Abstract
Purpose
This study was conducted to determine if prophylactic cranial irradiation (PCI) improves survival in locally advanced non–small-cell lung cancer (LA-NSCLC).
Patients and Methods
Patients with stage III NSCLC without disease progression after treatment with surgery and/or radiation therapy (RT) with or without chemotherapy were eligible. Participants were stratified by stage (IIIA v IIIB), histology (nonsquamous v squamous), and therapy (surgery v none) and were randomly assigned to PCI or observation. PCI was delivered to 30 Gy in 15 fractions. The primary end point of the study was overall survival (OS). Secondary end points were disease-free survival (DFS), neurocognitive function (NCF), and quality of life. Kaplan-Meier and log-rank analyses were used for OS and DFS. The incidence of brain metastasis (BM) was evaluated with the logistic regression model.
Results
Overall, 356 patients were accrued of the targeted 1,058. The study was closed early because of slow accrual; 340 of the 356 patients were eligible. The 1-year OS (P = .86; 75.6% v 76.9% for PCI v observation) and 1-year DFS (P = .11; 56.4% v 51.2% for PCI v observation) were not significantly different. The hazard ratio for observation versus PCI was 1.03 (95% CI, 0.77 to 1.36). The 1-year rates of BM were significantly different (P = .004; 7.7% v 18.0% for PCI v observation). Patients in the observation arm were 2.52 times more likely to develop BM than those in the PCI arm (unadjusted odds ratio, 2.52; 95% CI, 1.32 to 4.80).
Conclusion
In patients with stage III disease without progression of disease after therapy, PCI decreased the rate of BM but did not improve OS or DFS.
INTRODUCTION
Brain metastases (BM) in patients with non–small-cell lung cancer (NSCLC) are a devastating problem with profound impact on survival and quality of life (QoL). Although studies have shown that prophylactic cranial irradiation (PCI) is successful in decreasing the incidence of BM,1–3 preventative treatments for BM are rarely employed in clinical practice because of the lack of proven survival advantage and potential for toxicity.
Since the first prospective, randomized studies evaluating PCI for locally advanced non–small-cell lung cancer (LA-NSCLC) were conducted more than 2 decades ago, advances in surgical and radiation techniques have resulted in improved locoregional control of LA-NSCLC. Routine use of systemic therapy has resulted in decreased risk of nonbrain metastases but has limited impact on BM, which leaves the brain relatively undertreated.4–8 As patient survival lengthens, the risk of BM is increased. Several reviews have shown that longer survival for patients with LA-NSCLC5,9,10 is associated with an increased incidence of BM.
Recent studies employing multimodality therapy have reported median survival (MS) ranging from 20 to 43 months and 3-year survival rates of 34% to 37% for LA-NSCLC.6,11–16 These studies reported the brain to be one of the most frequent sites of initial failure. Overall, BM rates ranged from 22% to 55%, and brain as first site of relapse ranged from 16% to 43%.
This study was undertaken to reassess the use of PCI in the current era of lung cancer therapy. Since the original NSCLC PCI studies were completed, patients with LA-NSCLC are living longer with increasing incidence of BM and, thus, appear more likely to gain a survival advantage with PCI.
The Radiation Therapy Oncology Group (RTOG), with cooperation from the other US and Canadian cooperative groups, conducted a study of PCI for patients with LA-NSCLC after locoregional and systemic treatment. This study was designed to evaluate the impact of PCI on overall survival (OS), the incidence of brain metastases, disease-free survival (DFS), neurocognitive function (NCF) and QoL.
PATIENTS AND METHODS
Patient Selection
Patients with stage IIIA to IIIB NSCLC were study eligible if they had stable disease or better (ie, complete response or partial response) after potentially curative therapy, defined as high-dose thoracic radiation therapy (RT; ie, > 30 Gy) or surgery. Radiation could be given with or without neoadjuvant, adjuvant, or concurrent chemotherapy. Pre- or postoperative RT and/or chemotherapy were allowed. Therapy had to have been complete within 16 weeks of study entry. Patients were restaged with computed tomography (CT) scan of the chest and abdomen and magnetic resonance imaging (MRI) of the brain within 6 weeks of study entry. CT with contrast of the brain was allowed if MRI was contraindicated and if performance for pretreatment assessment was required for follow-up imaging.
Patients could have no evidence of progressive intrathoracic disease, brain metastases, or extracranial metastases. Any acute or subacute grade ≥ 3 toxicities from previous therapy had to decrease to grade ≤ 2 at the time of study entry.
Treatment and Follow-Up
Patients were stratified by stage (IIIA or IIIB), histology (nonsquamous or squamous), and therapy (surgery or none) and were randomly assigned to either PCI or observation. Patients randomly assigned to PCI were treated with 2 Gy per fraction, 5 days per week, to 30 Gy. Acute PCI toxicity was graded by using the National Cancer Institute Common Terminology Criteria (NCI-CTC) version 2.0. Late PCI toxicity was graded by using the RTOG/European Organisation for Research and Treatment of Cancer (EORTC) Late Toxicity Criteria. All patients had evaluation of NCF and QoL at baseline. NCF was reassessed at 3, 6, 12, 18, 24, 30, 36, and 48 months and then yearly. QoL was assessed, and brain imaging was performed at 6, 12, 24, 36, and 48 months and then yearly. Patients were observed in follow-up at 6 months from start of PCI, every 6 months for 2 years, and then yearly.
Study Design and End Points
The study was originally designed to test whether the OS rate of the PCI arm improved by 20% compared with the observation arm at 1 year. A total number of deaths of 527 was required with at least 12 months follow-up for each patient to have a statistical power of 80% with a one-sided significance level of .025. Given that 5% of patients are either retrospectively ineligible or not evaluable because they never start any therapy, a total of 529 patients per arm or 1,058 randomly assigned patients would have been required. The primary analysis of OS rate at 1 year was done when each patient had at least 12 months of follow-up. A failure event of OS was defined as death as a result of any cause.
The secondary objectives were to evaluate the effects of PCI on disease-free survival (DFS), brain metastasis, NCF, and QoL. A failure event of DFS was defined as the earliest event of death as a result of any cause, local progression, regional metastasis, distant metastasis, or second primary. The failure event of BM was defined as any evidence of metastatic disease in the brain. Time to event was measured from the date of random assignment to the date of failure or to the date of most recent follow-up if no failure occurred. The neurocognitive impact of PCI was evaluated by the Mini Mental Status Exam, Activities of Daily Living Scale,17 and Hopkins Verbal Learning Test.18 The EORTC Quality-of-Life Questionnaire-C3019 and Brain Module N2020 were used to evaluate QoL.
Statistical Methods
This analysis was undertaken after all patients had been potentially observed for a minimum of 12 months. The OS, DFS, and BM were estimated by the Kaplan-Meier method21 and were analyzed by using the stratified log-rank test22,23 and P = .025 (one-sided significance level). In addition, a Cox proportional hazards regression model 24 was used to calculate hazard ratios. The models included effects for treatment arm (observation v PCI [reference level; RL]). The 95% CI for the median time was calculated by using the method of Brookmeyer and Crowley.25 Logistic regression model 26 was used to model the incidence of brain metastases (presence v absence) associated with important prognostic variables at 1 year by controlling for the treatment group. All statistical comparisons were considered statistically significant with a P value of less than .05 (two sided). A Statistical Analysis System (SAS Institute, Cary, NC) software package was used for all statistical analyses.
RESULTS
The study opened to accrual on September 19, 2002, and closed on August 30, 2007. The study was closed early as a result of slow accrual. Targeted accrual was 1,058. Projected monthly accrual was 29 patients. Total accrual was 356 patients, with an average monthly accrual rate of six patients.
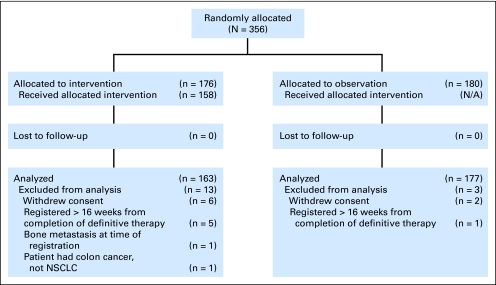
Among 356 patients entered onto this study; nine patients (seven from the PCI arm, and two from the observation arm) were ineligible, and seven patients (six from the PCI arm, and one from the observation arm) withdrew consent. Data from 340 eligible patients were analyzed as of November 2008.
At the time of this analysis, there were 150 patients alive with 23.8 months of median follow-up (range, 2 to 60.7 months) and there were 17 patients alive with less than 12 months of follow-up; three of these patients withdrew consent for follow-up. The pretreatment characteristics were evenly distributed between the two arms (Table 1) except for the Zubrod performance status (P = .03). The majority (96%) of patients had a Zubrod performance status of 0 to 1. A performance status of 0 (versus greater than 0) was not associated with OS, DFS, or incidence of BM. The median age was 62 years (range, 39 to 84 years). Approximately 62% of the patients were men. Approximately 34% of patients had squamous histology. Of the patients with nonsquamous histology, 49% had adenocarcinoma.
Table 1.
Pretreatment Demographic and Clinical Characteristics
| Variable | PCI (n = 163) |
Observation (n = 177) |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | |||||
| Median | 63 | 62 | .60 | ||
| Range | 39-84 | 39-83 | |||
| Sex | |||||
| Male | 102 | 63 | 110 | 62 | .93 |
| Female | 61 | 37 | 67 | 38 | |
| Zubrod performance status | |||||
| 0 | 77 | 47 | 105 | 59 | NA† |
| 1 | 76 | 47 | 68 | 38 | |
| 2 | 8 | 5 | 4 | 2 | |
| 3 | 1 | 1 | 0 | 0 | |
| Unknown | 1 | 1 | 0 | 0 | |
| 0 | 77 | 47 | 105 | 59 | .03 |
| > 0 | 86 | 53 | 72 | 41 | |
| Histology | |||||
| Squamous cell carcinoma | 51 | 31 | 60 | 34 | NA† |
| Adenocarcinoma | 53 | 33 | 60 | 34 | |
| Large-cell undifferentiated | 11 | 7 | 8 | 5 | |
| Combined/mixed | 0 | 0 | 4 | 2 | |
| Non–small-cell carcinoma, NOS | 44 | 27 | 43 | 24 | |
| Other | 4 | 2 | 2 | 1 | |
| Squamous | 51 | 31 | 64 | 36 | .34 |
| Nonsquamous | 112 | 69 | 113 | 64 | |
| Stage | |||||
| IIIA | 88 | 54 | 96 | 54 | .96 |
| IIIB | 75 | 46 | 81 | 46 | |
| Prior chemotherapy/RT | |||||
| Chemotherapy/RT | 147 | 90 | 160 | 90 | NA† |
| Chemotherapy alone | 3 | 2 | 3 | 2 | |
| RT alone | 13 | 8 | 14 | 8 | |
| Prior surgery | |||||
| No | 106 | 65 | 119 | 67 | .67 |
| Yes | 57 | 35 | 58 | 33 | |
| Entire prior therapy regimen | |||||
| RT and chemotherapy | 97 | 60 | 110 | 62 | NA† |
| RT only | 9 | 6 | 9 | 5 | |
| Surgery and chemotherapy | 3 | 2 | 3 | 2 | |
| Surgery and RT | 4 | 2 | 5 | 3 | |
| Surgery, RT, and chemotherapy | 50 | 31 | 50 | 28 | |
NOTE. Total No. of patients = 340.
Abbreviations: PCI, prophylactic cranial irradiation; NA, not applicable; NOS, not otherwise specified; RT, radiation therapy.
Age was tested with a t test; all others, with an χ2 test.
Insufficient cell counts.
Primary End Point
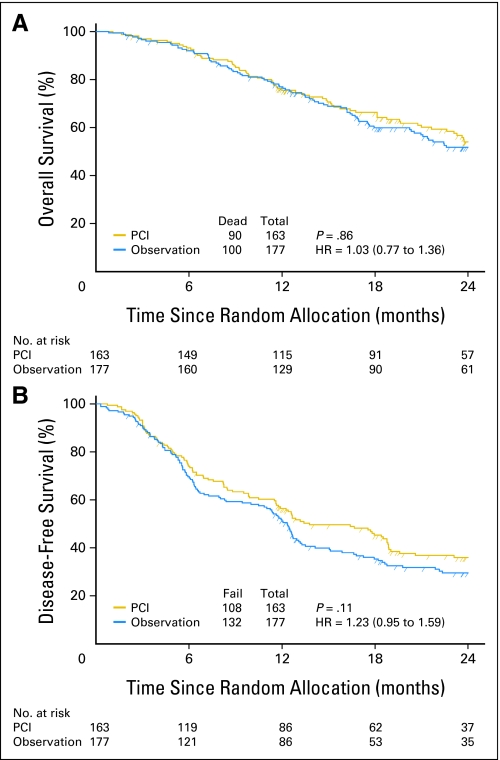
At the time of this analysis, 190 deaths had occurred of 340 evaluable patients (Fig 1). There was not a significant difference in OS at 1 year between the two arms. The 1-year OS rates were 75.6% and 76.9% for PCI and observation arms, respectively (Fig 2A). Estimated MS duration times were 25.8 and 24.8 months (P = .86) for PCI and observation arms, respectively (Table 2). The hazard ratio for observation arm versus PCI arm was 1.03 (95% CI, 0.77 to 1.36).
Fig 1.
CONSORT diagram. NSCLC, non–small-cell lung cancer.
Fig 2.
Overall survival and disease-free survival. PCI, prophylactic cranial irradiation; HR, hazard ratio.
Table 2.
Outcome Estimates
| Outcome by time | PCI (n = 163) |
Observation (n = 177) |
P* | ||||
|---|---|---|---|---|---|---|---|
| No. at Risk | Survival/Failure Estimate (%) | 95% CI (%) | No. at Risk | Survival/Failure Estimate (%) | 95% CI (%) | ||
| Overall survival | |||||||
| 6 months | 149 | 93.2 | 88.1 to 96.2 | 160 | 92.0 | 86.9 to 95.2 | .86 |
| 12 months | 115 | 75.6 | 68.1 to 81.5 | 129 | 76.9 | 69.9 to 82.5 | |
| MST, months | 25.8 | 24.8 | |||||
| 95% CI | 22.6 to 31.3 | 20.5 to 27.4 | |||||
| No. of patients who died | 90 | 100 | |||||
| HR of observation v PCI arm | 1.03 | ||||||
| 95% CI | 0.77 to 1.36 | ||||||
| Disease-free survival | |||||||
| 6 months | 119 | 74 | 66.5 to 80.1 | 121 | 69.7 | 62.2 to 75.9 | .11 |
| 12 months | 86 | 56.4 | 48.4 to 63.7 | 86 | 51.2 | 43.5 to 58.3 | |
| MDFST, months | 13.8 | 12.3 | |||||
| 95% CI | 11.6 to 18.8 | 10.5 to 13.1 | |||||
| No. of patients who experienced failure | 108 | 132 | |||||
| HR of observation v PCI arm | 1.23 | ||||||
| 95% CI | 0.95 to 1.59 | ||||||
| CNS metastasis | |||||||
| 6 months | 145 | 3.3 | 1.4 to 7.6 | 144 | 10.7 | 6.9 to 16.5 | .004 |
| 12 months | 109 | 7.7 | 4.3 to 13.4 | 113 | 18.0 | 12.9 to 24.9 | |
| MCNSMT, months | Not reached | Not reached | |||||
| 95% CI | |||||||
| No. of patients who experienced failure | 15 | 36 | |||||
| HR of observation v PCI arm | 2.35 | ||||||
| 95% CI | 1.29 to 4.30 | ||||||
NOTE. HR quantifies how much more or less risk patients at some level have than those at the reference level. CI that includes 1 indicates that no difference exists between the subgroups.
Abbreviations: PCI, prophylactic cranial irradiation; MST, median survival time; HR, hazard ratio; MDFST, median disease-free survival time; MCNSMT, median CNS metastasis time.
From stratified log-rank test.
DFS and BM
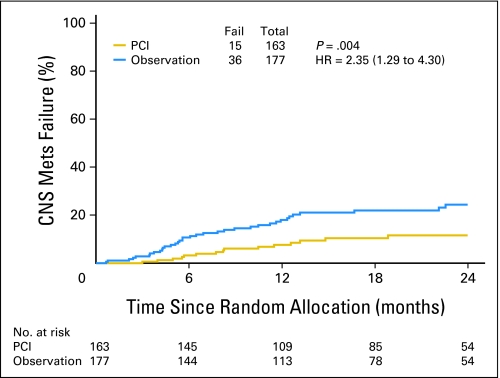
There were 240 DFS events at the time of analysis. There was not a significant difference in DFS between the arms. The 1-year DFS rates were 56.4% and 51.2% for PCI and observation arms, respectively (P = .11; Table 2; Fig 2B). The hazard ratio for the observation versus PCI arm was 1.23 (95% CI, 0.95 to 1.59). Fifty-one patients had BM at the time of analysis. The 1-year BM rates were 7.7% and 18.0% for PCI and observation arms, respectively (P = .004; Table 2; Fig 3). The hazard ratio for observation versus PCI arm was 2.35 (95% CI, 1.29 to 4.30). Results from a logistic regression model show that the unadjusted odds ratio of the incidence of BM was 2.52 (95% CI, 1.32 to 4.80; P = .005). Patients in the observation arm were 2.52 times more likely to have BM than those in the PCI arm.
Fig 3.
Brain metastases. Mets, metastasis; PCI, prophylactic cranial irradiation; HR, hazard ratio.
PCI-Related Toxicity
The worst nonhematologic acute grade 1, 2, 3, and 4 toxicities occurred in 14%, 34%, 4%, and 1%, respectively. Grade 1 acute toxicity was primarily constitutional and gastrointestinal, whereas grade 2 acute toxicity was constitutional and dermatologic. Six patients experienced grade 3 acute toxicity. This included four patients with grade 3 fatigue. Of these, two patients had grade 3 fatigue only, whereas one patient had both grade 3 fatigue and dyspnea and one patient had grade 3 fatigue, ataxia, and depression. One patient had grade 3 hematologic toxicity only, and one patient had unspecified grade 3 pain only. Acute grade 4 mood alteration/depression was reported in one patient. There was no late PCI toxicity greater than grade 3. Four patients reported grade 3 late toxicity, including dyspnea, syncope, weakness, and fatigue. The QoL and NCF analyses are the subject of a separate publication.
DISCUSSION
Prior randomized, controlled trials1–3 and several prospective studies without brain primary end points and retrospective studies evaluating PCI for NSCLC have been published and are summarized in Tables 3 and 4 with refs 27, 31, 33, 34, 32, 11.11,27–31 Studies have consistently shown a decrease and/or delay in BM with PCI of a magnitude similar to our study. PCI has not become part of standard management for LA-NSCLC because of concern for long-term toxicity and lack of a proven survival benefit. The lack of OS benefit in former studies has been attributed to poor locoregional and extracranial control and/or small study size. Improved RT techniques and the increased use of combined-modality therapy have resulted in improved patient survival but also more BMs. Thus, it was the ideal time to conduct a study powered to show a survival advantage and to prospectively study the long-term effects on NCF and QoL.
Table 3.
Randomized, Controlled Trials Evaluating PCI
| Study | No. of Patients | Primary Therapy | Dose (Gy) | CNS Metastases |
1-Year Survival (%) | Median Survival (months) | ||
|---|---|---|---|---|---|---|---|---|
| Observation (%) | PCI (%) | P | ||||||
| VALG 19811 | 281 | RT only(all NSCLC) | 20 (2 Gy × 10) | 13 | 6 | .038 | NA | 7-8 |
| MDACC 19843 | 97 | Multimodality (all NSCLC) | 30 (3 Gy × 10) | 27 | 4 | .002 | NA | NA |
| RTOG 19912 | 187 | RT only (nonsquamous) | 30 (3 Gy × 10) | 19 | 9 | .10 | 13 (2-year) | 8 |
| RTOG 2009 (this study) | 340 | Multimodality (all NSCLC) | 30 (2 Gy × 15) | 18 | 7.7 | .004 | 75 | 25 |
Abbreviations: PCI, prophylactic cranial irradiation; VALG, Veterans Affairs Lung Group; RT, radiation therapy; NSCLC, non–small-cell lung cancer; NA, not applicable; MDACC, MD Anderson Cancer Center; RTOG, Radiation Treatment Oncology Group.
Table 4.
Retrospective and Nonrandomized Prospective Trials Evaluating PCI
| Study | Primary Therapy | PCI Dose (Gy) | CNS Metastases |
Overall Survival Rates (%) | Median Survival (months) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observation |
PCI |
P | RR | 95% CI | |||||||
| % | No. of Patients/Total No. | % | No. of Patients/Total No. | ||||||||
| Jacobs 198730 | NA | 30 (2 Gy × 15) | 24 | 14/58 | 5 | 1/20 | .06 | NA | NA | 17 | |
| Skarin 198929 | Trimodality (all NSCLC) | 36 (2 Gy × 18) | 26 | 7/27 | 14 | 1/7 | NA | 31 at 3-5 years 32 | |||
| Strauss 199228 | Trimodality (nonsquam) | 30 (2 Gy × 15) | 12 | 5/41 | 0 | 0/13 | NA | 58 at 1 year 15.5 | |||
| Albain 199527 | Trimodality (all NSCLC) | 36 (2 Gy × 18) | 16 | 16/100 | 8 | 2/26 | .44 | NA | 37 at 2 years;27 at 3 years | 15 | |
| Stuschke 199911 | Trimodality (all NSCLC) | 30 (2 Gy × 15) | 54 | 15/28 | 13 | 6/47 | < .001 | 0.14 | 0.03 to 0.69 | 31 at 3 years | 20 |
| Pottgen 200731 | Trimodality (all NSCLC) | 30 (2 Gy × 15) | 34.7* | 7.8† | NA | 16-18 at 5 years | NA | ||||
Abbreviations: PCI, prophylactic cranial irradiation; RR, relapse rate; NA, not available; NSCLC, non–small-cell lung cancer; nonsquam, nonsquamous.
95% CI, 15.7% to 53.7%.
95% CI, 0% to 18.5%.
Unfortunately, despite securing commitment to participate from each of the North American Cooperative Groups, the study did not approach predicted accrual, which resulted in early closure. Despite early closure, this is the largest randomized, controlled trial to evaluate PCI in patients with LA-NSCLC to our knowledge and the only study of its kind in the era of combined-modality therapy. The study has shown that patients with LA-NSCLC who did not receive PCI were 2.52 times more likely to develop BM than patients who received PCI. It is possible that a survival advantage may become evident with longer follow-up. However, at the time of this analysis, the OS curves were superimposed. The DFS curves separated after 3 months in favor of PCI. More patients in the observation group had BM compared with the PCI group, which may explain the separation in the DFS curves.
Although the survival rate in our study is higher than in prior randomized, controlled trials, the BM rates were not as high as predicted on the basis of recent data from patients treated with combined-modality therapy. Studies employing multimodality therapy for LA-NSCLC with MS durations of greater that 20 months have reported the brain as one of the most frequent sites of initial disease failure, with overall brain failure rates of 22% to 55%, and the brain as first site of relapse in 16% to 43%.6,11–16
We expect BM to increase with continued follow-up, but it is not likely to reach the level anticipated in the design of this study. Most BMs occur within 2 years of diagnosis.6,10,12–14,32,33 Median time to relapse in the brain is 5.7 to 11.7 months.10,12–14,32 Gaspar et al33 reviewed timing of BM in patients treated on several Southwest Oncology Group studies. Only 17% of the BMs occurred more than 12 months after treatment. It is important to note that the outcomes of RTOG 0214 are measured from time of accrual, not from time of diagnosis. Time of accrual could be as long as 16 weeks after completion of therapy and is likely a minimum of 8 additional weeks from diagnosis.
The selection process in this study may have unintentionally selected patients with a low risk of BM. Primary treatment varied from radiation alone to chemotherapy, radiation, and surgery; therefore the primary therapeutic intervention alone does not explain the relatively long survival in this study. Long survival may be partially due to selection of patients with favorable prognostic factors, including low-volume clinical and subclinical disease that may predict for lower rates of BM. Additionally, many potential patients may not have been eligible because of disease failures occurring after the completion of therapy but before consideration of study enrollment.
There are patients for whom prevention of BM will result in cure and for whom treatment with PCI should be considered either on an individual basis or, preferably, on a clinical trial. Defining the cohort of high-risk patients is difficult, because it is dependent on reports that often have conflicting results. Although acute and late toxicities of PCI are acceptable and QoL is not different between the PCI and observation arms in this study, there was a decline in NCF. Therefore, it is important to identify patients who have a very low risk of BM for whom PCI is unnecessary and for whom even minimal NCF deficits are not acceptable.
Pretreatment factors that consistently predict for high rates of BM include histology,4,5,9,34 extent of disease,14,32,35 and young age.12,14,33 Serum carcinoembryonic antigen in adenocarcinoma of the lung36 and immunohistochemical staining for vascular endothelial growth factor C (VEGF-C) in LA-NSCLC37 have also been associated increased risk of BM.
Patients with more advanced disease are at higher risk for BM and if locoregional disease is controlled, these patients are the most likely to derive a survival benefit from PCI. Reviews of patients treated surgically have shown that mediastinal nodes ≥ 2 cm in diameter,14 stage IIIB versus IIIA,32 and greater number of lymph nodes and nodal regions35 predict for high rates of BM.
It is generally understood that BMs develop more often with adenocarcinoma and large-cell carcinoma than with squamous cell carcinoma.4,5,9,34 However, not all studies have shown a significant correlation3,6,11,14,31,32,38 and have shown that patients with locally advanced disease and control of disease with aggressive multimodality therapy are at high risk for BM regardless of histology. Although assessment of risk factors for failure was not reliable because of the low number of BM, nonsquamous histology in this study was the only factor other than observation that was associated with an increased risk of BM.
Several reviews have shown an association between younger age and increased risk of BM from lung cancer.12,14,33 Age younger than 50 to 60 years has been associated with an increased overall risk of BM14,33 and of brain as first site of failure.12 Other series have not shown an increased risk of BM with young age.13,32
Future studies assessing prophylactic treatment should focus on the highest risk patients, including those with individual or combination of poor-risk features, including adenocarcinoma, high-volume disease, young age, and predictive biomarkers. Appropriate timing of PCI is critical, as the patients at highest risk for failure are more likely to experience failure early. Attention should be paid to minimizing the risk of PCI with selective RT planning or neuroprotectants.
In conclusion, this study has shown that PCI decreases BM in patients with LA-NSCLC. Additional follow-up is necessary to assess a possible survival advantage of PCI and improve our understanding of the impact of PCI on failure patterns and NCF and QoL. However at this time, PCI is not recommended as standard therapy on the basis of this study or the available data, because there is no evidence of a survival benefit in patients with LA-NSCLC.
Footnotes
See accompanying article on page 279
Supported by Grants No. RTOG U10 CA21661, CCOP U10 CA37422, and Stat U10 CA32115 from the National Cancer Institute.
Presented at the 45th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009, Orlando, FL.
This article's contents are the sole responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00048997.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: James A. Bonner, Bristol-Myers Squibb (C), ImClone Systems (C), Oncolytics Biotech (U) Stock Ownership: None Honoraria: James A. Bonner, Bristol-Myers Squibb, ImClone Systems, Oncolytics Biotech Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Elizabeth M. Gore, Stuart J. Wong, Alexander Sun, James A. Bonner, Laurie E. Gaspar, Hak Choy
Provision of study materials or patients: Alexander Sun, James A. Bonner, Steven E. Schild, Laurie E. Gaspar, Jeffery A. Bogart, Maria Werner-Wasik, Hak Choy
Collection and assembly of data: Elizabeth M. Gore, Kyounghwa Bae
Data analysis and interpretation: Elizabeth M. Gore, Kyounghwa Bae, Alexander Sun, James A. Bonner, Laurie E. Gaspar
Manuscript writing: Elizabeth M. Gore, Kyounghwa Bae, Stuart J. Wong, Alexander Sun, James A. Bonner, Steven E. Schild, Laurie E. Gaspar, Jeffery A. Bogart, Maria Werner-Wasik, Hak Choy
Final approval of manuscript: Elizabeth M. Gore, Kyounghwa Bae, Stuart J. Wong, Alexander Sun, James A. Bonner, Steven E. Schild, Laurie E. Gaspar, Jeffery A. Bogart, Maria Werner-Wasik, Hak Choy
REFERENCES
- 1.Cox JD, Stanley K, Petrovich Z, et al. Cranial irradiation in cancer of the lung of all cell types. JAMA. 1981;245:469–472. [PubMed] [Google Scholar]
- 2.Russell AH, Pajak TE, Selim HM, et al. Prophylactic cranial irradiation for lung cancer patients at high risk for development of cerebral metastasis: Results of a prospective randomized trial conducted by the Radiation Therapy Oncology Group. Int J Rad Onc Biol Phys. 1991;21:637–643. doi: 10.1016/0360-3016(91)90681-s. [DOI] [PubMed] [Google Scholar]
- 3.Umsawasdi T, Valdivieso M, Chen TT, et al. Role of elective brain irradiation during combined chemoradiotherapy for limited disease non–small-cell lung cancer. J Neurooncol. 1984;2:253–259. doi: 10.1007/BF00253278. [DOI] [PubMed] [Google Scholar]
- 4.Andre F, Grunenwald D, Pujol JL, et al. Patterns of relapse of N2 non–small-cell lung carcinoma patients treated with preoperative chemotherapy. Cancer. 2001;91:2394–2400. [PubMed] [Google Scholar]
- 5.Cox JD, Scott CB, Byhardt RW, et al. Addition of chemotherapy to radiation therapy alters failure patterns by cell type within non–small-cell carcinoma of lung: Analysis of Radiation Therapy Oncology Group trials. Int J Radiat Oncol. 1999;43:505–509. doi: 10.1016/s0360-3016(98)00429-5. [DOI] [PubMed] [Google Scholar]
- 6.Law A, Karp DD, Dipetrillo T, et al. Emergence of increased cerebral metastasis after preoperative radiotherapy with chemotherapy in patients with locally advanced non–small-cell lung carcinoma. Cancer. 2001;92:160–164. doi: 10.1002/1097-0142(20010701)92:1<160::aid-cncr1304>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Arriagada R, Le Chevalier T, Quoix E, et al. ASTRO (American Society for Therapeutic Radiology and Oncology) plenary: Effect of chemotherapy on locally advanced non-small cell lung carcinoma—A randomized study of 353 patients: GETCB (Groupe d'Etude et Traitement des Cancers Bronchiques), FNCLCC9Federation Nationale des Centres de Lutte contre le Cancer) and the CEBI trialists. Int J Radiat Oncol Biol Phys. 1991;20:1183–1190. doi: 10.1016/0360-3016(91)90226-t. [DOI] [PubMed] [Google Scholar]
- 8.Komaki R, Scott CB, Sause WT, et al. Induction cisplatin/vinblastine and irradiation vs. irradiation in unresectable squamous cell lung cancer: Failure patterns by cell type in RTOG 88-08/ECOG 4588. Int J Radiat Oncol Biol Phys. 1997;39:537–544. doi: 10.1016/s0360-3016(97)00365-9. [DOI] [PubMed] [Google Scholar]
- 9.Komaki R, Cox JD, Stark R. Frequency of brain metastases in adenocarcinoma and large cell carcinoma of the lung: Correlation with survival. Int J Radiat Oncol Biol Phys. 1983;9:1467–1470. doi: 10.1016/0360-3016(83)90319-x. [DOI] [PubMed] [Google Scholar]
- 10.Komaki R, Scott CB, Byhardt R, et al. Failure patterns by prognostic group determined by recursive partitioning analysis (RPA) of 1547 patients on four Radiation Therapy Oncology Group (RTOG) studies in inoperable non–small-cell lung cancer. Int J Radiat Oncol Biol Phys. 1998;42:263–267. doi: 10.1016/s0360-3016(98)00213-2. [DOI] [PubMed] [Google Scholar]
- 11.Stuschke M, Eberhardt W, Pöttgen C, et al. Prophylactic cranial irradiation in locally advanced non–small-cell lung cancer after multimodality treatment: Long-term follow-up and investigations of late neuropsychologic effects. J Clin Oncol. 1999;17:2700–2709. doi: 10.1200/JCO.1999.17.9.2700. [DOI] [PubMed] [Google Scholar]
- 12.Carolan H, Sun AY, Bezjak A, et al. Does the incidence and outcome of brain metastases in locally advanced non–small-cell lung cancer justify prophylactic cranial irradiation or early detection? Lung Cancer. 2005;49:109–115. doi: 10.1016/j.lungcan.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Chen AM, Jahan TM, Jablons DM, et al. Risk of cerebral metastases and neurological death after pathological complete response to neoadjuvant therapy for locally advanced non–small-cell lung cancer: Clinical implications for the subsequent management of the brain. Cancer. 2007;109:1668–1675. doi: 10.1002/cncr.22565. [DOI] [PubMed] [Google Scholar]
- 14.Ceresoli GL, Reni M, Chiesa G, et al. Brain metastases in locally advanced non–small-cell lung carcinoma after multimodality treatment: Risk factors analysis. Cancer. 2002;95:605–612. doi: 10.1002/cncr.10687. [DOI] [PubMed] [Google Scholar]
- 15.Mamon HJ, Yeap BY, Jänne PA, et al. High risk of brain metastases in surgically staged IIIA non-small cell lung cancer patients treated with surgery, chemotherapy, and radiation. J Clin Oncol. 2005;23:1530–1537. doi: 10.1200/JCO.2005.04.123. [DOI] [PubMed] [Google Scholar]
- 16.Choi NC, Carey RW, Daly W, et al. Potential impact on survival of improved tumor downstaging and resection rate by preoperative twice-daily radiation and concurrent chemotherapy in stage IIIA non–small-cell lung cancer. J Clin Oncol. 1997;15:712–722. doi: 10.1200/JCO.1997.15.2.712. [DOI] [PubMed] [Google Scholar]
- 17.Wade DT. New York, NY: Oxford University Press; 1992. Measurement in Neurological Rehabilitation. [Google Scholar]
- 18.Benedict RHB, Schretlen D, Groninger L, et al. Hopkins Verbal Learning Test Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsych. 1998;12:43–55. [Google Scholar]
- 19.Osoba D, Aaronson N, Zee B, et al. Modification of the EORTC QLQ-C30 (version 2.0) based on content validity and reliability testing in large samples of patients with cancer: The Study Group on Quality of Life of the EORTC and the Symptom Control and Quality of Life Committees of the NCI of Canada Clinical Trials Group. Qual Life Res. 1997;6:103–108. doi: 10.1023/a:1026429831234. [DOI] [PubMed] [Google Scholar]
- 20.Osoba D, Aaronson NK, Muller M, et al. The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res. 1996;5:139–150. doi: 10.1007/BF00435979. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;35:457–481. [Google Scholar]
- 22.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Canc Chemo Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 23.Kim K, Tsiatis AA. Study duration for clinical trials with survival response and early stopping rule. Biometrics. 1990;46:81–92. [PubMed] [Google Scholar]
- 24.Cox DR. Regression models and life tables. J Royal Stat Soc B. 1972;34:187–200. [Google Scholar]
- 25.Brookmeyer R, Crowley JJ. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 26.Agresti A. New York, NY: Wiley & Sons; 1990. Categorical Data Analysis. [Google Scholar]
- 27.Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small cell lung cancer: Mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13:1880–1892. doi: 10.1200/JCO.1995.13.8.1880. [DOI] [PubMed] [Google Scholar]
- 28.Strauss GM, Herndon JE, Sherman DD, et al. Neoadjuvant chemotherapy and radiotherapy followed by surgery in stage IIIA non–small-cell carcinoma of the lung: Report of a Cancer and Leukemia Group B phase II study. J Clin Oncol. 1992;10:1237–1244. doi: 10.1200/JCO.1992.10.8.1237. [DOI] [PubMed] [Google Scholar]
- 29.Skarin A, Jochelson M, Sheldon T, et al. Neoadjuvant chemotherapy in marginally resectable stage III M0 non–small-cell lung cancer: Long-term follow-up in 41 patients. J Surg Oncol. 1989;40:266–274. doi: 10.1002/jso.2930400413. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs RH, Awan A, Bitran JD, et al. Prophylactic cranial irradiation in adenocarcinoma of the lung: A possible role. Cancer. 1987;59:2016–2019. doi: 10.1002/1097-0142(19870615)59:12<2016::aid-cncr2820591208>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 31.Pöttgen C, Eberhardt W, Grannass A, et al. Prophylactic cranial irradiation in operable stage IIIA non–small-cell lung cancer treated with neoadjuvant chemoradiotherapy: Results from a German multicenter randomized trial. J Clin Oncol. 2007;25:4987–4992. doi: 10.1200/JCO.2007.12.5468. [DOI] [PubMed] [Google Scholar]
- 32.Robnett TJ, Machtay M, Stevenson JP, et al. Factors affecting the risk of brain metastases after definitive chemoradiation for locally advanced non–small-cell lung carcinoma. J Clin Oncol. 2001;19:1344–1349. doi: 10.1200/JCO.2001.19.5.1344. [DOI] [PubMed] [Google Scholar]
- 33.Gaspar LE, Chansky K, Albain KS, et al. Time from treatment to subsequent diagnosis of brain metastases in stage III non-small cell lung cancer: A retrospective review by the Southwest Oncology Group. J Clin Oncol. 2005;23:2955–2961. doi: 10.1200/JCO.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 34.Perez CA, Pajak TF, Rubin P, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Cancer. 1987;59:1874–1881. doi: 10.1002/1097-0142(19870601)59:11<1874::aid-cncr2820591106>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 35.Wang S, Ye X, Ou W, et al. Risk of cerebral metastases for postoperative locally advanced non–small-cell lung cancer. Lung Cancer. 2009;64:238–243. doi: 10.1016/j.lungcan.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Arrieta O, Saavedra-Perez D, Kuri R, et al. Brain metastases development and poor survival associated with carcinoembryonic antigen (CEA) level in advanced non-small cell lung cancer: A prospective analysis. BMC Cancer. 2009;9:119–127. doi: 10.1186/1471-2407-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen G, Liu X, Wang Z, et al. Vascular endothelial growth factor C: The predictor of early recurrence in patients with N2 non-small cell lung cancer. Eur J Cardiothorac Surg. 2010;37:546–551. doi: 10.1016/j.ejcts.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Keith B, Vincent M, Stitt L, et al. Subsets more likely to benefit from surgery or prophylactic cranial irradiation after chemoradiation for localized non–small-cell lung cancer. Am J Clin Oncol. 2002;25:583–587. doi: 10.1097/00000421-200212000-00011. [DOI] [PubMed] [Google Scholar]