Abstract
Purpose
Acute graft-versus-host disease (aGVHD) is a major cause of morbidity and mortality after matched unrelated, related, or mismatched related donor hematopoietic stem-cell transplantation (HSCT). Improved GVHD prevention methods are needed. Pentostatin, an adenosine deaminase inhibitor, leads to lymphocyte depletion with low risk of myelosuppression. We hypothesized that addition of pentostatin to GVHD prophylaxis with tacrolimus and mini-methotrexate may improve outcomes, and we conducted a Bayesian adaptively randomized, controlled, dose-finding study, taking into account toxicity and efficacy.
Patients and Methods
Success was defined as the patient being alive, engrafted, in remission, without GVHD 100 days post-HSCT and no grade ≥ 3 GVHD at any time. Patients were randomly assigned to pentostatin doses of 0, 0.5, 1.0, 1.5, and 2.0 mg/m2 with drug administered on HSCT days 8, 15, 22, and 30. Eligible patients were recipients of mismatched related (n = 10) or unrelated (n = 137) donor HSCT.
Results
Median age was 47 years. Thirty-seven, 10, 29, 61, and 10 patients were assigned to the control and four treatment groups, respectively, with comparable baseline characteristics. Pentostatin doses of 1.0 and 1.5 mg/m2 had the highest success rates (69.0% and 70.5%) versus control (54.1%). The posterior probabilities that the success rates were greater with 1.5 mg/m2 or 1.0 mg/m2 versus control are 0.944 and 0.821, respectively. Hepatic aGVHD rates were 0%, 17.2%, and 11.1%, respectively, for 1.5 mg/m2, 1.0 mg/m2, and control groups. No grades 3 to 4 aGVHD occurred in 11 HLA-mismatched recipients in the 1.5 mg/m2 group.
Conclusion
Pentostatin increased the likelihood of success as defined here, and should be further investigated in larger randomized, confirmatory studies.
INTRODUCTION
Acute graft-versus-host disease (aGVHD) is a major cause of mortality and morbidity after allogeneic hematopoietic stem-cell transplantation (HSCT). High-resolution, allele-level HLA typing has improved the results, but even with donor-recipient high-resolution HLA matching at major histocompatibility complex class I (HLA-A, HLA-B, HLA-C) and class II (HLA-DRB1 and HLA-DQB1), the incidence of grades 2 to 4 aGVHD can be as high as 80% using cyclosporine- or tacrolimus-based prophylaxis.1–6 Outcomes after the development of grades 3 to 4 aGVHD are dismal, with a mortality rate of 60% to 80%. Improved prophylactic approaches are needed.7,8
Pentostatin is a purine analog that inhibits adenosine deaminase, leading to increased lymphocyte apoptosis and decreased interleukin-2 production.9,10 Preclinical data suggests that this drug induces T-lymphocyte functional impairment while sparing natural killer cell and humoral responses.11 In vitro and animal data support activity preventing GVHD, with minimal hematologic toxicity, making pentostatin appealing as peritransplantation therapy.12,13
Pentostatin has been used successfully to treat aGVHD and chronic GVHD (cGVHD).9,10,14,15 In a phase I dose-finding study, a 3-day schedule of the drug at 1.5 mg/m2/d was shown to have significant activity against steroid-refractory aGVHD, with 63% complete responses (CRs) and a 13% partial response rate.9 The drug was well tolerated with no significant hematologic adverse effects. In another study10 of heavily pretreated patients with steroid-refractory cGVHD, pentostatin at 4 mg/m2 given every 2 weeks for 12 doses led to an overall response rate of 55% and 2-year survival of 70%.
We hypothesized that the addition of pentostatin to our standard GVHD prophylaxis regimen (tacrolimus and mini-methotrexate) would reduce the incidence of aGVHD in the context of unrelated and HLA-mismatched related donor transplants. We performed a randomized dose-finding study, seeking to identify the optimal biologic effect of reducing aGVHD incidence. While preserving engraftment, we were not interested in just defining the maximum-tolerated dose, unlike classic phase I studies, but instead took both safety and efficacy into account. In addition, to understand both dose effect and drug effect, we included a control group. Unrelated or mismatched related donor transplantations are associated with higher aGVHD rates than matched related donor HSCT. To minimize patient GVHD risk heterogeneity, we therefore limited our study to this higher-risk population.
Our primary objective was to determine the dose most likely to produce success, which we defined as the patient being alive, in remission, with engraftment, without evidence of grade ≥ 2 aGVHD at 100 days after transplantation and no grade ≥ 3 aGVHD at any time. We used the classic definition of aGVHD (ie, occurring within the first 100 days after transplantation), hence the 100-day end point. Secondary objectives were to determine the safety of the drug in this patient population.
PATIENTS AND METHODS
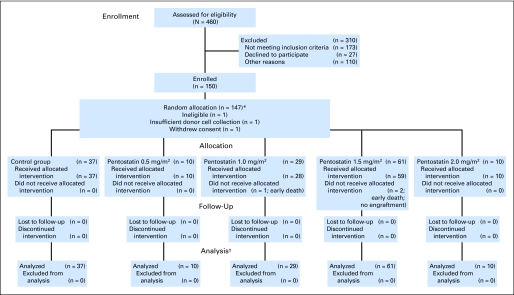
Recipients of unrelated or one-antigen mismatched related donor HSCT for treatment of high-risk and/or advanced hematologic malignancies were eligible. Patients were required to have normal renal, hepatic, pulmonary, and cardiac function. After two patients with myelofibrosis experienced engraftment failure, this disease became an exclusion criterion. Patients were stratified as high-risk if they had active disease at transplantation and/or any HLA mismatch, or low-risk if they were in CR from their malignancy and had no HLA mismatch (Fig 1). GVHD assessment was done according to the consensus criteria.16 The Appendix (online only) contains details.
Fig 1.
CONSORT diagram.
HLA Typing
High-resolution HLA typing was prospectively available for all donor-recipient pairs at HLA-DRB1 and HLA-DQB1 loci and in 93% of the pairs at HLA-A and HLA-B loci; the remaining pairs were retrospectively typed at the HLA-A and HLA-B loci by high-resolution methods, as previously described.17 All patients had intermediate-resolution HLA-C typing.
Treatment Plan
All patients received tacrolimus and methotrexate. Tacrolimus was given intravenously from day −2, targeting a blood level of 5 to 15 ng/mL. Methotrexate 5 mg/m2 was given intravenously on days +1, +3, and +6 (day +11 for control patients only). Pentostatin was given intravenously on days +8, +15, +22, and +30 after transplantation. There were five study arms: control (no pentostatin), and pentostatin at 0.5, 1.0, 1.5, or 2.0 mg/m2. Dose adjustments during treatment were made on the basis of creatinine level: if > 2.0 mg/dL, no pentostatin; if 1.5 to 2.0 mg/dL, there was a 75% dose reduction.
Statistical Design and Analysis
This study was a Bayesian adaptively randomized, five-arm, dose-finding study that took into account toxicity and efficacy. Throughout the trial, dose 0 was assigned with probability 20%, although the probability of assignment to any of the four active treatment doses changed during the trial according to the observed information about success by dose. A maximum of 150 patients were to be enrolled. Depending on accumulating results regarding infections, delay in engraftment, and any pentostatin-related grade 3 or greater toxicity, not every dose was available for administration. Initially, and in keeping with the phase I attitude, only the control and 0.5 mg/m2 doses were administered, and after three patients were treated on the 0.5 mg/m2 arm, with at least two of three of these patients having no drug-associated complications, the next higher dose became available for use. This decision process was repeated to determine whether each successive treatment arm would become available.
The primary efficacy end point was success, defined as the patient being alive, in CR, having engrafted neutrophils, and without grade ≥ 2 aGVHD 100 days after HSCT and no grade ≥ 3 aGVHD at any time. Patients who developed grades 1 to 2 aGVHD and responded to treatment with resolution of the manifestation at day 100 were considered a success. Patients with grades 3 to 4 aGVHD at any time were considered treatment failures regardless of response to GVHD treatment. Those treatment arms that evinced a better success rate received a greater proportion of the patients, with the degree of imbalance depending on the corresponding imbalance in observed success rates.
Specifically, the probability of success in each pentostatin dose group was compared with that in the control arm in the following manner. The observed success rate in the control arm was defined as p0. Similarly, p1, p2, p3, and p4 were the success rates in the 0.5, 1.0, 1.5, and 2.0 mg/m2 pentostatin treatment groups, respectively. Each time a patient entered the trial, we found P(pk > p0 current data) for k = 1, 2, 3, and 4. This is known as the posterior probability, since its calculation is performed after data or results from the study became available. The probability P(pk > p0), or prior probability, is estimated before data from the study are known, and it is based on prior information and/or past experience. Information regarding the prognosis of the patients (high-risk v low-risk) was also used in calculating the posterior probability. This probability was used in adapting the randomization allocation, making decisions on whether to close accrual in treatment arms, and selecting the best pentostatin dose. If at any time P(pk > p0 data) was > 0.99, we would stop the study and select dose k as the best pentostatin dose.
After 30 patients had been evaluated, we calculated the predictive probability that pk could ever be judged to be greater than p0, and we dropped treatment arm k if this predictive probability was < 0.05. The study would stop if all pentostatin treatment arms were dropped by this criterion, and pentostatin would have been declared ineffective at any of the doses studied. We repeated this process with each successive cohort of 10 patients.
The study had a false-positive rate of 0.05 for the null hypothesis in which none of the doses improved success rate over control (success rate anticipated to be 30% for low-risk patients and 15% for high-risk patients). This adaptive Bayesian design had power 0.70 to detect a dose that had a success rate of 60% for low-risk patients and 45% for high-risk patients.
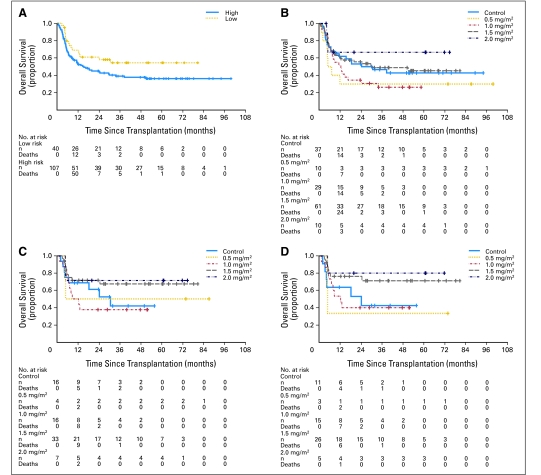
We used the product limit estimator of Kaplan and Meier to estimate overall survival and relapse-free survival, and we used log-rank statistics to compare treatment groups (Fig 2).18 We measured event times from the date of transplantation and used Fisher's exact test to compare aGVHD rates between each pentostatin arm and the control arm. Cumulative incidence of cGVHD was estimated considering early death as a competing risk.19 All analyses are by intention to treat.
Fig 2.
Kaplan-Meier plots of overall survival (A) by risk group. High-risk (n = 107) was defined as presence of active disease at the time of transplantation and/or any donor-recipient HLA mismatch, and low-risk (n = 40) was defined as complete remission at the time of transplantation and no HLA mismatch. (B) Overall survival by pentostatin dose assignment; control group (n = 37), 0.5 mg/m2 (n = 10), 1.0 mg/m2 (n = 29), 1.5 mg/m2 (n = 61), 2.0 mg/m2 (n = 10). (C) Survival of patients in complete response at the time of transplantation; control group (n = 16), 0.5 mg/m2 (n = 4), 1.0 mg/m2 (n = 16), 1.5 mg/m2 (n = 33), 2.0 mg/m2 (n = 7). (D) Overall survival of patients with myeloid disease in complete remission at the time of hematopoietic stem-cell transplantation; control group (n = 11), 0.5 mg/m2 (n = 3), 1.0 mg/m2 (n = 15), 1.5 mg/m2 (n = 26), 2.0 mg/m2 (n = 5). Comparison of pentostatin 1.5 mg/m2 v control: P = .071. All analyses were conducted by intention to treat.
RESULTS
A total of 150 patients were enrolled from November 2000 to December 2007. Three patients were removed from the study without treatment because of ineligibility (n = 1) and because of insufficient donor-cell collection (n = 1; both assigned to the 1.5 mg/m2 arm), although one patient (from the 2.0 mg/m2 arm) withdrew consent before treatment. Median follow-up was 13 months. Patient characteristics were similar across treatment groups (Table 1).
Table 1.
Patient Characteristics
| Characteristic | All Patients (N = 147) |
Dose (mg/m2) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control 0.0 (n = 37) |
Pentostatin |
|||||||||||
| 0.5 (n = 10) |
1.0 (n = 29) |
1.5 (n = 61) |
2.0 (n = 10) |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Age, years | ||||||||||||
| Median | 47 | 50 | 42 | 46 | 50 | 44 | ||||||
| Range | 18-72 | 18-69 | 23-55 | 20-71 | 22-72 | 31-59 | ||||||
| Sex | ||||||||||||
| Male | 83 | 56.5 | 22 | 59.5 | 7 | 70.0 | 18 | 62.1 | 33 | 54.1 | 3 | 30.0 |
| Female | 64 | 43.5 | 15 | 40.5 | 3 | 30.0 | 11 | 37.9 | 28 | 45.9 | 7 | 70.0 |
| Stem-cell source | ||||||||||||
| Peripheral blood | 28 | 19.0 | 8 | 21.6 | 0 | 8 | 27.6 | 11 | 18.0 | 1 | 10.0 | |
| Bone marrow | 119 | 81.0 | 29 | 78.4 | 10 | 100 | 21 | 72.4 | 50 | 82.0 | 9 | 90.0 |
| Conditioning regimen* | ||||||||||||
| BEAM | 2 | 1.4 | 0 | 1 | 10.0 | 0 | 0 | 1 | 10.0 | |||
| BU/FLU | 90 | 61.2 | 25 | 67.6 | 4 | 40.0 | 18 | 62.1 | 38 | 62.3 | 5 | 50.0 |
| BU/FLU/CLO | 3 | 2.0 | 1 | 2.7 | 0 | 0 | 2 | 3.3 | 0 | |||
| BU/CY | 14 | 9.5 | 2 | 5.4 | 3 | 30.0 | 4 | 13.8 | 3 | 4.9 | 2 | 20.0 |
| BU/MEL | 3 | 2.0 | 2 | 5.4 | 0 | 0 | 1 | 1.6 | 0 | |||
| CY/TBI | 14 | 9.5 | 4 | 10.8 | 0 | 1 | 3.5 | 7 | 11.5 | 2 | 20.0 | |
| FLU/MEL | 15 | 10.2 | 2 | 5.4 | 2 | 20.0 | 3 | 10.3 | 8 | 13.1 | 0 | |
| FLU/MEL/GO | 6 | 4.1 | 1 | 2.7 | 0 | 3 | 10.3 | 2 | 3.3 | 0 | ||
| BU-based | 112 | 76.2 | 30 | 81.1 | 8 | 80.0 | 22 | 75.9 | 44 | 72.1 | 8 | 80.0 |
| Regimen type | ||||||||||||
| Ablative | 112 | 76.2 | 28 | 75.7 | 7 | 70.0 | 24 | 82.8 | 45 | 73.8 | 8 | 80.0 |
| RIC | 35 | 23.8 | 9 | 24.3 | 3 | 30.0 | 5 | 17.2 | 16 | 26.2 | 2 | 20.0 |
| Donor | ||||||||||||
| Unrelated | 137 | 93.2 | 33 | 89.2 | 9 | 90.0 | 28 | 96.6 | 57 | 93.4 | 10 | 100 |
| Mismatched-related | 10 | 6.8 | 4 | 10.8 | 1 | 10.0 | 1 | 3.4 | 4 | 6.6 | 0 | |
| Antithymocyte globulin | 134 | 91.2 | 34 | 91.9 | 9 | 90.0 | 28 | 96.6 | 55 | 90.2 | 8 | 80.0 |
| Diagnosis | ||||||||||||
| AML | 84 | 57.1 | 15 | 40.5 | 6 | 60.0 | 23 | 79.3 | 35 | 57.4 | 5 | 50.0 |
| MDS | 21 | 14.3 | 8 | 21.6 | 0 | 1 | 3.5 | 12 | 19.7 | 0 | ||
| CML | 18 | 12.2 | 5 | 13.5 | 2 | 20.0 | 4 | 13.8 | 5 | 8.2 | 2 | 20.0 |
| ALL | 17 | 11.6 | 5 | 13.5 | 0 | 1 | 3.5 | 9 | 14.8 | 2 | 20.0 | |
| Lymphoma | 7 | 4.8 | 4 | 10.8 | 2 | 20.0 | 0 | 0 | 1 | 10.0 | ||
| HLA match | ||||||||||||
| 10/10 | 104 | 70.7 | 27 | 73.0 | 4 | 40.0 | 18 | 62.1 | 48 | 78.7 | 7 | 70.0 |
| 9/10 | 34 | 23.1 | 7 | 18.9 | 3 | 30.0 | 9 | 31.0 | 12 | 19.7 | 3 | 30.0 |
| 8/10 | 6 | 4.1 | 2 | 5.4 | 2 | 20.0 | 2 | 6.9 | 0 | 0 | ||
| 7/10 | 3 | 2.0 | 1 | 2.7 | 1 | 10.0 | 0 | 1 | 1.6 | 0 | ||
| Disease status at time of transplantation | ||||||||||||
| Complete remission | 67 | 45.6 | 15 | 40.5 | 4 | 40.0 | 14 | 48.3 | 29 | 47.5 | 5 | 50.0 |
| CML chronic phase | 9 | 6.1 | 1 | 2.7 | 0 | 2 | 6.9 | 4 | 6.6 | 2 | 20.0 | |
| Active disease | 71 | 48.3 | 21 | 56.8 | 6 | 60.0 | 13 | 44.8 | 28 | 45.9 | 3 | 30.0 |
| Risk group | ||||||||||||
| Low† | 40 | 27.2 | 8 | 21.6 | 0 | 9 | 31.0 | 19 | 31.2 | 4 | 40.0 | |
| High‡ | 107 | 72.8 | 29 | 78.4 | 10 | 100 | 20 | 69.0 | 42 | 68.9 | 6 | 60.0 |
| Graft characteristics (min-max) | ||||||||||||
| Total nucleated cells | ||||||||||||
| Median | 3.0 | 3.4 | 1.1 | 3.9 | 2.7 | 2.3 | ||||||
| Range | 0.2-554.1 | 0.6-21.3 | 0.8-4.4 | 0.3-22.8 | 0.2-16.3 | 0.6-5.54.1 | ||||||
| CD34 | ||||||||||||
| Median | 3.8 | 3.5 | 4.7 | 3.9 | 3.6 | 4.2 | ||||||
| Range | 1.1-29.3 | 1.4-12.7 | 1.7-6.9 | 1.3-29.3 | 1.1-12.4 | 1.6-7.7 | ||||||
| CD3 | ||||||||||||
| Median | 3.8 | 3.1 | 4.9 | 4.1 | 3.6 | 4.2 | ||||||
| Range | 0.9-29.3 | 1.4-12.7 | 0.9-6.9 | 1.3-29.3 | 1.1-12.4 | 1.6-7.7 | ||||||
Abbreviations: BEAM, carmustine, etoposide, cytarabine, melphalan; BU, busulfan; FLU, fludarabine; CLO, clofarabine; CY, cyclophosphamide; MEL, melphalan; TBI, total body irradiation; GO, gemtuzumab ozogamicin; RIC, reduced-intensity conditioning; AML, acute myeloid leukemia; MDS, myelodysplastic syndromes; CML, chronic myelogenous leukemia; ALL, acute lymphocytic leukemia.
Conditioning regimen: BU/MEL: busulfan 130 mg/m2 for 4 days, melphalan 70 mg/m2 for 2 days; FLU/MEL/MYLO: fludarabine 30 mg/m2 intravenously daily for 4 days, melphalan 140 mg/m2, gemtuzumab ozogamicin 2 mg/m2; BU/CY: busulfan 3.2 mg/kg intravenously daily for 4 days, cyclophosphamide 60 mg/kg for 2 days; BU/FLU: busulfan 130 mg/m2 intravenously daily for 4 days, fludarabine 40 mg/m2 intravenously daily for 4 days; CY/TBI: cyclophosphamide 120 mg/kg, total body irradiation at 12 Gy; BU/FLU/CLO: busulfan 130 mg/m2 intravenously daily for 4 days, fludarabine 40 mg/m2 for 4 days, clofarabine 40 mg/m2 intravenous daily for 4 days; BEAM: carmustine 300 mg/m2, etoposide 200 mg/m2 for 4 days, cytarabine 200 mg/m2 for4 days, melphalan 140 mg/m2 for 1 day; FLU/MEL: fludarabine 25 mg/m2 for 5 days, melphalan 70 mg/m2 for 2 days.
Low-risk: complete remission at the time of transplantation and no HLA mismatch.
High-risk: active disease at the time of transplantation or any HLA mismatch.
Treatment Success Rate
The success rates in the 1 mg/m2, 1.5 mg/m2, and control arms were 69%, 70.5%, and 54.1%, respectively (Table 2). The final posterior probability that the success rate in the 1.5 mg/m2 arm was higher than in the control arm was 0.944, and it was 0.821 for the 1 mg/m2 dose. Although the success rates for the 1.0 mg/m2 and 1.5 mg/m2 arms were similar, the 1.5 mg/m2 arm had twice as many patients assigned to it because of early treatment failures for patients assigned to the 1.0 mg/m2 dose. For example, three of the first six and four of the first 10 patients assigned to the 1.0 mg/m2 arm experienced treatment failure, whereas only one of the first six and two of the first 10 patients assigned to the 1.5 mg/m2 arm experienced treatment failure. At the end of the trial, the randomization probabilities were 0.342 (1.0 mg/m2) and 0.452 (1.5 mg/m2), with probabilities of 0.002 and 0.003 being assigned to the 0.5 mg/m2 and 2.0 mg/m2 dose levels, respectively.
Table 2.
Success Rates
| Patient Status | No. of Patients | Dose (mg/m2) |
Total (N = 147) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control 0.0 (n = 37) |
Pentostatin |
||||||||||||
| 0.5 (n = 10) |
1.0 (n = 29) |
1.5 (n = 61) |
2.0 (n = 10) |
||||||||||
| No.* | % | No.* | % | No.* | % | No.* | % | No.* | % | No.* | % | ||
| High-risk† | 107 | 14/29 | 48.3 | 3/10 | 30.0 | 13/20 | 65.0 | 28/42 | 66.7 | 3/6 | 50.0 | 61/107 | 57.0 |
| Low-risk‡ | 40 | 6/8 | 75.0 | 0 | 0.0 | 7/9 | 77.8 | 15/19 | 79.0 | 1/4 | 25.0 | 29/40 | 72.5 |
| Complete remission | 76 | 10/16 | 62.5 | 2/4 | 50.0 | 12/16 | 75.0 | 28/33 | 84.9 | 3/7 | 42.9 | 55/76 | 72.4 |
| Active disease | 71 | 10/21 | 47.6 | 1/6 | 16.7 | 8/13 | 61.5 | 15/28 | 53.6 | 1/3 | 33.3 | 35/71 | 49.3 |
| HLA match | 104 | 13/27 | 48.2 | 2/4 | 50 | 14/18 | 77.8 | 34/48 | 70.8 | 3/7 | 42.9 | 66/104 | 63.5 |
| HLA mismatch | 43 | 7/10 | 70.0 | 1/6 | 16.7 | 6/11 | 54.6 | 9/13 | 69.2 | 1/3 | 33.3 | 24/43 | 55.8 |
| All patients | 147 | 20/37 | 54.1 | 3/10 | 30.0 | 20/29 | 69.0 | 43/61 | 70.5 | 4/10 | 40.0 | 90/147 | 61.2 |
NOTE. Success is defined as patient is alive, in remission, without evidence of grade 2 acute graft-versus-host disease (aGVHD) at day 100, and absence of grades 3 to 4 GVHD at anytime.
Indicates number of events/patients at risk.
High-risk: active disease at the time of transplantation or any HLA mismatch.
Low-risk: complete remission at the time of transplantation and no HLA mismatch.
Engraftment
Eight patients experienced graft failure: control arm, n = 1 (2.7%) and pentostatin arms, n = 7 (6.4%). There were six primary graft failures and two secondary failures. Three graft failures occurred in the 1.5 mg/m2 arm and illustrate the learning curve associated with using the drug in this context. There were two additional primary graft failures: both patients had splenomegaly and a diagnosis of myelofibrosis/myelodysplasia (both received reduced-intensity conditioning). Subsequently, myelofibrosis became an exclusion criterion for the study. The two secondary graft failures occurred in patients receiving treatment doses of ganciclovir (one with mild renal dysfunction).
GVHD
Grades 1 to 4 aGVHD rates were 66.2% (92 of 139 engrafted patients), grades 2 to 4 were 43.9%, and grades 3 to 4 were 15.8% (Table 3). The median time to aGVHD was 27.5 days (range, 8 to 97 days). A nonsignificant trend toward less grade 2 to 4 and 3 to 4 aGVHD was seen in the pentostatin 1.5 mg/m2 arm (37.5% v 55.6%; P = .085) when compared with control (10.7% v 19.4%: P = .358). Four patients in the control group developed hepatic GVHD, as opposed to none of the 66 patients receiving pentostatin 1.5 mg/m2 or 2.0 mg/m2. Furthermore, among donor-recipient pairs with < 10/10 HLA match, no grades 3 to 4 aGVHD were seen in the pentostatin 1.5 mg/m2 or 2.0 mg/m2 groups (Table 3). There was no difference in the proportion of patients taking steroids at day 100 among different subgroups, but there was a higher response rate to steroids among patients with GVHD in the 1.5 mg/m2 arm. The cumulative incidence of cGVHD at 3.5 years was 43.6%, 40.0%, 46.2%, 46.8%, and 83.3% in the control and pentostatin 0.5, 1.0, 1.5, and 2.0 mg/m2 dose groups, respectively.
Table 3.
aGVHD Incidence and Distribution
| Variable | No. of Patients | Dose (mg/m2) |
All (n = 139) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control 0.0 (n = 36) |
Pentostatin |
||||||||||||||||
| 0.5 (n = 8) |
1.0 (n = 29) |
1.5 (n = 56) |
2.0 (n = 10) |
||||||||||||||
| No.* | % | No.* | % | P | No.* | % | P | No.* | % | P | No.* | % | P | No.* | % | ||
| Grades 2 to 4 aGVHD | 20 | 55.6 | 4 | 50.0 | .999 | 12 | 41.4 | .321 | 20 | 35.7 | .085 | 5 | 50 | .999 | 61 | 43.9 | |
| HLA 10/10 | 100 | 15/27 | 55.6 | 2/4 | 50 | 3/18 | 16.7 | 16/45 | 35.6 | 4/7 | 57.1 | 40/100 | 40.0 | ||||
| HLA < 10/10 | 39 | 5/9 | 55.6 | 2/5 | 40 | 9/11 | 81.8 | 4/11 | 36.4 | 1/3 | 33.3 | 21 | 53.8 | ||||
| Grades 3 to 4 aGVHD | 7 | 19.4 | 3 | 37.5 | .355 | 5 | 17.2 | .999 | 6 | 10.7 | .358 | 1 | 10 | .664 | 22 | 15.8 | |
| HLA 10/10 | 100 | 6/27 | 22.2 | 1/3 | 33.3 | 0/18 | 0 | 6/45 | 13.3 | 1/7 | 14.3 | 14 | 14.0 | ||||
| HLA < 10/10 | 39 | 1/9 | 11.1 | 2/5 | 40 | 5/11 | 45.5 | 0/11 | 0 | 0/3 | 0 | 8 | 20.5 | ||||
| Organs affected by grades 2 to 4 aGVHD | |||||||||||||||||
| Skin | 17 | 47.2 | 4 | 50 | 11 | 37.9 | 18 | 32.1 | 5 | 50 | 55 | 39.5 | |||||
| GI tract | 11 | 30.5 | 3 | 37.5 | 5 | 17.2 | 13 | 23.2 | 2 | 20 | 34 | 24.4 | |||||
| Liver | 4 | 11.1 | 2 | 25 | 5 | 17.2 | 0 | 0 | 11 | 7.9 | |||||||
| Time to grades 2 to 4 aGVHD, days | |||||||||||||||||
| Median | 33.5 | 21 | 20 | 23 | 27 | 25 | |||||||||||
| Range | 8-52 | 11-27 | 11-73 | 9-69 | 11-49 | 8-73 | |||||||||||
| Use of steroids on day 100 | 11/33 | 33.3 | 2/8 | 25 | .999 | 7/26 | 26.9 | .777 | 21/55 | 36.0 | .819 | 6/10 | 60.0 | .158 | 47/132 | 35.6 | |
| Response to steroids used for GVHD treatment | 9/22 | 40.9 | 1/4 | 25 | .999 | 14/17 | 82.4 | .020 | 31/40 | 77.5 | .006 | 4/9 | 44.4 | .999 | 59/92 | 64.1 | |
NOTE. P values are from Fisher's exact test for comparison with control arm.
Abbreviation: aGVHD, acute graft-versus-host disease.
Indicates number of events/patients at risk.
Toxicity
Overall, 90% of the intended doses of pentostatin were delivered. Among the engrafted patients, assignment to the treatment arms did not delay neutrophil or platelet engraftment (Table 4) or achievement of donor chimerism. The drug is potentially nephrotoxic, and the rates of mild (grades 2 to 3) renal toxicity were higher among patients receiving pentostatin when compared with controls (Table 4). Thrombotic-thrombocytopenic purpura has occurred in patients treated with pentostatin.20 In our study, we observed two cases in the control arm and five cases in the study arms. Rates of fungal, bacterial, and cytomegalovirus infection/reactivation were comparable in all study arms. In addition, rates of early death, disease recurrence, and no response to HSCT were similar (Table 4).
Table 4.
Engraftment, Mortality, and Toxicity
| Variable | Dose (mg/m2) |
Total (n = 147) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contro l0.0 (n = 37) |
Pentostatin |
|||||||||||
| 0.5 (n = 10) |
1.0 (n = 29) |
1.5 (n = 61) |
2.0 (n = 10) |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Engraftment | ||||||||||||
| Time to neutrophil engraftment, days* | ||||||||||||
| Median | 13 | 15 | 12 | 12 | 13 | 13 | ||||||
| Range | 8-23 | 10-20 | 10-22 | 8-24 | 11-25 | 8-25 | ||||||
| Time to platelet engraftment, days† | ||||||||||||
| Median | 18 | 21 | 15 | 15 | 22 | 16 | ||||||
| Range | 10-177 | 11-70 | 9-85 | 9-90 | 12-71 | 9-177 | ||||||
| Day-100 mortality | ||||||||||||
| Overall mortality | 5 | 13.5 | 2 | 20 | 4 | 13.8 | 6 | 9.8 | 0 | 17 | 11.6 | |
| Nonrelapse mortality | 3 | 8.1 | 1 | 10 | 3 | 10.3 | 5 | 8.2 | 0 | 12 | 8.2 | |
| Cause of death | ||||||||||||
| GVHD | 3 | 8.1 | 0 | 3 | 10.3 | 3 | 4.9 | 0 | 9 | 6.1 | ||
| Infection | 0 | 1 | 10.0 | 0 | 0 | 0 | 1 | 0.7 | ||||
| Relapse | 2 | 5.4 | 1 | 10.0 | 1 | 3.4 | 1 | 1.6 | 0 | 5 | 3.4 | |
| Other | 0 | 0 | 0 | 2 | 3.3 | 0 | 2 | 1.4 | ||||
| Day-100 relapse rate | 6 | 16.2 | 4 | 40 | 3 | 10.3 | 6 | 9.8 | 1 | 10 | 20 | 13.6 |
| No response to HSCT | 2 | 5.4 | 1 | 10.0 | 1 | 3.4 | 3 | 4.9 | 0 | 7 | 4.8 | |
| Transplantation-related toxicity | ||||||||||||
| Graft failure | 1 | 2.7 | 2 | 20 | 0 | 5* | 8.2 | 0 | 8 | 5.4 | ||
| Prolongation of neutrophil engraftment time beyond 21 days‡ | 4 | 10.8 | 0 | 0.0 | 2 | 6.9 | 2 | 3.3 | 1 | 10.0 | 9 | 6.1 |
| Early death (within 30 days from SCT) | 0 | 0 | 1 | 3.4 | 2 | 3.3 | 0 | 3 | 2.0 | |||
| Renal toxicity ‡ | ||||||||||||
| Creatinine elevation | ||||||||||||
| Grade 1 | 9 | 24.3 | 5 | 50.0 | 3 | 10.3 | 10 | 16.4 | 1 | 10.0 | 28 | 19.0 |
| Grade 2 | 2 | 5.4 | 1 | 10.0 | 1 | 3.4 | 8 | 13.1 | 3 | 30.0 | 15 | 10.2 |
| Grade 3 | 0 | 0 | 0 | 3 | 4.9 | 0 | 3 | 2.0 | ||||
| TTP/HUS | 2 | 5.4 | 0 | 0 | 3 | 4.9 | 2 | 20 | 7 | 4.8 | ||
| Infectious complications | ||||||||||||
| Bacterial | 24 | 64.9 | 6 | 60.0 | 17 | 58.6 | 38 | 62.3 | 7 | 70.0 | 92 | 62.6 |
| Viral | 23 | 62.2 | 4 | 40.0 | 15 | 51.7 | 7 | 70.0 | 89 | |||
| Fungal | 5 | 13.5 | 2 | 20.0 | 7 | 24.1 | 1 | 10.0 | 24 | 16.3 | ||
| Parasite | 0 | 0 | 1 | 3.4 | 0 | 2 | 1.4 | |||||
| CMV | 21 | 56.8 | 3 | 30.0 | 12 | 41.4 | 27 | 44.3 | 5 | 50.0 | 68 | 46.3 |
Abbreviations: GVHD, graft-verus-host disease; HSCT, hematopoietic stem-cell transplantation; SCT, stem-cell transplantation; TTP/HUS, thrombotic thrombocytopenic purpura/hemolytic uremic syndrome; CMV, cytomegalovirus.
The initial two graft failures had a diagnosis of chronic myelomonocytic leukemia and myelodysplastic syndromes with myelofibrosis and subsequently this was made an exclusion criteria. Thereafter, three additional cases of graft failures incurred in the pentostatin 1.5 mg/m2 arm.
Thirteen patients did not engraft platelets: two at control dose 0.0 mg/m2, three at dose 0.5 mg/m2, two at dose 1.0 mg/m2, six at dose 1.5 mg/m2.
In 0.5 mg/m2 dose group, one patient had renal failure in the context of multiple organ failure; in 1.5 mg/m2 dose group, one patient required dialysis.
Relapse and Survival
Twenty patients (13.6%) experienced relapse of their malignancy by 100 days after transplantation (Table 4). Eighty-one patients (55.1%) have died. The 5-year actuarial survival rate was 41%. The median survival for patients in CR treated in the pentostatin 1.5 mg/m2 arm (n = 29) was not reached, compared with 24 months for control patients (n = 15; P = .178). Furthermore, among patients with myeloid disease in CR, there was also a trend toward improved survival favoring patients treated with pentostatin 1.5 mg/m2 (n = 22) over the control arm (n = 10; P = .071). However, no survival advantage was observed for patients with active disease at HSCT (median overall survival of 13.6 months in the 1.5 mg/m2 arm v 18.4 months for controls; P = .46). Day-100 all-cause mortality was 11.6% for the whole cohort, and the nonrelapse mortality rate was 8.2% (Table 4).
DISCUSSION
We describe the first prospective adaptively randomized study to evaluate the role of pentostatin as a prophylactic aGVHD agent. We identified one and possibly two doses (1.5 mg/m2 and 1.0 mg/m2) that are promising with improved success rate. The absence of hepatic aGVHD in the 1.5 mg/m2 subgroup was notable. We consider the adaptive randomization method used here to have advantages in dose-finding, taking efficacy (engraftment, response) as well as toxicity (aGVHD, early death) into consideration in a single clinical trial for determining the optimal drug dose.21 Moreover, trial participants were more likely to be exposed to a dose that was not only less toxic but also more efficacious. We defined success as a composite of clinically meaningful and relevant end points (not only the absence of aGVHD). To the best of our knowledge, this type of design has never been used in the GVHD context.22 The overall success was 70.5% for the pentostatin 1.5 mg/m2 group versus 54.1% for controls, with reduced aGVHD rates. Furthermore, pentostatin was not associated with an increased relapse rate.
Grades 2 to 4 and 3 to 4 aGVHD rates of 56% and 19% in the control group were similar to the generally reported experience.17,23 Of note, the distribution of relevant baseline characteristics, such as proportion of patients with active disease, use of peripheral blood stem cells, use of myeloablative regimens, and use of antitymocyte globulin (ATG) was similar across treatment arms.
Pentostatin 1.5 mg/m2 was associated with no instances of grades 3 to 4 aGVHD in patients with < 10/10 HLA matched donor. No hepatic GVHD was observed in recipients of the higher pentostatin dose levels of 1.5 mg/m2 and 2 mg/m2. Similar to what has been documented in several aGVHD studies, cGVHD rates were comparable in the control and pentostatin arms. It is important to note, however, the low rates of cGVHD overall (including the control cohort). We do not have a clear explanation for that, but it may be related to the use of ATG and bone marrow as the stem-cell source in most transplants. In addition, although the proportion of patients given steroids to treat aGVHD on day 100 was similar across study groups, the response to treatment was significantly improved in the 1.5 mg/m2 group.
Acknowledging the limitations of subset analyses, there was a trend toward a survival advantage for patients in CR treated with pentostatin 1.5 mg/m2. We found a similar trend among patients with myeloid leukemia who had transplantations in CR (P = .071). This effect was evident only with longer follow-up and was not present in the subgroup of patients not in CR at HSCT. This potential advantage will need to be evaluated and confirmed in a larger cohort of patients.
Pentostatin is not devoid of risks, and a learning curve was evident in this study. Engraftment failure occurred in eight patients, including two patients with fibrotic marrows, suggesting that such patients should not be treated with this drug. Careful monitoring of renal function and avoidance of ganciclovir during pentostatin administration is also required. Nephrotoxicity is a well-known risk with the drug, and dose adjustments are necessary in the presence of renal failure.14,24
Infectious complications were equally distributed in the study and control arms. Although late infections and cytomegalovirus reactivation have been reported with higher pentostatin doses in GVHD treatment studies,10,14 we did not see such a trend here. Interestingly, we did not observe an increased relapse rate in the pentostatin arms, suggesting no major interference with the graft-versus-leukemia effect.
We conclude that pentostatin at a dose of 1.5 mg/m2 given prophylactically with tacrolimus and mini-methotrexate, leads to an increased success rate following unrelated donor or mismatched related allogeneic HSCT. Success was defined as the patient being alive, engrafted, in remission, and without signs and symptoms of aGVHD after HSCT. The 1.5 mg/m2 dose appeared more promising, considering the low rate of hepatic GVHD and the higher posterior probability of success when compared with controls. The drug also appeared to be active in the subgroup of mismatched unrelated donor transplantations as well. A prospective randomized trial comparing GVHD prophylaxis with and without this purine analog is warranted to definitively assess the efficacy of pentostatin in the setting of allogeneic HSCT.
Although this initial study with an adaptive randomization approach is no substitute for a large-scale, randomized phase III trial in disease-specific and conditioning therapy–specific populations, it was useful for obtaining estimates of the effectiveness of adding pentostatin to the GVHD prophylaxis armamentarium in an efficient manner. Our data can be used to support therapeutic decision making while awaiting the outcome of more definitive studies. Considering that ours was a phase I/II study, we had to incorporate a relatively short yet meaningful end point because of the logistics of conducting the trial. From a phase I perspective, 100 days was adequate time to detect toxicities and most direct drug effects. Our initial goal in the phase I portion was to define the dose and to innovatively use a statistical approach that allowed us to move seamlessly to the phase II portion of the trial incorporating efficacy in the measured outcome. Currently, our results support the use of pentostatin at a dose of 1.5 mg/m2 once weekly, as described, in patients with unrelated or one-antigen mismatched donors undergoing allogeneic HSCT for advanced hematologic malignancies. Future studies should use the defined pentostatin dose and provide longer-term end points.
Acknowledgment
We thank Morgani Rodrigues, MD, for contributing to data collection.
Appendix
Biopsies were obtained in all instances of graft-versus-host disease (GVHD) involving the skin, and in most cases of GI and hepatic GVHD. Biopsy specimens were read by pathologists who were unaware of study assignment. All GVHD cases were prospectively and retrospectively reviewed and staged by the primary attending physician, a research nurse, and two investigators (D.C. and M.D.L.). Patients who developed acute GVHD were treated with methylprednisone 2 mg/kg initially and then tapered as tolerated. The protocol was approved by the institutional review board, and all patients provided written informed consent. The MD Anderson Data Safety Monitoring Board monitored the conduct of the study.
Supportive Care
All patients received supportive care according to established routine or extant institutional protocols. Filgrastim was administered subcutaneously daily from day 7 after transplantation until absolute neutrophil count was > 500/μL for 3 days.
Footnotes
Supported in part by National Cancer Institute Grant No. P30 CA016672.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00506922.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Daniel Couriel, SuperGen Research Funding: Marcos J. de Lima, SuperGen Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Mark F. Munsell, Sergio A. Giralt, J. Kyle Wathen, Donald Berry, Richard E. Champlin, Marcos J. de Lima
Provision of study materials or patients: Borje S. Andersson, Daniel Couriel, Roy B. Jones, Elizabeth J. Shpall, Uday Popat, Paolo Anderlini, Sergio A. Giralt, Amin Alousi, Chitra Hosing, Richard E. Champlin, Marcos J. de Lima
Collection and assembly of data: Simrit Parmar, Doyle Bosque, Leandro de Padua Silva, Michael Westmoreland, Marcos J. de Lima
Data analysis and interpretation: Simrit Parmar, Mark F. Munsell, Marcelo Fernandez-Vina, Pedro Cano, J. Kyle Wathen, Richard E. Champlin, Marcos J. de Lima
Manuscript writing: Simrit Parmar, Borje S. Andersson, Daniel Couriel, Mark F. Munsell, Marcelo Fernandez-Vina, Roy B. Jones, Elizabeth J. Shpall, Uday Popat, Paolo Anderlini, Sergio A. Giralt, Amin Alousi, Pedro Cano, Doyle Bosque, Chitra Hosing, Leandro de Padua Silva, Michael Westmoreland, J. Kyle Wathen, Donald Berry, Richard E. Champlin, Marcos J. de Lima
Final approval of manuscript: Simrit Parmar, Borje S. Andersson, Daniel Couriel, Mark F. Munsell, Marcelo Fernandez-Vina, Roy B. Jones, Elizabeth J. Shpall, Uday Popat, Paolo Anderlini, Sergio A. Giralt, Amin Alousi, Pedro Cano, Doyle Bosque, Chitra Hosing, Leandro de Padua Silva, Michael Westmoreland, J. Kyle Wathen, Donald Berry, Richard E. Champlin, Marcos J. de Lima
REFERENCES
- 1.Hurley CK, Baxter Lowe LA, Logan B, et al. National Marrow Donor Program HLA-matching guidelines for unrelated marrow transplants. Biol Blood Marrow Transplant. 2003;9:610–615. doi: 10.1016/j.bbmt.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Madrigal JA, Scott I, Arguello R, et al. Factors influencing the outcome of bone marrow transplants using unrelated donors. Immunol Rev. 1997;157:153–166. doi: 10.1111/j.1600-065x.1997.tb00980.x. [DOI] [PubMed] [Google Scholar]
- 3.Ho VT, Kim HT, Liney D, et al. HLA-C mismatch is associated with inferior survival after unrelated donor non-myeloablative hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;37:845–850. doi: 10.1038/sj.bmt.1705315. [DOI] [PubMed] [Google Scholar]
- 4.Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104:1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 6.Teshima T, Matsuo K, Matsue K, et al. Impact of human leucocyte antigen mismatch on graft-versus-host disease and graft failure after reduced intensity conditioning allogeneic haematopoietic stem cell transplantation from related donors. Br J Haematol. 2005;130:575–587. doi: 10.1111/j.1365-2141.2005.05632.x. [DOI] [PubMed] [Google Scholar]
- 7.Goulmy E, Schipper R, Pool J, et al. Mismatches of minor histocompatibility antigens between HLA-identical donors and recipients and the development of graft-versus-host disease after bone marrow transplantation. N Engl J Med. 1996;334:281–285. doi: 10.1056/NEJM199602013340501. [DOI] [PubMed] [Google Scholar]
- 8.Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–2314. [PubMed] [Google Scholar]
- 9.Bolaños-Meade J, Jacobsohn DA, Margolis J, et al. Pentostatin in steroid-refractory acute graft-versus-host disease. J Clin Oncol. 2005;23:2661–2668. doi: 10.1200/JCO.2005.06.130. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsohn DA, Chen AR, Zahurak M, et al. Phase II study of pentostatin in patients with corticosteroid-refractory chronic graft-versus-host disease. J Clin Oncol. 2007;25:4255–4261. doi: 10.1200/JCO.2007.10.8456. [DOI] [PubMed] [Google Scholar]
- 11.Higman M, Vogelsang GB, Chen A. Pentostatin: Pharmacology, immunology, and clinical effects in graft-versus-host disease. Expert Opin Pharmacother. 2004;5:2605–2613. doi: 10.1517/14656566.5.12.2605. [DOI] [PubMed] [Google Scholar]
- 12.Epstein J, Bealmear PM, Kennedy DW, et al. Prevention of graft-versus-host disease in allogeneic bone marrow transplantation by pretreatment with 2′-deoxycoformycin. Exp Hematol. 1986;14:845–849. [PubMed] [Google Scholar]
- 13.Aye MT, Dunne JV. Effect of 2′-deoxycoformycin on erythroid, granulocytic, and T-lymphocyte colony growth. Blood. 1981;58:1043–1046. [PubMed] [Google Scholar]
- 14.Jacobsohn DA, Gilman AL, Rademaker A, et al. Evaluation of pentostatin in corticosteroid-refractory chronic graft-versus-host disease in children: A Pediatric Blood and Marrow Transplant Consortium study. Blood. 2009;114:4354–4360. doi: 10.1182/blood-2009-05-224840. [DOI] [PubMed] [Google Scholar]
- 15.Alousi AM, Weisdorf DJ, Logan BR, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: A randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114:511–517. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 17.Parmar S, Del Lima M, Zou Y, et al. Donor-recipient mismatches in MHC class I chain-related gene A in unrelated donor transplantation lead to increased incidence of acute graft-versus-host disease. Blood. 2009;114:2884–2887. doi: 10.1182/blood-2009-05-223172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Leach JW, Pham T, Diamandidis D, et al. Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome (TTP-HUS) following treatment with deoxycoformycin in a patient with cutaneous T-cell lymphoma (Sezary syndrome): A case report. Am J Hematol. 1999;61:268–270. doi: 10.1002/(sici)1096-8652(199908)61:4<268::aid-ajh9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Estey E. Do commonly used clinical trial designs reflect clinical reality? Haematologica. 2009;94:1435–1439. doi: 10.3324/haematol.2009.011411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin PJ, Bachier CR, Klingemann HG, et al. Endpoints for clinical trials testing treatment of acute graft-versus-host disease: A joint statement. Biol Blood Marrow Transplant. 2009;15:777–784. doi: 10.1016/j.bbmt.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: Clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 24.Margolis J, Grever MR. Pentostatin (Nipent): A review of potential toxicity and its management. Semin Oncol. 2000;27:9–14. [PubMed] [Google Scholar]