Abstract
One of the major attendant complications of multiple myeloma is renal injury, which contributes significantly to morbidity and mortality in this disease. Monoclonal immunoglobulin free light chains (FLCs) are usually directly involved, and tubulointerstitial renal injury and fibrosis are prominent histologic features observed in myeloma. The present study examined the role of monoclonal FLCs in altering the nuclear factor κ light chain enhancer of activated B cells (NF-κB) activity of renal epithelial cells. Human proximal tubule epithelial cells exposed to 3 different human monoclonal FLCs demonstrated Src kinase–dependent activation of the NF-κB pathway, which increased production of monocyte chemoattractant protein-1 (MCP-1). Tyrosine phosphorylation of inhibitor of κB kinases (IKKs) IKKα and IKKβ and a concomitant increase in inhibitor of κB (IκB) kinase activity in cell lysates were observed. Time-dependent, Src kinase–dependent increases in serine and tyrosine phosphorylation of IκBα and NF-κB activity were also demonstrated. Proteasome inhibition partially blocked FLC-induced MCP-1 production. These findings fit into a paradigm characterized by FLC-induced redox-signaling events that activated the canonical and atypical (IKK-independent) NF-κB pathways to promote a proinflammatory, profibrotic renal environment.
Introduction
Multiple myeloma, a malignant plasma cell disorder, has an incidence rate of approximately 1.1% among all malignancies and constitutes 12%-13% of hematologic malignancies in the United States.1 In a study examining newly diagnosed patients with multiple myeloma, the incidence of renal dysfunction, determined by serum creatinine elevation ≥ 1.3 mg/dL, was 48%. These same investigators showed that an increase in the serum creatinine concentration to ≥ 2.0 mg/dL portended a poor prognosis, with a 35% reduction in median survival compared with patients with normal serum creatinine concentrations.2 Mortality was accentuated if patients developed end-stage kidney disease in the setting of multiple myeloma. In one study that examined outcomes in 3298 patients, the 2-year all-cause mortality was 58% compared with 31% for patients with end-stage kidney disease from other causes.3 The immunoglobulin free light chain (FLC) is the culprit in most of these renal lesions, and the majority of patients with renal failure from monoclonal FLCs in this setting have tubulointerstitial renal disease.4 These studies place importance on maintaining or improving renal function and emphasize the need to focus not only on the reduction of FLCs during treatment, but also on understanding the underlying renal pathophysiology.
A major function of the kidney is to reclaim low-molecular-weight proteins that appear in the glomerular ultrafiltrate. FLCs are low-molecular-weight proteins that readily undergo glomerular filtration and are processed by the proximal tubule epithelium. Specifically, FLCs are absorbed into the proximal tubule by a receptor-mediated complex that consists of megalin and cubilin.5–8 Once endocytosed, proximal tubule epithelial cells hydrolyze the proteins and return the amino acid residues to the circulation. Under normal conditions, total serum FLC concentration is typically < 30 mg/L, and approximately 500 mg of FLCs are cleared daily by the kidney; however, in pathologic states such as multiple myeloma, serum levels exceeding 100 000 mg/L have been observed.9,10 In addition, unlike other proteins, this renal reclamation process is complicated by intracellular oxidative stress due to the production of hydrogen peroxide, which promotes cytotoxicity and also initiates signaling cascades that produce a pro-inflammatory state with elaboration of monocyte chemoattractant protein-1 (MCP-1) and interleukin-6 (IL-6).11,12 As is true for most progressive forms of renal disease regardless of underlying etiology, tubulointerstitial injury and fibrosis are invariant findings that contribute to a progressive loss of renal function.13 Thus, receptor-mediated endocytosis and metabolism of monoclonal FLCs generates an intrarenal pro-inflammatory environment that exacerbates ongoing renal injury and tubulointerstitial fibrosis, promoting functional progression of the kidney disease.
The intracellular signaling process is known to be mediated through oxidative activation of c-Src, the 60-kDa product of c-src, and activation of nuclear factor κB (NF-κB), but it is not clear how these signaling events are linked. The present study was therefore designed to determine the mechanism of activation of the NF-κB pathway by FLCs.
Methods
Cells and reagents
Human proximal tubular epithelial cells.
Human kidney-2 (HK-2) cells, which have been characterized previously by Ryan et al,14 were obtained from ATCC. Monolayers of HK-2 cells were grown on 6-well plates (Costar; Corning) that were precoated with 5 μg/cm2 of rat tail collagen type 1 (Invitrogen), and incubated at 37°C with 5% CO2/95% air in keratinocyte serum-free medium (GIBCO) supplemented with recombinant human epidermal growth factor (5 ng/mL) and bovine pituitary extract (50 μg/mL). Medium was exchanged at 48-hour intervals, and cells were not used beyond 25-30 passages.
Human immunoglobulin FLCs.
Three unique monoclonal FLCs, 1 κ and 2 λ, labeled κ2, λ2, and λ3, were purified using standard methods from the urine of patients who had multiple myeloma and light-chain proteinuria.15 These patients had clinical evidence of significant renal damage that was presumed to be cast nephropathy, although renal biopsy was not performed. The FLCs were endotoxin-free and were observed to generate hydrogen peroxide and promote intracellular oxidative stress in HK-2 cells in culture.11 Experiments were also performed using polyclonal κ and λ FLCs purified from healthy blood donor sera.
Commercial reagents.
4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyramidine (PP2; EMD Biosciences), 10μM, served as a potent selective chemical inhibitor of Src kinase activity.16 Pyrrolidine dithiocarbamate (PDTC; Sigma-Aldrich), 200μM, served as a cell-permeable, selective inhibitor of NF-κB.17,18 bortezomib (PS-341; Millennium Pharmaceuticals), a proteasome inhibitor,19 was used in concentrations between 0 and 200nM. Rabbit polyclonal antibody to RelA (p65; Abcam) and Alexa Fluor 488–labeled goat anti–rabbit immunoglobulin G (IgG; Molecular Probes) were used in the confocal laser scanning microscopy experiments. Antibodies directed against IKKα, IKKβ, IκBα, phospho-IκBα (S32/36), and phospho-c-Src (Y416) were obtained from Cell Signaling Technology. Anti–phosphotyrosine antibodies were also obtained commercially (Millipore). IKKβ kinase activity was quantified using an in vitro kinase assay with biotinylated IκBα as a substrate (HTScan IKKβ kinase assay kit; Cell Signaling Technology). MCP-1 was quantified with a sandwich enzyme immunoassay (human MCP-1 enzyme-linked immunosorbent assay kit, R&D Systems). NF-κB activity in nuclear extracts was quantified using a kit (NF-κB filter plate assay; Signosis). Release of hydrogen peroxide into the medium was determined using a kit (Amplex red hydrogen peroxide/peroxidase assay kit; Invitrogen), as described previously.11,12 Release of cellular lactate dehydrogenase into the medium was determined using a kit (Roche Diagnostics).
Experimental approach
At the start of the experiment, the medium was exchanged for keratinocyte serum-free medium containing FLCs (1 mg/mL), and cells were incubated for up to 24 hours with the FLCs before analysis. The FLC concentration (1 mg/mL) was within the expected concentration range to which proximal tubule cells are exposed based on the serum levels found in patients with multiple myeloma10,20 and the estimated glomerular sieving coefficients for these low-molecular-weight proteins.21,22 The pharmacologic inhibitors PP2, PDTC, and PS-341 were added just before the addition of the FLCs.
For immunofluorescence microscopy, cells were grown on glass slides precoated with type 1 collagen. After 24 hours of incubation with the FLCs (κ2 and λ2), the cells were fixed for 5-10 minutes in acetone and then incubated with the anti–p65 antibody for 1 hour at room temperature. Cells were then incubated with the secondary antibody, Alexa Fluor 488-labeled goat anti–rabbit IgG, at a 1/1000 dilution for 1 hour. 4′,6-Diamidino-2-phenylindole, dihydrochloride was used to stain nuclei. The slides were examined using confocal laser scanning microscopy (model LSM 710 confocal microscope; Carl Zeiss MicroImaging) provided through the High Resolution Imaging Facility at the University of Alabama at Birmingham.
For immunoprecipitation studies, Western blot analyses, and IKKβ kinase assays, cells were lysed in radioimmunoprecipitation assay buffer containing a protease inhibitor cocktail (Complete; Roche). Cell lysates were clarified by centrifugation and lysates were stored at −70°C until they were assayed. Total soluble protein in lysates was determined using a kit (BCA protein assay kit; Pierce). For coimmunoprecipitation experiments, the lysates were initially incubated with 1.0 μg of a control IgG that corresponded to the host species of the primary antibody and 20 μL of agarose-conjugated protein A (Santa Cruz Biotechnology), 25% vol/vol, at 4°C for 30 minutes. After centrifugation and transfer of the supernatant, the lysates were incubated with 1 μg of primary antibody for 2 hours at 4°C. After the addition of 20 μL of agarose-conjugated protein A suspension, the solutions were incubated at 4°C on a rocker platform overnight. The pellets were washed 3 times with ice-cold radioimmunoprecipitation assay buffer and then boiled in sodium dodecyl sulfate sample buffer containing dithiothreitol (6.0 mg/mL). The proteins were resolved on 12% sodium dodecyl sulfate-polyacrylamide gels and transferred to polyvinylidene fluoride membranes for Western blotting, which proceeded in the standard fashion.23–25
To quantify NF-κB activity, after incubation for 4 or 24 hours in medium containing κ2 or λ3 FLCs, 1 mg/mL, the cells were harvested and nuclear extracts were prepared using a nuclear extraction kit (Signosis). The nuclear extracts were then used to determine NF-κB activity following the directions provided by the manufacturer.
Statistical analysis
Data were expressed as means ± SEM. Significant differences among datasets were determined by analysis of variance with standard post-hoc testing (GraphPad InStat version 3.1a for Macintosh, GraphPad Software). A P value less than .05 was assigned statistical significance.
Results
Incubation of human proximal tubule epithelial cells with human monoclonal FLCs promotes the appearance of RelA (p65) in the nucleus and the production of MCP-1
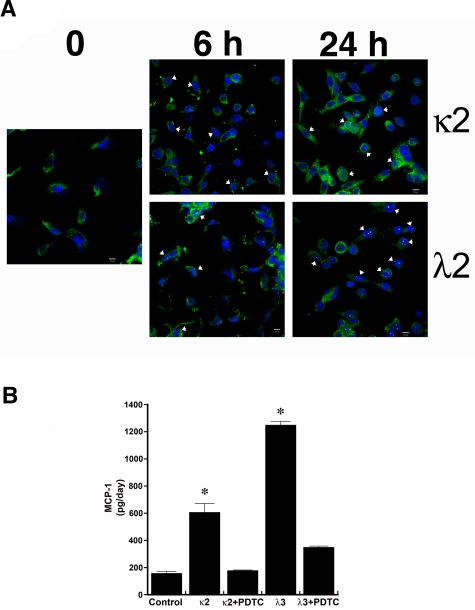
Confocal microscopy demonstrated nuclear colocalization of RelA (p65) during incubation of HK-2 cells with κ2 and λ2 FLCs, 1 mg/mL (Figure 1A). Consistent with prior studies,11,12 both κ2 and λ3 FLCs increased the production of MCP-1 by HK-2 cells; the addition of the NF-κB inhibitor PDTC inhibited this response (Figure 1B). In contrast, no statistically significant changes were observed in the release of hydrogen peroxide or MCP-1 into the medium among the different conditions of vehicle, polyclonal κ or λ FLCs at 1 or 5 mg/mL, and equimolar concentrations of delipidated human serum albumin at 3 or 15 mg/mL (n = 6 per group).
Figure 1.
Incubation of human proximal tubular epithelial cells with 2 different monoclonal FLCs (κ2 and λ2) increases the production of MCP-1 through activation of the NF-κB pathway. (A) Confocal laser scanning microscopy using the LSM 710 confocal microscope (Carl Zeiss MicroImaging) and the accompanying LSM 710 ZEN software demonstrated nuclear localization (arrowheads) of RelA (p65) in cells within 6 hours of exposure to κ2 and λ2 FLCs (1 mg/mL). The white bar represents 10 μm. (B) Increase in MCP-1 production induced by 24-hour incubation of renal epithelial cells with the FLCs was inhibited by PDTC, an inhibitor of NF-κB. *P < .05 compared with samples incubated in medium alone (control) and in medium containing the corresponding FLCs and PDTC; n = 6 experiments in each group.
Human FLCs induce an Src kinase–dependent tyrosine phosphorylation of IKKα and IKKβ
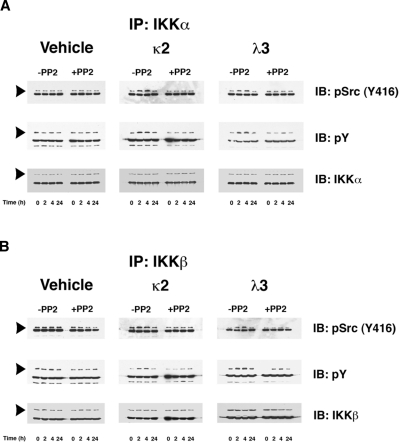
Prior studies demonstrated that Src-kinase activity, particularly c-Src, increases in proximal tubular cells during incubation with FLCs.12 Coimmunoprecipitation studies have demonstrated that active c-Src associates in a time-dependent fashion with both IKKα and IKKβ; the addition of the Src kinase inhibitor PP2 prevented this interaction (Figure 2A-B top panels). Interaction was observed at 2 and 4 hours of incubation and returned to baseline by 24 hours. In an Src-kinase-dependent manner, both IKKα and IKKβ were tyrosine phosphorylated (Figure 2A-B middle panels), an event known to activate these enzymes by c-Src.26,27 Active IKKα and IKKβ form hetero- and homodimers, but activated IKKβ in particular appears to be the primary kinase responsible for phosphorylation of IκBα on serine residues at positions 32 and 36.28–31 An in vitro IKKβ kinase assay, which used biotinylated IκBα as a substrate, confirmed a 1.5-2–fold increase in IKKβ enzyme activity at 2 and 4 hours in lysates from cells incubated with κ2 and λ3 FLCs compared with time-controlled experiments, with a return to baseline by 24 hours. The increase in IKKβ activity was inhibited by the addition of PP2.
Figure 2.
FLCs promote a time-dependent coimmunoprecipitation of IKKα and IKKβ with activated c-Src, which induces tyrosine phosphorylation of these IκB kinases. Renal epithelial cells were incubated with 2 different FLCs (κ2 and λ3) and vehicle. At different time points (0, 2, 4, and 24 hours), the experiments were stopped and cell lysates were produced to immunoprecipitate either IKKα (A) or IKKβ (B). These experiments demonstrated a time-dependent association with activated (phosphorylated) c-Src (top panels) in lysates obtained from cells exposed to the monoclonal FLCs but not medium alone. The approximate 2- to 4-fold increase in association of activated c-Src with the IκB kinases was prevented by the addition of PP2. The middle panels of (A) and (B) demonstrate a contemporaneous 2- to 4-fold increase in tyrosine phosphorylation of the IκB kinases in those cells exposed to the FLCs but not vehicle. The bottom panels demonstrated the presence of similar amounts of IKKα (A) or IKKβ (B) in the immune precipitate. The other major band in the gels represents the heavy-chain component of the antibody used to precipitate the protein complexes.
Human FLCs promote an Src kinase–dependent serine and tyrosine phosphorylation of IκBα
Incubation of cells with κ2 and λ3 FLCs promoted a time-dependent, Src kinase–dependent phosphorylation of serine residues at positions 32 and 36 of IκBα (Figure 3 top panels), indicating activation of the canonical NF-κB pathway.32–34 In addition, we also observed a time-dependent, PP2-inhibitable tyrosine phosphorylation of IκBα (Figure 3 middle panels), which also directly promotes the activation of NF-κB independently of the IKK complex.35 These findings indicated that c-Src regulates the NF-κB pathway through several posttranslational modifications of upstream molecules in this system.
Figure 3.
FLCs (κ2 and λ3) induce Src kinase-dependent serine and tyrosine phosphorylation of IκBα in renal epithelial cells. Renal epithelial cells were incubated with 2 different FLCs (κ2 and λ3) and vehicle. At different time points (0, 2, 4, and 24 hours), the experiments were stopped and cell lysates were produced to immunoprecipitate IκBα. Incubation of cells with the FLCs κ2 and λ3, but not the vehicle, promoted a > 2-fold increase in serine phosphorylation of IκBα (top panels), indicating activation of the canonical NF-κB pathway. This effect, which was observed as early as after the first 2 hours of incubation, was inhibited by the Src kinase inhibitor PP2. Both FLCs, but not the vehicle, generated a 4-fold increase in tyrosine phosphorylation of IκBα (middle panels); this was also inhibited by PP2. The bottom panels demonstrate the presence of IκBα in all of the samples studied. The other major band in the gels represents the heavy-chain component of the antibody used to precipitate the protein complexes.
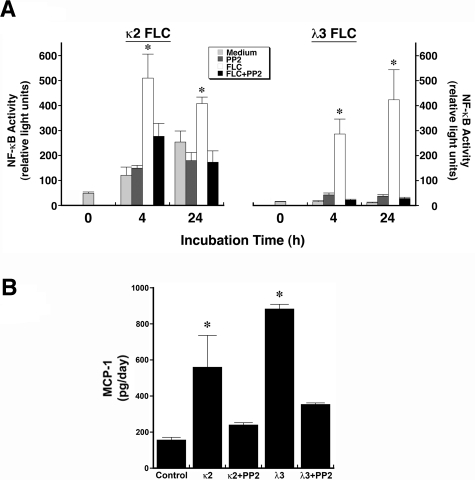
FLC-induced nuclear NF-κB activity and MCP-1 production are inhibited by Src kinase inhibition
NF-κB activity was quantified in nuclear extracts of cells incubated for 4 or 24 hours in medium containing κ2 or λ3 FLCs. Consistent with the immunofluorescence studies (Figure 1A), increased NF-κB activity was observed at 4 and 24 hours with both FLCs; coincubation with PP2 inhibited the increase in activity (Figure 4A). The observed increase in MCP-1 production by cells incubated with FLCs (κ2 and λ3) was inhibited by the addition of PP2 (Figure 4B).
Figure 4.
Incubation of renal epithelial cells with 2 different FLCs (κ2 and λ3) increase nuclear NF-κB activity and promote MCP-1 production in an Src kinase–dependent fashion. (A) Nuclear lysates were obtained from HK-2 cells after 4 and 24 hours of incubation in medium containing 2 different FLCs (κ2 and λ3). Nuclear NF-κB activity was quantified using a filter plate assay, which consisted initially of incubating nuclear extracts with a biotinylated NF-κB–specific DNA-binding sequence. This NF-κB DNA complex was captured on a filter plate to remove the unbound probes and was then denatured and hybridized onto precoated microwells. Bound NF-κB DNA complexes were detected with streptavidin–horseradish peroxidase and quantified using a luminometer. Results, expressed in relative light units, showed an increase in nuclear NF-κB activity at 4 and 24 hours of incubation of renal epithelial cells with κ2 and λ3 FLCs compared with medium alone. The addition of PP2 to the medium inhibited the FLC-induced increase in activity. *P < .05 compared with contemporaneous samples incubated in medium alone, PP2, and corresponding FLCs and PP2; n = 6-12 experiments in each group. (B) Increase in production of MCP-1 after overnight incubation of renal epithelial cells with the κ2 and λ3 FLCs; the addition of PP2 prevented this FLC-induced increase in MCP-1. *P < .05 compared with control and samples treated with PP2 and corresponding FLCs; n = 6 experiments in each group
PS-341 partially inhibits FLC-induced MCP-1 production
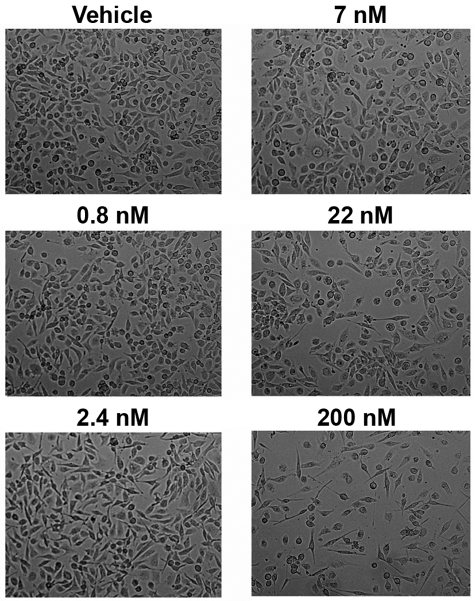
PS-341 inhibits the proteasome, a feature that prevents the degradation of IκBα after serine phosphorylation, and thereby inhibits NF-κB activity.36,37 Experiments were therefore performed to determine whether PS-341 inhibited FLC-induced production of MCP-1 by proximal tubular cells. In initial studies, the dose of PS-341 was titrated between 0.8 and 200nM to minimize cytotoxicity. The cells tolerated PS-341 at a concentration of 7nM in the medium (Figure 5); release of cellular lactate dehydrogenase into the medium did not differ between vehicle- and PS-341–treated (7nM) cells (0.315 ± 0.22 vs 0.281 ± 0.016; P > .05). PS-341 (7nM) partially inhibited the MCP-1 production that occurred when cells were incubated with κ2 and λ2 FLCs (Figure 6).
Figure 5.
Exposure of renal epithelial cells to PS-341 in concentrations < 22nM does not produce cytotoxicity. To determine the sensitivity of HK-2 cells to PS-341, cells were incubated overnight in medium containing PS-341 in concentrations between 0.8 and 200nM. Although concentrations up to 7mM were well tolerated, cell loss was observed at 22 and 200nM.
Figure 6.
Overnight coincubation of PS-341 (7nM) with FLCs (κ2 and λ2) inhibits renal epithelial cell production of MCP-1. HK-2 cells were incubated overnight in medium that contained PS-341 (7mM) and 2 different FLCs (κ2 and λ2). While PS-341 did not affect MCP-1 production by cells incubated in medium alone, FLC-induced MCP-1 production was partially inhibited. *P < .05 compared with sample containing the corresponding FLCs; n = 6 in each group
Discussion
The complexities of the NF-κB pathway have been elucidated in some detail in previous studies.32–34 The canonical NF-κB signaling pathway is initiated by activation of the IKK complex, which consists of IKKα, IKKβ, and NEMO (IKKγ). This complex phosphorylates IκB, releasing the NF-κB complex and permitting nuclear translocation of this transcription factor and the induction of gene transcription. The NF-κB pathway may be activated by a variety of events, including tyrosine phosphorylation of members of the IKK complex, IKKα and IKKβ,26,27 or IκBα.35 Tyrosine phosphorylation of IκBα activates NF-κB independently of the IKK complex (termed the atypical pathway).33,35 In prior studies from this laboratory, monoclonal FLCs were found to promote MCP-1 and IL-6 production through intracellular redox signaling events provoked by elaboration of hydrogen peroxide.11,12 A critical mediator was c-Src, which was revealed using RNA interference and pharmacologic inhibitors12; findings from those studies also supported direct oxidation of c-Src, which as Giannoni et al have showed,38 facilitates c-Src activation. The present studies demonstrated that, in the setting of FLC-induced activation of proximal tubular epithelium: (1) the NF-κB pathway was activated, nuclear translocation of RelA (p65) was observed, and MCP-1 production was mitigated by inhibitors of NF-κB and c-Src; (2) active (phosphorylated) c-Src coimmunoprecipitated with both IKKα and IKKβ and promoted a time-dependent tyrosine phosphorylation of these enzymes; (3) IκBα was activated by the canonical pathway, as demonstrated by serine phosphorylation, but was also tyrosine phosphorylated in an Src kinase–dependent fashion; and (4) the increase in NF-κB activity was inhibited by Src inhibition. The combined findings demonstrate that c-Src activation is upstream of NF-κB and activates, through tyrosine phosphorylation, both the canonical and atypical (IKK-independent) NF-κB pathways (Figure 7). Finally, not all FLCs appear to activate the proximal tubule, because exposure of HK-2 cells to a mixture of polyclonal κ and λ FLCs did not generate hydrogen peroxide or promote MCP-1 production. Along with prior data demonstrating that the cellular response to various monoclonal FLCs differs in intensity,11 the combined data suggest that the underlying mechanism integrally involves the physicochemical composition of the FLCs.
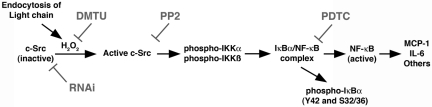
Figure 7.
Diagram depicting the proposed signal transduction pathway that is initiated after endocytosis of FLCs into renal epithelial cells and leads to activation of the canonical and atypical NF-κB pathways. Data supporting this pathway have been published previously11,12 and are also found in the present study. RNAi, RNA interference; DMTU, 1,3-dimethyl-2-thiourea.
The present study has potential clinical relevance. Renal failure, a major complication of multiple myeloma, shortens life span and heralds a poor prognosis in these patients.2 Monoclonal immunoglobulin FLCs are the culprit in most of these renal lesions, and the majority of patients with renal failure from FLCs in this setting have tubulo-interstitial renal disease.4 Endocytosis of monoclonal FLCs into the proximal tubular epithelium promotes the intrarenal generation of chemokines such as MCP-1, which contributes to the proinflammatory, profibrotic process in the interstitium. Particularly relevant to myeloma, activation of the NF-κB pathway also promotes the production of other cytokines, especially IL-6. In addition, other signaling pathways, including p38 mitogen-activated protein kinase, are involved in this process.12,39–42 Clinical evidence suggests that bortezomib (PS-341)–based therapies provide effective treatment of myeloma, even in patients with advanced renal failure.43,44 A recent nonrandomized trial showed that a bortezomib-based chemotherapeutic regimen produced a high response rate in patients who had myeloma and FLC-induced acute kidney injury, with improvement in renal function noted in 62%.45 The results of the present study suggest that proteasome inhibitors such as bortezomib may afford additional therapeutic benefit in those patients who have myeloma and associated renal dysfunction, perhaps by interfering with NF-κB activation36,37 and renal epithelial cell production of MCP-1 and IL-6. However, the reduction in FLC-induced MCP-1 production by this proteasome inhibitor was less than complete. The reason for this partial response was not conclusively determined, but was perhaps related to the discovery that FLCs generated multiple activation points in the NF-κB cascade. Unlike phosphorylation of serine residues at positions 32 and 36, which promote ubiquitination and degradation of IκBα,46 tyrosine phosphorylation of IκBα can permit activation of NF-κB without proteasomal degradation of IκBα, rendering proteasome inhibition ineffective in completely preventing NF-κB activation.32,35 Finally, other pharmacologic interventions, including the use of novel agents such as pituitary adenylate cyclase-activating polypeptide-38, which inhibits FLC-induced NF-κB activity and proinflammatory cytokine and chemokine production,41 or perhaps an Src-kinase inhibitor, may provide additional efficacy in those patients who have myeloma and associated kidney disease from overproduction of monoclonal FLCs.
Until a cure for multiple myeloma is found, the attendant renal complications that contribute significantly to morbidity and mortality in this disease should be considered in therapeutic plans. Even small elevations in the serum creatinine concentration reflect significant renal disease. Although the findings were limited to in vitro experiments, the present study emphasizes both the challenges in providing effective renoprotective therapy and additional potential avenues to pursue in the management of patients with renal dysfunction from multiple myeloma.
Acknowledgments
We thank Mr Shawn Williams at the University of Alabama-Birmingham High Resolution Imaging Facility for assistance with experiments that used the confocal laser scanning microscope. We also thank Binding Site Ltd for their unrestricted support of K.B.'s clinical fellowship and for their expertise in protein chemistry.
This research was supported by the National Institutes of Health, George M. O'Brien Kidney and Urological Research Centers Program grants R01 DK46199 and P30 DK079337 and by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: W.-Z.Y. designed and performed essential experiments, analyzed findings, and assisted with and approved the final version of the manuscript; P.-X.W. designed and performed essential experiments, analyzed findings, and assisted with and approved the final version of the manuscript; K.J.A. assisted with experiments, analyzed findings, and assisted with and approved the final version of the manuscript; K.B. performed experiments, analyzed findings, and assisted with and approved the final version of the manuscript; and P.W.S. planned experiments, analyzed findings from every experiment, coordinated the research activities, and finalized and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul W. Sanders, MD, Division of Nephrology/Department of Medicine, 642 Lyons-Harrison Research Bldg, 1530 Third Ave S, University of Alabama at Birmingham, Birmingham, AL 35294-0007; e-mail: psanders@uab.edu.
References
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50(1):7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 3.Abbott KC, Agodoa LY. Multiple myeloma and light chain-associated nephropathy at end-stage renal disease in the United States: patient characteristics and survival. Clin Nephrol. 2001;56(3):207–210. [PubMed] [Google Scholar]
- 4.Sanders PW. Myeloma and secondary involvement of the kidney in dysproteinemias. In: Wilcox CS, editor. Therapy in nephrology and hypertension: a companion to Brenner and Rector's the kidney. 5th ed. London, United Kingdom: WB Saunders; 2008. pp. 461–468. [Google Scholar]
- 5.Batuman V, Dreisbach AW, Cyran J. Light-chain binding sites on renal brush-border membranes. Am J Physiol. 1990;258(5 Pt 2):F1259–F1265. doi: 10.1152/ajprenal.1990.258.5.F1259. [DOI] [PubMed] [Google Scholar]
- 6.Batuman V, Guan S. Receptor-mediated endocytosis of immunoglobulin light chains by renal proximal tubule cells. Am J Physiol. 1997 Apr;272(4 Pt 2):F521–F530. doi: 10.1152/ajprenal.1997.272.4.F521. [DOI] [PubMed] [Google Scholar]
- 7.Batuman V, Verroust PJ, Navar GL, et al. Myeloma light chains are ligands for cubilin (gp280). Am J Physiol. 1998;275(2 Pt 2):F246–F254. doi: 10.1152/ajprenal.1998.275.2.F246. [DOI] [PubMed] [Google Scholar]
- 8.Klassen RB, Allen PL, Batuman V, Crenshaw K, Hammond TG. Light chains are a ligand for megalin. J Appl Physiol. 2005;98(1):257–263. doi: 10.1152/japplphysiol.01090.2003. [DOI] [PubMed] [Google Scholar]
- 9.Bradwell AR, Mead GP, Carr-Smith HD. Bradwell AR. Serum free light chain analysis. 3rd ed. Birmingham: The Binding Site; 2005. Diseases with increased polyclonal free light chains. pp. 175–194. [Google Scholar]
- 10.Mead GP, Carr-Smith HD, Drayson MT, Morgan GJ, Child JA, Bradwell AR. Serum free light chains for monitoring multiple myeloma. Br J Haematol. 2004;126(3):348–354. doi: 10.1111/j.1365-2141.2004.05045.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang PX, Sanders PW. Immunoglobulin light chains generate hydrogen peroxide. J Am Soc Nephrol. 2007;18(4):1239–1245. doi: 10.1681/ASN.2006111299. [DOI] [PubMed] [Google Scholar]
- 12.Basnayake K, Ying WZ, Wang PX, Sanders PW. Immunoglobulin light chains activate tubular epithelial cells through redox signaling. J Am Soc Nephrol. 2010;21(7):1165–1173. doi: 10.1681/ASN.2009101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20(1):1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 14.Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45(1):48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- 15.Sanders PW, Herrera GA, Galla JH. Human Bence Jones protein toxicity in rat proximal tubule epithelium in vivo. Kidney Int. 1987;32(6):851–861. doi: 10.1038/ki.1987.286. [DOI] [PubMed] [Google Scholar]
- 16.Hanke JH, Gardner JP, Dow RL, et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271(2):695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 17.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175(5):1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams J, Behnke M, Chen S, et al. Potent and selective inhibitors of the proteasome: dipeptidyl boronic acids. Bioorg Med Chem Lett. 1998;8(4):333–338. doi: 10.1016/s0960-894x(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 20.Bradwell AR. 5th ed. Birmingham, United Kingdom: The Binding Site; 2008. Serum free light chain analysis. [Google Scholar]
- 21.Pesce AJ, Clyne DH, Pollak VE, Kant SK, Foulkes EC, Selenke WM. Renal tubular interactions of proteins. Clin Biochem. 1980;13(5):209–215. doi: 10.1016/s0009-9120(80)80025-7. [DOI] [PubMed] [Google Scholar]
- 22.Baylis C, Falconer-Smith J, Ross B. Glomerular and tubular handling of differently charged human immunoglobulin light chains by the rat kidney. Clin Sci (Lond) 1988;74(6):639–644. doi: 10.1042/cs0740639. [DOI] [PubMed] [Google Scholar]
- 23.Ying WZ, Xia H, Sanders PW. Nitric oxide synthase (NOS2) mutation in Dahl/Rapp rats decreases enzyme stability. Circ Res. 2001;89(4):317–322. doi: 10.1161/hh1601.094625. [DOI] [PubMed] [Google Scholar]
- 24.Ying WZ, Sanders PW. Accelerated ubiquitination and proteasome degradation of a genetic variant of inducible nitric oxide synthase. Biochem J. 2003;376(Pt 3):789–794. doi: 10.1042/BJ20031058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ying WZ, Zhang HG, Sanders PW. EGF receptor activity modulates apoptosis induced by inhibition of the proteasome of vascular smooth muscle cells. J Am Soc Nephrol. 2007;18(1):131–142. doi: 10.1681/ASN.2006040333. [DOI] [PubMed] [Google Scholar]
- 26.Huang WC, Chen JJ, Inoue H, Chen CC. Tyrosine phosphorylation of I-kappa B kinase alpha/beta by protein kinase C-dependent c-Src activation is involved in TNF-alpha-induced cyclooxygenase-2 expression. J Immunol. 2003;170(9):4767–4775. doi: 10.4049/jimmunol.170.9.4767. [DOI] [PubMed] [Google Scholar]
- 27.Ten RM, McKinstry MJ, Trushin SA, Asin S, Paya CV. The signal transduction pathway of CD23 (Fc epsilon RIIb) targets I kappa B kinase. J Immunol. 1999;163(7):3851–3857. [PubMed] [Google Scholar]
- 28.Amir RE, Haecker H, Karin M, Ciechanover A. Mechanism of processing of the NF-kappa B2 p100 precursor: identification of the specific polyubiquitin chain-anchoring lysine residue and analysis of the role of NEDD8-modification on the SCF(beta-TrCP) ubiquitin ligase. Oncogene. 2004;23(14):2540–2547. doi: 10.1038/sj.onc.1207366. [DOI] [PubMed] [Google Scholar]
- 29.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 30.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 31.Mercurio F, Murray BW, Shevchenko A, et al. IkappaB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Mol Cell Biol. 1999;19(2):1526–1538. doi: 10.1128/mcb.19.2.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perkins ND. Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene. 2006;25(51):6717–6730. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- 33.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8(1):49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8(11):837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 35.Imbert V, Rupec RA, Livolsi A, et al. Tyrosine phosphorylation of I kappa B-alpha activates NF-kappa B without proteolytic degradation of I kappa B-alpha. Cell. 1996;86(5):787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 36.Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61(7):3071–3076. [PubMed] [Google Scholar]
- 37.Nasr R, El-Sabban ME, Karam JA, et al. Efficacy and mechanism of action of the proteasome inhibitor PS-341 in T-cell lymphomas and HTLV-I associated adult T-cell leukemia/lymphoma. Oncogene. 2005;24(3):419–430. doi: 10.1038/sj.onc.1208212. [DOI] [PubMed] [Google Scholar]
- 38.Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol. 2005;25(15):6391–6403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sengul S, Zwizinski C, Simon EE, Kapasi A, Singhal PC, Batuman V. Endocytosis of light chains induces cytokines through activation of NF-kappaB in human proximal tubule cells. Kidney Int. 2002;62(6):1977–1988. doi: 10.1046/j.1523-1755.2002.00660.x. [DOI] [PubMed] [Google Scholar]
- 40.Sengul S, Zwizinski C, Batuman V. Role of MAPK pathways in light chain-induced cytokine production in human proximal tubule cells. Am J Physiol Renal Physiol. 2003;284(6):F1245–F1254. doi: 10.1152/ajprenal.00350.2002. [DOI] [PubMed] [Google Scholar]
- 41.Arimura A, Li M, Batuman V. Potential protective action of pituitary adenylate cyclase-activating polypeptide (PACAP38) on in vitro and in vivo models of myeloma kidney injury. Blood. 2006;107(2):661–668. doi: 10.1182/blood-2005-03-1186. [DOI] [PubMed] [Google Scholar]
- 42.Li M, Balamuthusamy S, Simon EE, Batuman V. Silencing megalin and cubilin genes inhibits myeloma light chain endocytosis and ameliorates toxicity in human renal proximal tubule epithelial cells. Am J Physiol Renal Physiol. 2008;295(1):F82–F90. doi: 10.1152/ajprenal.00091.2008. [DOI] [PubMed] [Google Scholar]
- 43.Jagannath S, Barlogie B, Berenson JR, et al. Bortezomib in recurrent and/or refractory multiple myeloma. Initial clinical experience in patients with impared renal function. Cancer. 2005;103(6):1195–1200. doi: 10.1002/cncr.20888. [DOI] [PubMed] [Google Scholar]
- 44.Chanan-Khan AA, Kaufman JL, Mehta J, et al. Activity and safety of bortezomib in multiple myeloma patients with advanced renal failure: a multicenter retrospective study. Blood. 2007;109(6):2604–2606. doi: 10.1182/blood-2006-09-046409. [DOI] [PubMed] [Google Scholar]
- 45.Ludwig H, Adam Z, Hajek R, et al. Light chain-induced acute renal failure can be reversed by bortezomib-doxorubicin-dexamethasone in multiple myeloma: results of a phase ii study. J Clin Oncol. 2010;28(30):4635–4641. doi: 10.1200/JCO.2010.28.1238. [DOI] [PubMed] [Google Scholar]
- 46.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267(5203):1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]