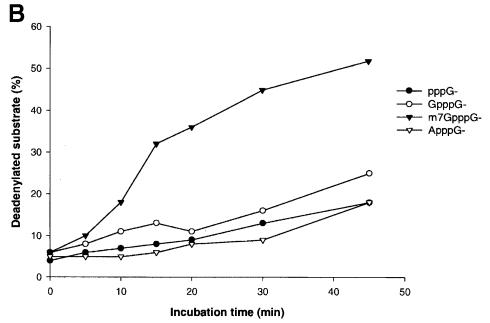
As PARN is the nuclease responsible for poly(A) shortening in HeLa cell cytoplasmic extracts (Körner and Wahle, 1997), the cap dependence of deadenylation catalyzed by purified PARN was examined. The calf thymus PARN preparation described earlier (Körner and Wahle, 1997) exhibited cap-dependent deadenylation activity with two different substrate RNAs tested (Figure 2; data not shown). m7GpppG-capped RNA directed a 4-fold higher rate of product accumulation compared with GpppG-capped RNA in reactions containing purified PARN (Figure 2). Interestingly, PARN was also able to invade the non-adenylate portion of the m7GpppG-capped RNA, which was resistant to degradation in GpppG-capped RNA substrates (Figure 2A). Presumably, this reflects a higher affinity of the enzyme for the m7GpppG-capped RNA rather than a reduced specificity for poly(A). Substrates that either contained an ApppG cap or lacked a 5′ cap entirely were deadenylated at the same rate as GpppG-capped substrates.
Fig. 2. Purified PARN prefers correctly capped RNA. (A) The 50 μl reaction mixtures were assembled on ice containing 15 fmol of bovine PARN and 40 fmol of polyadenylated (A130) β-globin 3′-UTR RNA with an m7GpppG, GpppG, ApppG dinucleotide or pppG at the 5′ end of the transcript. The reactions were started by the addition of enzyme and incubation at 37°C. Then 7 μl aliquots were withdrawn at the time points indicated in the figure, and decay products were separated on a 6% denaturing polyacrylamide gel and analyzed on a PhosphorImager. Fully deadenylated β-globin RNA is indicated by an arrowhead, and the sizes of the DNA marker are shown at the side of the M lane. (B) Quantitation of the accumulation of fully deadenylated RNA in (A). The appearance of deadenylated substrate at each time point, as part of the total radioactivity in each lane, was quantitated on a PhosphorImager and plotted versus incubation time.
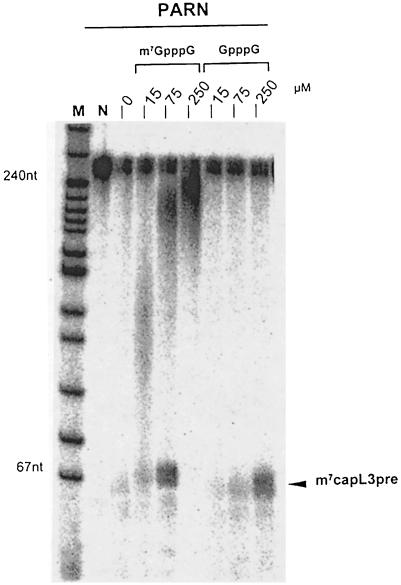
When purified PARN was assayed by the release of acid-soluble nucleotides from a radiolabeled poly(A) tail, the m7GpppG-capped RNA was also deadenylated at a 4-fold higher rate relative to RNA containing an unmethylated cap (data not shown). Purified PARN was also inhibited by free cap, the methylated form again being more efficient (Figure 3). In the reaction lacking free cap, the amount of PARN used was sufficient to deadenylate all of the accessible substrate and degrade most of it further; only a small amount of completely deadenylated RNA remained, in addition to a completely resistant fraction. With increasing inhibition by m7GpppG, both the completely deadenylated RNA and partially deadenylated intermediates became visible. At the highest concentration of m7GpppG, little deadenylation was observed. In contrast, 250 μM unmethylated GpppG was less inhibitory than 15 μM m7GpppG (Figure 3).
Fig. 3. Inhibition of purified PARN by free m7GpppG. A reaction mixture was assembled on ice containing 95 fmol of m7G-capped and polyadenylated (A160) L3 pre-RNA and 60 fmol of bovine PARN in 160 μl of reaction buffer. The mix was divided into 20 μl aliquots and m7GpppG or GpppG was added at the concentrations indicated. The reactions were started by incubation at 37°C. After 90 min, they were stopped and products analyzed on a denaturing 8% polyacrylamide gel. The sizes of DNA markers are indicated. The position of fully deadenylated L3 pre-RNA (m7capL3pre) is indicated by an arrowhead. Note that the amount of PARN used was sufficient to degrade most of the body of the RNA; fully deadenylated RNA became visible upon partial inhibition by intermediate concentrations of free cap (see the text).
It was demonstrated previously that anti-PARN antibody microinjected into Xenopus oocytes inhibits default deadenylation during meiotic maturation and that human PARN can substitute for the amphibian nuclease that catalyzes this reaction in vivo (Körner et al., 1998). In order to determine the relevance of the 5′ cap for deadenylation in vivo and at the same time provide an additional test for the role of Xenopus PARN in default deadenylation, we examined the cap dependence of default deadenylation in vivo and of poly(A) removal directed by the Xenopus PARN in vitro.
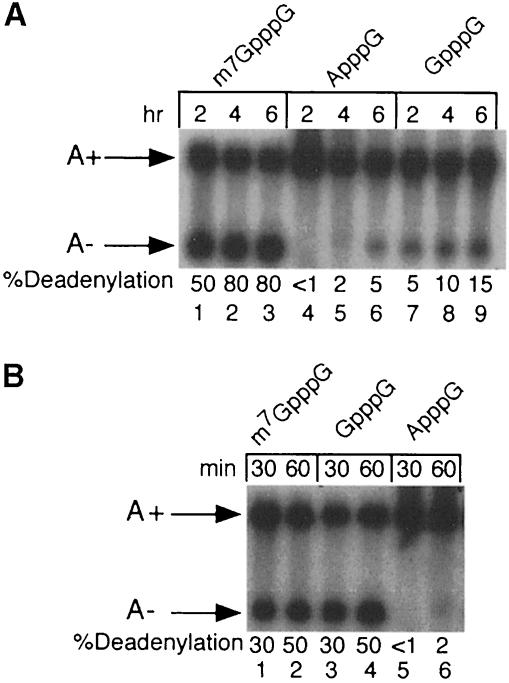
Radiolabeled substrate RNAs containing 5′ m7GpppG, GpppG or ApppG caps were microinjected into progesterone-matured oocytes and assayed for deadenylation. ApppG was used to generate a stable ‘uncapped’ RNA as transcripts containing free 5′-terminal phosphates are degraded rapidly in microinjected oocytes (Drummond et al., 1985). As shown in Figure 4A, >50% of m7GpppG -capped RNA was deadenylated 2 h after injection and >80% was deadenylated by 6 h. In contrast, only 15% of the non-methylated GpppG-capped RNA was deadenylated 6 h after injection and <5% of RNA containing an ApppG 5′ terminus was deadenylated. Thus, the presence of an m7GpppG cap significantly stimulates default deadenylation in mature oocytes.
Fig. 4. Cap-dependent deadenylation by Xenopus PARN. (A) In vivo deadenylation in mature oocytes. Gel-purified, radiolabeled G52 RNA containing 5′-terminal m7GpppG (lanes 1–3), ApppG (lanes 4–6) or GpppG (lanes 7–9) structures was injected into progesterone-matured Xenopus oocytes. RNA was isolated from oocytes 2, 4 or 6 h post-injection and analyzed by electrophoresis in 6% polyacrylamide–7 M urea gels. (B) In vitro deadenylation in Xenopus oocyte extracts. Oocyte extracts were incubated at 25°C for the indicated times with gel-purified, radiolabeled G52 RNA containing 5′-terminal m7GpppG (lanes 1 and 2), GpppG (lanes 3 and 4) or ApppG (lanes 5 and 6) structures.
As seen with mammalian extracts, deadenylation in extracts derived from mature Xenopus oocytes was also cap dependent (Figure 4B). RNAs containing either methylated m7GpppG- or non-methylated GpppG-capped RNAs were deadenylated with comparable efficiencies. This lack of distinction was not due to the methylation of GpppG-capped RNAs in these extracts (data not shown). However, a substrate RNA containing the non-physiological ApppG cap was 20-fold less efficient as an in vitro deadenylation substrate. We conclude that both mammalian and amphibian PARNs exhibit a marked dependence on the 5′ cap, with most experiments showing a preference for a bona fide m7GpppG cap structure.
PARN is a cap-binding protein
The cap dependence of deadenylation in both cell-free extracts and in Xenopus oocytes in vivo could reflect either an intrinsic cap-binding activity of PARN or a requirement for cap-binding proteins as cofactors for this nuclease. Neither of two characterized cap-binding proteins, eIF4E (Gingras et al., 1999) or the 20 kDa subunit of the nuclear cap-binding complex (Izaurralde et al., 1994), were detectable by immunoblot analysis of purified bovine PARN. Less than 1 pmol of eIF4E was present for 7 pmol of PARN, as determined by comparison with a purified eIF4E standard. No standard was available for the 20 kDa subunit of the nuclear cap-binding complex, but strong signals were easily obtained from nuclear extract whereas the protein was not detectable in the purified nuclease (data not shown).
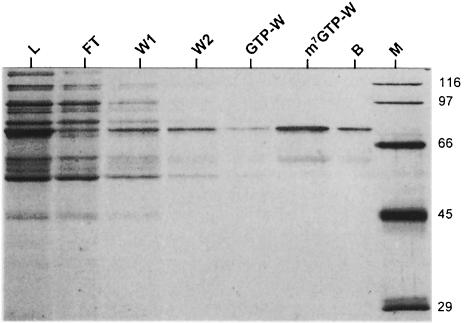
In order to determine whether PARN itself has intrinsic cap-binding activity, the ability of partially purified PARN to bind m7GTP–Sepharose was assayed. A significant and selective depletion of PARN was evident by a comparison of the loaded and the supernatant fractions after incubation with m7GTP–Sepharose (Figure 5). A small amount of PARN was released from the m7GTP resin with buffer containing no nucleotide, and negligible PARN was released by a subsequent wash with buffer containing GTP. In contrast, most of the enzyme was eluted with m7GTP and some remaining protein with SDS (Figure 5). If binding to the beads had been mediated by an associated cap-binding factor, stoichiometric amounts of this putative protein should have been present in the eluate. This was not the case. Only a small amount of a 60 kDa protein was detectable. In the PARN preparation used for this experiment, a 60 kDa polypeptide reacted with anti-PARN antibody in a Western blot. Thus, the only other protein showing specific binding to the m7GTP resin was probably a proteolytic fragment of PARN. Interestingly, deadenylation catalyzed by bacterially expressed recombinant PARN was not cap dependent, and binding of recombinant PARN to m7GTP or m7GpppG was much weaker by several criteria, suggesting that post-translational modifications may be required for cap recognition by this enzyme (data not shown).
Fig. 5. Binding of bovine PARN to m7GTP–Sepharose. The experiment was carried out with a partially purified fraction of PARN as described in Materials and methods. Lane L (load), starting material; lane FT (flow-through), unbound proteins in the supernatant; lanes W1 and W2, washes with nucleotide-free buffer; lane GTP-W, GTP wash; lane m7GTP-W, m7GTP wash; lane B, beads; lane M, molecular weight markers. Proteins were detected by silver staining. Note that only 50% of the total sample was loaded in lanes L and FT, whereas the entire samples were loaded in the other lanes.
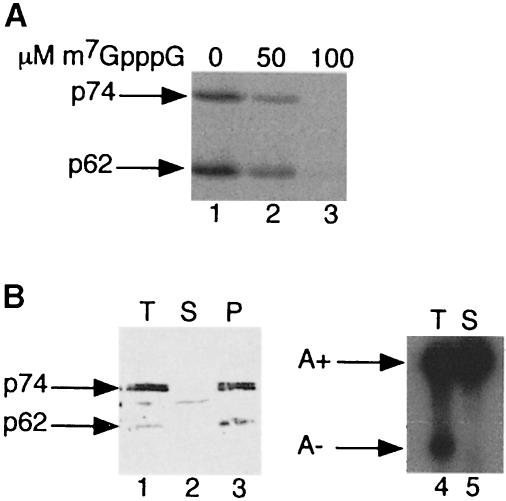
The ability of Xenopus PARN present in extracts derived from mature Xenopus oocytes to bind the 5′ cap was also tested. Xenopus oocytes contain two isoforms of PARN: a 74 kDa species that initially is localized to the oocyte nucleus and catalyzes default deadenylation at maturation and a 62 kDa protein that is exclusively cytoplasmic (Körner et al., 1998). Both polypeptides could be cross-linked photochemically to RNA containing radioactive label exclusively in the m7GpppG cap (Figure 6A). This cap binding was specific as it was competed by the presence of free m7GpppG. Both p74 and p62 PARN isoforms could be depleted quantitatively from oocyte extracts with m7GTP–Sepharose (Figure 6B, lanes 1–3). This depletion correlated with the loss of default deadenylation activity in vitro (Figure 6B, lanes 4 and 5). We conclude that both amphibian and mammalian PARNs not only exhibit a strong cap dependence in catalyzing deadenylation, but that cap binding is an inherent and evolutionarily conserved property of this enzyme.
Fig. 6. Binding of Xenopus PARN to m7GpppG cap. (A) Photochemical cross-linking of Xenopus p74 and p62 PARN isoforms to [32P]cap-labeled RNA. Total mature oocyte extract was incubated with 20 fmol of G52 RNA containing a radiolabeled m7GpppG cap in the presence of 0, 50 or 100 μM m7GpppG. After UV irradation and digestion with RNase A, samples were immuno- precipitated with anti-PARN 205-5 antibody and analyzed by 10% SDS–PAGE. (B) m7GTP–Sepharose depletion of Xenopus PARN. Total oocyte extract was incubated with m7GTP–Sepharose for 2 h at 4°C. Fractions corresponding to two oocyte equivalents of total (T), depleted supernatant (S) and bound (P) protein eluted with 0.5 mM m7GpppG were analyzed by immunoblot probed with anti-PARN 205-5 antibody (lanes 1–3). In addition, total (T) and m7GTP–Sepharose-depleted extract was analyzed for deadenylation activity by incubation for 1 h at 25°C with radiolabeled m7GpppG-capped G52 substrate RNA.
Discussion
Here we have presented evidence that PARN-dependent deadenylation of mRNA in vivo and in vitro is stimulated by a 5′ cap structure. PARN itself is responsible for the cap dependence of deadenylation by binding to m7GpppG. While most experiments revealed a preference for the genuine methylated cap structure, this was not seen in Xenopus oocyte extracts. A possible explanation might be that m7GpppG-capped RNA is a better substrate for PARN but at the same time is protected more efficiently by other cap-binding proteins. Our results suggest that the ‘closed loop’ configuration in which the 5′ cap and the 3′ poly(A) tail are juxtaposed is the optimal substrate not only for the assembly of a translation initiation complex but also for deadenylation.
In HeLa cell extracts, capped RNA was deadenylated with the previously described pattern of intermediates caused by partial protection through PABP1 binding (Körner and Wahle, 1997), whereas uncapped RNA or RNA containing a non-physiological cap did not show these decay intermediates. This difference might be caused by an increased affinity of PABP1 for the m7GpppG-capped RNA owing to its indirect interaction with the cap mediated by eIF4E and eIF4G. In this case, one would expect the uncapped RNA, being protected less stringently by PABP1, to be deadenylated faster than the capped RNA. However, uncapped and m7GpppG-capped RNAs exhibited comparable stabilities in HeLa cell extracts, and ApppG-capped RNA was, in fact, the most stable. In Xenopus oocyte extracts, the ApppG cap had an even stronger stabilizing effect, and deadenylation intermediates were not detected with m7GpppG-capped RNAs, presumably owing to the limited amount of PABP1 present (Wormington et al., 1996). Thus, differences in protection by PABP1 are unlikely to account for the differences in decay. Instead, m7GpppG-capped RNA appeared to decay mainly through deadenylation by PARN, whereas uncapped or non-physiologically capped RNA was degraded primarily through other pathways without detectable intermediates. Experiments with purified PARN confirmed that deadenylation is indeed cap dependent.
The pattern of deadenylation observed here with capped, polyadenylated substrate RNAs might be considered evidence for a processive reaction mechanism, as full-length substrate and completely deadenylated product co-existed. However, the substrate RNAs are likely to be heterogeneous due to incomplete capping, partially blocked 3′ ends (Körner and Wahle, 1997) and, possibly, conformational heterogeneity. Thus, conclusions regarding the processivity of PARN should not be drawn from the experiments presented here. When homopolymeric poly(A) containing a 5′ monophosphate is used as the substrate, PARN acts distributively (Körner and Wahle, 1997). Additional experiments are required to determine whether this distributive behavior is altered by the interaction of PARN with the cap or with other non-adenylate residues within the substrate.
Cap-dependent deadenylation does not appear to require either of the two known cap-binding proteins, eIF4E or the nuclear cap-binding complex. The sequence of human PARN contains a possible match (amino acids 56–72) to the eIF4E-binding site of eIF4G (Morley et al., 1997), although the invariant tyrosine required for the interaction with eIF4E (Marcotrigiano et al., 1999) is not present. Immunoblot analysis of purified PARN did not reveal the presence of eIF4E, and no detectable eIF4E co-purified with PARN on m7GTP–Sepharose. Also, the addition of recombinant eIF4E did not enhance the cap dependence of PARN activity (C.G.Körner, unpublished data). Recombinant eIF4E did not inhibit PARN, as would be expected should these two proteins compete directly for cap binding. It is possible that in the absence of eIF4G (Haghigat and Sonenberg, 1997; Ptushkina et al., 1998) and phosphoryl– ation (Minich et al., 1994), the cap-binding affinity of eIF4E is so weak that it cannot compete with PARN.
The two primary targets of mRNA decay, the 5′ cap and 3′ poly(A) tail, also play crucial roles in translation initiation by mediating multiple interactions through their respective binding proteins, eIF4E and PABP1. Thus, one would expect actively translated mRNAs to be stabilized via protection of both targets. A translation-dependent protection of the cap by Pab1p has in fact been demonstrated (Caponigro and Parker, 1995; Wickens et al., 1997), although it is unclear how precisely the protection is mediated (Schwartz and Parker, 1999). Similarly, one would expect translation to protect mRNA against the attack of PARN, by preventing the enyzme's access to the cap and by stabilizing the binding of PABP1, which is inhibitory to PARN under physiological conditions (Wormington et al., 1996; Körner and Wahle, 1997). As the cap accelerates deadenylation but is not absolutely required, binding of the translation initiation complex may be expected to modulate the rate of deadenylation and, thus, mRNA stability. In this respect, it is interesting that destabilizing AU-rich elements in the 3′-UTR of unstable mRNAs, which are known to stimulate deadenylation, have been shown to repress translation (Kruys et al., 1989). A number of additional experiments, primarily in yeast, support the idea of a stabilizing influence of translation (Muhlrad et al., 1995; Muckenthaler et al., 1997; LaGrandeur and Parker, 1999; Schwartz and Parker, 1999). However, as the yeast genome contains no obvious homolog of PARN, it remains to be determined whether cap-dependent deadenylation exists in this organism and contributes to the translation dependence of mRNA stability. Even in mammalian cells, a role for PARN in cytoplasmic mRNA decay has not been demonstrated.
It should be noted that translation-mediated mRNA stabilization is not supported by all experiments. Inhibition of translation by insertion of a stable stem–loop structure into the 5′-UTR of mammalian mRNAs often resulted in the stabilization of an unstable mRNA, although this effect has not been observed uniformly (Jacobson and Peltz, 1996). The reason for these apparent discrepancies is unknown. However, one has to consider the possibility that the insertion of a stable RNA secondary structure in the 5′-UTR may not only inhibit translation but may also affect the extent to which the cap is protected by the translation initiation complex. A stable RNA structure inserted close to the cap might even directly inhibit PARN independently of its influence on translation.
The data presented here are consistent with the idea that the translational status of an mRNA is a major factor in determining its half-life (Schwartz and Parker, 1999). The influence of translation and in particular of the initiation complex on deadenylation has to be tested directly. It will be of particular interest to determine whether destabilizing elements present within the 3′-UTR of mRNAs work by directly recruiting one or more of the enzymes catalyzing mRNA decay or, alternatively, accelerate decay as an indirect consequence of translational repression.
Materials and methods
RNA
pSP6L3pre and pSP6glob (Körner and Wahle, 1997) were linearized with RsaI or EcoRI, respectively, and transcribed in vitro by SP6 RNA polymerase (Boehringer) in the presence of 50 μCi of [α-32P]UTP, 600 μM GpppG or m7GpppG (New England Biolabs), 100 μM GTP and UTP, 500 μM CTP and ATP. Polyadenylated transcripts were prepared as described (Körner and Wahle, 1997). Non-radioactive transcripts with homogeneously labeled poly(A) tails, used for the trichloroacetic acid (TCA) precipitation assay, were made with 125-fold molar excess of ATP over transcript and 50 μCi of [α-32P]ATP. Capped and polyadenylated G52 RNAs (Varnum and Wormington, 1990) were synthesized with SP6 RNA polymerase and uniformly labeled with [α-32P]UTP as described above except that reactions contained 50 μM GTP and 1 mM m7GpppG, GpppG or ApppG. Unlabeled G52 RNA was synthesized as described (Wormington, 1991). A 5 pmol aliquot of gel-purified G52 RNA was incubated for 2 h at 37°C in a 20 μl reaction containing: 5 U of guanylyltransferase, 50 μCi of [α-32P]GTP (800 Ci/mmol), 0.5 mM S-adenosyl-l-methionine, 50 mM Tris–HCl pH 7.9, 2 mM MgCl2, 0.1 mM EDTA, 5 mM KCl, 2.5 mM dithiothreitol (DTT), 40 U of RNasin and 0.1 U of pyrophosphatase. RNAs typically were labeled to a specific activity of 2 × 103 d.p.m./fmol. Incorporation of the methylated cap was confirmed by thin-layer chromatography of nuclease P1 digestion products on polyethyleneimine–cellulose plates developed with saturated ammonium sulfate/1 M NaOAc/isopropanol (81:18:2) using m7GpppG and GpppG as standards detected by UV shadowing. All RNAs were gel purified prior to use.
Proteins
PARN was the preparation described previously (Körner and Wahle, 1997). Antibodies to eIF4E and recombinant eIF4E were gifts from Martina Muckenthaler and Matthias Hentze, EMBL, Heidelberg. Antibodies to the nuclear cap-binding complex were a gift from Iain Mattaj, EMBL, Heidelberg.
Deadenylation and photochemical cross-linking assays
Deadenylation assays were performed as described (Körner and Wahle, 1997) with the substrate and enzyme concentrations indicated in the figure legends. The reaction buffers contained 100 mM KCl for assays with purified enzyme and 120 mM potassium acetate for assays with HeLa cytoplasmic extract. Extracts derived from progesterone-matured Xenopus oocytes were prepared as described (Varnum et al., 1992). In brief, 100 mature oocytes were homogenized by repeated pipeting in 100 μl of buffer containing: 10% (v/v) glycerol, 10 mM HEPES–KOH pH 7.2, 70 mM KCl, 1 mM MgCl2, 2 mM DTT, 0.1 mM EDTA. Homogenates were centrifuged at 14 000 r.p.m. for 5 min, to remove yolk platelets and pigment granules. Deadenylation reactions containing 10 μl of clarified supernatant and 5 fmol of uniformly labeled G52 RNA were performed as described (Wormington et al., 1996). Reaction products were electrophoresed on 6% polyacrylamide–7 M urea gels, visualized and quantified using a Molecular Dynamics PhosphorImager. Photochemical cross-linking reactions contained 10 μl of clarified supernatant, 5 fmol of [32P]cap-labeled G52 RNA and 0, 50 or 100 μM unlabeled m7GpppG competitor. After incubation for 10 min at 25°C, reactions were transferred to ice and UV irradiated for 10 min in a Stratalinker 1800. Samples were digested with 40 μg of RNase A for 30 min at 37°C, immunoprecipitated with anti-PARN 205-5 antiserum and analyzed by SDS–PAGE (10% polyacrylamide) as described (Körner et al., 1998). Cross-linked proteins were visualized by phosphoimagery.
Oocyte manipulation and microinjection
Stage VI oocytes were obtained and used for microinjection as described (Körner et al., 1998). In brief, defolliculated oocytes were isolated from collagenase-treated dissociated ovarian fragments and maintained at 18°C in modified Barth's saline. Oocytes were incubated for 12 h in the presence of 10 μg/ml of progesterone in order to induce maturation, which was ascertained by the appearance of a white spot on the animal hemisphere. Mature oocytes were injected cytoplasmically with 5–10 fmol of uniformly radiolabeled G52 RNA containing either m7GpppG, GpppG or ApppG caps. Total RNA was isolated at various times after injection and analyzed as described (Körner et al., 1998).
Binding of PARN to m7GTP–Sepharose
A 100 μl aliquot of a side fraction (#34) from the bovine PARN purification described (Körner and Wahle, 1997) was diluted in 500 μl of wash buffer [50 mM Tris–HCl pH 7.9, 100 mM KCl, 1 mM EDTA, 1 mM DTT, 0.2% (v/v) NP-40, 0.4 μg/ml leupeptin, 0.7 μg/ml pepstatin, 0.5 mM phenylmethylsulfonyl fluoride (PMSF)]. Then 300 μl of this mixture were incubated with 150 μl of packed m7GTP–Sepharose beads (Pharmacia), pre-washed with wash buffer, on a rotating wheel at 4°C for 2 h. The beads were pelleted by a quick centrifugation. They were washed successively twice with 300 μl of wash buffer, then with 300 μl of wash buffer containing 0.1 mM GTP and 300 μl of wash buffer containing 0.1 mM m7GTP (Sigma). A 150 μl aliquot of each of the loaded material and the first supernatant and the entire volume of each of the washes was TCA precipitated with 10 μg of yeast RNA (Boehringer) as a carrier. Pellets were resuspended in 15 μl of SDS–gel loading buffer, separated on a 9% SDS–polyacrylamide gel and silver stained. To examine cap binding of Xenopus PARN, mature oocyte extracts were incubated with m7GTP–Sepharose beads as described above and depleted supernatants were assayed for deadenylation activity in vitro. Fractions corresponding to total extract, depleted supernatant and bound protein eluted with 0.5 mM m7GpppG were separated by SDS–PAGE (12.5% polyacrylamide) and analyzed by immunoblot analysis with anti-PARN 205-5 antiserum as described (Körner et al., 1998).
Acknowledgments
Acknowledgements
We thank Matthias Hentze, Iain Mattaj and Martina Muckenthaler for reagents and Elsebet Lund for advice. This work was supported by NSF grant MCB-9808196 to M.W. and by grants from the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie (to E.W.).
Note added in proof
Cap-dependence of mRNA deadenylation has independently been observed by Jeff Wilusz and colleagues [M.Gao, D.T.Fritz, L.P.Ford and J.Wilusz (2000) Interaction between a poly(A)-specific ribonuclease and the 5′ cap influences mRNA deadenylation rates in vitro. Mol. Cell, in press].
References
- Astrom J., Astrom, A. and Virtanen, A. (1991) In vitro deadenylation of mammalian mRNA by a HeLa cell 3′ exonuclease. EMBO J., 10, 3067–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrom J., Astrom, A. and Virtanen, A. (1992) Properties of a HeLa cell 3′ exonuclease specific for degrading poly(A) tails of mammalian mRNA. J. Biol. Chem., 267, 18154–18159. [PubMed] [Google Scholar]
- Audic Y., Omilli, F. and Osborne, H.B. (1997) Postfertilization deadenylation of mRNAs in Xenopus laevis embryos is sufficient to cause their degradation at the blastula stage. Mol. Cell. Biol., 17, 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashkirov V.I., Scherthan, H., Solinger, J.A., Buerstedde, J.-M. and Heyer, W.-D. (1997) A mouse cytoplasmic exoribonuclease (mXRN1p) with preference for G4 tetraplex substrates. J. Cell Biol., 136, 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beelman C.A. and Parker, R. (1995) Degradation of mRNA in eukaryotes. Cell, 81, 179–183. [DOI] [PubMed] [Google Scholar]
- Boeck R., Tarun, S., Rieger, M., Deardorff, J.A., Müller-Auer, S. and Sachs, A.B. (1996) The yeast Pan2 protein is required for poly(A)-binding protein-stimulated poly(A)-nuclease activity. J. Biol. Chem., 271, 432–438. [DOI] [PubMed] [Google Scholar]
- Bouvet P., Omilli, F., Arlot-Bonnemains, Y., Legagneux, V., Roghi, C., Bassez, T. and Osborne, H.B. (1994) The deadenylation conferred by the 3′ untranslated region of a developmentally controlled mRNA in Xenopus embryos is switched to polyadenylation by deletion of a short sequence element. Mol. Cell. Biol., 14, 1893–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C.E. and Sachs, A.B. (1998) Poly(A) tail length control in Saccharomyces cerevisiae occurs by message-specific deadenylation. Mol. Cell. Biol., 18, 6548–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C.E., Tarun, S.Z., Boeck, R. and Sachs, A.B. (1996) PAN3 encodes a subunit of the Pab1p-dependent poly(A) nuclease in Saccharomyces cerevisiae. Mol. Cell. Biol., 16, 5744–5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiting K., Körner, C., Ulrich, B., Wahle, E. and Horsthemke, B. (1999) The human gene for the poly(A)-specific ribonuclease (PARN) maps to 16p13 and has a truncated copy in the Prader–Willi/Angelman syndrome region on 15q11–q13. Cytogenet. Cell Genet., 87, 125–131. [DOI] [PubMed] [Google Scholar]
- Caponigro G. and Parker, R. (1995) Multiple functions for the poly(A)-binding protein in mRNA decapping and deadenylation in yeast. Genes Dev., 9, 2421–2432. [DOI] [PubMed] [Google Scholar]
- Caponigro G. and Parker, R. (1996) Mechanisms and control of mRNA turnover in Saccharomyces cerevisiae. Microbiol. Rev., 60, 233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-Y. and Shyu, A.-B. (1995) AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem. Sci., 20, 465–470. [DOI] [PubMed] [Google Scholar]
- Chernokalskaya E., Dubell, A.N., Cunningham, K.S., Hanson, M.N., Dompenciel, R.E. and Schoenberg, D.R. (1998) A polysomal ribonuclease involved in the destabilization of albumin mRNA is a novel member of the peroxidase gene family. RNA, 4, 1537–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller J.M., Gray, N.K. and Wickens, M.P. (1998) mRNA stabilization by poly(A) binding protein is independent of poly(A) and requires translation. Genes Dev., 12, 3226–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couttet P., Fromont-Racine, M., Steel, D., Pictet, R. and Grange, T. (1997) Messenger RNA deadenylylation precedes decapping in mammalian cells. Proc. Natl Acad. Sci. USA, 94, 5628–5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio E., Preiss, T. and Hentze, M.W. (1999) Translation driven by an eIF4G core domain in vivo. EMBO J., 18, 4865–4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond D.R., Armstrong, J. and Colman, A. (1985) The effect of capping and polyadenylation on the stability, movement and translation of synthetic messenger RNAs in Xenopus oocytes. Nucleic Acids Res., 13, 7375–7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C.A. and Wickens, M. (1990) Poly(A) removal during oocyte maturation: a default reaction selectively prevented by specific sequences in the 3′ UTR of certain maternal mRNAs. Genes Dev., 4, 2287–2298. [DOI] [PubMed] [Google Scholar]
- Gillian-Daniel D.L., Gray, N.K., Aström, J., Barkoff, A. and Wickens, M. (1998) Modifications of the 5′ cap of mRNAs during Xenopus oocyte maturation: independence from changes in poly(A) length and impact on translation. Mol. Cell. Biol., 18, 6152–6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras A.-C., Raught, B. and Sonenberg, N. (1999) eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem., 68, 913–963. [DOI] [PubMed] [Google Scholar]
- Haghigat A. and Sonenberg, N. (1997) eIF4G dramatically enhances the binding of eIF4E to the mRNA 5′-cap structure. J. Biol. Chem., 272, 21677–21680. [DOI] [PubMed] [Google Scholar]
- Imataka H., Gradi, A. and Sonenberg, N. (1998) A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. EMBO J., 17, 7480–7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaurralde E., Lewis, J., McGuigan, C., Jankowska, M., Darzynkiewicz, E. and Mattaj, I. (1994) A nuclear cap binding protein complex involved in pre-mRNA splicing. Cell, 78, 657–668. [DOI] [PubMed] [Google Scholar]
- Jacobs Anderson J.S. and Parker, R. (1998) The 3′ to 5′ degradation of yeast mRNAs is a general mechanism for mRNA turnover that requires the SKI2 DEVH box protein and 3′ to 5′ exonucleases of the exosome complex. EMBO J., 17, 1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A. (1996) Poly(A) metabolism and translation: the closed-loop model. In Hershey,J.W.B., Mathews,M.B. and Sonenberg,N. (eds), Translational Control. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 451–480. [Google Scholar]
- Jacobson A. and Peltz, S.W. (1996) Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu. Rev. Biochem., 65, 693–739. [DOI] [PubMed] [Google Scholar]
- Körner C. and Wahle, E. (1997) Poly(A) tail shortening by a mammalian poly(A)-specific 3′-exoribonuclease. J. Biol. Chem., 272, 10448–10456. [DOI] [PubMed] [Google Scholar]
- Körner C.G., Wormington, M., Muckenthaler, M., Schneider, S., Dehlin, E. and Wahle, E. (1998) The deadenylating nuclease (DAN) is involved in poly(A) tail removal during the meiotic maturation of Xenopus oocytes. EMBO J., 17, 5427–5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruys V., Marinx, O., Shaw, G., Deschamps, J. and Huez, G. (1989) Translational blockade imposed by cytokine-derived UA-rich sequences. Science, 245, 852–855. [DOI] [PubMed] [Google Scholar]
- LaGrandeur T. and Parker, R. (1999) The cis acting sequences responsible for the differential decay of the unstable MFA2 and stable PGK1 transcripts in yeast include the context of the translational start codon. RNA, 5, 420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legagneux V., Omilli, F. and Osborne, H.B. (1995) Substrate-specific regulation of RNA deadenylation in Xenopus embryo and activated egg extracts. RNA, 1, 1001–1008. [PMC free article] [PubMed] [Google Scholar]
- Marcotrigiano J., Gingras, A.-C., Sonenberg, N. and Burley, S.K. (1999) Cap-dependent translation initiation in eukaryotes is regulated by a molecular mimic of eIF4G. Mol. Cell, 3, 707–716. [DOI] [PubMed] [Google Scholar]
- Minich W.B., Balasta, M.L., Goss, D.J. and Rhoads, R.E. (1994) Chromatographic resolution of in vivo phosphorylated and nonphosphorylated eukaryotic translation initiation factor eIF-4E: increased cap affinity of the phosphorylated form. Proc. Natl Acad. Sci. USA, 91, 7668–7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Petfalski, E., Shevchenko, A., Mann, M. and Tollervey, D. (1997) The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′–5′ exoribonuclease activities. Cell, 91, 457–466. [DOI] [PubMed] [Google Scholar]
- Morley S.J., Curtis, P.S. and Pain, V.M. (1997) eIF4G: translation's mystery factor begins to yield its secrets. RNA, 3, 1085–1104. [PMC free article] [PubMed] [Google Scholar]
- Muckenthaler M., Gunkel, N., Stripecke, R. and Hentze, M.W. (1997) Regulated poly(A) tail shortening in somatic cells mediated by cap-proximal translational repressor proteins and ribosome association. RNA, 3, 983–995. [PMC free article] [PubMed] [Google Scholar]
- Muhlrad D. and Parker, R. (1994) Premature translational termination triggers mRNA decapping. Nature, 370, 578–581. [DOI] [PubMed] [Google Scholar]
- Muhlrad D., Decker, C.J. and Parker, R. (1994) Deadenylation of the unstable mRNA encoded by the yeast MFA2 gene leads to decapping followed by 5′–3′ digestion of the transcript. Genes Dev., 8, 855–866. [DOI] [PubMed] [Google Scholar]
- Muhlrad D., Decker, C.J. and Parker, R. (1995) Turnover mechanism of the stable yeast PGK1 mRNA. Mol. Cell. Biol., 15, 2145–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero L.J., Ashe, M.P. and Sachs, A.B. (1999) The yeast poly(A)-binding protein Pab1p stimulates in vitro poly(A)-dependent and cap-dependent translation by distinct mechanisms. EMBO J., 18, 3153–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piron M., Vende, P., Cohen, J. and Poncet, D. (1998) Rotavirus RNA-binding protein NSP3 interacts with eIF4GI and evicts the poly(A) binding protein from eIF4. EMBO J., 17, 5811–5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss T. and Hentze, M.W. (1998) Dual function of the messenger RNA cap structure in poly(A)-tail-promoted translation in yeast. Nature, 392, 516–520. [DOI] [PubMed] [Google Scholar]
- Ptushkina M., von der Haar, T., Vasilescu, S., Frank, R., Birkenhäger, R. and McCarthy, J.E.G. (1998) Cooperative modulation by eIF4G of eIF4E binding to the mRNA 5′ cap in yeast involves a site partially shared by p20. EMBO J., 17, 4798–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J.D. (1996) Dynamics of poly(A) addition and removal during development. In Hershey,J., Sonenberg,N. and Mathews,M. (eds), Translational Control. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 481–503. [Google Scholar]
- Ross J. (1995) mRNA stability in mammalian cells. Microbiol. Rev., 59, 423–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Echevarria M.J., Czaplinski, K. and Peltz, S.W. (1996) Making sense of nonsense in yeast. Trends Biochem. Sci., 21, 433–438. [DOI] [PubMed] [Google Scholar]
- Schwartz D.C. and Parker, R. (1999) Mutations in translation initiation factors lead to increased rates of deadenylation and decapping of mRNAs in Saccharomyces cerevisiae. Mol. Cell. Biol., 19, 5247–5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu A.-B., Belasco, J.G. and Greenberg, M.E. (1991) Two distinct destabilizing elements in the c-fos message trigger deadenylation as a first step in rapid mRNA decay. Genes Dev., 5, 221–231. [DOI] [PubMed] [Google Scholar]
- Tarun S.Z. and Sachs, A.B. (1996) Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J., 15, 7168–7177. [PMC free article] [PubMed] [Google Scholar]
- Tarun S.Z., Wells, S.E., Deardorff, J.A. and Sachs, A.B. (1997) Translation initiation factor eIF4G mediates in vitro poly(A) tail-dependent translation. Proc. Natl Acad. Sci. USA, 94, 9046–9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharun S. and Parker, R. (1999) Analysis of mutations in the yeast mRNA decapping enzyme. Genetics, 151, 1273–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum S.M. and Wormington, W.M. (1990) Deadenylation of maternal mRNAs during Xenopus oocyte maturation does not require specific cis-sequences: a default mechanism for translational control. Genes Dev., 4, 2278–2286. [DOI] [PubMed] [Google Scholar]
- Varnum S.M., Hurney, C.A. and Wormington, W.M. (1992) Maturation-specific deadenylation in Xenopus oocytes requires nuclear and cytoplasmic factors. Dev. Biol., 153, 283–290. [DOI] [PubMed] [Google Scholar]
- Voeltz G.K. and Steitz, J.A. (1998) AUUUA sequences direct mRNA deadenylation uncoupled from decay during Xenopus early development. Mol. Cell. Biol., 18, 7537–7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells S.E., Hillner, P.E., Vale, R.D. and Sachs, A.B. (1998) Circularization of mRNA by eukaryotic translation initiation factors. Mol. Cell, 2, 135–140. [DOI] [PubMed] [Google Scholar]
- Wickens M., Anderson, P. and Jackson, R.J. (1997) Life and death in the cytoplasm: messages from the 3′-end. Curr. Opin. Genet. Dev., 7, 220–232. [DOI] [PubMed] [Google Scholar]
- Wilson T. and Treisman, R. (1988) Removal of poly(A) and consequent degradation of c-fos mRNA facilitated by 3′ AU-rich sequences. Nature, 336, 396–399. [DOI] [PubMed] [Google Scholar]
- Wormington M. (1991) Preparation of synthetic mRNAs and analyses of translational efficiency in microinjected Xenopus oocytes. Methods Cell Biol., 36, 167–183. [DOI] [PubMed] [Google Scholar]
- Wormington M., Searfoss, A.M. and Hurney, C.A. (1996) Overexpression of poly(A) binding protein prevents maturation-specific deadenylation and translational inactivation in Xenopus oocytes. EMBO J., 15, 900–909. [PMC free article] [PubMed] [Google Scholar]