Abstract
Purpose
Repair of a lacerated flexor digitorum profundus (FDP) tendon underneath or just distal to the A4 pulley can be technically challenging, and success may be confounded by tendon triggering and scarring to the pulley. The purpose of this study was to quantify the effect of partial and complete A4 pulley release in the context of a lacerated and repaired FDP tendon just distal to the A4 pulley.
Methods
Tendon biomechanics were tested in six cadaveric hands secured to a rigid frame permitting measurement of tendon excursion, tendon force, and finger range of motion. After control testing, each finger underwent laceration and repair of the FDP tendon at the distal margin of the A4 pulley using a six-strand core suture technique and epitendinous repair. Testing was then repeated after the following interventions: 1) Intact A4 pulley 2) Release of the distal half of the A4 pulley, 3) Complete release of the A4 pulley, and 4) Continued proximal release of the sheath to the distal edge of A2 (release of C2, A3, and C1 pulleys). Release of the pulleys was performed by incision; no tissue was removed from the specimens.
Results
From full extension to full flexion, average profundus tendon excursion for all intact digits was 37.9 ± 1.5 mm, and tendon repair resulted in average tendon shortening of 1.6 ± 0.4 mm. Flexion lag increased from less than 1 mm to over 4 mm with venting of the A4 pulley, complete A4 release, and proximal sheath release, respectively. Compared to the intact state, repair of the tendon with an intact A4 pulley, release of half the A4 pulley, complete A4 release, and proximal sheath release resulted in percent increases in work of flexion (WOF) of 11.5 ± 3.1%, 0.83 ± 2.8%, 2.6 ± 2.4%, and 3.25 ± 2.2%, respectively (p<0.05).
Conclusions
After FDP laceration and repair in the region of the A4 pulley, WOF did not increase by more than 3% from control conditions after partial or complete A4 pulley release, and WOF was significantly less than performing a repair and leaving the A4 pulley intact.
Keywords: biomechanics, finger injury, muscle, pulley release, tendon repair
INTRODUCTION
The nine finger flexor pulleys maintain the course of the digital flexor tendons adjacent to the phalanges. This positioning of the tendons is critical for maintaining normal finger function1. The relative importance of each pulley (Palmar aponeurotic [PA], A1-5, and C1-3) has been extensively studied, and most studies support the concept that the A2 and A4 pulleys are the most crucial to maintain normal digital function by preventing bowstringing and loss of finger flexion2–5. Consequently, early recommendations advocated repair or reconstruction of the pulleys6. Subsequent biomechanical research examining excursion change7–9 or changes in the work of flexion (WOF)2 after a pulley release reinforced the idea that A2 and A4 pulleys cannot be sacrificed without significant loss of motion or strength. In response, a variety of pulley reconstructive techniques were developed10–11. Flexor tenorrhaphy is technically challenging, and repair site entrapment by the pulley system can compromise the outcome12–13. The most common indications for pulley release during repair are to facilitate tendon exposure, permit smooth tendon gliding of the repaired tendon, accommodate post-surgical edema, or prevent postoperative tendon entrapment by adhesions. These indications tend to be especially true with smaller pulleys (A4) and smaller tendons (digitus minimus14).
In the mid-1990s, Tang and Tomiano et al. investigated the simulated range of digital motion using cadaveric hands and suggested that partial pulley release actually reduces WOF while maintaining normal or near normal tendon excursion and range of motion15–16. However, these studies were performed in normal functioning tendons undisturbed by laceration and subsequent repair. Further, recent studies in chicken models suggest that partial pulley release, and in some cases completely dividing the pulley, may actually improve tendon gliding and decrease the risk of tendon rupture after repair17–19. Additional studies have shown that partial excision of the A2 and A4 tendon pulleys can be tolerated without a significant change in finger flexion15, 20–21, and clinical reports of Kwai Ben and Elliot and Tang advocate complete A4 pulley excision to prevent tendon catching14, 22. Given that, in humans, tendon repair under or distal to the A4 pulley is often technically challenging, and that outcomes may be compromised by triggering and tethering of the repair due to adhesions to the A4 pulley, these recent studies provide a rational basis for the intentional release of the A4 pulley associated with flexor tendon repair. The purpose of this study was to examine the effect of partial and complete A4 pulley release, as well as sheath (C2, A3, and C1 pulleys) release, in the context of a lacerated and repaired human flexor digitorum profundus (FDP) tendon at the level of the A4 pulley.
METHODS
Experimental Specimens
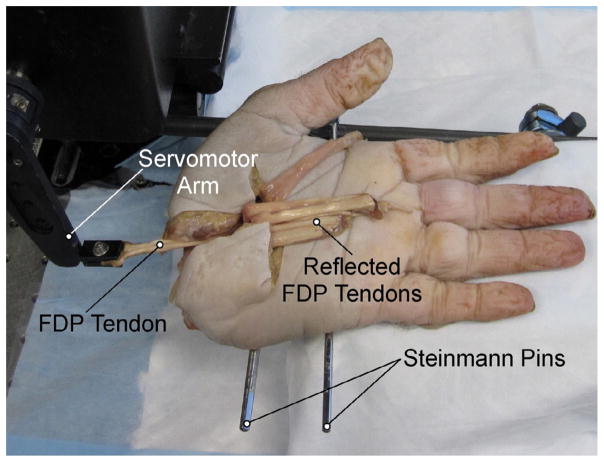
Tendon properties were tested in six adult fresh frozen human cadaveric hands thawed overnight to room temperature. Hands were severed at the carpometacarpal joint with removal of the thumb. With the exception of the FDP tendon to each digit, all skin and soft tissue were removed proximal to the midpalmar crease. Tendons to individual digits (index, middle, ring, and small) were identified and freed from adjacent tendon sheaths and connective tissue. Two Steinman pins were drilled through three or four metacarpals and hands were secured to a rigid custom frame, palm up, to permit measurement of tendon excursion, tendon force, and finger range of motion of each FDP tendon to each digit. Hands were rested on an adjustable solid support to prevent digital hyperextension due to gravity.
Experimental Method
Each FDP tendon was clamped to a dual mode servo-motor (Aurora Scientific, Inc., Model 310, Aurora, Ontario, Canada), which allowed continuous recording of length and force. Initial tendon position was defined with the finger fully extended, and the tendon was pulled proximally (simulating active flexion) at a rate of 300 mm/min to the point of full flexion, confirmed by visual contact between the finger-tip and palmar skin (Fig. 1). An initial trial with intact tendons and pulleys was recorded to provide baseline values of excursion, force of flexion, and WOF needed to flex the finger. Then, flexor profundus tendons were completely transversely lacerated uniformly at the distal margin of the A4 pulley and repaired by a single experienced board certified hand surgeon with a certificate of added qualifications. Repairs were performed with a modified Tsai six-strand repair23 using a single 4-0 fiberwire suture (Arthrex; Naples, Florida, USA) and augmented with a standardized Silfverskiold cross-stitch24 epitendinous repair with 6-0 prolene (Ethicon; San Angelo, TX, USA). Fingers then underwent the same testing protocol after each of the following interventions: 1) Intact A4 pulley, 2) Release of the distal half of the A4 pulley, 3) Complete release of the A4 pulley, and 4) Continued proximal release of the sheath (“proximal sheath release”) to the distal edge of A2 (release of C2, A3, and C1 pulleys). In all conditions, the pulley was centrally and longitudinally incised and no tissue was removed in the process. The skin was reapproximated and sutured with 4-0 nylon (Ethicon; USA) after each intervention. The surgical site was irrigated with Lactated Ringer’s solution to prevent drying.
Figure 1.
Mechanical testing apparatus. The cadaveric hand was disarticulated proximal to the base of the metacarpals, and skin was removed proximal to the level of the proximal wrist crease. Two Steinman pins were drilled through at least 3 metacarpals and securely fixed to a custom brace. The tendon was then clamped to a dual-servo motor for excursion/force testing.
Raw data obtained from the servomotor were tendon force and tendon excursion, from which the following values were calculated:
Tendon excursion (mm): Excursion required for full digital flexion, confirmed by contact between the fingertip and the palmar skin. The control excursion distance was defined as the minimum excursion distance required for full flexion, measured in the repaired condition.
Work of flexion (mJ): Area under the force-excursion curve.
Flexion lag (mm): The distance between the fingertip and the palmar skin when the tendon was pulled to the control excursion distance.
Two trials were conducted and recorded for each condition after a single conditioning trial. Final values were recorded as the average of the two test trials.
Load to Failure Biomechanical Testing
Load to failure (N) was determined for each tendon after in vivo testing by removing the complete tendon with its repaired segment as well as one cm each of the native proximal and distal tendon. Tendon end regions were clamped into a materials testing device (Model 5565A, Instron, Inc., Grove City, PA) and, after three repeated preconditioning trials of 1% strain, tendons were elongated to failure while sampling force at 100Hz.
Statistical Analysis
Data were analyzed by two-way ANOVA (finger and treatment as grouping factors) with significance level (α) set to 0.05. Post hoc comparisons were made between treatments using multiple paired comparisons. For purposes of analysis, fingers within each hand were treated as a repeated measure. All data are presented as a mean ± SEM.
RESULTS
A total of 18 digits (4 index, 4 long, 5 ring, 5 small) were successfully tested from a total of six adult hands (1 female, 5 male; range 42–71 years). A total of 22 digits were initially available for testing. Reasons for excluding digits included an inability to fully flex the finger (n=3), and in one case, the tendon slipped from the clamp during testing, thus resulting in a total of 18 digits for analysis.
There were no complications during the laceration or surgical repair of any of the tendons. In all cases, exposure was adequate for the repair without damage to surrounding pulley structures or soft tissue restraints. In some cases, thin connections between tendon and bone were released to assist with exposure during the repair. Tendon repair resulted in an average tendon shortening of 1.6 ± 0.4 mm (n=18 digits).
Baseline average excursion of the FDP tendons for all intact digits was 37.9 ± 1.5 mm, and average baseline WOF was 69.5 ± 8.8 mJ. Absolute excursion and force values were variable among digits due to differences in size and soft tissue characteristics (Table 1). Because the relative effects were very consistent across all digits, summary data are presented in terms of percent changes from baseline.
Table 1.
Work of flexion, force of flexion, and flexion lag for each digit type
| Digit | Tendon Treatment | Pulley Treatment | Work of Flexion (mJ) | Force of Flexion (N) | Flexion Lag (mm) |
|---|---|---|---|---|---|
| Index (n=4) | Control |
Baseline | 72.2 ± 25.4 | 6.6 ± 2.4 | |
| With Tendon Repaired | Intact A4 | 70.2 ± 19.7 | 6.5 ± 2.1 | ||
| Half A4 | 63.7 ± 16.0 | 5.7 ± 1.4 | 0.5 ± 0.3 | ||
| A4 Removed | 62.8 ± 15.2 | 5.0 ± 1.4 | 2.5 ± 0.7 | ||
| No sheath | 66.8 ± 17.4 | 6.2 ± 1.8 | 1.8 ± 0.7 | ||
| Long (n=4) | Control |
Baseline | 110.6 ± 5.6 | 11.9 ± 0.7 | |
| With Tendon Repaired | Intact A4 | 128.4 ± 11.3 | 13.9 ± 1.2 | ||
| Half A4 | 110.6 ± 6.7 | 12.6 ± 1.5 | 0.2 ± 0.1 | ||
| A4 Removed | 114.7 ± 8.3 | 12.2 ± 1.2 | 1.9 ± 0.1 | ||
| No sheath | 113.5 ± 7.4 | 11.8 ± 1.3 | 4.8 ± 0.8 | ||
| Ring (n=5) | Control |
Baseline | 69.5 ± 7.8 | 7.4 ± 1.0 | |
| With Tendon Repaired | Intact A4 | 83.8 ± 9.5 | 7.5 ± 0.7 | ||
| Half A4 | 75.8 ± 9.9 | 6.7 ± 0.8 | 0.3 ± 0.2 | ||
| A4 Removed | 74.7 ± 8.2 | 6.9 ± 0.8 | 1.6 ± 0.6 | ||
| No sheath | 73.1 ± 8.3 | 7.0 ± 0.8 | 4.1 ± 0.5 | ||
| Small (n=5) | Control |
Baseline | 34.68 ± 8.95 | 4.03 ± 1.00 | |
| With Tendon Repaired | Intact A4 | 36.16 ± 9.23 | 3.96 ± 0.96 | ||
| Half A4 | 34.12 ± 9.35 | 3.62 ± 0.83 | 0.25 ± 0.11 | ||
| A4 Removed | 35.20 ± 9.10 | 3.70 ± 0.79 | 1.60 ± 0.58 | ||
| No sheath | 35.92 ± 9.58 | 3.83 ± 0.89 | 3.30 ± 0.58 | ||
Summarized data including work of flexion, force of flexion, and excursion distance for each digit type.
Data are presented as mean ± SEM.
signifies p<0.05 across different pulley/sheath conditions.
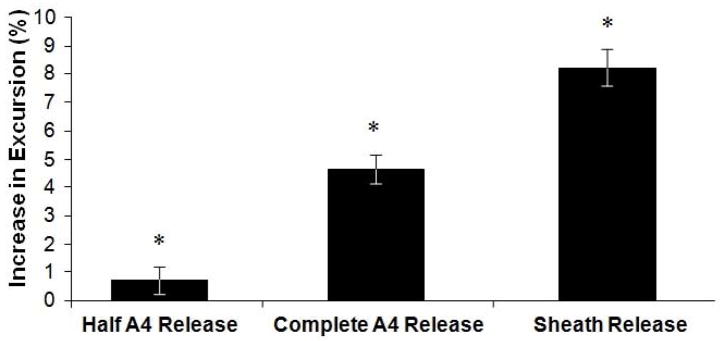
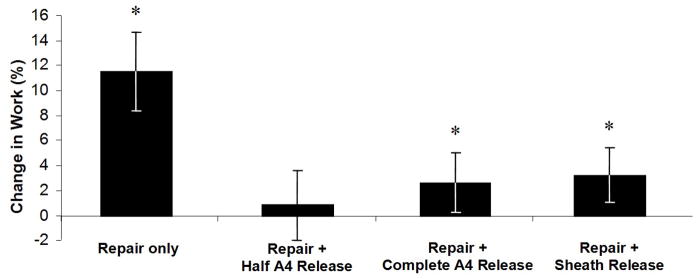
There was a general trend towards increasing excursion necessary to achieve full flexion with each incremental release of the A4 pulley and sheath after tendon repair. Release of the A4 pulley, complete A4 release, and proximal sheath release resulted in percent increases of excursion necessary to achieve full flexion of 0.7 ± 0.5%, 4.6 ± 0.5%, and 8.2 ± 0.7%, respectively (Fig. 2; p<0.05 compared to intact A4). WOF increased after tendon repair with the A4 pulley intact and decreased towards baseline levels after release of one half of the A4 pulley. Repair of the tendon with an intact A4 pulley, release of half the A4 pulley, complete A4 release, and proximal sheath release resulted in percent increases in WOF of 11.5 ± 3.1%, 0.83 ± 2.8%, 2.6 ± 2.4%, and 3.25 ± 2.2%, respectively (Fig. 3; p<0.05 compared to baseline condition).
Figure 2.
Excursion represented as the percent change from the control condition for all fingers. Error bars represent the SEM. *signifies p<0.05 across all conditions.
Figure 3.
Work of flexion represented as the percent change from the repaired condition with A4 pulley intact. Error bars represent the SEM. *signifies p<0.05 across all conditions.
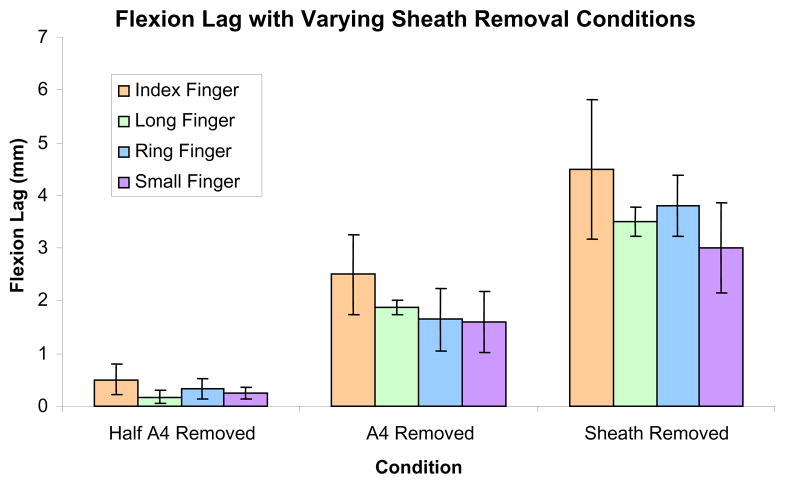
To simulate the clinical result of changes in tendon and pulley properties, flexion lag was measured between the fingertip and palmar skin. As expected, flexion lag increased from less than 1 mm to over 4 mm with venting of the A4 pulley, complete A4 release, and proximal sheath release (Fig. 4). There was no significant difference between digits (p=0.064), and statistical power (1-β) was approximately 0.74 to detect a 1 mm flexion lag.
Figure 4.
Flexion lag under each pulley/sheath condition for each of the four fingers. Data are represented as a mean for each type of finger and error bars represent the SEM.
After mechanical testing was complete, the load to complete failure of the suture repair was obtained for all tendons. The mean load to failure was very high (49.8 ± 2.7 N) compared to forces measured during digital manipulation (highest average force = 16.3 N; Table 1). In all cases, failure occurred secondary to suture failure across the repair site without any evidence of tendon rupture.
DISCUSSION
Historically, intentional sacrifice of the A4 pulley has been performed with caution due to its presumed importance in flexor tendon function25. Multiple studies have validated the significance of the A2 and A4 pulleys with respect to effective finger flexion, and more recent studies have appropriately focused on WOF as a relevant outcome measure2–5. Peterson et al. concluded that A2 and A4 are the most and second most important pulleys, respectively, based on their findings that loss of the A4 pulley resulted in a 20% increased WOF. As a result, hand surgery dogma suggests that sacrifice of either the A2 or A4 pulleys should be avoided, if possible, and reconstruction should be performed in cases of pulley laceration or rupture. However, conclusions drawn from Peterson’s study may be limited by the methodology. Specifically, the study involved the use of nonhuman primate hands, which may not mimic the biomechanics of human hands. In addition, their study did not provide statistical analysis making it impossible to define significant differences, if any, between conditions. Finally, their study primarily investigated the condition in which the flexor tendon was intact, and pulley integrity was the experimental variable. Thus, the clinical question remains: What pulley conditions are associated with the least WOF and would consequently lead to the best clinical outcomes in the context of flexor tendon repair?
The results of our study demonstrated an approximate 3% increase in WOF of a repaired FDP tendon with loss of the A4 pulley. Although our results demonstrated statistically significant percent changes in WOF after each intervention, the absolute difference in work was small. In fact, the amount of work required to move the tendon within the sheath is nearly negligible in the context of the physiological potential of the FDP muscle bellies. Given that each belly of the FDP can produce ~45N of peak force and each has an excursion of at least 3 cm26, each belly can produce ~1.3 J of energy during a single contraction. Thus, the changes demonstrated in our study between control and repaired conditions, ranging between 2–18 mJ for each finger, are probably not clinically significant.
Tang and Xie sequentially released digital flexor pulleys and tested the excursion of flexor tendons in a setting similar to that used in this study27. They reported a 19% average excursion increase in 11 digits when flexed to 110° after release of the A3 and A4 pulleys and the synovial sheath between the two pulleys. In contrast, we find an 8.2% increase in excursion after the proximal sheath release, which includes the release performed by Tang and Xie, as well as release of the sheath between A3 and A2. This discrepancy may be explained by the fact that our testing was performed with fingers fully flexed to the palm, not limited to 110°, suggesting that changes in pulley function may depend upon finger position and arc of motion.
Our results are in agreement with reports that, compared to the potential for triggering or entrapment of the repair at the level of the pulley, loss of the A4 pulley and sheath may be mechanically well-tolerated15, 27. This lends support to recent clinical reports suggesting that release of the A4 pulley may, in some cases, lead to better outcomes than leaving the A4 pulley intact14, 22.
When repairing flexor tendons and choosing whether or not a pulley can be sacrificed, hand surgeons must balance the risk of decreased finger flexion (based on the biomechanics of digital flexor tendons and pulleys) with the risk of gliding interference and scar tissue formation beneath the pulley (due to the pathological state of flexor tendon injury). Results from our study suggest that the decreased WOF and excursion after loss of at least half the A4 pulley, and likely the complete pulley, can be easily overcome by the intrinsic ability of the FDP muscle bellies. In this study, we measured flexion lag under each pulley and sheath release condition to evaluate whether the loss of excursion would result in a clinically significant loss of full flexion. Even if the FDP muscle belly were unable to compensate for the loss of excursion, our results indicate that loss of half the A4 pulley and complete A4 release resulted in an average flexion lag of 0.3 mm and 1.8 mm, respectively. Our clinical experience has been that if there is scarring or triggering with entrapment of a repair site under the A4 pulley, the outcome is very poor, due to essentially no distal joint flexion. This result is worse than would be anticipated with repair and A4 release, even if the muscle were unable to compensate for the necessary added excursion induced by tendon bowstringing to achieve full flexion.
This study has the following limitations. The use of a cadaveric model is not equivalent to performing in vivo clinical studies to evaluate surgical outcomes after release of the flexor pulleys. This study cannot account for time dependent changes and other factors that occur in a living model, such as scar formation and adhesions, tendon swelling, weakening of the tendon at the site of repair, compensation by the muscle bellies in response to changes in excursion lengths and forces, or changes in the A3 pulley structure and strength following the release of A4. We believe, however, that those types of conditions in the living model would have likely amplified the differences in WOF between each condition and probably would have made the scenario of A4 release appear even better when compared to the repaired tendon with A4 left intact. In addition, the “control” condition performed on the intact finger did not include incision and repair of the skin, as was performed after all trial conditions. Our study design of performing the control testing prior to any skin incisions helped assure that our primary outcome, WOF, would be compared to the “normal” condition as closely as possible. However, as a result, subsequent skin incision with suture repair (as was performed with every intervention) may have changed the tendon gliding mechanics of our model and influenced those results. In addition, the WOF may decrease with each additional run, and, because our baseline comparison values were performed first, WOF under the sheath release conditions may be underestimated. We believe that these effects were comparatively small and remained constant for all intervention conditions.
The results from this study are clinically significant. We suggest that when faced with a choice between performing an A4 pulley release (and forgoing immediate A4 pulley reconstruction) at the time of FDP repair versus the potential for long-term complications secondary to an intact or repaired pulley, hand surgeons can release the A4 pulley. Our findings suggest that both WOF and tendon excursion may not suffer from any clinically relevant changes after release of the A4 pulley. In contrast, our clinical experience suggests that repair of the pulley over a repaired tendon is often associated with a high rate of tendon scarring and loss of DIP flexion. We believe these results can provide hand surgeons with information that will help guide intraoperative decision-making and improve patient outcomes.
Acknowledgments
The authors acknowledge the Department of Veterans Affairs, and NIH grant R24HD050837.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE REFERENCES
- 1.Doyle JR, Blythe WF. Anatomy of the flexor tendon sheath and pulleys of the thumb. J Hand Surg. 1977;2A:149–151. doi: 10.1016/s0363-5023(77)80101-9. [DOI] [PubMed] [Google Scholar]
- 2.Peterson WW, Manske PR, Bollinger BA, Lesker PA, McCarthy JA. Effect of pulley excision on flexor tendon biomechanics. J Orthop Res. 1986;4:96–101. doi: 10.1002/jor.1100040112. [DOI] [PubMed] [Google Scholar]
- 3.Doyle JR. Anatomy of the flexor tendon sheath and pulley system: a current review. J Hand Surg. 1989;14A:349–351. doi: 10.1016/0363-5023(89)90110-x. [DOI] [PubMed] [Google Scholar]
- 4.Lin GT, Amadio PC, An KN, Cooney WP. Functional anatomy of the human digital flexor pulley system. J Hand Surg. 1989;14A:949–956. doi: 10.1016/s0363-5023(89)80043-7. [DOI] [PubMed] [Google Scholar]
- 5.Rispler D, Greenwald D, Shumway S, Allan C, Mass D. Efficiency of the flexor tendon pulley system in human cadaver hands. J Hand Surg. 1996;21A:444–450. doi: 10.1016/S0363-5023(96)80361-3. [DOI] [PubMed] [Google Scholar]
- 6.Lister GD. Incision and closure of the flexor sheath during primary tendon repair. Hand. 1983;15:123–135. doi: 10.1016/s0072-968x(83)80001-1. [DOI] [PubMed] [Google Scholar]
- 7.Brand PW, Cranor KC, Ellis JC. Tendon and pulleys at the metacarpophalangeal joint of a finger. J Bone Joint Surg. 1975;57A:779–784. [PubMed] [Google Scholar]
- 8.Hunter J. Anatomy of flexor tendons - Pulley, vincular, synovia, and vascular structures. Kaplan’s Functional and Surgical Anatomy of the Hand. 3. Philadelphia: JB Lippincott; 1984. pp. 65–92. [Google Scholar]
- 9.Manske PR, Lesker PA. Palmar aponeurosis pulley. J Hand Surg. 1983;8A:259–263. doi: 10.1016/s0363-5023(83)80154-3. [DOI] [PubMed] [Google Scholar]
- 10.Boyer MI. Flexor tendon biology. Hand Clin. 2005;21:159–166. doi: 10.1016/j.hcl.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Tang JB, Wang B, Chen F, Pan CZ, Xie RG. Biomechanical evaluation of flexor tendon repair techniques. Clin Orthop Relat Res. 2001:252–259. doi: 10.1097/00003086-200105000-00033. [DOI] [PubMed] [Google Scholar]
- 12.Ketchum LD, Martin NL, Kappel DA. Experimental evaluation of factors affecting the strength of tendon repairs. Plast Reconstr Surg. 1977;59:708–719. doi: 10.1097/00006534-197705000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Strickland JW. Flexor tendon injuries. Part 2. Flexor tendon repair. Orthop Rev. 1986;15:701–721. [PubMed] [Google Scholar]
- 14.Tang JB. Indications, methods, postoperative motion and outcome evaluation of primary flexor tendon repairs in Zone 2. J Hand Surg Vol. 2007;32B:118–129. doi: 10.1016/J.JHSB.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Tomaino M, Mitsionis G, Basitidas J, Grewal R, Pfaeffle J. The effect of partial excision of the A2 and A4 pulleys on the biomechanics of finger flexion. J Hand Surg. 1998;23B:50–52. doi: 10.1016/s0266-7681(98)80218-0. [DOI] [PubMed] [Google Scholar]
- 16.Tang JB. The double sheath system and tendon gliding in zone 2C. J Hand Surg. 1995;20B:281–285. doi: 10.1016/s0266-7681(05)80078-6. [DOI] [PubMed] [Google Scholar]
- 17.Tang JB, Xie RG, Cao Y, Ke ZS, Xu Y. A2 pulley incision or one slip of the superficialis improves flexor tendon repairs. Clin Orthop Relat Res. 2007;456:121–127. doi: 10.1097/01.blo.0000246564.96208.b0. [DOI] [PubMed] [Google Scholar]
- 18.Cao Y, Tang JB. Strength of tendon repair decreases in the presence of an intact A2 pulley: biomechanical study in a chicken model. J Hand Surg. 2009;34A:1763–1770. doi: 10.1016/j.jhsa.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Tang JB, Cao Y, Wu YF, Wang GH. Effect of A2 pulley release on repaired tendon gliding resistance and rupture in a chicken model. J Hand Surg. 2009;34A:1080–1087. doi: 10.1016/j.jhsa.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Savage R. The mechanical effect of partial resection of the digital fibrous flexor sheath. J Hand Surg. 1990;15B:435–442. doi: 10.1016/0266-7681(90)90086-j. [DOI] [PubMed] [Google Scholar]
- 21.Mitsionis G, Bastidas JA, Grewal R, Pfaeffle HJ, Fischer KJ, Tomaino MM. Feasibility of partial A2 and A4 pulley excision: effect on finger flexor tendon biomechanics. J Hand Surg. 1999;24A:310–314. doi: 10.1053/jhsu.1999.0310. [DOI] [PubMed] [Google Scholar]
- 22.Kwai Ben I, Elliot D. “Venting” or partial lateral release of the A2 and A4 pulleys after repair of zone 2 flexor tendon injuries. J Hand Surg. 1998;23B:649–654. doi: 10.1016/s0266-7681(98)80020-x. [DOI] [PubMed] [Google Scholar]
- 23.Gill RS, Lim BH, Shatford RA, Toth E, Voor MJ, Tsai TM. A comparative analysis of the six-strand double-loop flexor tendon repair and three other techniques: a human cadaveric study. J Hand Surg. 1999;24A:1315–1322. doi: 10.1053/jhsu.1999.1315. [DOI] [PubMed] [Google Scholar]
- 24.Silfverskiold KL, Andersson CH. Two new methods of tendon repair: an in vitro evaluation of tensile strength and gap formation. J Hand Surg. 1993;18A:58–65. doi: 10.1016/0363-5023(93)90246-Y. [DOI] [PubMed] [Google Scholar]
- 25.Green DP. Green’s operative hand surgery. 5. Vol. 2. Philadelphia, Pa: Elsevier/Churchill Livingstone; 2005. pp. 230–233. [Google Scholar]
- 26.Jacobson MD, Raab R, Fazeli BM, Abrams RA, Botte MJ, Lieber RL. Architectural design of the human intrinsic hand muscles. J Hand Surg. 1992;17A:804–809. doi: 10.1016/0363-5023(92)90446-v. [DOI] [PubMed] [Google Scholar]
- 27.Tang JB, Xie RG. Effect of A3 pulley and adjacent sheath integrity on tendon excursion and bowstringing. J Hand Surg. 2001;26A:855–861. doi: 10.1053/jhsu.2001.27768. [DOI] [PubMed] [Google Scholar]