Abstract
Transforming growth factor-β1 (TGF-β) can be tumor suppressive, but it can also enhance tumor progression by stimulating the complex process of epithelial-to-mesenchymal transdifferentiaion (EMT). The signaling pathway(s) that regulate EMT in response to TGF-β are not well understood. We demonstrate the acquisition of a fibroblastoid morphology, increased N-cadherin expression, loss of junctional E-cadherin localization, and increased cellular motility as markers for TGF-β–induced EMT. The expression of a dominant-negative Smad3 or the expression of Smad7 to levels that block growth inhibition and transcriptional responses to TGF-β do not inhibit mesenchymal differentiation of mammary epithelial cells. In contrast, we show that TGF-β rapidly activates RhoA in epithelial cells, and that blocking RhoA or its downstream target p160ROCK, by the expression of dominant-negative mutants, inhibited TGF-β–mediated EMT. The data suggest that TGF-β rapidly activates RhoA-dependent signaling pathways to induce stress fiber formation and mesenchymal characteristics.
INTRODUCTION
Transforming growth factor-β1 (TGF-β) regulates growth, differentiation, and epithelial transformation in the multistep processes of tumorigenesis, wound healing, and embryogenesis. Based on studies using cultured cells, transgenic mice, and human tumors, an emerging model suggests the TGF-β signaling pathway acts as a tumor suppressor, but it can act as a promoter of tumor progression during the later stages of tumorigenesis (McLeod et al., 1990; Han et al., 1993; Pierce et al., 1995; Cui et al., 1996; Oft et al., 1998; Portella et al., 1998). Although a role for TGF-β as an autocrine transforming and morphogenic factor in epithelial cells is well established, our understanding of its intracellular signaling mechanisms is limited. This report identifies intracellular TGF-β effectors and new mechanistic insight into TGF-β–mediated fibroblastic conversion of mammary epithelial cells, a process likely involved in tumor invasion and metastasis.
TGF-β signals through an activated heteromeric complex of type I and type II serine/threonine kinase receptors (Wrana et al., 1994). Subsequent cytoplasmic signaling involves the phosphorylation of ligand-specific SMAD proteins, Smad2 and/or Smad3, that act as intermediates for transcriptional regulation and cell cycle arrest in conjunction with Smad4 in epithelial cells (reviewed in Kretzschmar and Massague, 1998). RhoA, Rac1, and Jun N-terminal kinase (JNK) can promote SMAD-mediated signaling, whereas negative regulators of SMAD-mediated transcription include Smad7, RhoB, and calmodulin (reviewed in Engel et al., 1998b). Smad7 specifically prevents the access and subsequent phosphorylation of Smad2 and Smad3 to the activated TGF-β receptor complex before downstream amplification of the signaling cascade (Nakao et al., 1997). Additionally, the activation of the H-Ras oncogene leads to suppression of SMAD signaling (Calonge and Massague, 1999; Kretzschmar et al., 1999). Parallel TGF-β–mediated transcriptional regulation has been shown to include the mitogen-activated protein kinase family (Atfi et al., 1997; Engel et al., 1999) and the potentiation of phosphatidylinositol 3-kinase (PI3-kinase) activity (Krymskaya et al., 1997; Higaki and Shimokado, 1999). The initiation of multiple signaling pathways downstream of the activated receptor complex results in the pleotropic effects of TGF-β.
Acquisition of a spindle-shaped morphology, delocalization of E-cadherin from cell junctions, and elevated N-cadherin expression are hallmarks of mesenchymal phenotypic conversion of mammary epithelia in cell culture and in tumor invasion (Nieman et al., 1999). As regulators of the actin cytoskeleton and cadherin junctions, the Rho family of small GTPases is commonly implicated in these processes (Bishop and Hall, 2000). Microinjection of RhoA, Rac1, or Cdc42 into fibroblasts triggers the formation of stress fibers, lamellipodia, or filopodia, respectively (Ridley and Hall, 1992; Ridley et al., 1992). Rac1 and Cdc42 are involved in the establishment and maintenance of epithelial intercellular adhesions (Braga et al., 1997; Hordijk et al., 1997; Takaishi et al., 1997; Zhong et al., 1997); in contrast, RhoA activation is implicated in the reversion of the epithelioid phenotype toward a migratory, fibroblastoid morpholology of NIH3T3 cells (Sander et al., 1999). The activated GTP-bound form of RhoA associates specifically with multiple protein kinases. Among these, p160 Rho-associated coiled-coil–containing protein kinase (p160ROCK) regulates actin stress fiber formation and integrin activation (Ishizaki et al., 1997). Thus, data indicate an active role for small GTPases in the maintenance and dynamic regulation of intercellular adhesion as well as having a cytoskeleton-independent role in cell transformation, both alone and in the context of H-Ras activation.
Because TGF-β treatment of mammary epithelial cells recapitulates this transition in culture, we explored the involvement of small GTPases and candidate downstream effectors in TGF-β–induced EMT. We show here that EMT induced by TGF-β requires RhoA signaling. Importantly, we demonstrate that TGF-β rapidly activates RhoA in epithelial nontransformed mouse mammary cell line (NMuMG), mink lung cell line (Mv1Lu), pancreatic tumor cell line (BxPc3), and primary mouse keratinocytes, but not in fibroblastic NIH3T3 cells or TGF-β type I receptor deficient mink lung cells (R1B).
MATERIALS AND METHODS
Reagents and Constructs
TGF-β1 was supplied by R&D Systems (Minneapolis, MN), LY294002 and curcumin was purchased from Sigma (St. Louis, MO). SCH51344 was a gift from Dr. C.C. Kumar (State University of New York at Stony Brook, NY) (Walsh et al., 1997) and LPA (1-oleoyl) was from Avanti Polar Lipids (Alabaster, AL). The 3TP-Lux-reporter construct was obtained from J. Massagué (Memorial Sloan-Kettering Cancer Center, New York, NY). The cDNA constructs encoding Smad3-FLAG (Dr. Rik Derynck, University of California, San Francisco, CA), Smad7-FLAG (Dr. Peter ten Dijke, Ludwig Institute for Cancer Research, Uppsala, Sweden), JNK1APF, N17Rac1 (Dr. Lynn Cross, National Institutes of Health, Bethesda, MD), N19-RhoA (Dr. Lynn Cross), QL-RhoA (Dr. Lynn Cross), KD-IA p160ROCK (Dr. Shuh Narumiya, Kyoto University, Kyoto, Japan), and green fluorescent protein (GFP) (Dr. Fred H. Kant, Columbia University, New York, NY) were subcloned into the pBabe retroviral vector.
Thymidine Incorporation and Luciferase Reporter Assay
NMuMG cells (American Type Culture Collection, Manassas, VA) stably expressing Smad3 and Smad7 as well as the parental NMuMG cell line were plated in 24-well plates for thymidine incorporation and 3TP-Lux reporter assays. To assay cell growth, cells were treated for 24 h with TGF-β and loaded with [3H]thymidine 2 h before harvesting. The cells were washed and lysates measured with a scintillation counter. Transcriptional activation was tested in cells transfected with the 3TP-Lux luciferase (firefly) reporter construct cDNA in conjunction with a cytomegalovirus-driven renela luciferase plasmid (Promega, Madison, WI). Dual-luciferase assays were performed on lysed cells as indicated by the manufacturer, Promega, and measured on a Monolight 2010 luminometer (Analytical Luminescence Laboratory, San Diego, CA). The ratios of firefly and renela luciferase measurements were calculated in normalizing the reporter data in relative luminescent units.
Retroviral Transduction and Cell Culture
Phoenix packaging cells (Kinsella and Nolan, 1996) were transfected with retroviral constructs with Superfect (Qiagen, Chatsworth, CA) according to manufacturer recommendations to produce culture supernatants containing virus. We established matched isogenic clones from the NMuMG parent cell line. Each of the lines selected for further studies were TGF-β sensitive for growth inhibition and EMT (Bhowmick and Moses, unpublished data). NMuMG cells were infected with virus by culturing the cells for 18 h in 1:1 Phoenix conditioned media: fresh DMEM, 10% fetal calf serum, 10 μg/ml insulin, supplemented with 4 ng/ml Polybrene (Sigma). The cells were subjected to various treatments, assaying for protein expression, or cultured in puromycin-containing media for the establishment of stable cell lines 48 h after transduction.
Immunofluorescent Detection
Cells grown on coverslips to be stained for E- or N-cadherin (Transduction Laboratories, Lexington, KY) were fixed in ice-cold 100% methanol and subsequently permeabilized in phosphate-buffered saline containing 0.1% Triton X-100 for 10 min each step. Nonspecific sites were blocked with 3% milk; diluted primary antibody (1:1000) was incubated for 1 h, and visualized using secondary antibody conjugated to Cy2 or Cy3 (Sigma) fluorescence on a Zeiss Axovert fluorescence microscope. F-actin was stained by fixing the cells in 4% paraformaldehyde followed by incubation with Texas Red-conjugated phalloidin (Molecular Probes).
Western Blotting and p160ROCK Kinase Assay
Cells transfected using Superfect (Qiagen) or retrovirally transduced were washed in ice-cold phosphate-buffered saline, lysed (in 50 mM HEPES [pH 7.5], 150 mM NaCl, 0.2 mM vanadate, 1 mM MgCl2, 1 mM CaCl2, 10% glycerol, 1% NP-40, and 0.1% SDS), briefly sonicated, and clarified by centrifugation. Protein (10 μg) was separated on a 10% SDS-polyacrylamide gel, transferred to nitrocellulose, and immunoblotted with appropriate antibodies. Respective secondary horseradish peroxidase-conjugated antibodies (Amersham Pharmacia Biotech, Piscataway, NJ) were used, and were visualized with an enhanced chemiluminescence system (Amersham Pharmacia Biotech). Activity of p160ROCK was determined by immunoprecipitating myc-tagged p160ROCK from 200 μg of total cell lysate for incubation with 10 μg of histone, 5 mM [32P]ATP in 50 mM HEPES (pH 7.4) and 0.5 mM MgCl2 for 8 min at 30°C. The reaction was stopped by the addition of Laemmli sample buffer and separated on a 15% polyacrylamide gel for visualization by autoradiography.
GTPase Activity Assays
The biochemical activity assays were performed essentially as described previously by Reid et al. (Ren et al., 1999). A glutathione S-transferase (GST) fusion protein of the Rho binding domain (RBD, a kind gift from Dr. Martin A. Schwartz, Scripps Institute, La Jolla CA), rhotekin (Reid et al., 1996), was used (Ren et al., 1999). For each measurement, one 100-mm dish of cells was lysed in 1% NP-40, 50 mM Tris, pH 7.4, 10% glycerol, 100 mM NaCl, and 10 mM MgCl2. The GST-RBD precoupled to agarose-glutathione beads (Sigma) was used to precipitate GTP-bound RhoA from cleared lysates of cells for 30 min at 4°C for subsequent immunoblotting for RhoA, similar to that described previously (Ren et al., 1999). A GST fusion protein of the PAK3 binding domain (PBD, a kind gift from Dr. Gary M. Bokoch, Scripps Institute) was used to capture GTP-bound Rac1 and Cdc42 for subsequent visualization by immunoblotting in a similar manner (Benard et al., 1999).
RESULTS
EMT in mammary epithelial cells is characterized by the acquisition of spindle morphology and increased motility, with changes in cadherin expression and localization. We used pharmacological and genetic approaches to affect candidate-signaling pathways in TGF-β–mediated EMT. SMAD- and RhoGTPase-dependent pathways figured prominently given their previously demonstrated involvement in TGF-β signal transduction.
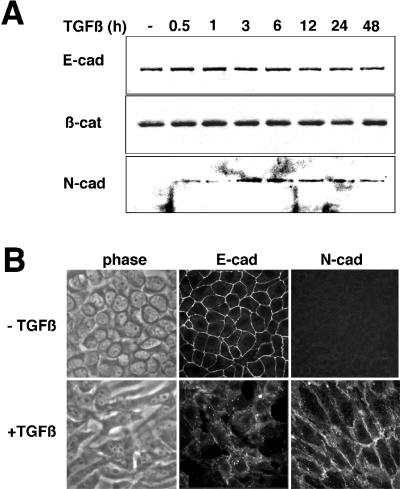
TGF-β–mediated morphological changes of NMuMG cells were accompanied by a loss of E-cadherin junctional localization as reported previously (Miettinen et al., 1994; Piek et al., 1999) as well as our finding of the emergence of N-cadherin expression at cell margins (Figure 1). Recently, a relationship between N-cadherin expression with elevated motility and invasive characteristics of mammary tumor cells has been reported (Nieman et al., 1999). Interestingly, immunoblot analysis showed no change in E-cadherin or cadherin-associated β-catenin expression levels in response to TGF-β. However, N-cadherin expression, absent from epithelioid NMuMG cells, was observed after 3 h of TGF-β treatment and remained elevated through the 48-h time course examined (Figure 1A). Examination of TGF-β–treated cells showed disruption of cell-cell adhesions and a change in cell morphology to a spindle shape in association with the loss of E-cadherin junctional localization and the appearance of N-cadherin staining at the plasma membrane (Figure 1B). Concomitant acquisition of actin stress fibers was also observed (Piek et al., 1999) (see below). However, the cells undergoing EMT remained sensitive to TGF-β growth inhibition during fibroblastic conversion (Figure 2C). These data suggest that TGF-β initiates a program of EMT that correlates with changes in cadherin expression and localization.
Figure 1.
NMuMG cells treated with TGF-β undergo EMT. (A) Immunoblotting of NMuMG cells incubated with 5 ng/ml TGF-β for 0–48 h shows nearly equivalent cellular expression of E-cadherin and β-catenin through the time course, whereas N-cadherin expression is induced by 3 h of TGF-β treatment and remains elevated throughout the time course. (B) Epifluorescent and corresponding phase contrast images show that the fibroblastic cell morphology of cells treated with 5 ng/ml TGF-β for 24 h have a loss of immunodetectable E-cadherin at the cell junctions with a gain of N-cadherin expression at cell margins.
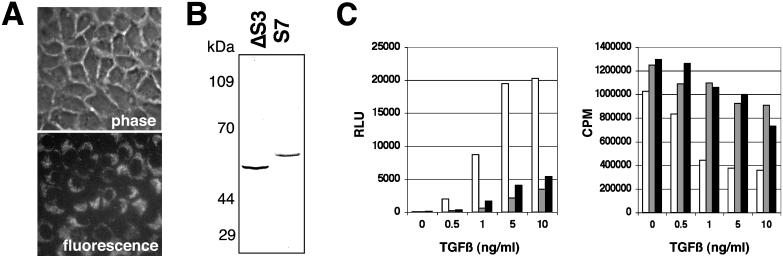
Figure 2.
The down-regulation of SMAD signaling by retroviral transduction inhibits TGF-β–mediated responses. (A) Retroviral infection of the GFP cDNA demonstrates the high transduction efficiency of the NMuMG cells. GFP-retrovirus infected cells were observed by phase contrast and epifluorescence microscopy. (B) Expression of FLAG-tagged Smad3 or Smad7 was detected in retrovirally transduced NMuMG by immunoblotting for the epitope tag. (C) Cells stably transduced with GFP (open bar), Smad3 (gray bar), and Smad7 (closed bar) retrovirus were treated with TGF-β for 24 h and assayed for 3TP-Lux reporter activity (left) and thymidine incorporation (right).
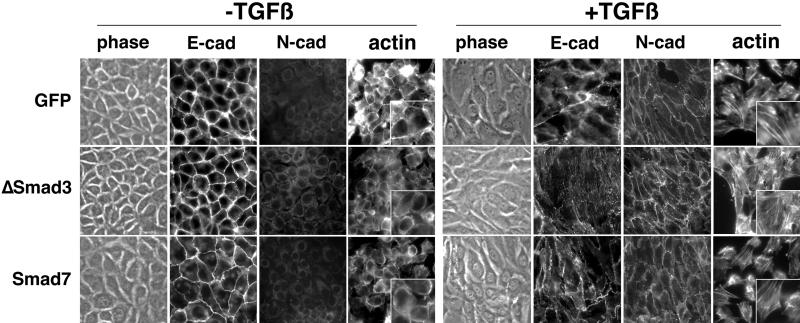
We initially addressed the role of SMADs in TGF-β–mediated EMT by the overexpression of antagonists of the SMAD signaling pathway. Stable retroviral infections with GFP (control), FLAG-Smad3 (a Smad3 deletion of the C-terminal–activating phosphorylation site that behaves in a dominant-negative manner; Zhang et al., 1996), and FLAG-Smad7 (an inhibitor of SMAD signaling; Nakao et al., 1997) cDNA were expressed and confirmed by epifluorescence microscopy and immunoblotting (Figure 2, A and B). The transduced cells displayed ≥80% inhibition of TGF-β transcriptional activation of the 3TP-Lux promoter, and diminished responsiveness to TGF-β–mediated growth inhibition as expected from previous reports (Zhang et al., 1996; Nakao et al., 1997; Itoh et al., 1998) (Figure 2C). However, Smad3- or Smad7-expressing cells treated with TGF-β acquired a fibroblastic morphology with a concomitant loss of junctional E-cadherin staining and gain of plasma membrane N-cadherin localization similar to control cells infected with retrovirus containing the GFP gene (Figure 3). These data suggest that TGF-β–induced EMT is unaffected by decreased SMAD signaling as reflected by SMAD-dependent growth and transcriptional responses. Additionally, TGF-β–mediated stress fiber formation was not inhibited by the down-regulation of SMAD signaling.
Figure 3.
The down-regulation of SMAD signaling does not block TGF-β–mediated EMT. NMuMG cells stably expressing GFP, Smad3, or Smad7 were treated with TGF-β (5 ng/ml for 24 h at 37°C), stained for E- or N-cadherin, and visualized by antimouse Cy2 fluorescence and phase contrast microscopy. F-actin localization was determined by Texas Red-phalloidin. The panels (400×) are magnified as insets (800×).
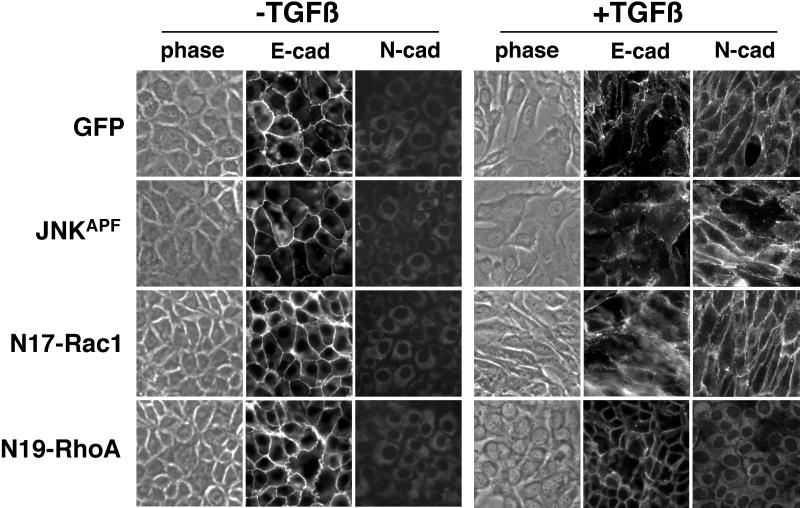
To identify TGF-β effectors that contribute to EMT, we next blocked signaling pathways that have been previously attributed to changes in cellular morphology through the retroviral transduction of dominant-negative RhoA, Rac1, and JNK. Transient expression of dominant-negative N17Rac1 or JNKAPF in NMuMG cells did not alter EMT induction by TGF-β; however, dominant-negative N19-RhoA blocked the mesenchymal transition (Figure 4). E-cadherin expression was maintained at the cell junctions and N-cadherin expression was not observed in cells transduced with N19-RhoA retrovirus after 24 h of 5-ng/ml TGF-β treatment. Assays for G-protein activation suggested the efficacy of retroviral expression of RhoA and Rac1 mutants (Figures 5 and 6). Pharmacological inhibition of the transcription factor activator protein-1 (downstream of JNK) with curcumin, and specific Rac1 inhibition with 5 M SCH51344 (Walsh et al., 1997) did not block TGF-β–mediated EMT; however, protein synthesis inhibition, with 50 μg/ml cycloheximide did block TGF-β–mediated EMT (our unpublished data). Thus, the recruitment of RhoA-dependent signaling pathways and nascent protein synthesis are involved in TGF-β–mediated EMT.
Figure 4.
The expression of dominant-negative RhoA (N19-RhoA) inhibits TGF-β–mediated EMT. Forty-eight hours after retroviral infection with N19-RhoA, dominant-negative JNK (JNKAPF), dominant-negative Rac1 (N17-Rac1), or GFP cDNA as a control, NMuMG cells were treated with TGF-β (5 ng/ml for 24 h at 37°C). Phase contrast and immunofluorescent E- and N-cadherin localization was observed. Expression of dominant-negative N17-RhoA blocked TGF-β induction of EMT, whereas expression of N17-Rac1 and JNKAPF did not.
Figure 5.
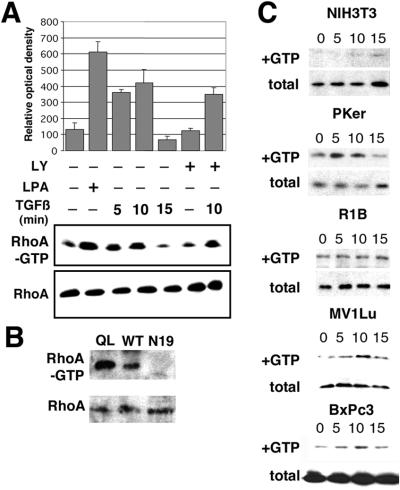
TGF-β treatment activates RhoA in epithelial cells. NMuMG cells cultured in serum-free medium were incubated with 10 ng/ml TGF-β for 0–15 min at 37°C in the presence or absence of 10 μg/ml LY294002 (LY) added 1 h before TGF-β treatment. (A) GTP loaded RhoA was measured by adsorbing cell lysates to GST-RBD beads and immunoblotted for RhoA. The densitometry measurements were normalized to total RhoA content of nonadsorbed cell lysate and represented by the bar graph (n = 4 ± SD). As a positive control, NMuMG cells treated for 5 min with LPA were assayed. TGF-β induces the rapid accumulation of RhoA-GTP in a PI3-kinase independent manner. (B) Further controls to show the GTP-loading potential of QL-RhoA– and N19-RhoA–transduced NMuMG cells assayed for RhoA activity. (C) NIH-3T3, primary keratinocytes (Pker), R1B, Mv1Lu, and BxPc3 cells were similarly assayed for RhoA activation. Only epithelial cells with intact TGF-β type I receptor exhibited TGF-β–mediated RhoA-GTP accumulation.
Figure 6.
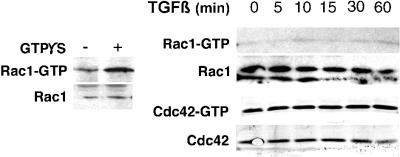
Rac1 and Cdc42 activation by TGF-β in NMuMG cells. TGF-β–treated (10 ng/ml for 0–60 min at 37°C) NMuMG cells were adsorbed to PBD-GST or run directly on a 12% acrylamide gel and immunoblotted sequentially for Rac1 and Cdc42. As a control, permeabilized cells incubated with GTPS were assayed for Rac1 activation (left). TGF-β–mediated induction of Rac1 and Cdc42 activation was negligible in NMuMG cells.
We (Engel et al., 1999) and others (Atfi et al., 1997) have demonstrated the role of RhoA in TGF-β transcriptional signaling, as a positive cofactor for the JNK and SMAD signaling pathways. However, no direct evidence for RhoA activation by TGF-β has been presented. Thus, we performed assays for the activation state of Rho-GTPases by measuring GTP-bound RhoA, as described by Ren et al. (1999), with NMuMG, Mv1Lu, R1B, primary mouse keratinocytes, BxPc3, and NIH3T3 cells in response to treatment with TGF-β or lysophosphatic acid (LPA) as positive control (Figure 5). The incubation of cells with LPA for 5 min resulted in a maximal 5-fold increase in RhoA-GTP over untreated cells (Figure 5A, lane 2). TGF-β treatment of the NMuMG cells showed nearly a 4-fold accumulation of RhoA-GTP. Another control included the observation of elevated levels of GTP-RhoA in constitutively active QL-RhoA and diminished GTP-RhoA signal in N19-RhoA retrovirally transduced NMuMG cells (Figure 5B). TGF-β treatment of NMuMG, Mv1Lu cells, primary mouse keratinocytes, or BxPc3 cells exhibited RhoA activation within 5 min, with a maximal 4–6-fold increase in GTP-bound RhoA was observed at 10 min. This was followed by a rapid decrease in RhoA activity to baseline levels by 15 min of TGF-β incubation. There was little change in the level of expression of RhoA in each of the epithelial cell types that display TGF-β–mediated changes in morphology. However, NIH3T3 and R1B cells exhibited little or no change in TGF-β–induced RhoA activation over basal levels (Figure 5C). TGF-β treatment for 48 h showed similar expression levels of RhoA (Bhowmick and Moses, unpublished data). We further found that a 1 h pretreatment with LY294002 (a specific PI3-kinase inhibitor) was largely ineffective in blocking the TGF-β–mediated RhoA activation, suggesting the rapid increase in RhoA-GTP is independent of PI3-kinase activity (Figure 5A, lanes 6 and 7). The transient retroviral expression of a constitutively active RhoA (QL-RhoA) in NMuMG cells did not result in spontaneous mesenchymal transition, nor did it effect TGF-β–mediated EMT (Bhowmick and Moses, unpublished data). This indicates that RhoA activation is necessary, but not sufficient for the induction of EMT in these cells.
The evidence of TGF-β–mediated PI3-kinase (Krymskaya et al., 1997) and RhoA activity suggested the potential for the activation of other GTPases such as Rac1 or Cdc42. We tested this possibility by GTP-Rac1 and GTP-Cdc42 affinity precipitation with GST-PAK3 binding domain, after immunoblotting for Rac1 or Cdc42 (Benard et al., 1999). GTPS loading of NMuMG cells showed appreciable Rac1 activation (as a control); however, neither GTPase exhibited detectable activation by TGF-β over basal levels (Figure 6). TGF-β is observed to activate RhoA but not Rac1 or Cdc42 in NMuMG cells.
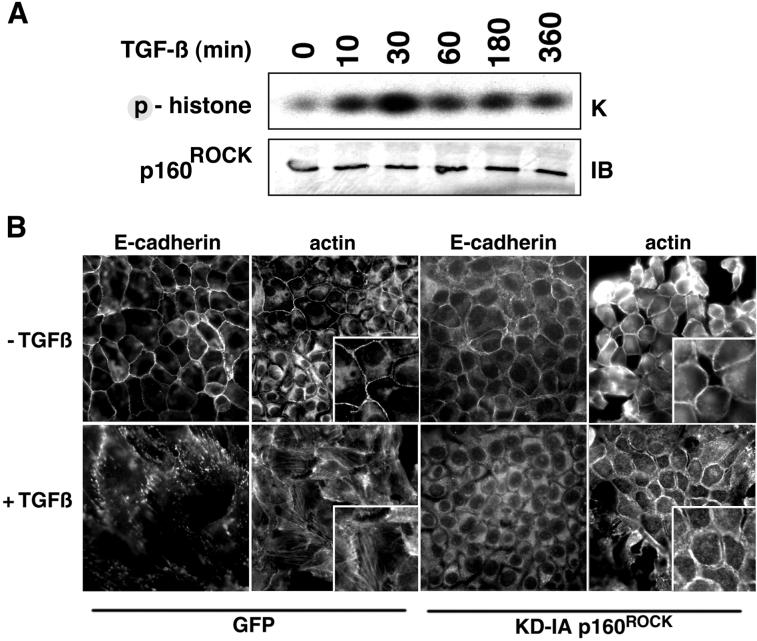
Thus, our findings suggest the potential involvement of RhoA-selective downstream targets in TGF-β–mediated responses. We focused on the action of p160ROCK as a potential mediator of TGF-β–induced component of EMT, namely, the regulation of actin cytoskeleton and adherens junction integrity and NMuMG cell motility. Myc-tagged p160ROCK cDNA was transfected into NMuMG cells and subjected to in vitro kinase assays after TGF-β stimulation by using histone as substrate. Elevated p160ROCK activity was detected by 10 min, peaking at a 4-fold level above control by 30 min of TGF-β treatment (Figure 7A). The introduction of a p160ROCK cDNA, encoding a kinase dead protein unable to interact with Rho (KD-IA; Ishizaki et al., 1997), into NMuMG cells provided further insight into the role of p160ROCK in TGF-β signaling. Cells retrovirally transduced with KD-IA p160ROCK cDNA manifested E-cadherin delocalization from adherens junctions, but did not acquire stress fibers and did not adopt a mesenchymal phenotype in response to TGF-β (Figure 7B). Phalloidin staining showed notable cell-cell junctions in the untreated cells, whereas a gap between cells was observed in TGF-β–treated cells that maintained cortical actin expression. A p160ROCK inhibitor, HA1077 [1-5-(isoquinolinesulfonyl)-homopiperazine HCl; Alexis Biochemicals], at 1 μM concentration also inhibited stress fiber formation and the fibroblastic morphology induced by TGF-β (unpublished data). These data suggest that regulation of actin cytoskeletal organization and E-cadherin junction integrity diverge at RhoA, with the former involving p160ROCK, and the latter requiring other signaling factors.
Figure 7.
TGF-β regulation of p160ROCK kinase activity is involved in the EMT of NMuMG cells. (A) Myc-tagged p160ROCK was immunoprecipitated from cells treated with TGF-β (10 ng/ml for 0–360 min at 37°C) and phosphorylation of histone was resolved by electrophoreses followed by autoradiography. Expression of myc-tagged p160ROCK was determined by Western blotting. TGF-β induces p160ROCK kinase activity. (B) Cells retrovirally transduced with either GFP (as a control) or KD-IA p160ROCK cDNA were treated with 5 ng/ml TGF-β for 18 h. Subsequently, cells were fixed and stained with for E-cadherin or F-actin. The panels (400×) are magnified as insets (800×). The data suggest p160ROCK activity is involved in TGF-β–mediated actin stress fiber formation, but not E-cadherin delocalization.
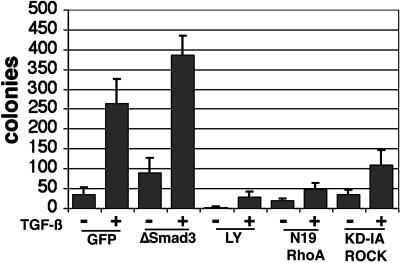
We next carried out motility studies to correlate the changes in cell morphology observed in EMT with their migratory potential. Previous studies showed that PI3-kinase and RhoA signaling could be independently involved in the migratory phenotype of fibroblasts (Price et al., 1999; O'Connor et al., 2000). NMuMG cells transduced with GFP (as a control), Smad3, N19-RhoA, or KD-IA p160ROCK retrovirus incubated with or without TGF-β or LY294002 were measured for their migration through 8 μM pore size polycarbonate filters. A 6-fold increase in cell migration was observed in TGF-β–treated over control, nontreated cells (Figure 8). The expression of Smad3 failed to antagonize either basal or TGF-β–mediated NMuMG cell motility. The TGF-β–induced migration was almost completely inhibited by treatment with 10 M LY294002. And the expression of N19-RhoA antagonized the motility of the cells, as did KD-IA p160ROCK to a lesser extent.
Figure 8.
TGF-β–induced motility of NMuMG cells can be antagonized by blocking the PI3-kinase or RhoA signaling pathways. Retrovirally transduced NMuMG cells allowed to attach to 8 μM polycarbonate filters (Costar, Cambridge, MA) for 18 h, were transferred to wells with fresh media supplemented with 10% serum, with or without 5 ng/ml TGF-β and/or 10 nM LY294002 (LY). The bar graph represents colonies present after 48 h in the presence or absence of 5 ng/ml TGF-β in at least two experiments done in triplicate. Blocking PI3-kinase and RhoA signaling limited TGF-β–mediated motility of NMuMG cells.
DISCUSSION
There is now considerable evidence that autocrine/paracrine TGF-β action is important in developmental processes (Brown et al., 1996) as well as in the invasion and metastatic spread of carcinoma cells. The NMuMG cells that we used were isolated from normal mammary glands and do not form malignant lesions when injected into nude mice (Hynes et al., 1985). However, H-Ras-transformed fibroblastoid NMuMG cells are E-cadherin negative and become fully invasive in vitro and in vivo (Van den Broecke et al., 1996). Oft et al. (1996) reported that fully polarized mammary epithelial cells are converted to a fibroblastoid morphology by TGF-β. Using a dominant-negative type II TGF-β receptor construct, this same group derived evidence indicating that autocrine TGF-β stimulation was necessary for invasion and metastasis (Oft et al., 1998). In transgenic mice with keratinocyte targeted TGF-β expression, outgrowth of benign papillomas was inhibited, consistent with TGF-β's growth inhibitory role. However, those tumors that escaped growth inhibition by TGF-β manifested a higher rate of malignant conversion, often characterized by an invasive, spindle cell phenotype (Cui et al., 1996). This phenotype required TGF-β receptor function (Portella et al., 1998), suggesting separation in signal transduction pathways downstream of the receptor complex. Clinical data from hereditary nonpolyposis colon cancer patients support the hypothesis that TGF-β signaling is important in metastasis. Hereditary nonpolyposis colon cancer patients frequently develop proximal colon cancers with microsatellite instability (MSI) (Thibodeau et al., 1993). Approximately 90% of colon carcinomas with MSI have inactivating mutations of TRII (Parsons et al., 1995), and MSI is significantly correlated with a reduced incidence of metastases and increased patient survival (Gryfe et al., 2000; Thibodeau et al., 1993), suggesting that complete loss of TRII in carcinomas results in less aggressive tumors. In addition, cells from Smad3 knockout mice have a diminished growth inhibitory response to TGF-β (Datto et al., 1999; Yang et al., 1999), yet these mice exhibit accelerated wound healing and develop invasive, metastasizing colorectal carcinomas (Zhu et al., 1998; Ashcroft et al., 1999). TGF-β signal transduction has been traditionally associated with SMAD signaling for the regulation of gene expression and growth inhibition. Our results point to an alternative signaling pathway for TGF-β via RhoA activation in the positive regulation of EMT.
TGF-β regulates at least two components of EMT progression through RhoA-dependent pathways, the regulation of the actin cytoskeleton and stability of adherens junctions. Both changes occur in the presence of inhibitors of SMAD or JNK signaling (Figures 3 and 4). However, TGF-β–induced SMAD- and JNK-mediated transcriptional activation are both suggested to be dependent on RhoA activity (Atfi et al., 1997; Engel et al., 1999). They do not, however, exclude a role for SMAD signaling in EMT, as determined by the expression pattern of E-cadherin, N-cadherin, actin, and cellular motility. Indeed, others have reported that overexpression of Smad2 and Smad3 in the context of concomitant expression of constitutively active TGF-β type I receptor induces EMT of NMuMG cells (Piek et al., 1999). Because dominant-negative constructs rarely abolish endogenous protein activity, it may be that EMT requires significantly lower Smad3 activity than that required for the activation of 3TP-Lux or growth inhibition.
Our data suggest TGF-β stimulation of the RhoA/p160ROCK signaling pathway is necessary for the acquisition of stress fibers and a fibroblastic morphology in NMuMG and primary mouse keratinocytes. Additionally, the expression of dominant-negative N19-RhoA in NMuMG cells blocked TGF-β–mediated EMT. The observed rapid GTP loading of RhoA in Mv1Lu cells correlates with the previously recognized loss of cell-cell adhesion and acquisition of actin stress fibers in these cells upon TGF-β treatment (Azuma et al., 1996). Furthermore, the lack of stimulation of RhoA activity by TGF-β in R1B cells illustrates the need for the TGF-β type I receptor in RhoA signaling. Interestingly, NIH-3T3 fibroblasts, shown to be growth stimulated by TGF-β (Li et al., 1993), exhibited no detectable GTP-RhoA accumulation in response to TGF-β treatment. This can be a result of number potential reasons, including the differential expression of RhoA-modifying proteins such as farnesyltransferase or guanine-nucleotide-exchange factors (GEFs). Interestingly, BxPc3 cells, a pancreatic metastatic tumor line not growth inhibited by TGF-β with a homologous deletion in the Smad4 gene, is TGF-β responsive for RhoA activation. This clearly indicates a bifurcation of signaling pathways downstream of the receptor.
Recent observations show that NIH-3T3 fibroblasts can be converted to a more epithelial morphology by elevated Rac1 activity, and their fibroblastic morphology reverted by the restoration of RhoA activity through the expression of constitutively active V14RhoA (Sander et al., 1999). Interestingly, neither V14-RhoA nor N19-RhoA affects Rac1 activity, but Rac1 activation down-regulates RhoA activity (Sander et al., 1999). Although inhibiting Rho-GTPases by C3 microinjection is known to disrupt E-cadherin cytoskeletal links in adherens junctions and blocks the assembly of new adherens junctions (Hall, 1998), the expression of N19-RhoA did not produce the same results (Figure 4). This is possibly due to redundant functions of RhoA with other Rho proteins that N19-RhoA does not inhibit. For example, the RhoB protein is reportedly stabilized in the cytoplasm by TGF-β (Engel et al., 1998a). The complex role of Rho proteins in the regulation of cadherin-mediated adhesion is yet to be elucidated (Braga et al., 1999).
We found Rac1 signaling may not be a direct signaling partner for TGF-β–mediated EMT in NMuMG cells through the use of N17-Rac1 expression, SCH51344 (Rac1 inhibitor (Walsh et al., 1997), and assaying for Rac1 activation. However, our studies do not rule out the role of Rac1 and Cdc42 in the TGF-β–mediated EMT in an indirect role in the complex process of cytoskeletal reorganization. The GEFs are thought to mediate the replacement of GTPase-bound GDP with GTP. The rapid response in epithelial cells would suggest a potential role for TGF-β in the direct regulation of such GEFs. Although there are as many as 30 GEF family members identified to date (Bishop and Hall, 2000), the results show a specific TGF-β–mediated activation of RhoA, but not Rac1 or Cdc42, to suggest that a RhoA-specific GEF is potentially stimulated by the activated TGF-β receptor complex.
The role of PI3-kinase in the regulation of the interactions between the plasma membrane and cytoskeleton is a subject of current study by many groups. The dual capacity for the activation of RhoA and PI3-kinase (Krymskaya et al., 1997) by TGF-β makes this cytokine an important target for the study of the complex process of EMT. We found that both RhoA GTP loading is independent of PI3-kinase activity and inhibiting PI3-kinase can inhibit the motility of in NMuMG cells. Unfortunately, the cooverexpression of a constitutively active RhoA (QL-RhoA) and PI3-kinase (Myr-p110) is not feasible, because the constitutive activation of RhoA results in the rapid initiation of apoptosis (Subauste et al., 2000; Watanabe and Akaike, 1999). To adequately mimic TGF-β signaling, a transitory activation of RhoA is required or increased GTP exchange (see references in Van Aelst and D'Souza-Schorey, 1997).
The elegant studies of Vasioukhin et al. (2000) have given us insight into the mechanism of adhesion junction formation by actin polymerization and reorganization. In epithelial cells plasma membrane spanning E-cadherin is physically tethered to the actin cytoskeleton by β-catenin, α-catenin, and in turn several actin-binding proteins. Clustering of E-cadherin at the cell junctions provide the proper conformation and/or density of α-catenin to bind actin and establish a continuous epithelial sheet (Vasioukhin et al., 2000). Our studies indicate that TGF-β signaling is capable of destabilizing E-cadherin junctions by regulating actin organization through RhoA/p160ROCK induction. The results from the interference of p160ROCK activity by the expression of KD-IA p160ROCK suggest a dichotomous role for RhoA in TGF-β–mediated EMT, as a dynamic regulator of adhesion junctions and the complex mechanism of cellular morphology. The loss of adherens junctions in TGF-β–treated KD-IA-p160ROCK-expressing cells resulted in a discontinuity in the epithelial sheet. Actin localization indicated distinct staining of cell borders that had not formed cell-cell contacts. Because TGF-β treatment does not cause E-cadherin expression levels to change appreciably in NMuMG cells, we can speculate that E-cadherin relocalizes to the cytoplasm in a p160ROCK-independent manner. Thus, the organization of actin at the cell borders does not necessarily presuppose the formation of adherens junctions. The role of other RhoA effectors in TGF-β–mediated actin cytoskeletal organization remains to be determined. Likely other TGF-β/RhoA downstream signals such as those involved in vesicular trafficking of E-cadherin from the plasma membrane are involved in the disassembly of adherens junctions. The identified interactions among RhoA, E-cadherin, and actin (Braga et al., 1999) suggest various methods of TGF-β/RhoA regulation of cadherin junctions.
In summary we show that 1) TGF-β activates RhoA and p160ROCK, 2) N19-RhoA blocks TGF-β–mediated EMT, and 3) p160ROCK inhibition blocks actin cytoskeleton rearrangement and motility induced by TGF-β. These findings indicate a signaling cascade involving RhoA in TGF-β–induced EMT. The biological activity of TGF-β–mediated RhoA signaling is not limited to its capacity to mediate the actin reorganization observed in many cell types. RhoA is currently established as a positive regulatory factor in cell-cell contacts, secretion, vesicular trafficking, and transformation. These studies further suggest TGF-β signaling pathways for growth inhibition and tumor suppression may be separable from those pathways involved in EMT. Hence, it may be possible to generate antagonists of the EMT pathways useful for inhibiting tumor invasion and metastasis without blocking the desirable tumor suppressive effects of TGF-β.
ACKNOWLEDGMENTS
We are grateful to Drs. William Grady, Brian Law, and Bart Lutterbach for their critical reading of the manuscript. Fluorescence and phase contrast microscopy images were acquired through the use of the Vanderbilt University Medical Center Cell Imaging Core Resource (supported by National Institutes of Health Grants CA-68485 and DK-20593). This work was supported by a National Institutes of Health training grant CA-09592 and Department of Defense, US Army Medical Research and Materiel Command Grant BC-991184 (to N.A.B.), Public Health Service Grants CA-42572 and CA-85492 (to H.L.M.), CA-62212 (to C.L.A.), Department of Defense, US Army Medical Research and Materiel Command Grant DAMD-17-98-1-8262 (to C.L.A.), and Vanderbilt-Ingram Cancer Center support Grant CA-68485.
REFERENCES
- Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, Roberts AB. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- Atfi A, Djelloul S, Chastre E, Davis R, Gespach C. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor β-mediated signaling. J Biol Chem. 1997;272:1429–1432. doi: 10.1074/jbc.272.3.1429. [DOI] [PubMed] [Google Scholar]
- Azuma M, Tamatani T, Fukui K, Yuki T, Motegi K, Sato M. Different signaling pathways involved in transforming growth factor-β1-induced morphological change and type IV collagen synthesis in simian virus-40-immortalized normal human salivary gland duct and myoepithelial cell clones. Arch Oral Biol. 1996;41:413–424. doi: 10.1016/0003-9969(96)00003-9. [DOI] [PubMed] [Google Scholar]
- Benard V, Bohl BP, Bokoch GM. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348:241–255. [PMC free article] [PubMed] [Google Scholar]
- Braga VM, Del Maschio A, Machesky L, Dejana E. Regulation of cadherin function by Rho and Rac: modulation by junction maturation and cellular context. Mol Biol Cell. 1999;10:9–22. doi: 10.1091/mbc.10.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CB, Boyer AS, Runyan RB, Barnett JV. Antibodies to the type II TGF-β receptor block cell activation and migration during atrioventricular cushion transformation in the heart. Dev Biol. 1996;174:248–257. doi: 10.1006/dbio.1996.0070. [DOI] [PubMed] [Google Scholar]
- Calonge MJ, Massague J. Smad4/DPC4 silencing and hyperactive Ras jointly disrupt transforming growth factor-β antiproliferative responses in colon cancer cells. J Biol Chem. 1999;274:33637–33643. doi: 10.1074/jbc.274.47.33637. [DOI] [PubMed] [Google Scholar]
- Cui W, Fowlis DJ, Bryson S, Duffie E, Ireland H, Balmain A, Akhurst RJ. TGF-1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell. 1996;86:531–542. doi: 10.1016/s0092-8674(00)80127-0. [DOI] [PubMed] [Google Scholar]
- Datto MB, Frederick JP, Pan L, Borton AJ, Zhuang Y, Wang XF. Targeted disruption of Smad3 reveals an essential role in transforming growth factor β-mediated signal transduction. Mol Cell Biol. 1999;19:2495–2504. doi: 10.1128/mcb.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel ME, Datta PK, Moses HL. RhoB is stabilized by transforming growth factor β and antagonizes transcriptional activation. J Biol Chem. 1998a;273:9921–9926. doi: 10.1074/jbc.273.16.9921. [DOI] [PubMed] [Google Scholar]
- Engel ME, Datta PK, Moses HL. Signal transduction by transforming growth factor-β: a cooperative paradigm with extensive negative regulation. J Cell Biochem Suppl. 1998b;31:111–122. doi: 10.1002/(SICI)1097-4644(1998)72:30/31+<111::AID-JCB15>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Engel ME, McDonnell MA, Law BK, Moses HL. Interdependent SMAD, and JNK signaling in transforming growth factor-β-mediated transcription. J Biol Chem. 1999;274:37413–37420. doi: 10.1074/jbc.274.52.37413. [DOI] [PubMed] [Google Scholar]
- Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, Redston M, Gallinger S. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342:69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Han EK, Guadagno TM, Dalton SL, Assoian RK. A cell cycle and mutational analysis of anchorage-independent growth: cell adhesion and TGF-β1 control G1/S transit specifically. J Cell Biol. 1993;122:461–471. doi: 10.1083/jcb.122.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki M, Shimokado K. Phosphatidylinositol 3-kinase is required for growth factor-induced amino acid uptake by vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:2127–2132. doi: 10.1161/01.atv.19.9.2127. [DOI] [PubMed] [Google Scholar]
- Hordijk PL, ten Klooster J P, van der Kammen R A, Michiels F, Oomen L C, Collard J G. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- Hynes NE, Jaggi R, Kozma SC, Ball R, Muellener D, Wetherall NT, Davis BW, Groner B. New acceptor cell for transfected genomic DNA: oncogene transfer into a mouse mammary epithelial cell line. Mol Cell Biol. 1985;5:268–272. doi: 10.1128/mcb.5.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- Itoh S, Landstrom M, Hermansson A, Itoh F, Heldin CH, Heldin NE, ten Dijke P. Transforming growth factor β1 induces nuclear export of inhibitory Smad7. J Biol Chem. 1998;273:29195–29201. doi: 10.1074/jbc.273.44.29195. [DOI] [PubMed] [Google Scholar]
- Kinsella TM, Nolan GP. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum Gene Ther. 1996;7:1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Timokhina I, Massague J. A mechanism of repression of TGF-β/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar M, Massague J. SMADs: mediators and regulators of TGF-β signaling. Curr Opin Genet Dev. 1998;8:103–111. doi: 10.1016/s0959-437x(98)80069-5. [DOI] [PubMed] [Google Scholar]
- Krymskaya VP, Hoffman R, Eszterhas A, Ciocca V, Panettieri RA., Jr TGF-β1 modulates EGF-stimulated phosphatidylinositol 3-kinase activity in human airway smooth muscle cells. Am J Physiol. 1997;273:L1220–L1227. doi: 10.1152/ajplung.1997.273.6.L1220. [DOI] [PubMed] [Google Scholar]
- Li PM, Fukazawa H, Yamamoto C, Mizuno S, Tanaka K, Hori M, Yaginuma S, Saito T, Uehara Y. Method of identifying inhibitors of oncogenic transformation: selective inhibition of cell growth in serum-free medium. Oncogene. 1993;8:1731–1735. [PubMed] [Google Scholar]
- McLeod C, Thornley A, Veale R, Scott E. The anchorage-dependent and -independent growth of a human SCC cell line: the roles of TGF alpha/EGF and TGF β. Br J Cancer. 1990;61:267–269. doi: 10.1038/bjc.1990.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin C H, ten Dijke P. Identification of Smad7, a TGF-β-inducible antagonist of TGF-β signaling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147:631–644. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor KL, Nguyen BK, Mercurio AM. RhoA function in lamellae formation and migration is regulated by the alpha64 integrin and cAMP metabolism. J Cell Biol. 2000;148:253–258. doi: 10.1083/jcb.148.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oft M, Heider KH, Beug H. TGF-β signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol. 1998;8:1243–1252. doi: 10.1016/s0960-9822(07)00533-7. [DOI] [PubMed] [Google Scholar]
- Oft M, Peli J, Rudaz C, Schwarz H, Beug H, Reichmann E. TGF-β1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 1996;10:2462–2477. doi: 10.1101/gad.10.19.2462. [DOI] [PubMed] [Google Scholar]
- Parsons R, Myeroff LL, Liu B, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Microsatellite instability and mutations of the transforming growth factor β type II receptor gene in colorectal cancer. Cancer Res. 1995;55:5548–5550. [PubMed] [Google Scholar]
- Piek E, Moustakas A, Kurisaki A, Heldin C, ten Dijke P. TGF-β type I receptor/ALK-5 and smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J Cell Sci. 1999;112:4557–4568. doi: 10.1242/jcs.112.24.4557. [DOI] [PubMed] [Google Scholar]
- Pierce DF, Jr, Gorska AE, Chytil A, Meise KS, Page DL, Coffey RJ, Jr, Moses HL. Mammary tumor suppression by transforming growth factor β 1 transgene expression. Proc Natl Acad Sci USA. 1995;92:4254–4258. doi: 10.1073/pnas.92.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portella G, Cumming SA, Liddell J, Cui W, Ireland H, Akhurst RJ, Balmain A. Transforming growth factor β is essential for spindle cell conversion of mouse skin carcinoma in vivo: implications for tumor invasion. Cell Growth Differ. 1998;9:393–404. [PubMed] [Google Scholar]
- Price JT, Tiganis T, Agarwal A, Djakiew D, Thompson EW. Epidermal growth factor promotes MDA-MB-231 breast cancer cell migration through a phosphatidylinositol 3′-kinase and phospholipase C-dependent mechanism. Cancer Res. 1999;59:5475–5478. [PubMed] [Google Scholar]
- Reid T, Furuyashiki T, Ishizaki T, Watanabe G, Watanabe N, Fujisawa K, Morii N, Madaule P, Narumiya S. Rhotekin, a new putative target for Rho bearing homology to a serine/threonine kinase, PKN, and rhophilin in the rho-binding domain. J Biol Chem. 1996;271:13556–13560. doi: 10.1074/jbc.271.23.13556. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior J. Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subauste MC, Von Herrath M, Benard V, Chamberlain CE, Chuang TH, Chu K, Bokoch GM, Hahn KM. Rho family proteins modulate rapid apoptosis induced by cytotoxic T lymphocytes and Fas. J Biol Chem. 2000;275:9725–9733. doi: 10.1074/jbc.275.13.9725. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Van den Broecke C, Vleminckx K, De Bruyne G, Van Hoorde L, Vakaet L, Van Roy F, Mareel M. Morphotypic plasticity in vitro and in nude mice of epithelial mouse mammary cells (NMuMG) displaying an epithelioid (e) or a fibroblastic (f) morphotype in culture. Clin Exp Metastasis. 1996;14:282–296. doi: 10.1007/BF00053902. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Walsh AB, Dhanasekaran M, Bar-Sagi D, Kumar CC. SCH 51344-induced reversal of RAS-transformation is accompanied by the specific inhibition of the RAS and RAC-dependent cell morphology pathway. Oncogene. 1997;15:2553–2560. doi: 10.1038/sj.onc.1201424. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Akaike T. Possible involvement of caspase-like family in maintenance of cytoskeleton integrity. J Cell Physiol. 1999;179:45–51. doi: 10.1002/(SICI)1097-4652(199904)179:1<45::AID-JCP6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-β receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, Roberts AB, Deng C. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-β. EMBO J. 1999;18:1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Feng X, We R, Derynck R. Receptor-associated Mad homologues synergize as effectors of the TGF-β response. Nature. 1996;383:168–172. doi: 10.1038/383168a0. [DOI] [PubMed] [Google Scholar]
- Zhong C, Kinch MS, Burridge K. Rho-stimulated contractility contributes to the fibroblastic phenotype of Ras-transformed epithelial cells. Mol Biol Cell. 1997;8:2329–2344. doi: 10.1091/mbc.8.11.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Richardson JA, Parada LF, Graff JM. Smad3 mutant mice develop metastatic colorectal cancer. Cell. 1998;94:703–714. doi: 10.1016/s0092-8674(00)81730-4. [DOI] [PubMed] [Google Scholar]