Abstract
Transplantation of cells, tissues, and organs from one individual to another can incite the production of antibodies specific for foreign antigens, especially major histocompatibility antigens, in the graft. Antibodies specific for a graft provide an index of immunity and a potential trigger for injury and rejection. However, the index of immunity can sometimes miss antibody-mediated rejection and besides causing injury the antibodies against a graft can also protect a graft from injury by blocking immune recognition, called enhancement, regulating activation of complement, and inducing changes in the graft that resist damage. Reviewed here are potential limitations in the use of antibodies as an index of immunity and the ways antibodies cause and/or prevent injury.
No subject in the field of transplantation immunology arouses more interest today than the subject of antibodies in transplantation. Antibodies cause the most vexing types of rejection observed after transplantation of organs (Figure 1) and the presence of these antibodies against a given donor, ascertained by a cross-match test prior to transplantation, constitutes a relative or absolute barrier to transplantation of the kidney or heart. Antibodies comprise the most challenging barrier to transplantation of animal organs into humans, i.e., xenotransplantation (Cascalho and Platt, 2001), which might otherwise address the severe shortage of human organs available for transplantation. Antibodies can also protect grafts from injury and provide a more or less incisive glimpse at the immunological response to transplantation and the state of tissue injury. And, antibodies have provided key insights into fundamental components of the immune system and the mechanisms by which those components function. This communication summarizes current knowledge and the limits of current knowledge about how antibodies determine the fate of transplants. Reviews of B cell responses to transplantation and non-cognate functions of B cells can be found elsewhere (Balin et al., 2009; Zarkhin et al., 2010).
Figure 1.
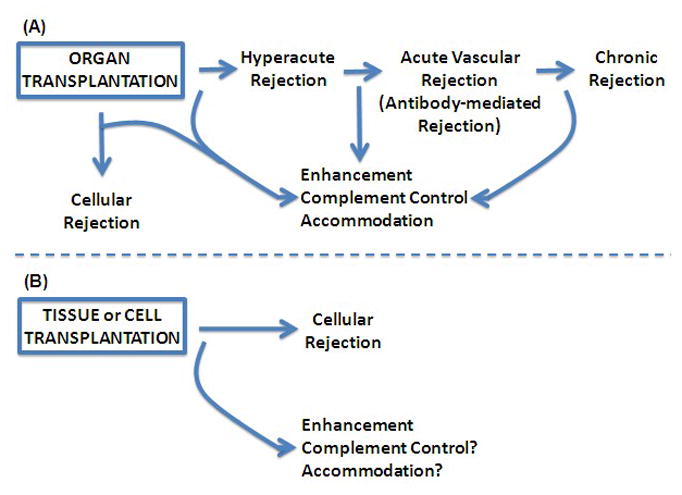
The distinct biological outcomes of organ, cell, and tissue grafts. Organ, tissue, and cell transplants all stimulate cellular and humoral immunity. The impact of immunity on these types of grafts differs greatly, however. To the largest extent, the impact depends on the way grafts receive a vascular supply. (A) Organ Transplants. Organ transplants have a foreign vascular tree. These grafts can be attacked by antibodies binding to foreign endothelial cells or by cellular immunity. Antibodies binding at the time of transplantation can cause hyperacute rejection, and antibodies produced after transplantation can cause acute vascular rejection (also called humoral rejection and antibody-mediated rejection) or chronic rejection. (B) Tissue and cell transplants. Tissue and cell transplants receive a vascular supply by in-growth of recipient blood vessels. The recipient blood vessels are not targeted by allo-reactive antibodies. Although small amounts of allo-reactive antibody and complement may diffuse beyond vascular spaces, these usually do not cause discernible damage. However, tissue and cell transplants are fully susceptible to cellular rejection. Sometimes humoral immunity prevents graft injury. Allo-reactive antibodies can induce enhancement which prevents cellular and possibly humoral rejection, and can induce accommodation which prevents humoral and possibly cellular rejection. Antibodies also control activation of complement.
The Impact of Antibodies on Transplants
The impact of antibodies on transplants depends to the greatest extent on the way in which transplants connect with the circulation of the recipient. Organ transplants are connected to the circulation by direct anastomosis between blood vessels of the graft and blood vessels of the recipient. Organ transplants thus have a vascular tree lined by foreign endothelial cells. The foreign blood vessels in organ transplants can be attacked by antibodies of the recipient that are present in the circulation at the time of transplantation or that arise following transplantation. Binding of antibodies of the recipient to foreign blood vessels in a transplant activates complement and recruits phagocytic cells leading to vascular injury and the types of rejection indicated in Figure 1. To a large extent, the injury caused by antibodies, complement, and phagocytic cells on blood vessels in a foreign organ depends on how quickly complement is activated and phagocytes are recruited and whether the blood vessels are protected by mechanisms discussed below.
The mechanisms of antibody-mediated injury have been reviewed in detail (Murata and Baldwin, 2009). Binding of antibody to endothelium of a graft triggers activation of complement and recruitment of phagocytic cells. Within minutes, these can cause loss of heparan sulfate proteoglycan, expression of P-selectin, and retraction of endothelial cells, allowing interaction of platelets with underlying matrix (Platt and Saadi, 1999; Saadi and Platt, 1998; Saadi et al., 2004). These early events probably cause the condition referred to as hyperacute rejection.
During the next period of hours, bound antibodies, activated complement, and activated phagocytic cells change the physiology of blood vessels in ways that promote coagulation, thrombosis, inflammation, and immunity. These changes, which lead to endothelial cell activation, cause a type of rejection variously called “antibody-mediated rejection,” “acute humoral rejection,” or “acute vascular rejection.” While antibodies and phagocytic cells may cause some aspects of this condition independently, acute vascular rejection is marked by the presence of C4d and C3d, which are fragments of complement components C4 and C3 and are covalently attached to endothelial cells in the graft (Colvin, 2007). Bound antibodies fix C1qrs complexes that cleave and activate C4 and C2, which in turn cleave and activate C3. These reactions ultimately generate catalytically inactive C4d and C3d as markers of the complement reaction. Although C4d and C3d can be generated independent of antibodies (e.g., by the lectin pathway of complement activation), the deposition of these fragments is generally taken as evidence that complement-fixing antibodies had bound to cells on which the fragments are detected.
Over periods of weeks to months, bound antibodies, activated complement, leukocytes, and perhaps other factors induce ‘chronic’ changes in blood vessels which lead to manifestations of “chronic rejection.” The pathogenesis of chronic rejection is a subject of intense investigation and heated debate. Besides vascular injury, it may be caused by infection, metabolic abnormality, and ischemia. Regardless of the inciting factor(s), chronic rejection has been associated with the presence of a fragment of complement (C4d) on endothelial cells (Regele et al., 2002), suggesting that antibodies are among those factors.
In contrast to the outcome of organ transplants, cell and tissue transplants appear scarcely to be affected by antibodies and complement (Platt, 1998). As the only example, we showed that transplants consisting of xenogeneic hepatocytes in recipients with very high levels of antibodies specific for the xenografts survive and function (Nagata et al., 2007). Because antibodies are mainly confined to the blood (IgM 90% and IgG 75%), and because the size of the extra-vascular interstitial spaces far exceeds the volume of blood (extracellular volume exceeds intravascular volume by roughly six-fold), the concentration of antibodies outside of blood vessels is far lower than the concentration inside vascular spaces. Likewise, complement resides mainly in vascular spaces. Hence, antibodies have far more biological impact on intravascular targets than on extra-vascular targets. And while transplants consisting of isolated cells and tissues such as pancreatic islets or skin provoke intense humoral immune responses, these transplants seem inured to humoral immunity but susceptible to cellular immunity (Figure 1).
The ability of cell and tissue grafts to stimulate production of antibodies specific for cells of the same origin as the graft provided seminal evidence that grafts are immunogenic and that the antigens expressed by grafts are inherited traits (Gorer, 1937). Yet, the limited ability of these antibodies to attack cell and tissue transplants raised questions about whether (Woglom, 1929) and how immunity could explain the loss of foreign grafts. Demonstration that immunity to tissues could be transferred by cells but not serum (Mitchison, 1953) clarified this concept.
The question of whether antibodies only mark or also mediate immunity remains a challenging one in medicine today. Antibodies against components of nuclei, insulin, and other components of beta cells and even against the surfaces of extra-vascular cells are commonly observed and taken as evidence of autoimmunity. Yet, many people who have autoantibodies do not manifest autoimmune disease and when disease is present the role of autoantibodies can be difficult to determine. Hopefully, the technologies developed to identify antibody mediated disease of transplants such as detection of C4d and C3d can be usefully applied for evaluation of other immunological disorders. Early reports suggest that C3d and C4d on the surface of erythrocytes might predict the intensity of disease in systemic lupus erythematosus (Yang et al., 2009) and certain dermatoses (Magro and Dyrsen, 2008).
Antibodies and the Prediction of Graft Outcome
Whether or not antibodies in the circulation of graft recipients damage transplants (Figure 1), they do predict outcome of transplantation. Antibodies generated by transplantation of allogeneic tumor cells provided the first definitive evidence that the transplants were immunogenic and that capacity of a tumor to be so recognized and destroyed was inherited (Gorer, 1937). These antibodies and the susceptibility to rejection eventually allowed the identification and mapping of the major histocompatibility gene complex (Dausset and Nenna, 1952; Gorer et al., 1948). Even today, the presence of these antibodies against a given donor, detected by a cross-match test, or against a set of potential donors, determined by one of several “panel reactive antibody” assays, is taken as evidence of prior sensitization and susceptibility to rejection (Cinti et al., 2009; Susal et al., 2009).
The analysis of donor reactive and panel reactive antibodies has proven so useful that a number of technologies for simplifying and standardizing these assays have been developed (Tinckam, 2009; Vlad et al., 2009). One would typically determine whether a potential transplant recipient has antibodies specific for a given transplant donor, and, if those antibodies are detected, organs from that donor could not be transplanted into the recipient but would be directed to other potential recipients. Thus, in the case of an individual awaiting transplantation, especially kidney transplantation, one would typically determine the panel reactive antibody (sometimes called PRA) level (i.e., the fraction of individuals in the population against whom a potential recipient has alloantibodies) to determine what fraction of potential donors would be excluded. Present approaches to determining panel reactivity include testing of serum for antibodies against a panel of cells obtained from individuals of known histocompatibility types and testing binding to beads coated with histocompatibility antigens of a known type isolated from transformed cells. The use of a panel of cells has the advantage that histocompatibility antigens are expressed in a ‘natural’ setting on the surface of leukocytes, and the disadvantage that the precise specificities of antibodies that bind to those cells are not immediately apparent. The use of isolated antigen allows precise determination of specificity of antibodies and standardizes the assays in one or in many centers, but the disadvantage is that some antibodies that bind to isolated molecules might not bind to cell surfaces. Research in a number of centers is currently aimed at determining whether one or the other approach better predicts graft outcome and avoids unnecessary exclusion of organs from a given potential donor in a given recipient.
Regardless of the assay used, antibodies detected in the blood may not represent antibodies acting on the graft. Antibodies of the highest affinity will bind to accessible antigens in the graft, potentially hindering by steric means the binding of antibodies of lower affinity. Thus, putatively allo-reactive antibodies detected in the circulation may actually represent those antibodies that cannot bind or cannot compete for binding to the graft.
Detecting humoral immunity after transplantation may have as much importance as detecting it before transplantation. Allo-reactive antibodies developed after transplantation cause antibody-mediated and chronic rejection -- conditions that are difficult, sometimes impossible, to reverse. To the extent that the challenge in treating antibody-mediated and chronic rejection reflects the advanced stage of these conditions at the time of diagnosis, early detection might allow more effective intervention. However, early detection can be difficult because affected individuals often have little or no alloantibody in the circulation. The paradox of antibody-mediated rejection occurring in the apparent absence of anti-graft antibodies is explained by the extraordinary ability of organs to absorb and metabolize antibodies directed against graft blood vessels. Xenografts, against which more than 1% of circulating immunoglobulin is directed, absorb nearly all xenoreactive antibodies until antibody mediated rejection has nearly destroyed the graft. Allografts also can absorb most or all alloantibodies so that antibodies against the graft may be difficult to detect until rejection is well established and perhaps irreversible (Carpenter et al., 1976). Hence, anti-donor antibodies detected after transplantation by the technologies mentioned above may really mark graft damage rather than initiation of a humoral immune response. The possibility that such “donor specific antibodies,” or DSA, represent late rather than early markers of rejection is important given the high level of interest in this diagnostic index (Sis et al., 2010; Takemoto et al., 2004). Markers of humoral immunity other than antibodies might be needed to detect and treat antibody-mediated injury early in the course.
Antibodies Associated with Favorable Outcome of Grafts
While transplantation immunologists and clinicians have focused on involvement of antibodies in rejection of organ transplants and on therapeutics that would suppress antibody production, antibody responses to transplantation are not always damaging and sometimes may limit or prevent graft damage. Table 1 lists some ways antibodies can prevent injury to grafts. Figure 2 explains how antibodies might protect grafts from injury. These mechanisms are discussed in the sections below. Besides these protective properties of antibodies, one might consider that under some conditions, B cells can suppress cellular immune responses and perhaps in this way limit graft injury independent of the antibodies produced (Balin et al., 2009; Yanaba et al., 2008).
Table 1.
Conditions in which antibodies can improve graft outcome
| Condition | Antibody Target | Mechanism | Reference |
|---|---|---|---|
| Enhancement | MHC | blocking | Morris, 1980 |
| Complement control | none | diversion of complement | Frank et al., 1992 |
| Accommodation | unknown | protection of target | Koch et al., 2004 |
Figure 2.
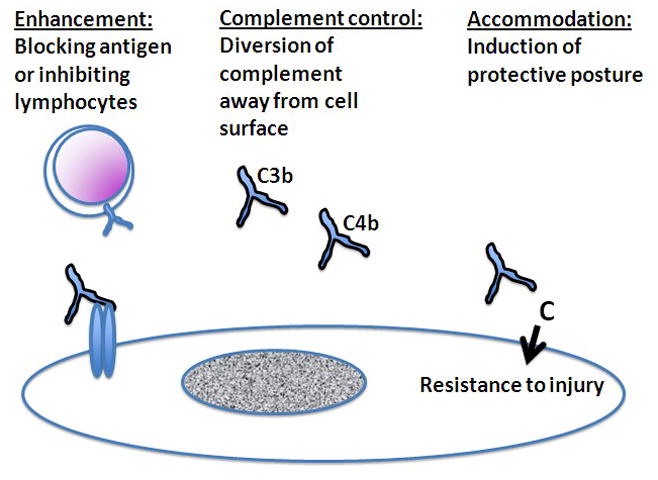
Mechanisms of antibody-mediated protection. Besides causing injury to grafts, antibodies can protect grafts from injury. The mechanisms of antibody mediated protection include enhancement, complement control, and accommodation. In enhancement, antibodies bind to and block target antigens or interact with lymphocytes in ways that suppress lymphocyte functions. In complement control, antibodies serve as alternative targets for activation of complement (C3b and C4b), thus diverting complement away from cell surfaces. In accommodation, antibodies activate complement (C) on cell surfaces and in so doing change the biology of cells in ways that make the cells resist injury.
Enhancement
The early years of the 20th century brought excitement about the possibility that immunity evoked by immunization with non-viable cancer cells and extracts might be exploited to treat cancer (Woglom, 1929). Contrary to this hope, immunity, when generated, was directed toward histocompatibility antigens and not against tumor specific antigens and, strikingly, sometimes immunity seemed to “enhance” rather than retard tumor growth (Snell et al., 1946). Later work revealed that it was antibodies that conferred this protection (Kaliss, 1969; Kaliss and Molomut, 1952) and that the protective antibodies could be raised by sensitization with normal tissues and could protect grafts of normal tissue, sometimes indefinitely (Carpenter et al., 1976; Stuart et al., 1968).
While the phenomenon of enhancement, as such, may reflect various mechanisms listed in Table 1, most evidence suggests that enhancement results from the “blocking” of immunological recognition (Morris, 1980) and/or suppression of T cell responses (Figure 2) (Cruse et al., 2002). Such blocking of recognition or suppression of response to recognition might be exerted when immunity is initially elicited (afferent inhibition) or when immunity attacks the graft (efferent inhibition) (Cruse et al., 2002). The importance of blocking of immune recognition rather than generalized immunosuppression as a mechanism of enhancement would be suggested by the specificity of the phenomenon for foreign histocompatibility antigens in in vitro and in vivo assays. Also implicating blocking is the fact that enhancement depended on the dosage of antigen rather than the dosage of antibody (i.e., small amounts of antibody were as effective as large amounts of antibody and transplants of cross-bred F1 animals to animals of a parental strain were more susceptible to enhancement than transplants between entirely disparate strains). Of course, antibodies have long been known to block humoral immunity against isolated antigens but enhancement seemed different because it was cellular immunity rather than humoral immunity that was blocked. Enhancement can be generated in large animals and may even restrain immunity against clinical allografts (Morris, 1980).
Perhaps because blocking of antigen is not readily assayed or because the control of T cell responses by modern immunosuppression obscures enhancement, this subject is rarely, if ever, discussed. Recent workshops on diagnosis and mechanisms of graft injury fail even to mention enhancement as a potential benefit of antibody responses. Perhaps the emergence of more effective approaches to controlling B cell responses will bring unexpected results and these results will once more ignite interest in this subject.
Complement control
Twenty years ago, immunoglobulin, previously considered a key complement activator, was found to control complement-mediated tissue damage (Basta et al., 1989). For example, administration of γ-globulin protected animals from complement mediated hemolytic anemia (Basta et al., 1989) and prevented hyperacute rejection of cardiac xenografts, arguably the most explosive and devastating immunological conditions (Magee et al., 1995).
Regulation of complement by immunoglobulin may reflect the operation of a number of mechanisms (Wagner and Frank, 2010). Immunoglobulin can block Fc receptors and inhibit inflammatory agonists of various types. Immunoglobulin can also serve as a target for activated C3 and C4 (Figure 2). Whereas, activated C3 and C4 form covalent bonds with adjacent cell surfaces they also form bonds with immunoglobulin in the vicinity of cells. Binding of C3 and C4 to immunoglobulin diverts the complement cascade away from cell surfaces; presumably higher concentrations of immunoglobulin shift the balance of the reaction from the cell surface to the plasma. Because complement is subject to intrinsic control on cell surfaces, a relatively subtle shift in the reaction away from the cell might effectively prevent cellular injury.
Regulation of complement by immunoglobulin may explain some of the effectiveness of immunoglobulin in preventing antibody-mediated rejection in pre-sensitized subjects (Wagner and Frank, 2010). Conversely, depletion of complement by plasmapheresis potentially deprives the treated individual of this control unless γ-globulin is administered. Similarly, mice lacking immunoglobulin might exhibit heightened susceptibility to complement-mediated processes.
Besides diverting complement activation away from cells, immunoglobulin can stimulate the production of cell surface complement inhibitors. Attachment of immunoglobulin and some other proteins to cell surfaces can stimulate expression of CD59 (Dalmasso et al., 2000), a protein that inhibits the membrane attack complex. To what extent control of complement in this way modifies complement-mediated injury in vivo is not known but experiments using cultured cells indicate that this mechanism may prevent complement-mediated lysis. Immunoglobulin can also attach to and protect the glycocalyx of cells (Parker et al., 1998), thus maintaining a negative cell surface charge that also controls activation of complement. Finally, subclasses of IgG that fix complement poorly can block binding of IgG subclasses that fix complement (Yu et al., 1996), thus inhibiting activation of complement on cell surfaces in the course of specific immunological reactions.
Obviously a fuller understanding of the molecular basis of complement control by immunoglobulin could offer important insights and a therapeutic avenue in transplantation and other fields. One can envision production of recombinant immunoglobulin or immunoglobulin fragments with defined regulatory properties might improve the outcome of transplants or other tissues.
Accommodation
During the 1980’s, transplantation of kidneys across blood group-A and -B barriers (e.g., blood group A kidney into a recipient that produces anti-blood group A antibodies) allowed some to receive transplants who would otherwise be excluded (Alexandre et al., 1985; Chopek et al., 1987). Previously such transplants were avoided because antibodies against blood groups expressed in an organ, like antibodies against histocompatibility antigens, could lead to hyperacute or acute vascular rejection. Although the recipients of the incompatible organ transplants were depleted of anti-blood group antibodies by plasmapheresis or other means, the antibodies were expected to return and hence the transplants were thought likely to be injured if not destroyed (the transplantations were performed only because the recipients experienced complications of dialysis and had positive cross matches against other potential donors). As expected, the anti-blood group antibodies did eventually return to the circulation but surprisingly the incompatible transplants often functioned for extended periods of time. Although the survival and well being of the grafts in the face of anti-graft antibodies might be ascribed to various mechanisms, such as loss of antigen or enhancement, the mechanism that best fitted the circumstances was acquired resistance to immunological injury, a condition later called accommodation (Bannett et al., 1989; Platt et al., 1990). Survival of grafts in the face of anti-HLA antibodies was subsequently described (Jeannet et al., 1981), although the significance of accommodation in this setting remains a point of controversy (Hourmant et al., 2005; Piazza et al., 2006).
While aside from ABO-incompatible transplantation, accommodation is sometimes considered infrequent, and there is reason to think it may be more common than supposed (Lynch and Platt, 2008). Because normal, well-perfused organs can absorb enormous amounts of antibody, donor specific antibody may be nearly undetectable in the circulation of recipients of accommodated grafts. Consistent with this concept, many recipients of renal transplants can be shown to have detectable donor-reactive antibodies (Mizutani et al., 2005). In a still broader context, accommodation may explain why anti-DNA antibodies do not inevitably cause manifestations of systemic lupus erythematosus (Andrejevic et al., 2007; Vaile et al., 2000). How to detect accommodation in the absence of donor specific antibodies is therefore an important, still unanswered question.
Accommodation protects grafts (and other tissues) from injury that might be inflicted in immune and inflammatory reactions (Figure 2); but how exactly that protection arises and how it manifests are important questions that are still unanswered. Accommodation has been associated with protection against complement-mediated injury (Dalmasso et al., 2000). Resistance against complement may recruit pathways that not only protect cells from injury by complement or cytotoxic lymphocytes but also facilitate control of intracellular organisms (Koch et al., 2004). For example, an agent that controls replication of the hepatitis C virus also heightens expression of heme oxygenase-1 (Bonifaz et al., 2009), a protein typically expressed by accommodated cells. On the other hand, accommodation may incur biological disadvantages in the form of changes in cell metabolism or loss of function (Platt and Nath, 1998). Thus, pathways and products associated with accommodation might contribute to chronic rejection of transplanted organs (Tang and Platt, 2007).
Accommodation and tolerance together explain more fully than either alone can avoidance of inadvertent injury when the immune system protects against environmental threats. Tolerance chiefly reflects structural and/or functional lacunae in immunological recognition such that foreign organisms and their products are recognized and attacked but autologous cells and substances are not. Accommodation refers to alterations of the target tissue. Because the immune system can and often does recognize autologous cells and substances, as in the positive selection of T cells or production of “natural” auto-reactive antibodies (Lleo et al., 2010), accommodation allows such recognition to occur for the control of intracellular microorganisms without excessive biological cost. With this perspective, one can imagine that autoimmune disease may in some instances reflect failure of accommodation as much as it reflects autoimmunity. And, approaches to detecting failure of accommodation and a way of restoring it might be sought as novel avenues of immune management. In transplantation, the challenge in overcoming or limiting humoral immunity to transplantation antigens encourages efforts to promote accommodation of organ grafts.
Concluding Remarks
Antibodies provide the most sensitive, specific, and easily detected index of immunity to transplantation. Accordingly, much attention has focused on the devising of new assays for allo-reactive antibodies. While these assays will surely prove immensely valuable, they may also be found on some occasions to mislead to the detriment of patients and fundamental knowledge.
Absence of antibodies in the circulation of a transplant recipient does not prove that the recipient lacks humoral immunity to the graft. Humoral rejection is sometimes diagnosed when antibody and complement are detected in graft tissue, while anti-graft antibody is not detectable in the blood. This circumstance may explain why plasmapheresis and administration of γ-globulin are occasionally found to improve graft function in the absence of circulating anti-graft antibodies.
On the other hand, newly discovered anti-graft antibodies in the blood may not constitute early evidence of humoral rejection. Because graft can absorb large amounts of antibody, an abrupt increase in the level of anti-graft antibody may be a late sign of rejection. Surely earlier and more sensitive indices of humoral response to transplantation are needed.
Finally, antibodies against a graft are not all inimical to the well-being of the graft. Some antibodies or some level of antibodies may actually suppress immunity and/or protect the graft. In the absence of better indices of enhancement, complement control, and accommodation, one should hesitate to apply intrusive therapies for humoral rejection in the absence of worsened graft function and/or pathological evidence of rejection. In short, while antibodies can cause injury and rejection of transplants, antibodies can also oppress rejection and limit graft injury. This dichotomy between toxicity and protection recapitulates a dichotomy observed also for inflammatory cytokines, ischemia, and other biologically active substances and processes.
Acknowledgments
Work in the author’s laboratory is supported by grants from the National Institutes of Health (HL52297, HL79067)
References
- Alexandre GPJ, Squifflet JP, De Bruyere M, Latinne D, Moriau M, Ikabu N, Carlier M, Pirson Y. Splenectomy as a prerequisite for successful human ABO-incompatible renal transplantation. Transplant Proc. 1985;17:138–43. [Google Scholar]
- Andrejevic S, Bonaci-Nikolic B, Sefik-Bukilica M, Petrovic R. Clinical and serological follow-up of 71 patients with anti-mitochondrial type 5 antibodies. Lupus. 2007;16(10):788–93. doi: 10.1177/0961203307081913. [DOI] [PubMed] [Google Scholar]
- Balin S, Platt J, Cascalho M. Non cognate function of b cells in transplantation. Transpl Int. 2009;22(6):593–8. doi: 10.1111/j.1432-2277.2008.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannett AD, Mcalack RF, Morris M, Chopek M, Platt JL. ABO incompatible renal transplantation: A qualitative analysis of native endothelial tissue ABO antigens after transplant. Transplant Proc. 1989;21:783–5. [PubMed] [Google Scholar]
- Basta M, Kirshbom P, Frank MM, Fries LF. Mechanism of therapeutic effect on high-dose intravenous immunoglobulin: Attenuation of acute, complement-dependent immune damage in a guinea pig model. J Clin Invest. 1989;84:1974–81. doi: 10.1172/JCI114387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifaz V, Shan Y, Lambrecht RW, Donohue SE, Moschenross D, Bonkovsky HL. Effects of silymarin on hepatitis C virus and haem oxygenase-1 gene expression in human hepatoma cells. Liver Int. 2009;29(3):366–73. doi: 10.1111/j.1478-3231.2008.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter CB, D’apice AJ, Abbas AK. The role of antibodies in the rejection and enhancement of organ allografts. Adv Immunol. 1976;22:1–65. doi: 10.1016/s0065-2776(08)60547-7. [DOI] [PubMed] [Google Scholar]
- Cascalho M, Platt JL. The immunological barrier to xenotransplantation. Immunity. 2001;14:437–46. doi: 10.1016/s1074-7613(01)00124-8. [DOI] [PubMed] [Google Scholar]
- Chopek MW, Simmons RL, Platt JL. ABO-incompatible renal transplantation: Initial immunopathologic evaluation. Transplant Proc. 1987;19:4553–7. [PubMed] [Google Scholar]
- Cinti P, Pretagostini R, Lai Q, Tamburro ML, Rossi M, Poli L, Berloco P. Alloantibodies and outcomes of deceased donor kidney allografts. Hum Immunol. 2009;70(8):651–4. doi: 10.1016/j.humimm.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Colvin RB. Antibody-mediated renal allograft rejection: Diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18(4):1046–56. doi: 10.1681/ASN.2007010073. [DOI] [PubMed] [Google Scholar]
- Cruse JM, Lewis RE, Dilioglou S. Immunological enhancement revisited. Exp Mol Pathol. 2002;73(2):112–27. doi: 10.1006/exmp.2002.2458. [DOI] [PubMed] [Google Scholar]
- Dalmasso AP, Benson BA, Johnson JS, Lancto C, Abrahamsen MS. Resistance against the membrane attack complex of complement induced in porcine endothelial cells with a gal alpha(1–3)gal binding lectin: Up-regulation of CD59 expression. J Immunol. 2000;164(7):3764–73. doi: 10.4049/jimmunol.164.7.3764. [DOI] [PubMed] [Google Scholar]
- Dausset J, Nenna A. Presence d’une leuco-agglutinine dans le serum d’un can d’agranulocytose chronique. Comptes Rendus des Seances de la Societe de Biologie et de ses Filiales (Paris) 1952;146:1539–41. [PubMed] [Google Scholar]
- Frank MM, Basta M, Fries LF. The effects of intravenous immune globulin on complement-dependent immune damage of cells and tissues. Clin Immunol Immunopathol. 1992;62:s82–6. doi: 10.1016/0090-1229(92)90045-p. [DOI] [PubMed] [Google Scholar]
- Gorer P. The genetic and antigentic basis of tumor transplantation. J Pathol Bacteriol. 1937;44:691–7. [Google Scholar]
- Gorer PA, Lyman S, Snell GD. Studies on the genetic and antigenic basis of tumour transplantation. Linkage between a histocompatibility gene and “fused” in mice. Proc R Soc Lond B Biol Sci. 1948;135:499–505. [Google Scholar]
- Hourmant M, Cesbron-Gautier A, Terasaki PI, Mizutani K, Moreau A, Meurette A, Dantal J, Giral M, Blancho G, Cantarovich D, Karam G, Follea G, Soulillou JP, Bignon JD. Frequency and clinical implications of development of donor-specific and non-donor-specific HLA antibodies after kidney transplantation. J Am Soc Nephrol. 2005;16(9):2804–12. doi: 10.1681/ASN.2004121130. [DOI] [PubMed] [Google Scholar]
- Jeannet M, Benzonana G, Arni I. Donor-specific B and T lymphocyte antibodies and kidney graft survival. Transplantation. 1981;31(3):160–3. doi: 10.1097/00007890-198103000-00003. [DOI] [PubMed] [Google Scholar]
- Kaliss N. Immunological enhancement. Int Rev Exp Pathol. 1969;8:241–76. [PubMed] [Google Scholar]
- Kaliss N, Molomut N. The effect of prior injections of tissue antiserums on the survival of cancer homoiografts in mice. Cancer Res. 1952;12(2):110–2. [PubMed] [Google Scholar]
- Koch CA, Khalpey ZI, Platt JL. Accommodation: Preventing injury in transplantation and disease. J Immunol. 2004;172(9):5143–8. doi: 10.4049/jimmunol.172.9.5143. [DOI] [PubMed] [Google Scholar]
- Lleo A, Invernizzi P, Gao B, Podda M, Gershwin ME. Definition of human autoimmunity --autoantibodies versus autoimmune disease. Autoimmun Rev. 2010;9(5):A259–66. doi: 10.1016/j.autrev.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Lynch RJ, Platt JL. Accommodation in organ transplantation. Curr Opin Organ Transplant. 2008;13:165–70. doi: 10.1097/MOT.0b013e3282f6391e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Collins BH, Harland RC, Lindman BJ, Bollinger RR, Frank MM, Platt JL. Immunoglobulin prevents complement mediated hyperacute rejection in swine-to-primate xenotransplantation. J Clin Invest. 1995;96:2404–12. doi: 10.1172/JCI118297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro CM, Dyrsen ME. The use of C3d and C4d immunohistochemistry on formalin-fixed tissue as a diagnostic adjunct in the assessment of inflammatory skin disease. J Am Acad Dermatol. 2008;59(5):822–33. doi: 10.1016/j.jaad.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Mitchison NA. Passive transfer of transplantation immunity. Nature. 1953;171(4345):267–8. doi: 10.1038/171267b0. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Terasaki P, Rosen A, Esquenazi V, Miller J, Shih RN, Pei R, Ozawa M, Lee J. Serial ten-year follow-up of HLA and MICA antibody production prior to kidney graft failure. Am J Transplant. 2005;5(9):2265–72. doi: 10.1111/j.1600-6143.2005.01016.x. [DOI] [PubMed] [Google Scholar]
- Morris PJ. Suppression of rejection of organ allografts by alloantibody. Immunol Rev. 1980;49:93–125. doi: 10.1111/j.1600-065x.1980.tb00428.x. [DOI] [PubMed] [Google Scholar]
- Murata K, Baldwin WM., 3rd Mechanisms of complement activation, C4d deposition, and their contribution to the pathogenesis of antibody-mediated rejection. Transplant Rev (Orlando) 2009;23(3):139–50. doi: 10.1016/j.trre.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata H, Nishitai R, Shirota C, Zhang J-L, Koch CA, Cai J, Awwad M, Schuurman H-J, Christians U, Abe M, Baranowska-Kortylewicz J, Platt JL, Fox IJ. Prolonged survival of porcine hepatocytes in cynomolgus monkeys. Gastroenterology. 2007;132:321–9. doi: 10.1053/j.gastro.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Parker W, Holzknecht ZE, Song A, Blocher BA, Bustos M, Reissner KJ, Everett ML, Platt JL. Fate of antigen in xenotransplantation: Implications for acute vascular rejection and accommodation. Am J Pathol. 1998;152:829–39. [PMC free article] [PubMed] [Google Scholar]
- Piazza A, Poggi E, Ozzella G, Borrelli L, Scornajenghi A, Iaria G, Tisone G, Adorno D. Post-transplant donor-specific antibody production and graft outcome in kidney transplantation: Results of sixteen-year monitoring by flow cytometry. Clin Transpl. 2006;2006:323–336. [PubMed] [Google Scholar]
- Platt JL. New directions for organ transplantation. Nature. 1998;392(Suppl 6679):11–7. doi: 10.1038/32023. [DOI] [PubMed] [Google Scholar]
- Platt JL, Nath KA. Heme oxygenase: Protective gene or trojan horse. Nat Med. 1998;4(12):1364–5. doi: 10.1038/3947. [DOI] [PubMed] [Google Scholar]
- Platt JL, Saadi S. The role of complement in transplantation. Mol Immunol. 1999;36:965–71. doi: 10.1016/s0161-5890(99)00119-4. [DOI] [PubMed] [Google Scholar]
- Platt JL, Vercellotti GM, Dalmasso AP, Matas AJ, Bolman RM, Najarian JS, Bach FH. Transplantation of discordant xenografts: A review of progress. Immunol Today. 1990;11:450–6. doi: 10.1016/0167-5699(90)90174-8. [DOI] [PubMed] [Google Scholar]
- Regele H, Bohmig GA, Habicht A, Gollowitzer D, Schillinger M, Rockenschaub S, Watschinger B, Kerjaschki D, Exner M. Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: A contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol. 2002;13(9):2371–80. doi: 10.1097/01.asn.0000025780.03790.0f. [DOI] [PubMed] [Google Scholar]
- Saadi S, Platt JL. Endothelial cell responses to complement activation. In: Volanakis JE, Frank MM, editors. The Human Complement System in Health and Disease. Marcel Dekker, Inc: New York, New York, USA; 1998. pp. 335–353. [Google Scholar]
- Saadi S, Takahashi T, Holzknecht RA, Platt JL. Pathways to acute humoral rejection. American J Pathol. 2004;164(3):1073–80. doi: 10.1016/S0002-9440(10)63194-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Baldwin WM, 3rd, Bracamonte ER, Broecker V, Cosio F, Demetris AJ, Drachenberg C, Einecke G, Gloor J, Glotz D, Kraus E, Legendre C, Liapis H, Mannon RB, et al. Banff ‘09 meeting report: Antibody mediated graft deterioration and implementation of banff working groups. Am J Transplant. 2010;10:464–71. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- Snell GD, Cloudman AM, Failor E, Douglass P. Inhibition and stimulation of tumor homoiotransplants by prior injections of lyophilized tumor tissue. J Natl Cancer Inst. 1946;6:303–16. [PubMed] [Google Scholar]
- Stuart FP, Saitoh T, Fitch FW. Rejection of renal allografts: Specific immunologic suppression. Science. 1968;160(835):1463–5. doi: 10.1126/science.160.3835.1463. [DOI] [PubMed] [Google Scholar]
- Susal C, Dohler B, Opelz G. Presensitized kidney graft recipients with HLA class I and II antibodies are at increased risk for graft failure: A collaborative transplant study report. Hum Immunol. 2009;70(8):569–73. doi: 10.1016/j.humimm.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Takemoto SK, Zeevi A, Feng S, Colvin RB, Jordan S, Kobashigawa J, Kupiec-Weglinski J, Matas A, Montgomery RA, Nickerson P, Platt JL, Rabb H, Thistlethwaite R, Tyan D, Delmonico FL. National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant. 2004;4(7):1033–41. doi: 10.1111/j.1600-6143.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- Tang AH, Platt JL. Accommodation of grafts: Implications for health and disease. Hum Immunol. 2007;68(8):645–51. doi: 10.1016/j.humimm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinckam K. Histocompatibility methods. Transplant Rev (Orlando) 2009;23(2):80–93. doi: 10.1016/j.trre.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Vaile JH, Dyke L, Kherani R, Johnston C, Higgins T, Russell AS. Is high titre ANA specific for connective tissue disease? Clin Exp Rheumatol. 2000;18(4):433–8. [PubMed] [Google Scholar]
- Vlad G, Ho EK, Vasilescu ER, Colovai AI, Stokes MB, Markowitz GS, D’agati VD, Cohen DJ, Ratner LE, Suciu-Foca N. Relevance of different antibody detection methods for the prediction of antibody-mediated rejection and deceased-donor kidney allograft survival. Hum Immunol. 2009;70(8):589–94. doi: 10.1016/j.humimm.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Wagner E, Frank MM. Therapeutic potential of complement modulation. Nat Rev Drug Discov. 2010;9(1):43–56. doi: 10.1038/nrd3011. [DOI] [PubMed] [Google Scholar]
- Woglom WH. Immunity to transplantable tumors. Cancer Rev. 1929;4:129–214. [Google Scholar]
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–50. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Yang DH, Chang DM, Lai JH, Lin FH, Chen CH. Usefulness of erythrocyte-bound c4d as a biomarker to predict disease activity in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2009;48(9):1083–7. doi: 10.1093/rheumatology/kep161. [DOI] [PubMed] [Google Scholar]
- Yu PB, Holzknecht ZE, Bruno D, Parker W, Platt JL. Modulation of natural IgM binding and complement activation by natural IgG antibodies. J Immunol. 1996;157:5163–8. [PubMed] [Google Scholar]
- Zarkhin V, Chalasani G, Sarwal MM. The yin and yang of B cells in graft rejection and tolerance. Transplant Rev (Orlando) 2010;24(2):67–78. doi: 10.1016/j.trre.2010.01.004. [DOI] [PubMed] [Google Scholar]


