Abstract
Bovine pancreatic ribonuclease A (RNase A) is a much studied enzyme that efficiently catalyzes the cleavage of RNA. The active site of RNase A contains two histidine residues with imidazole groups positioned to act as a general base (H12) and a general acid (H119) during catalysis of RNA cleavage. Recombinant DNA techniques were used to produce mutant enzymes in which either H12 or H119 was replaced with an alanine residue. Each mutation resulted in a 104-fold decrease in the value of kcat/Km for cleaving either poly(C) or UpA. Thus, H12 and H119 each lower by 5–6 kcal/mol the free energy of the rate-limiting transition state during RNA cleavage. The value of kcat/Km for cleavage of UpOC6H4-p-NO2 was decreased by 104-fold by replacing H12 but was unaffected by replacing H119. This result provides the first direct evidence that H119 acts as a general acid during catalysis by RNase A.
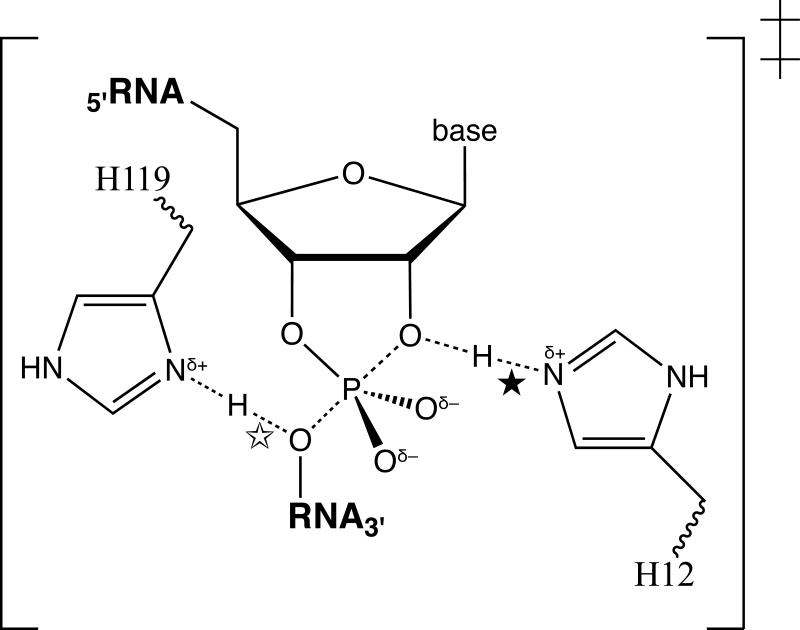
Bovine pancreatic ribonuclease A (RNase A; E.C. 3.1.27.5) has been one of the most studied of all enzymes.1 RNase A efficiently catalyzes the cleavage of RNA.2 Early X-ray diffraction analyses revealed that the active site of RNase A contains two histidine residues, histidine 12 (H12) and histidine 119 (H119).3 The results of chemical modification4 and pH–rate5 studies are consistent with an enzymatic reaction mechanism in which the rate-limiting transition state for RNA cleavage is similar to that shown in Figure 1. In this mechanism, the imidazole side chain of H12 acts as a general base by deprotonating the 2′ oxygen, and that of H119 acts as a general acid by protonating the 5″ oxygen. These two residues have evoked much interest in bioorganic chemistry,6 as well as in protein chemistry and enzymology.1 Indeed, no residue other than H12 and H119 need be invoked to explain the classic bell shape of the pH–rate profile5 for catalysis by this enzyme. Here, we report the explicit value of this general acid and this general base to catalysis by RNase A.
Figure 1.
Putative structure of the transition state for the RNase A-catalyzed cleavage of RNA. Proposed interactions with H12 (★) and H119 (☆) are indicated.
We used recombinant DNA techniques to produce mutant ribonucleases in which either H12 or H119 was changed to an alanine residue.7 This change effectively substitutes a proton for the imidazole group of each residue. We then determined the ability of the resulting mutant enzymes, H12A RNase A and H119A RNase A, to catalyze the cleavage of three phosphodiester substrates: polycytidylic acid [poly(C)], uridylyl(3′→5′)adenosine (UpA), and uridine 3′-(p-nitrophenylphosphate) (UpOC6H4-p-NO2).9
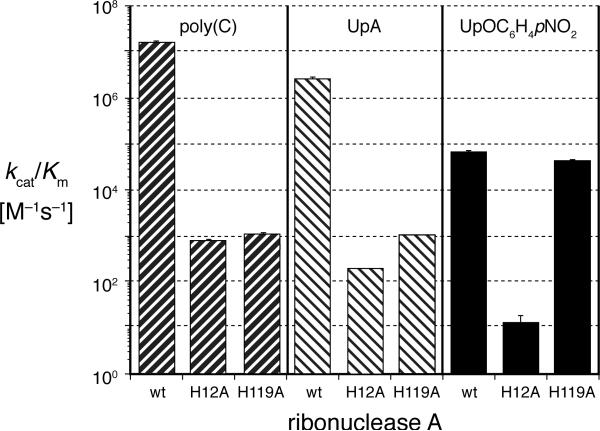
The values of the steady-state kinetic parameters for cleavage of poly(C), UpA, and UpOC6H4-p-NO2 by the wild-type and mutant ribonucleases are given in Table 1 and Figure 2. The second-order rate constant, kcat/Km, is proportional to the association constant of an enzyme and the rate-limiting transition state during catalysis.10 As shown in Figure 2, eliminating the imidazole group of H12 decreased the affinity of the enzyme for this transition state by 104-fold during cleavage of poly(C), UpA, and UpOC6H4-p-NO2.11 Eliminating the imidazole group of H119 decreased this affinity by 104-fold during cleavage of poly(C) and by almost 104-fold during cleavage of UpA.12 In contrast, this change had no significant effect on the rate of cleavage of UpOC6H4-p-NO2.
Table 1.
Steady-State Kinetic Parameters for Cleavage of Ribonucleotides by Wild-Type and Mutant Ribonucleasesa
| RNase A | substrateb | kcat (s–1) | Km (mM) | kcat/Km (M–1 s–1) | |
|---|---|---|---|---|---|
| wild-type | poly(C) | (4.1 ± 0.1) × 102 c | 0.034 ± 0.002c | (1.5 ± 0.1) × 107 c | 1.0 |
| H12A | poly(C) | 0.073 ± 0.006 | 0.105 ± 0.025 | (7.3 ± 0.2) × 102 | (4.9 ± 0.9) × 10–5 |
| H119A | poly(C) | 0.24 ± 0.03 | 0.21 ± 0.03 | (1.1 ± 0.1) × 103 | (7.3 ± 0.8) × 10–5 |
| wild-type | UpA | (1.40 ± 0.15) × 103 c | 0.62 ± 0.09c | (2.3 ± 0.4) × 106 c | 1.0 |
| H12A | UpA | 0.15 ± 0.02 | 0.86 ± 0.17 | (1.7 ± 0.1) × 102 | (7.4 ± 1.4) × 10–5 |
| H119A | UpA | 0.76 ± 0.10 | 0.80 ± 0.15 | (9.5 ± 0.5) × 102 | (4.1 ± 0.7) × 10–4 |
| wild-type | UpOC6H4-p-NO2 | 18.8 ± 0.6 | 0.33 ± 0.05 | (5.7 ± 0.6) × 104 | 1.0 |
| H12A | UpOC6H4-p-NO2 | 0.0029 ± 0.0001 | 0.275 ± 0.041 | (1.1 ± 0.1) × 101 | (1.9 ± 0.3) × 10–4 |
| H119A | UpOC6H4-p-NO2 | 27 ± 1 | 0.76 ± 0.08 | (3.6 ± 0.1) × 104 | 0.63 ± 0.11 |
All reactions were performed at 25 °C in 50 mM MES buffer, pH 6.0, containing 0.1 M NaCl. Steady-state kinetic parameters were determined by fitting the initial velocity data to a hyperbolic curve using the program HYPERO.22
Cleavage of poly(C) and UpOC6H4-p-NO2 were monitored at 250 nm (Δε250 = 2380 M–1 cm–1) and 330 nm (Δε330 = 6200 M–1 cm–1), respectively; cleavage of UpA was monitored at 265 nm in the presence of excess adenosine deaminase23 (Δε265 = –6000 M–1 cm–1).
Data from ref 8.
Figure 2.
Values of kcat/Km for the cleavage reaction catalyzed by wild-type and mutant ribonucleases.
The value of the imidazole group of H119 to catalysis depends on the pKa of the conjugate acid of the leaving groups. Cleavage of poly(C) and UpA is accelerated dramatically by the side chain of H119. The nucleotide and nucleoside leaving groups in these substrates have conjugate acids with pKa ≈ 14.8.13 In contrast, the cleavage of UpOC6H4-p-NO2 is unaffected by the side chain of H119. The p-nitrophenolate leaving group in substrate has a conjugate acid with pKa = 7.14.14 Together, these data provide the first direct evidence that the role of H119 is to protonate the leaving group during RNA cleavage. This result also illustrates how a capable catalyst for cleavage of an activated model substrate (e.g., UpOC6H4-p-NO2) can lack a component important for cleavage of an unactivated substrate (e.g., RNA).
The results for UpOC6H4-p-NO2 illuminate the mechanism of catalysis by RNase A. Breslow has proposed that RNase A catalyzes RNA cleavage via a phosphorane intermediate.6b In the Breslow mechanism, H119 is proposed to both protonate a non-bridging oxygen of the phosphate anion and deprotonate this same oxygen in the phosphorane intermediate.15 Yet, wild-type and H119A RNase A cleaved UpOC6H4-p-NO2 at the same rate (Figure 2). Thus our data argue against the participation of H119 in the formation of a phosphorane, at least during the cleavage of UpOC6H4-p-NO2.16
The steady-state kinetic parameters of wild-type, H12A, and H119A RNase A are consistent with H12 acting as a general base and show that H119 acts as a general acid during the cleavage of RNA. Further, the observed rate enhancements agree with those expected for acid–base catalysis by H12 and H119. For example, suppose a water molecule replaces the imidazole group in the mutant enzymes such that the interactions marked by ★ or ☆ in Figure 1 are now to the oxygen or a hydrogen, respectively, of H2O. The rate enhancements then derived from the Brfnsted equation are and , where and 17 and and . The Brønsted equation therefore predicts that general base catalysis provides a 107.5β-fold rate enhancement, and general acid catalysis provides a 109.5α-fold rate enhancement. Values of α and β tend to be approximately 0.5 for proton transfers between oxygen and nitrogen.18 Thus, the rate enhancements predicted with this simple model are similar to those observed (Figure 2).
The side chains of H12 and H119 each bind to the rate-limiting transition state during RNA cleavage with an apparent free energy of ΔGapp = 5–6 kcal/mol.19 This free energy is related20 to the strength of the interaction marked by ★ or ☆ in Figure 1. For many reasons, however, neither of these interactions can be assigned an explicit free energy. For example, mutagenesis may have altered the structure of the transition state or its position on the reaction coordinate.10,18,20 Also, catalysis by wild-type RNase A may be limited in part by a diffusive step rather than a chemical interconversion.21 Studies to illuminate these and other aspects of catalysis by this venerable enzyme are underway.
ACKNOWLEDGMENTS
We thank Dr. A. C. Hengge for advice in the syntheses of UpA and UpOC6H4-p-NO2. This work was supported by grant GM44783 (NIH). J.E.T. was supported by Cellular and Molecular Biology Training Grant GM07215 (NIH). R.T.R. is a Presidential Young Investigator (NSF), Searle Scholar (Chicago Community Trust), and Shaw Scientist (Milwaukee Foundation).
References
- 1.For reviews, see: Richards FM, Wyckoff HW. Enzymes. 1971;4:647–806. Karpeisky M. Ya., Yakovlev GI. Soviet Scientific Review, Section D. Vol. 2. Harvard Academic Press; New York: 1981. pp. 145–257. Blackburn P, Moore S. Enzymes. 1982;15:317–433. Wlodawer A. In: Biological Macromolecules and Assemblies, Vol. II, Nucleic Acids and Interactive Proteins. Jurnak FA, McPherson A, editors. Wiley; New York: 1985. pp. 393–439. Beintema JJ. Life Chem. Rep. 1987;4:333–389. Eftink MR, Biltonen RL. In: Hydrolytic Enzymes. Neuberger A, Brocklehurst K, editors. Elsevier; New York: 1987. pp. 333–376.
- 2.The enzyme also catalyzes (albeit inefficiently, see: Thompson JE, Venegas FD, Raines RT. Biochemistry. doi: 10.1021/bi00189a047. in press. the hydrolysis of the 2′,3′-cyclic phosphodiester product of RNA cleavage.
- 3.a Kartha G, Bello J, Harker D. Nature. 1967;213:862–865. doi: 10.1038/213862a0. [DOI] [PubMed] [Google Scholar]; b Avey HP, Boles MO, Carlisle CH, Evans SA, Morris SJ, Palmer RA, Woolhouse BA, Shall S. Nature. 1967;213:557–562. doi: 10.1038/213557a0. [DOI] [PubMed] [Google Scholar]
- 4.a Crestfield AM, Stein WH, Moore S. Arch. Biochem. Biophys. 1962;(Suppl. 1):217–222. [PubMed] [Google Scholar]; b Lennette EP, Plapp BV. Biochemistry. 1979;18:3938–3946. doi: 10.1021/bi00585a015. [DOI] [PubMed] [Google Scholar]; c Pincus M, Thi LL, Carty RP. Biochemistry. 1975;14:3653–3661. doi: 10.1021/bi00687a022. [DOI] [PubMed] [Google Scholar]
- 5.a Herries DG, Mathias AP, Rabin BR. Biochem. J. 1962;85:127–134. doi: 10.1042/bj0850127. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Findlay D, Mathias AP, Rabin BR. Biochem. J. 1962;85:139–144. doi: 10.1042/bj0850139. [DOI] [PMC free article] [PubMed] [Google Scholar]; c delRosario EJ, Hammes GG. Biochemistry. 1969;8:1884–1889. doi: 10.1021/bi00833a017. [DOI] [PubMed] [Google Scholar]; d Eftink MR, Biltonen RL. Biochemistry. 1983;22:5123–5134. doi: 10.1021/bi00291a011. [DOI] [PubMed] [Google Scholar]
- 6.a Breslow R, Labelle M. J. Am. Chem. Soc. 1986;108:2655–2659. [Google Scholar]; b Anslyn E, Breslow R. J. Am. Chem. Soc. 1989;111:4473–4482. [Google Scholar]; c Breslow R. Acc. Chem. Res. 1991;24:317–324. [Google Scholar]; d Breslow R, Anslyn E, Huang D-L. Tetrahedron. 1991;47:2365–2376. [Google Scholar]; e Breslow R, Xu R. J. Am. Chem. Soc. 1993;115:10705–10713. [Google Scholar]
- 7.Mutations in the cDNA that codes for RNase A were made by oligonucleotide-mediated site-directed mutagenesis Kunkel TA, Roberts JD, Zakour RA. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. Mutant cDNAs were expressed in Escherichia coli under the control of the T7 RNA polymerase promoter, and the resulting proteins were purified by standard methods (ref 8).
- 8.delCardayré SB, Raines RT. Biochemistry. 1994;33:6031–6037. doi: 10.1021/bi00186a001. [DOI] [PubMed] [Google Scholar]
- 9.Poly(C) was from Sigma Chemical (St. Louis, MO), and was purified by precipitation from aqueous ethanol (70% v/v). UpA was synthesized by the methods of Ogilvie Ogilvie KK, Beaucage SL, Schifman AL, Theriault NY, Sadana KL. Can. J. Chem. 1978;56:2768–2780. and Carruthers Beaucage SL, Carruthers MH. Tetrahedron Lett. 1981;22:1859–1862. UpOC6H4-p-NO2 was synthesized by the method of Williams Davis AM, Regan AC, Williams A. Biochemistry. 1988;27:9042–9047. doi: 10.1021/bi00425a024.
- 10.Wolfenden R. Annu. Rev. Biophys. Bioeng. 1976;5:271–306. doi: 10.1146/annurev.bb.05.060176.001415. [DOI] [PubMed] [Google Scholar]
- 11.The kinetic parameters in Table 1 for UpOC6H4-p-NO2 cleavage are about 2-fold higher than those reported previously Davis AM, Regan AC, Williams A. Biochemistry. 1988;27:9042–9047. doi: 10.1021/bi00425a024. This disparity may result from a difference in the pH of the assay solutions (6.0 vs 7.5).
- 12.Replacing H119 of bovine seminal ribonuclease (a homolog of RNase A) with an aspartate residue decreases kcat/Km by (4 × 103)-fold for UpA cleavage Kim J-S, Raines RT. unpublished results. The H13A and H114A mutants of human angiogenin (another homolog of RNase A) each have >104-fold less activity for RNA cleavage than does wild-type human angiogenin Shapiro R, Vallee BL. Biochemistry. 1989;28:7401–7408. doi: 10.1021/bi00444a038.
- 13.CH3OCH2CH2OH has pKa = 14.8 Ballinger P, Long FA. J. Am. Chem. Soc. 1960;82:795–798.
- 14.Fickling MM, Fischer A, Mann BR, Packer J, Vaughan J. J. Am. Chem. Soc. 1959;81:4226–4230. [Google Scholar]
- 15.In the Breslow mechanism, H119 is also proposed to protonate the leaving group during breakdown of the phosphorane intermediate. This role is probably unnecessary, however, if p-nitrophenolate is the leaving group.
- 16.Gerlt and Gassman have proposed the existence of the same phosphorane intermediate as in the Breslow mechanism Gerlt JA, Gassman PG. Biochemistry. 1993;32:11943–11952. doi: 10.1021/bi00096a001. In the Gerlt and Gassman mechanism, however, H119 serves only to protonate the 5″ oxygen, a role that is not in conflict with our results.
- 17.Markley JM. Acc. Chem. Res. 1975;8:70–79. [Google Scholar]
- 18.Jencks WP. Chem. Rev. 1985;85:511–527. references therein. [Google Scholar]
- 19.At 25 °C, ΔGapp = (1.364 kcal/mol) × log(rate enhancement).
- 20.Fersht AR. Biochemistry. 1988;27:1577–1580. doi: 10.1021/bi00405a027. [DOI] [PubMed] [Google Scholar]
- 21.For example, see: Blacklow SC, Raines RT, Lim WA, Zamore PD, Knowles JR. Biochemistry. 1988;27:1158–1167. doi: 10.1021/bi00404a013.
- 22.Cleland WW. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- 23.Ipata PL, Felicioli RA. FEBS Lett. 1968;1:29–31. doi: 10.1016/0014-5793(68)80010-9. [DOI] [PubMed] [Google Scholar]