Abstract
Purpose
A phase I/II trial was performed to evaluate the safety and immunogenicity of a novel vaccination with α-type 1 polarized dendritic cells (αDC1) loaded with synthetic peptides for glioma-associated antigen (GAA) epitopes and administration of polyinosinic-polycytidylic acid [poly(I:C)] stabilized by lysine and carboxymethylcellulose (poly-ICLC) in HLA-A2+ patients with recurrent malignant gliomas. GAAs for these peptides are EphA2, interleukin (IL)-13 receptor-α2, YKL-40, and gp100.
Patients and Methods
Twenty-two patients (13 with glioblastoma multiforme [GBM], five with anaplastic astrocytoma [AA], three with anaplastic oligodendroglioma [AO], and one with anaplastic oligoastrocytoma [AOA]) received at least one vaccination, and 19 patients received at least four vaccinations at two αDC1 dose levels (1 × or 3 × 107/dose) at 2-week intervals intranodally. Patients also received twice weekly intramuscular injections of 20 μg/kg poly-ICLC. Patients who demonstrated positive radiologic response or stable disease without major adverse events were allowed to receive booster vaccines. T-lymphocyte responses against GAA epitopes were assessed by enzyme-linked immunosorbent spot and HLA-tetramer assays.
Results
The regimen was well-tolerated. The first four vaccines induced positive immune responses against at least one of the vaccination-targeted GAAs in peripheral blood mononuclear cells in 58% of patients. Peripheral blood samples demonstrated significant upregulation of type 1 cytokines and chemokines, including interferon-α and CXCL10. Nine (four GBM, two AA, two AO, and one AOA) achieved progression-free status lasting at least 12 months. One patient with recurrent GBM demonstrated sustained complete response. IL-12 production levels by αDC1 positively correlated with time to progression.
Conclusion
These data support safety, immunogenicity, and preliminary clinical activity of poly-ICLC-boosted αDC1-based vaccines.
INTRODUCTION
Early phase studies of glioma vaccines have shown feasibility and encouraging preliminary clinical activity.1–9 The ultimate success of glioma vaccines likely depends critically on the further refinement of strategies to promote type 1 immunity10–12 and to target multiple glioma-associated antigen (GAA) epitopes, given the marked antigenic heterogeneity of gliomas.13,14
To this end, this study employed two novel strategies based on our own preclinical studies. First, we implemented culture methods for α-type 1 polarized DCs (αDC1) that are able to produce high levels of interleukin (IL) -12 and induce long-lived type 1 T-cell responses against tumor-associated antigens more efficiently than standard mature DCs.15,16 Second, we incorporated administration of the immunoadjuvant polyinosinic-polycytidylic acid [poly(I:C)] stabilized by lysine and carboxymethylcellulose (poly-ICLC), which has been shown to be safe through extensive evaluations in patients with malignant glioma,17–19 to enhance the efficacy of GAA-targeting vaccinations, as we demonstrated in glioma-bearing mice.20,21
The HLA-A2–restricted epitopes included two that we had previously identified, an IL-13Rα2-derived analog peptide (IL-13Rα2345-353:1A9V)22,23 and EphA2883-891,24,25 as well as two additional epitopes, YKL-40201-21026,27 and gp100209-217:M2.28
Thus, this study is the first to evaluate αDC1 loaded with GAA epitopes in combination with poly-ICLC in humans. We hypothesized that this regimen would prove to be safe, and would induce potent antiglioma immune responses.
PATIENTS AND METHODS
Patients
Patients with recurrent malignant glioma were enrolled with informed consent and approvals by the institutional review board and US Food and Drug Administration (BB-IND#12415). Clinical characteristics of patients are summarized in Table 1 and Appendix Figure A1 (online only). Enrollment criteria included: histologic diagnosis of glioblastoma multiforme (GBM) or anaplastic glioma (AG) including anaplastic astrocytoma (AA), anaplastic oligodendroglioma (AO), or anaplastic oligoastrocytoma (AOA); up to two previous recurrences; ≥ 18 years old; Karnofsky performance status ≥ 60; adequate liver and renal function and HLA-A2+. Minimum doses of corticosteroid (dexamethasone up to 4 mg/d) were permitted. Twenty-two patients were enrolled and received at least one vaccination. Nineteen of 22 patients completed the scheduled initial four immunizations; three patients (patients 4, 11, and 13) were withdrawn from the protocol due to early tumor progression. Nine patients completed five additional booster vaccinations. Immunologic and safety data are presented on patients who had at least four vaccinations (n = 19), and at least one vaccination (n = 22), respectively.
Table 1.
Demographics and Clinical Characteristics of Participating Patients
| Characteristic | DC Dose Level (No. of DC/dose) |
Total No. of Patients (n = 22) | % | |
|---|---|---|---|---|
| 1 (1 × 107) | 2 (3 × 107) | |||
| Received at least one vaccine | 11 | 11 | 22 | |
| Completed at least four vaccines | 10 | 9 | 19 | 86 |
| Female (received at least four vaccines) | 5 | 4 | 9 | 47 |
| Median age, years | 52 | 46 | 48 | |
| Range | 37-71 | 28-63 | 28-71 | |
| Tumor histology | ||||
| AA | 3 | 2 | 5 | 23 |
| AO | 1 | 2 | 3 | 14 |
| AOA | 1 | 0 | 1 | 4 |
| GBM | 6 | 7 | 13 | 59 |
| No. of previous recurrences | ||||
| 0 | 7 | 4 | 11 | 50 |
| 1 | 2 | 5 | 7 | 32 |
| 2 | 2 | 2 | 4 | 18 |
Abbreviations: DC, dendritic cells; AA, anaplastic astrocytoma; AO, anaplastic oligodendroglioma; AOA, anaplastic oligoastrocytoma; GBM, glioblastoma multiforme.
Clinical Trial Design
This single institution phase I/II study was designed to assess toxicity and the induction of immune and preliminary clinical responses of vaccinations with GAA-loaded αDC1 and administration of poly-ICLC (Hiltonol; Oncovir, Washington, DC). The first course of vaccines consisted of four ultrasound-guided intranodal (IN) administrations of 1 × or 3 × 107 αDC1/injection every 2 weeks rotating between right and left inguinal and axillary lymph node clusters to minimize the potential effects of injection-induced trauma in the microenvironment of the lymph nodes (Data Supplement Fig DS1, online only). The first 10 evaluable patients received 1 × 107 αDC1/injection (dose level 1); the subsequent nine received 3 × 107 αDC1/injection (dose level 2). The sample size justification is provided in the Data Supplement (Study Design Parameters; online only). All patients received intramuscular injections with poly-ICLC (20 μg/kg) twice/wk for 8 weeks starting on day 1. Patients exhibiting stable disease or tumor regression without major adverse events (AEs) after the fourth vaccination were eligible for additional vaccinations. Starting at week 13, these patients were treated with the same dose of additional vaccinations every 4 weeks to a maximum of five vaccine injections and intramuscular poly-ICLC starting on the day of the first additional vaccine and twice/wk (first booster phase). Patients not demonstrating major AEs or tumor progression after the first booster phase were offered the same dose of additional vaccines (every 3 months) and poly-ICLC (every week) for up to 3 years from the first vaccination (second booster phase).
Toxicity Assessment and Stopping Rules
The trial was monitored continuously for treatment-related AEs using the National Cancer Institute Common Toxicity Criteria version 3.0. The following were considered to be a dose-limiting toxicity (DLT) if they were judged possibly, probably, or definitely associated with treatment: ≥ grade 2 hypersensitivity; ≥ grade 3 nonhematologic/metabolic toxicity; ≥ grade 3 hematologic (except for lymphopenia) or metabolic toxicity that did not subside after 4 weeks cessation of poly-ICLC. Stopping rules were implemented such that a dose level was considered excessively toxic, warranting that accrual be halted, if at any time the observed rate of DLT was ≥ 33% and at least two DLTs had been observed.
Peptides
HLA-A2–restricted peptides used in these studies were: ALPFGFILV (IL-13Rα2345-353:1A9V)23; TLADFDPRV (EphA2883-891)24; IMDQVPFSV (GP100209-217:M2)29; and SIMTYDFHGA (YKL-40201-210). αDC1 were also loaded with aKXVAAWTLKAAaZC (a pan-DR epitope [PADRE]), a non-natural epitope optimized for helper T-cell response.30 The peptides were synthesized by automated solid-phase peptide synthesis in the University of Pittsburgh Peptide Synthesis Facility. Peptides were tested in multiple quality-assurance studies including purity, sterility, identity, potency, pyrogenicity, and stability.
Vaccine Preparation
For the DC culture, monocytes were obtained from the leukapheresis product and purified by the Elutra System (CardianBCT, Lakewood, CO). The monocytes were cultured in antibiotic-free culture medium (CellGenix Technologie Transfer GmbH, Antioch, IL) supplemented with 1,000 U/mL granulocyte-macrophage colony-stimulating factor and 1,000 U/mL IL-4 in sterile cartridges, using the Replicell System (Aastrom Biosciences, Ann Arbor, MI). The immature DC (iDC) were harvested on day 6 and cryopreserved. Before each vaccination, aliquots of frozen iDCs were thawed, further matured, and polarized with clinical grade IL-1β (10 ng/mL), tumor necrosis factor-α (10 ng/mL), interferon (IFN) -α (3,000 U/mL), IFN-γ (1,000 U/mI), and poly-I:C (20 μg/mI) at 37°C in 5% CO2 for 48 hours and loaded with GAA peptides (10 μg/mL) for 4 to 6 hours. Two hours before harvest, the PADRE peptide was added to the cultures. Criteria for release of αDC1 included: sterility by Gram stain and bacteriologic culture; negative Mycoplasma; endotoxin lower than 5.0 EU/kg of body weight; greater than 70% expression of both CD86 and HLA-DR on αDC1.
Enzyme-Linked Immunosorbent Spot Assays
Enzyme-linked immunosorbent spot (ELISPOT) assays were performed as described previously5,31 with slight modifications. Briefly, peripheral blood mononuclear cells (PBMC) samples drawn and cryopreserved at each visit for vaccine (before the vaccine) as well as weeks 0, 9, and 33 were evaluated simultaneously after in vitro stimulation with autologous, irradiated PBMC loaded with wild-type IL-13Rα345-353,22 EphA2883-891, GP100209-217, and YKL-40202-211 for a week. A positive ELISPOT response was defined as a two-fold increase in spot-forming T cells over the prevaccine level and at least 10 spots/20,000 cells for at least two consecutive postvaccine time points against any antigen.
Tetramer Assays
Phycoerythrin-conjugated HLA-A*0201/ALPFGFILV (IL-13Rα2-tetramer), HLA-A*0201/IMDQVPFSV (gp100-tetramer), and HLA-A*0201/TLADFDPRV (EphA2-tetramer) were produced by the National Institute of Allergy and Infectious Disease tetramer facility (Emory University Vaccine Center, Atlanta, GA) using the peptide synthesized by the University of Pittsburgh Peptide Production Facility. Fluorescein isothiocyanate–conjugated antihuman CD8 was obtained from BD Biosciences (Sparks, MD). A single timepoint-positive response for a peptide was defined to be (0.1+B)% of all CD8+ cells positive by tetramer assay,32,33 where B is the percent positive at baseline, which was lower than 0.01% in all cases. A patient was considered to have responded if he/she had two consecutive single timepoint responses for any peptide.
Cytokine and Chemokine Assays
Total RNA samples were obtained from PBMC using the PAXgene Blood RNA System (PreAnalytix, Hombrechtikon, Switzerland). Reverse-transcriptase polymerase chain reaction (RT-PCR) was performed in triplicate, and values were standardized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and relative expression of mRNAs was calculated using the ΔΔCT method.34 The Luminex-based assay was performed in serum samples as previously described.35 Pretested, multiplex plates (Invitrogen, Carlsbad, CA) included standard curves and cytokine standards (R&D Systems, Minneapolis, MN). In situ hybridization with radiolabeled cRNA probe for CXCL10 was performed as described,36 with autoradiographic exposure times of 14 days.
Radiologic Response Monitoring and Other Clinical End Points
Tumor size was assessed at weeks 9, 17, 25, and 33, and every 3 months thereafter using magnetic resonance imaging (MRI) scans with contrast enhancement. Response was evaluated by McDonald criteria by gadolinium (Gd) -enhanced T1 weighted images, area of signal prolongation on T2 weighted images, or a combination of both, on the basis of the appearance of the pretreatment MRI. Overall survival was defined by the interval from study entry to date of death. MRI scans were used to evaluate time to progression (TTP).
RESULTS
Summary of Clinical Toxicities
Treatment-related AEs are listed for all 22 patients in Data Supplement Table DS1 (online only). There were no grade 3 or 4 toxicities, no deaths on study, and no DLT at any dose through the first booster phase. No incidences of autoimmunity were encountered. Toxicity profiles were comparable across dose levels (data not shown). Grade 1 or 2 injection site reactions were the most common (82%). Grade 1 flu-like symptoms, including fatigue (73%), myalgia (32%), fever (23%), chills/rigors (18%), and headache (32%), were common and usually limited to 24 hours after each vaccine. Grade 2 lymphopenia was recorded in one patient (5%).
IL-12 Production by αDC1 and Induction of Epitope-Specific Immune Responses Against GAAs
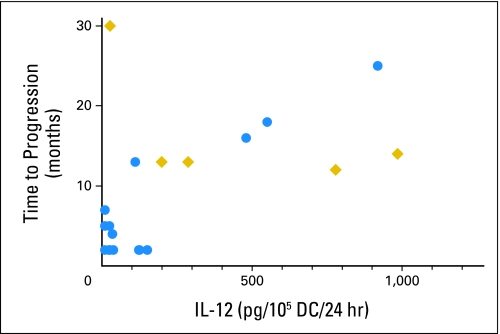
As presented in Appendix Figure A1, CD40L-induced IL-12 p70 production levels by αDC1 varied substantially between patients, and positively correlated with TTP (P = .0255; Fig 1), but not with IFN-γ ELISPOT response, patients' age, or tumor types (data not shown).
Fig 1.
Interleukin (IL) -12 production levels positively correlated with time to progression. P = .0255 is based on Cox regression followed by likelihood-ratio test. Circles indicate patients who have already experienced disease progression; diamonds represent patients who have not experienced recurrence to date. DC, dendritic cells.
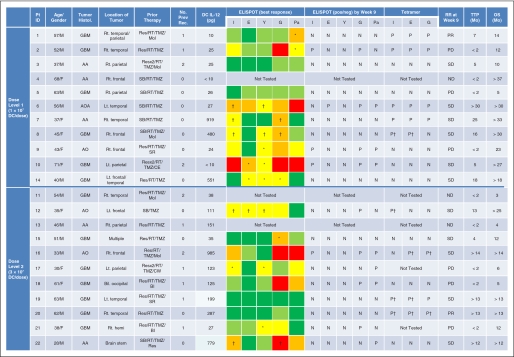
All 19 patients who completed the initial course of four vaccinations had PBMCs available for immunologic monitoring. Insufficient PBMC were obtained from patients 17, 21, and 22 to perform both ELISPOT and tetramer assays, and functional ELISPOT assays were prioritized. The scheduled first four vaccines induced immune reactivity to at least one of the vaccine-targeted GAAs in six of 10 and five of nine patients in dose levels 1 and 2, respectively, by either IFN-γ ELISPOT or tetramer assays (Appendix Fig A1). In patients 6, 7, 8, 16, 19, 20, and 22, some readouts reached the criteria for positive response after booster vaccines (indicated by † in Appendix Fig A1). In summary, 11 (58%) of 19 evaluable patients showed positive responses after the initial four vaccinations, and three additional subjects (patients 8, 19, and 20; 16%) showed positive responses only after booster vaccines.
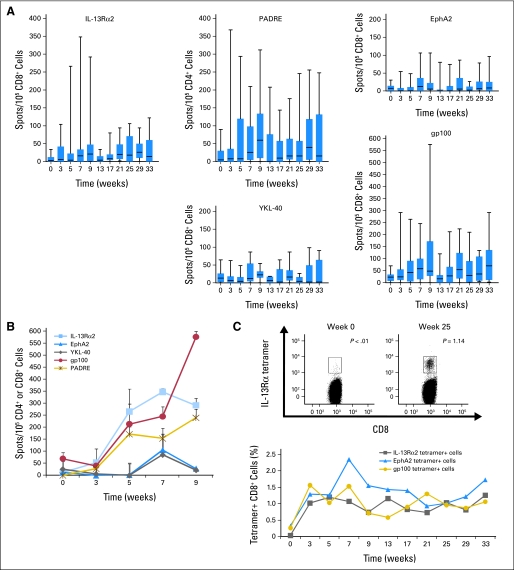
Positive response rates (either by tetramer or ELISPOT) did not show significant differences across the two αDC1 doses per Fisher's exact test. Furthermore, the magnitudes of ELISPOT response, based on the summation of positive spots from weeks 3 through 9, were comparable across the two αDC1 dose levels (Wilcoxon test). Therefore, we present the time course of IFN-γ ELISPOT responses by combining results from both dose levels (Fig 2A). The gp100 epitope demonstrated the highest magnitude of response among the GAA peptides tested (P < .001 against IL-13Rα2-, EphA2-, and YKL-40-derived peptides by Wilcoxon test). For the other epitopes, booster vaccines appeared to improve the induction of specific responses. A temporary decline of responses was typically observed at week 13, which may reflect that some patients who demonstrated positive responses by week 9 did not participate in the booster phase due to tumor progression (patients 2, 9, 18, and 21) or lymphopenia (patient 10), resulting in overall reduction of response when data are pooled for all patients. Patient 10 demonstrated the highest magnitude of IFN-γ ELISPOT responses against IL-13Rα2- and gp100-derived epitopes as well as PADRE (Fig 2B) but tetramer analyses on this patient yielded no responses (Appendix Fig A1). Patient 6, who demonstrated stable disease for longer than 30 months, developed durable and high level responses in tetramer (Fig 2C) and ELISPOT assays.
Fig 2.
Glioma-associated antigen (GAA) –specific T-cell responses evaluated by interferon (IFN) -γ enzyme-linked immunosorbent spot (ELISPOT) and tetramer analyses. (A) Time course for IFN-γ ELISPOT assays for all evaluated patients with box plots (boxes = 25th to 75th percentiles; vertical lines = minimum to maximum). Numbers at the bottom of each time point in the panel for YKL-40 are the number of assessable patients at the time shown. These numbers also pertain to the other GAAs and a pan-DR epitope (PADRE). (B) IFN-γ ELISPOT analysis for patient 10. (C) Patient 6 showed durable tetramer responses, which were analyzed for up to 33 weeks. Examples of histograms for positive tetramer responses against the interleukin (IL) -13Rα2-epitope are shown.
Induction of Type-1 Cytokine and Chemokine Responses
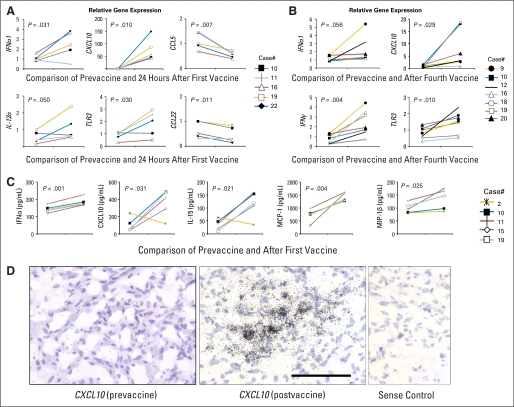
RT-PCR analyses of PBMC (Appendix Fig A2A, A2B, online only) revealed upregulation of mRNA expression for several type 1 cytokines and chemokines, specifically IFN-α1, CXCL10, and TLR3, at both postfirst vaccine and postfourth vaccine. IFN-γ was found to be upregulated after the fourth vaccine, but not after the first vaccine, suggesting that the IFN-γ upregulation may be associated with the induction of adaptive, rather than innate, immune response. CCL22, which is known to attract regulatory T cells,37 and CCL5 levels decreased in paired analyses of postfirst vaccine samples. Perforin, Granzyme B, cyclo-oxygenase2, and forkhead box protein p3 levels did not change significantly (data not shown).
A panel of cytokines and chemokines was evaluated at protein levels in available prevaccine and postvaccine serum samples from five patients (Appendix Fig A2C). Among them, IFN-α, CXCL10, IL-15, MCP-1, and MIP-1β were significantly upregulated in postvaccine sera. IL-17 was under detectable ranges in both RT-PCR and serum analyses (data not shown).
In addition, three of five available tumors resected due to postvaccine radiographic progression expressed mRNA for CXCL10, which is a critical chemokine for effective trafficking of CD8+ T-cells to brain tumor sites (Appendix Fig A2D for a representative case).10–11,38 These data suggest that this regimen induces systemic, polyfunctional immune responses in generally immunosuppressed patients with malignant glioma.
Clinical Outcomes
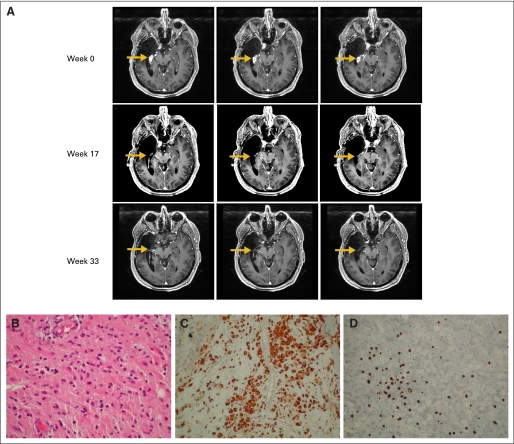
Two patients (patients 1 and 20) experienced objective clinical tumor regressions (response rate, 9%). Both patients were nonresponders by ELISPOT, but tetramer responders. Patient 20 with recurrent GBM demonstrated complete response based on disappearance of the Gd-enhanced mass at week 17 postvaccine compared with the baseline MRI, which has been durable and ongoing to date for at least 13 months since initiation of treatment (Appendix Fig A3A, online only). Patient 1 with recurrent GBM exhibited a partial response at week 9. After two booster vaccines, the Gd-enhanced lesion enlarged. Biopsy of the lesion, however, revealed intensive infiltration of CD8+ T cells and CD68+ macrophages and no evidence of mitotically active tumor (Appendix Figs A3B to A3D). Then, this patient received one additional vaccine before exhibiting recurrence at 7 months after the initial vaccine. Nine patients (41%; four and five with GBM and AG, respectively) are progression free for at least 12 months. Five patients are currently progression-free (Appendix Fig A1) and still receiving booster vaccines. Median TTP were 4 and 13 months for GBM and AG, respectively (Data Supplement Fig DS2, online only).
DISCUSSION
To our knowledge, this is the first clinical evaluation of αDC1-based vaccines loaded with novel GAA-derived peptides, in combination with poly-ICLC. Our findings demonstrate safety and immunogenicity as well as preliminary efficacy of the approach.
With regard to the manufacturing of αDC1, since the publication of the original αDC1 preparation method16 and during optimization studies for αDC1 production from patients with glioma, lipopolysaccharide was eliminated to achieve the US Food and Drug Administration–mandated endotoxin level on vaccine release. Our preclinical studies also indicated that prostaglandin E2 should not be used for optimal type-1 maturation of DC.39,40 Although suppressed immune status,41 as well as decreased numbers and functions of monocytes,42,43 have been reported in patients with glioma, we had no major difficulties generating adequate numbers of αDC1. Although the ability to produce IL-12 varied substantially among the patients, surface maturation markers, such as CD83, were all strongly positive in all DC preparations (data not shown), supporting that these cells were properly matured. Based on our data showing a positive correlation between αDC1 production of IL-12 and TTP, further studies are warranted to determine which factors in patients with cancer influence the IL-12 production of αDC1, and whether IL-12 production is a surrogate measure of DC potency and vaccine efficacy in future trials.
This study is the first to document in vivo induction of specific CD8+ T-cell responses against three of four GAA epitopes, IL-13Rα2345-353:1A9V,23,44,45 EphA2883-891,24,46 and YKL-40201-210.26,27 With regard to the expression of these GAAs in our series, although reresection of the recurrent tumor is not often clinically indicated in these patients, we summarized available immunohistochemistry data for seven patients in Data Supplement Table DS2. These preliminary data suggest that expression of gp100 may be very low in primary high-grade gliomas, consistent with published results.47 We included the gp100 peptide to assess the potency of the vaccine regimen because this was the only epitope that was previously demonstrated for its potent immunogenicity in humans.29,48 Now that the immunogenicity of the other GAAs have been shown, we may not include the gp100 epitope in future designs due to its low expression levels in primary gliomas.
We still have to elucidate the mechanisms underlying the observed divergence in tetramer versus ELISPOT responses. This may be because ELISPOT assays were performed following 1 week restimulation of PBMC whereas tetramer assays were performed without restimulation. Two clinical responders (patients 1 and 20) demonstrated tetramer but not ELISPOT responses. Although we cannot make conclusive comments with only two patients as to whether tetramer responses would better correlate with clinical success than ELISPOT, we are currently developing multicolor flow-cytometric analyses for evaluation of polyfunctional tetramer-reactive T cells (eg, tetramer-positive cells that also express multiple effector molecules, such as IFN-γ, TNF-α, and IL-2).49 These new technologies may lead us to better understand critical immune response indicators that may help us to predict clinical responses.
Although analyses of sentinel lymph nodes have reported higher positive rates and magnitudes of vaccine-induced responses than PBMCs,50,51 we focused our analyses on PBMCs to avoid invasive lymph node biopsy and to save the central site of vaccine-induced responses. Although our data did not clearly demonstrate correlations between GAA-specific responses and clinical outcomes, this may be because αDC1 vaccines promote the T-cell expression of receptors against tumor-associated chemokines,11,15,52 thereby facilitating their translocation from the circulation to tissues.
Our RT-PCR and serum analyses indicate upregulation of some type-1 cytokines and chemokines by the current regimen. This observation is intriguing, given that our preclinical studies have demonstrated critical contributions of these molecules to the efficacy of glioma vaccines.10–12,20,38 Further studies are warranted to determine if one or more of these molecules can serve as biomarkers of response in patients receiving glioma vaccines.
The choice of DC administration route may critically impact the efficacy of vaccines. We employed ultrasound-guided IN administration based on previous studies showing feasibility53 and superior T-cell response induction54 of the IN route of DC administration compared with intravenous and intradermal routes. In our study, IN administration was feasible and well-tolerated, warranting further development with this approach.
The postvaccine biopsy on patient 1 after the increase of Gd-enhancing signals revealed remarkable immune cell infiltration, but not mitotically active tumor cells, suggesting that the condition was pseudotumor progression. This implies that MRI-based evaluation of TTP requires particularly careful interpretation of imaging data and, potentially, pathologic confirmation of true versus pseudotumor progression.
Although the relatively high number of patients with AG probably contributed to the high rate of 12-month progression-free survival, our preliminary data on TTP in patients with GBM are not inferior to the results of studies that provided the basis for recent US Food and Drug Administration approval of the use of bevacizumab in these patients.55–57 These data, particularly the complete response in a patient with GBM, support larger studies of αDC1-based vaccines loaded with novel GAA epitopes.
Supplementary Material
Acknowledgment
Presented in part at the 5th International Meeting on Dendritic Cell Vaccination and Other Strategies to Tip the Balance of the Immune System, July 16-18, 2007, Bamberg, Germany; at the 12th Annual Scientific Meeting for Society for Neuro-Oncology, November 15-18, 2007, Dallas, TX; at the 13th Annual Scientific Meeting for Society for Neuro-Oncology, November 20-23, 2008, Las Vegas, NV; at the Mobilizing Cellular Immunity for Cancer Therapy Keystone Symposium, January 13, 2009, Snowbird Resort, UT; at the 3rd Quadrennial Meeting of the World Federation of Neuro-Oncology, May 11-14, 2009, Yokohama, Japan; at the Joint Meeting of the Society for Neuro-Oncology and the American Association of Neurological Surgeons and the Congress of Neurological Surgeons Section on Tumors, October 22-24, 2009, New Orleans, LA; at the Annual Meeting of the International Society for Biological Therapy of Cancer, October 29-31, 2009, Washington, DC; at the 101st Annual Meeting for American Association for Cancer Research, April 20, 2010, Washington, DC; and at the Neurex Workshop, Brain Tumours: From Current Concepts to Emerging Therapeutic Approaches, June 3-4, 2010, Strasbourg, France.
We thank Clinical Research Services for regulatory management; Mitchell Tublin for vaccine injections; physicians who referred their patients; Joseph Kiss for leukapheresis; Jennifer Mabold, CRNP, and Clinical Translational Science Institute for patient care; Mitsugu Fujita, Beth Fallert-Junecko, and Heather McDonald for technical assistance; and patients and their families.
Appendix
Fig A1.
Patient demographics, immune and clinical responses. Results of interferon (IFN) - γ enzyme-linked immunosorbent spot (ELISPOT) assays are summarized as a heat map. Each color indicates a range of positive spots per 105 CD8+ cells at the time point that indicated the maximum response for each patient: dark green for less than 25, light green for 25 to 49, yellow for 50 to 99, orange for 100 to 199, and red for greater than 200. (*) Only a single point, but not two or more consecutive points, demonstrated 50 or more spots/105 cells (thus not positive). (†) Positive only after booster vaccines. Pt, patient; ID, identification number; Histol, histology; No Prev Rec, number of previous recurrences; DC IL-12, production of IL-12 p70 by αDC1 (pg/105 cells/24 hours); RR, radiologic response; TTP, time to progression; OS, overall survival; Mo, month; M, male; GBM, glioblastoma multiforme; Rt, right; Temp, temporal; Pa, parietal; Res, resection; RT, radiation therapy; TMZ, temozolomide; Mol, molecularly targeted therapy; I, IL-13α2; E, EphA2; Y, YKL-40; G, gp100; Pa, PADRE; N, negative; P, positive; PR, partial response; PD, progressive disease; AA, anaplastic astrocytoma; SD, stable disease; F, female; SB, stereotactic biopsy; ND, not determined due to early progression before week 9; AOA, anaplastic oligoastrocytoma; Lt, left; AO, anaplastic oligodendroglioma; SR, stereotactic radiosurgery; CE, carboplatin and etoposide; CW, carmustine-releasing wafer; Bil, bilateral; BI, bevacizumab and irinotecan; Hemi, hemispheric.
Fig A2.
Induction of type-1 cytokine and chemokine responses. Line graphs represent paired relative gene expression by reverse transcriptase polymerase chain reaction on one day before the first vaccination compared to (A) 24 hours or (B) 9 weeks after first vaccine. (C) Luminex analyses were performed in pre-first and post-fourth vaccine serum samples. Values indicate concentrations of cytokine/chemokines by pg/mL. (A-C), Numbers in each panel indicate P values based on paired t test using the means of (A and B) ΔΔCT value or (C) concentration for each patient. (D) Patient 1 demonstrated increase in the size of gadolinium-enhanced lesion after two booster vaccines and underwent surgical resection of the lesion. In situ hybridization detected mRNA for CXCL10 (dark spots) in the postvaccine tissue but not in the initially resected tumor (prevaccine). None of two other prevaccine tissues demonstrated positive CXCL10 messages (data not shown). The scale bar equals 100 μm. Hematoxylin and eosin staining was performed for background. IL, interleukin; IFN, interferon.
Fig A3.
Patients with clinical response. (A) Patient 20 demonstrated complete radiologic response of gadolinium (Gd) -enhancing tumor (arrows) on magnetic resonance imaging on weeks 17 and 33 (three consecutive slices shown for each time point). (B-D) After two booster vaccines, patient 1 demonstrated enlargement of Gd-enhanced lesion. Resected tissue revealed (B) no evidence of mitotically active tumor but remarkable infiltration of (C) CD68+ macrophages and (D) CD8+ T cells. Original magnifications × 20 for B-D.
Footnotes
See accompanying article on page 337
Supported by Grants No. 2P01NS40923 (H.O., I.F.P.), 1R21CA117152 (H.O.), and P01 CA132714 (H.O., P.K., D.L.B., T.A.R.) from the National Institutes of Health; Musella Foundation for the overall conduct of the study; and Toray Industry for providing funds for the YKL-40 peptide.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00766753.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Andres M. Salazar, Oncovir (C) Consultant or Advisory Role: Frank S. Lieberman, Roche, Genentech (C) Stock Ownership: Andres M. Salazar, Oncovir Honoraria: None Research Funding: Frank S. Lieberman, AstraZeneca Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Hideho Okada, Pawel Kalinski, Ian F. Pollack, Andres M. Salazar
Administrative support: Teresa E. Donegan
Provision of study materials or patients: Teresa E. Donegan, Arlan H. Mintz, Johnathan A. Engh, David L. Bartlett, Charles K. Brown, Herbert Zeh, Matthew P. Holtzman, Andres M. Salazar
Collection and assembly of data: Hideho Okada, Ryo Ueda, Aki Hoji, Gary Kohanbash, Teresa E. Donegan, Todd A. Reinhart, Theresa L. Whiteside, Lisa H. Butterfield, Ronald L. Hamilton, Frank S. Lieberman
Data analysis and interpretation: Hideho Okada, Pawel Kalinski, Ryo Ueda, Aki Hoji, Gary Kohanbash, Teresa E. Donegan, Todd A. Reinhart, Theresa L. Whiteside, Lisa H. Butterfield, Ronald L. Hamilton, Douglas M. Potter, Frank S. Lieberman
Manuscript writing: Hideho Okada, Pawel Kalinski, Ryo Ueda, Aki Hoji, Gary Kohanbash, Teresa E. Donegan, Arlan H. Mintz, Johnathan A. Engh, David L. Bartlett, Charles K. Brown, Herbert Zeh, Matthew P. Holtzman, Todd A. Reinhart, Theresa L. Whiteside, Lisa H. Butterfield, Ronald L. Hamilton, Douglas M. Potter, Ian F. Pollack, Andres M. Salazar, Frank S. Lieberman
Final approval of manuscript: Hideho Okada, Pawel Kalinski, Ryo Ueda, Aki Hoji, Gary Kohanbash, Teresa E. Donegan, Arlan H. Mintz, Johnathan A. Engh, David L. Bartlett, Charles K. Brown, Herbert Zeh, Matthew P. Holtzman, Todd A. Reinhart, Theresa L. Whiteside, Lisa H. Butterfield, Ronald L. Hamilton, Douglas M. Potter, Ian F. Pollack, Andres M. Salazar, Frank S. Lieberman
REFERENCES
- 1.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 2.Wheeler CJ, Black KL, Liu G, et al. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 2008;68:5955–5964. doi: 10.1158/0008-5472.CAN-07-5973. [DOI] [PubMed] [Google Scholar]
- 3.De Vleeschouwer S, Fieuws S, Rutkowski S, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14:3098–3104. doi: 10.1158/1078-0432.CCR-07-4875. [DOI] [PubMed] [Google Scholar]
- 4.Izumoto S, Tsuboi A, Oka Y, et al. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J. Neurosurg. 2008;108:963–971. doi: 10.3171/JNS/2008/108/5/0963. [DOI] [PubMed] [Google Scholar]
- 5.Okada H, Lieberman FS, Walter KA, et al. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of patients with malignant gliomas. J Transl Med. 2007;5:67. doi: 10.1186/1479-5876-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okada H, Lieberman FS, Edington HD, et al. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of recurrent glioblastoma: Preliminary observations in a patient with a favorable response to therapy. J Neurooncol. 2003;64:13–20. doi: 10.1007/BF02700016. [DOI] [PubMed] [Google Scholar]
- 7.Kikuchi T, Akasaki Y, Abe T, et al. Vaccination of glioma patients with fusions of dendritic and glioma cells and recombinant human interleukin 12. J Immunother. 2004;27:452–459. doi: 10.1097/00002371-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Yamanaka R, Homma J, Yajima N, et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: Results of a clinical phase I/II trial. Clin Cancer Res. 2005;11:4160–4167. doi: 10.1158/1078-0432.CCR-05-0120. [DOI] [PubMed] [Google Scholar]
- 9.Sampson JH, Archer GE, Mitchell DA, et al. An epidermal growth factor receptor variant III–targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther. 2009;8:2773–2779. doi: 10.1158/1535-7163.MCT-09-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura F, Dusak JE, Eguchi J, et al. Adoptive transfer of Type 1 CTL mediates effective anti-central nervous system tumor response: Critical roles of IFN-inducible protein-10. Cancer Res. 2006;66:4478–4487. doi: 10.1158/0008-5472.CAN-05-3825. [DOI] [PubMed] [Google Scholar]
- 11.Fujita M, Zhu X, Ueda R, et al. Effective Immunotherapy against murine gliomas using type 1 polarizing dendritic cells: Significant roles of CXCL10. Cancer Res. 2009;69:1587–1595. doi: 10.1158/0008-5472.CAN-08-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki K, Zhu X, Vasquez C, et al. Preferential expression of very late antigen-4 on type 1 CTL cells plays a critical role in trafficking into central nervous system tumors. Cancer Res. 2007;67:6451–6458. doi: 10.1158/0008-5472.CAN-06-3280. [DOI] [PubMed] [Google Scholar]
- 13.Pollack IF, Finkelstein SD, Woods J, et al. Expression of p53 and prognosis in children with malignant gliomas. N Engl J Med. 2002;346:420–427. doi: 10.1056/NEJMoa012224. [DOI] [PubMed] [Google Scholar]
- 14.Smith JS, Tachibana I, Passe SM, et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst. 2001;93:1246–1256. doi: 10.1093/jnci/93.16.1246. [DOI] [PubMed] [Google Scholar]
- 15.Watchmaker PB, Berk E, Muthuswamy R, et al. Independent regulation of chemokine responsiveness and cytolytic function versus CD8+ T cell expansion by dendritic cells. J Immunol. 2010;184:591–597. doi: 10.4049/jimmunol.0902062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mailliard RB, Wankowicz-Kalinska A, Cai Q, et al. α-type-1 polarized dendritic cells: A novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 17.Butowski N, Chang SM, Junck L, et al. A phase II clinical trial of poly-ICLC with radiation for adult patients with newly diagnosed supratentorial glioblastoma: A North American Brain Tumor Consortium (NABTC01-05) J Neurooncol. 2009;91:175–182. doi: 10.1007/s11060-008-9693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butowski N, Lamborn KR, Lee BL, et al. A North American Brain Tumor Consortium phase II study of poly-ICLC for adult patients with recurrent anaplastic gliomas. J Neurooncol. 2009;91:183–189. doi: 10.1007/s11060-008-9705-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salazar AM, Levy HB, Ondra S, et al. Long-term treatment of malignant gliomas with intramuscularly administered polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose: An open pilot study. Neurosurgery. 1996;38:1096–1103. [PubMed] [Google Scholar]
- 20.Zhu X, Nishimura F, Sasaki K, et al. Toll like receptor-3 ligand poly-ICLC promotes the efficacy of peripheral vaccinations with tumor antigen-derived peptide epitopes in murine CNS tumor models. J Transl Med. 2007;5:10. doi: 10.1186/1479-5876-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu X, Fallert-Junecko B, Fujita M, et al. Poly-ICLC promotes the infiltration of effector T cells into intracranial gliomas via induction of CXCL10 in IFN-α and IFN-γ dependent manners. Cancer Immunol Immunother. 2010;59:1401–1409. doi: 10.1007/s00262-010-0876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okano F, Storkus WJ, Chambers WH, et al. Identification of a novel HLA-A*0201 restricted cytotoxic T lymphocyte epitope in a human glioma associated antigen, interleukin-13 receptor 2 chain. Clin Cancer Res. 2002;8:2851–2855. [PubMed] [Google Scholar]
- 23.Eguchi J, Hatano M, Nishimura F, et al. Identification of interleukin-13 receptor alpha2 peptide analogues capable of inducing improved antiglioma CTL responses. Cancer Res. 2006;66:5883–5891. doi: 10.1158/0008-5472.CAN-06-0363. [DOI] [PubMed] [Google Scholar]
- 24.Hatano M, Eguchi J, Tatsumi T, et al. EphA2 as a glioma-associated antigen: A novel target for glioma vaccines. Neoplasia. 2005;7:717–722. doi: 10.1593/neo.05277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatano M, Kuwashima N, Tatsumi T, et al. Vaccination with EphA2-derived T cell-epitopes promotes immunity against both EphA2-expressing and EphA2-negative tumors. J Transl Med. 2004;2:40. doi: 10.1186/1479-5876-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelloski CE, Mahajan A, Maor M, et al. YKL-40 expression is associated with poorer response to radiation and shorter overall survival in glioblastoma. Clin Cancer Res. 2005;11:3326–3334. doi: 10.1158/1078-0432.CCR-04-1765. [DOI] [PubMed] [Google Scholar]
- 27.Nutt CL, Betensky RA, Brower MA, et al. YKL-40 is a differential diagnostic marker for histologic subtypes of high-grade gliomas. Clin Cancer Res. 2005;11:2258–2264. doi: 10.1158/1078-0432.CCR-04-1601. [DOI] [PubMed] [Google Scholar]
- 28.Liu G, Ying H, Zeng G, et al. HER-2, gp100, and MAGE-1 are expressed in human glioblastoma and recognized by cytotoxic T cells. Cancer Res. 2004;64:4980–4986. doi: 10.1158/0008-5472.CAN-03-3504. [DOI] [PubMed] [Google Scholar]
- 29.Salgaller ML, Marincola FM, Cormier JN, et al. Immunization against epitopes in the human melanoma antigen gp100 following patient immunization with synthetic peptides. Cancer Res. 1996;56:4749–4757. [PubMed] [Google Scholar]
- 30.Alexander J, Sidney J, Southwood S, et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751–761. doi: 10.1016/s1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 31.Kirkwood JM, Lee S, Moschos SJ, et al. Immunogenicity and antitumor effects of vaccination with peptide vaccine+/-granulocyte-monocyte colony-stimulating factor and/or IFN-alpha2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group phase II trial E1696. Clin Cancer Res. 2009;15:1443–1451. doi: 10.1158/1078-0432.CCR-08-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber J, Boswell W, Smith J, et al. Phase 1 trial of intranodal injection of a Melan-A/MART-1 DNA plasmid vaccine in patients with stage IV melanoma. J Immunother. 2008;31:215–223. doi: 10.1097/CJI.0b013e3181611420. [DOI] [PubMed] [Google Scholar]
- 33.Melanoma Study Group of the Mayo Clinic Cancer C. Celis E. Overlapping human leukocyte antigen class I/II binding peptide vaccine for the treatment of patients with stage IV melanoma: Evidence of systemic immune dysfunction. Cancer. 2007;110:203–214. doi: 10.1002/cncr.22744. [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Szczepanski MJ, Czystowska M, Szajnik M, et al. Triggering of toll-like receptor 4 expressed on human head and neck squamous cell carcinoma promotes tumor development and protects the tumor from immune attack. Cancer Res. 2009;69:3105–3113. doi: 10.1158/0008-5472.CAN-08-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fallert BA, Reinhart TA. Improved detection of simian immunodeficiency virus RNA by in situ hybridization in fixed tissue sections: Combined effects of temperatures for tissue fixation and probe hybridization. J Virol Methods. 2002;99:23–32. doi: 10.1016/s0166-0934(01)00378-0. [DOI] [PubMed] [Google Scholar]
- 37.Muthuswamy R, Urban J, Lee JJ, et al. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. 2008;68:5972–5978. doi: 10.1158/0008-5472.CAN-07-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu X, Fallert-Junecko BA, Fujita M, et al. Poly-ICLC promotes the infiltration of vaccine-induced T cells into intracranial gliomas via induction of CXCL10 in IFN-α and IFN-γ dependent manners. Cancer Immunol Immunother. 2010;59:1401–1409. doi: 10.1007/s00262-010-0876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalinski P, Hilkens CM, Snijders A, et al. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- 40.Kalinski P, Schuitemaker JH, Hilkens CM, et al. Final maturation of dendritic cells is associated with impaired responsiveness to IFN-gamma and to bacterial IL-12 inducers: Decreased ability of mature dendritic cells to produce IL-12 during the interaction with Th cells. J Immunol. 1999;162:3231–3236. [PubMed] [Google Scholar]
- 41.Okada H, Kohanbash G, Zhu X, et al. Immunotherapeutic approaches for glioma. Crit Rev Immunol. 2009;29:1–42. doi: 10.1615/critrevimmunol.v29.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodrigues JC, Gonzalez GC, Zhang L, et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010;12:351–365. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogden AT, Horgan D, Waziri A, et al. Defective receptor expression and dendritic cell differentiation of monocytes in glioblastomas. Neurosurgery. 2006;59:902–909. doi: 10.1227/01.NEU.0000233907.03070.7B. [DOI] [PubMed] [Google Scholar]
- 44.Debinski W, Gibo DM. Molecular expression analysis of restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol Med. 2000;6:440–449. [PMC free article] [PubMed] [Google Scholar]
- 45.Debinski W, Gibo DM, Hulet SW, et al. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin Cancer Res. 1999;5:985–990. [PubMed] [Google Scholar]
- 46.Liu F, Park PJ, Lai W, et al. A genome-wide screen reveals functional gene clusters in the cancer genome and identifies EphA2 as a mitogen in glioblastoma. Cancer Res. 2006;66:10815–10823. doi: 10.1158/0008-5472.CAN-06-1408. [DOI] [PubMed] [Google Scholar]
- 47.Saikali S, Avril T, Collet B, et al. Expression of nine tumour antigens in a series of human glioblastoma multiforme: Interest of EGFRvIII, IL-13Ralpha2, gp100 and TRP-2 for immunotherapy. J. Neurooncol. 2007;81:139–148. doi: 10.1007/s11060-006-9220-3. [DOI] [PubMed] [Google Scholar]
- 48.Monsurro V, Nagorsen D, Wang E, et al. Functional heterogeneity of vaccine-induced CD8(+) T cells. J Immunol. 2002;168:5933–5942. doi: 10.4049/jimmunol.168.11.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Connell KA, Bailey JR, Blankson JN. Elucidating the elite: Mechanisms of control in HIV-1 infection. Trends Pharmacol Sci. 2009;30:631–637. doi: 10.1016/j.tips.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Chianese-Bullock KA, Pressley J, Garbee C, et al. MAGE-A1-, MAGE-A10-, and gp100-derived peptides are immunogenic when combined with granulocyte-macrophage colony-stimulating factor and montanide ISA-51 adjuvant and administered as part of a multipeptide vaccine for melanoma. J Immunol. 2005;174:3080–3086. doi: 10.4049/jimmunol.174.5.3080. [DOI] [PubMed] [Google Scholar]
- 51.Slingluff CL, Jr, Petroni GR, Olson W, et al. Helper T-cell responses and clinical activity of a melanoma vaccine with multiple peptides from MAGE and melanocytic differentiation antigens. J Clin Oncol. 2008;26:4973–4980. doi: 10.1200/JCO.2008.17.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalinski P, Okada H. Polarized dendritic cells as cancer vaccines: Directing effector-type T cells to tumors. Semin Immunol. 2010;22:173–182. doi: 10.1016/j.smim.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilliet M, Kleinhans M, Lantelme E, et al. Intranodal injection of semimature monocyte-derived dendritic cells induces T helper type 1 responses to protein neoantigen. Blood. 2003;102:36–42. doi: 10.1182/blood-2002-07-2274. [DOI] [PubMed] [Google Scholar]
- 54.Bedrosian I, Mick R, Xu S, et al. Intranodal administration of peptide-pulsed mature dendritic cell vaccines results in superior CD8+ T-cell function in melanoma patients. J Clin Oncol. 2003;21:3826–3835. doi: 10.1200/JCO.2003.04.042. [DOI] [PubMed] [Google Scholar]
- 55.Cohen MH, Shen YL, Keegan P, et al. FDA drug approval summary: Bevacizumab (Avastin(R)) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14:1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 56.Vredenburgh JJ, Desjardins A, Herndon JE, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clinical Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 57.Vredenburgh JJ, Desjardins A, Herndon JE, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.