Abstract
Background
Separate health-related quality of life (HRQL) instruments exist for asthma and rhinitis. The Rhinasthma questionnaire, originally developed in Italian, is a unique measure designed for use where both conditions coexist.
Objective
We sought to assess the performance and validity of a new adaptation of the Rhinasthma for use in English-speaking populations.
Methods
We analyzed cross-sectional data from an ongoing study of adults with asthma and rhinitis (n=450), asthma alone (n=75), or rhinitis alone (n=20). Subjects were administered an English translation of the original 30-item Rhinasthma questionnaire. Health status measures simultaneously assessed include the Short Form (SF)-12, EuroQol (EQ)-5D, and Marks Asthma Quality-of-Life (AQoL).
Results
Variable cluster analysis of the original 30 item instrument identified 5 discrete item clusters corresponding to the following domains: nasal (5 items), eye (4 items), respiratory (5 items), activity restriction (9 items), treatment burden (5 items). Two other items were removed due to poor item-cluster correlations. Subjects with concomitant asthma and rhinitis had greater HRQL impairment, as measured by the Rhinasthma, than subjects with either asthma or rhinitis alone. The Rhinasthma correlated significantly (p<0.05) with the SF-12, EQ-5D, and Marks AQoL in the anticipated direction consistent with the underlying constructs. In multiple logistic regression, poorer Rhinasthma HRQL was associated with significantly (p<0.05) increased odds of both asthma- and rhinitis-related disability even after taking into account physical health status as measured by the SF-12.
Conclusion
The 28-item English adaptation of Rhinasthma performs well in assessing HRQL in patients with asthma, rhinitis, or both conditions combined.
INTRODUCTION
As many as 80% or more of asthmatics suffer from symptoms of rhinitis (1–4) and those with rhinitis alone frequently develop asthma over time.(4–7) Studies of patient-centered outcomes in either asthma or rhinitis that ignore their co-existence may face critical shortcomings. Indeed, an increasing body of evidence supports the construct that asthma and rhinitis represent different manifestations of a common underlying airway inflammatory disorder.(8) Consistent with this construct, studies show that nasal inflammation is present in asthmatics without rhinitis (9) and that bronchial mucosal inflammation is observed in patients with rhinitis, but without clinical asthma.(10, 11)
The assessment of health-related quality of life (HRQL) in either asthma or rhinitis has traditionally been performed using separate disease-specific instruments.(12, 13) In populations where asthma and rhinitis coexist to varying degrees, however, the use of any single disease-specific measure may fail to capture the true burden of illness. Rhinitis-specific measures tend to focus on the impact of eye and nasal symptoms, whereas asthma-specific measures focus predominantly on the effects of lower respiratory tract. Although the co-administration of 2 separate instruments is possible, such an approach poses limitations. For example, the comparison and interpretation of results from different disease-specific measures is often complicated by overlapping item content and differences in scaling. Furthermore, the administration of 2 separate instruments is unwieldy, increasing respondent burden and fatigue.(14–16)
To address the challenges associated with using separate instruments for 2 overlapping conditions, Baiardini and collaborators developed the Rhinasthma questionnaire for use in populations with asthma and/or rhinitis.(17) Rather than requiring respondents to attribute specific symptoms or problems to either their asthma or rhinitis, this integrated HRQL measure allows for the 2 conditions to be treated as different manifestations of the same disease spectrum. The Rhinasthma was originally developed in Italian,(17) but has subsequently been administered in Finnish, Swedish, and German.(18, 19) Aside from original validation efforts, however, its construct validity has never been fully reassessed within larger populations or with respect to other types of health status measures. Furthermore, the psychometric integrity of the instrument has been not been previously examined in an English-speaking population.
The aims of the present study were two-fold. First, we sought to develop an English-language adaptation of the Rhinasthma instrument and to identify item clusters that facilitate the interpretation of effects pertinent to asthma or rhinitis. Second, we sought to provide further evidence of the instrument’s construct validity by examining its psychometric performance within a large, well-described population with mixed airway disease among which multiple other types of health status measures were simultaneously assessed.
METHODS
Subject Recruitment
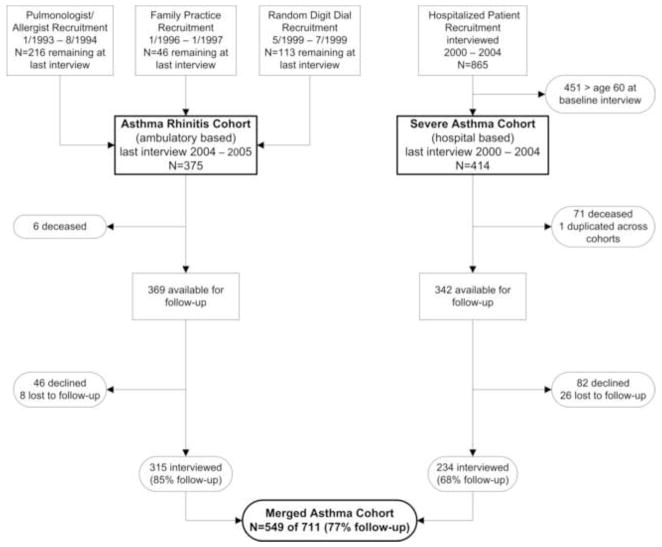
The study cohort reflects a merger of 2 different study groups separately recruited and previously studied independently. The flow of subject recruitment, retention, and integration is illustrated in Figure 1. Study of the merged cohort was approved by the University of California San Francisco Committee on Human Research.
Figure 1.
Flow of subject recruitment, retention, and integration the Asthma Rhinitis Cohort and Severe Asthma Cohort. Of the 549 subjects in the Merged Asthma Cohort, 189 (35%) were originally recruited from pulmonary and allergy specialty practices, 38 (7%) from family medicine practices, 88 (16%) by random digit dial, and 234 (42%) based on prior hospitalization for asthma.
In the first of the 2 parent study groups, the Asthma Rhinitis Cohort (ARC), recruitment occurred in three phases. Subjects with asthma were recruited initially through a random sample of pulmonary and allergy specialty practices in northern California beginning in 1992,(20, 21) followed by second phase of recruitment from family medicine specialty practices in 1996.(22) Eligibility was based on standard clinical criteria for asthma. In 1999, a third phase of subjects with either asthma or rhinitis was recruited by random-digit dial sampling in the same northern California catchment area.(23) For this sample, eligibility was based on respondent report of a physician’s diagnosis of asthma, or (for rhinitis) allergic rhinitis, chronic sinusitis, hay fever, or chronic post nasal drip. Subjects in all three of these sampling phases were between age 18 and 50 at the time of enrollment and those with concomitant diagnoses of chronic bronchitis or emphysema were ineligible. Although the subjects recruited through physicians underwent up to three interviews prior to recruitment of the random-digit dial group, all three phases were merged together to form a cohort with telephone interviews at 18 to 24 month intervals thereafter. From this merger, retention over 2 subsequent interview waves was 76% and 90%, respectively, leaving 375 for potential follow-up.
In the second study group, the Severe Asthma Cohort (SAC), we used data from a prospective cohort study of adult members of Northern California Kaiser Permanente, which provides health care to 25–30% of the region’s population with demographic characteristics representative of the general population except for at the extremes of income distribution.(24, 25) Recruitment methods have been reported previously.(26, 27) Beginning in April 2000 and for the next 4-years, eligibility was as follows: any recent Kaiser hospitalization for asthma for a Kaiser member aged ≥18 years with an International Classification of Diseases (9th revision ICD-9) code 493.xx as the primary discharge diagnosis or as a secondary code linked to an acute asthma-related respiratory condition. Those with a primary or secondary discharge diagnosis of chronic bronchitis, emphysema, or COPD were excluded. Beginning in April 2000 we attempted to recruit all intensive care unit (ICU) cases surviving to discharge and meeting these criteria; beginning in September 2000 this was expanded to include every fourth hospitalized, non-ICU case. Enrollment continued over a 4-year period through March 2004. The complete cohort included 865 subjects (53% completion rate among eligible subjects). All subjects studied reported a physician diagnosis of asthma at the time of baseline telephone interview. There were no further interviews in this cohort until the present study. To sample an age range at follow-up for the SAC similar to that of the ARC, we limited follow-up to those aged ≤60 years at baseline (n=414).
Subject Interviews
All participants in the combined cohort underwent structured telephone interviews administered by trained personnel using computer-assisted interviewing software. The interviews were approximately 45 minutes in duration and were conducted in English, although Spanish language assistance was available when needed. (Two of 549 interviews were conducted with the assistance of a Spanish translator.) The interviews included information on demographics, occupation, smoking, clinical symptoms, asthma and rhinitis medication usage, and activities limitations, in addition to the study measures described below.
Study Measures
Rhinasthma questionnaire
The original Rhinasthma instrument is comprised of 30 items; each item utilizes a 5-point Likert response scale (1 = ‘not at all’; 5 = ‘very much’) to rate the impact of each problem during the previous 2 weeks.(17) Individual responses are totaled and transformed to a scale from 0 to 100; higher scores indicate poorer HRQL. The original 30-item Rhinasthma was developed and validated in Italian.(17) For this study, the Rhinasthma was translated into English (standard American usage) using a 3-step translation process certified by the International Organization for Standardization (ISO) and performed by separate native-speaking, American Translators Association-certified medical linguists (TransPerfect; San Francisco, CA).
Measures of asthma-specific quality of life and asthma severity
Asthma-specific HRQL was assessed using the Marks Asthma Quality of Life (AQoL) questionnaire, a validated 20-item questionnaire. This was administered only to those subjects with a diagnosis of asthma (with or without concomitant rhinitis).(28) Higher Mark AQoL scores indicate poorer HRQL. Asthma severity was assessed using a previously validated measure, the Severity of Asthma Score (SAS). The SAS integrates recent and chronic severity, incorporating current symptoms and recent medication use, as well as longer term indicators of severity such as prior hospitalization and intubation and past and chronic exogenous corticosteroid administration.(22, 29, 30) Higher scores indicate greater asthma severity.
Measures of general health status
General health status was assessed using 2 well-established generic health measures: the Short Form (SF)-12 and EuroQol (EQ)-5D utility index. The physical component summary (PCS) of the SF-12 was used as a measure of general physical health status.(31) Scores are weighted to correspond to a population mean ± standard deviation of 50 ± 10; higher scores indicate better health status. The EQ-5D, like the SF-12, is a generic measure of general health status.(32) Its utility index is comprised of 5 items. The combination of individual item responses generates a single summary index, or ‘utility’, using preference-based weights elicited from the general population. A utility of 1.0 corresponds to perfect health and a value of zero represents death (negative values reflect health states considered worse than death).
Statistical Analysis
We used variable cluster analysis to assess the content validity of the Rhinasthma by identifying clusters of related items within the original 30-item scale. Variable cluster analysis offers an alternative to traditional multivariate methods for scale creation, such as factor analysis. It borrows from the factor analysis literature, while also utilizing concepts from hierarchical clustering. A key advantage of variable cluster analysis is that it yields non-overlapping item clusters that are relatively easy to interpret, in contrast to traditional methods, which frequently require multidimensional rotation to reduce cross-loading on multiple factors.(33) We implemented variable cluster analysis using PROC VARCLUS in SAS version 9.2 (SAS Institute; Cary, NC). VARCLUS uses iterative splitting and factor analytic methods to divide a group of variables into discrete clusters until a stopping criterion is reached. For this analysis we used a combined stopping criterion of second Eigenvalue of <1.0 and an increase of <5% in the proportion of variance explained with the next potential iteration. Items that correlated poorly with other items within the same cluster (i.e., item-cluster R2 <0.40) were eliminated. Variable cluster analysis was repeated to confirm that the same cluster solution was consistently identified. Each cluster was individually assessed for face validity based on its item content and then thematically labeled by consensus among the study investigators. Scores for each cluster were calculated by summing the responses to individual items and transforming to a scale from 0 to 100. The overall total Rhinasthma score was achieved by summing all of the retained items and similarly rescaling to 0 to 100.
Characteristics among subjects with asthma and rhinitis, asthma alone, and rhinitis alone were compared using analysis of variance (ANOVA) for continuous data and Fisher’s exact test for categorical data. “Known groups” validity (a form of construct validity determined by the degree to which an instrument distinguishes groups known to vary with regard to the measured construct) was assessed using ANOVA followed by Tukey’s Honestly Significant Difference (HSD) test to perform pairwise testing. Cronbach’s alpha was used to assess the internal consistency of the overall scale, as well as each individual cluster. Convergent validity (a form of construct validity determined by the degree to which an instrument correlates with instruments measuring related constructs) was assessed using Spearman correlations to examine the relationship between the Rhinasthma and other established health status measures. Convergent validity of specific clusters was also assessed in relation to conceptually-related components of other measures. For example, the cluster of Rhinasthma items pertaining to respiratory issues was compared directly with the breathlessness component of the asthma-specific Marks AQoL.
Multiple logistic regression was used to examine the relationship between the Rhinasthma-28 and disability after taking into account general health status, as measured by the SF-12 PCS. Disability, defined as 1 or more days of restricted activity during the past month, was specified as the dependent variable. Separate models were constructed for disability due to: any health problem, asthma specifically, and rhinitis specifically. Adjusted odds ratios (95% confidence interval [CI]) were reported per 1 standard effect (SE) size change in the direction of poorer health status. Additional models including covariates for age, sex, and race were also constructed.
RESULTS
Study participation
We interviewed 549 (77%) of 711 eligible subjects. Follow-up differed significantly (p<0.01) between the 2 parent study groups from which subjects were recruited; 85% follow-up for the ARC and 68% for the SAC (Table 1). Overall, those interviewed were approximately four years older and less likely to be current or former smokers compared to those not interviewed (p<0.01). We ultimately excluded four subjects because of missing data for key variables (1 for Rhinasthma and 3 for SF-12), leaving 545 subjects for analysis.
Table 1.
Subject characteristics by interview status
| Characteristics | Follow-up status |
P | |
|---|---|---|---|
| Interviewed (n=549) | Not interviewed (n=162) | ||
| Original cohort prior to integration, n (row %) | <0.01 | ||
| Asthma Rhinitis Cohort (ARC) | 315 (85) | 54 (15) | |
| Severe Asthma Cohort (SAC) | 234 (68) | 108 (32) | |
| Age in years, mean ± SD | 52.4 ± 9 | 48.5 ± 11 | <0.01 |
| Female, n (column %) | 407 (74) | 114 (70) | 0.34 |
| White, non-Hispanic, n (column %)* | 327 (60) | 86 (57) | 0.51 |
| Education, high school or less, n (column %) | 104 (19) | 28 (17) | 0.63 |
| Smoking status, n (column %) | <0.01 | ||
| Current | 53 (10) | 27 (17) | |
| Former | 186 (34) | 65 (40) | |
| Severity-of-Asthma Score (SAS), mean ± SD | 9.5 ± 5.4 | 10.4 ± 4.6 | 0.054 |
| SF-12 PCS, mean ± SD | 41.1 ± 12.1 | 41.8 ± 11.9 | 0.51 |
Race-ethnicity data missing for 10 subjects
SF-12 PCS = Short Form-12 Physical Component Summary
Subject characteristics
Of 545 subjects included in this analysis, 450 (82.6%) reported a physician diagnosis of both asthma and rhinitis, 75 (13.8%) asthma only, and 20 (3.7%) rhinitis only. Subject characteristics are shown in Table 2. Overall, subjects were predominantly white, non-Hispanic females. Statistically significant differences in income (p=0.01) and health-related unemployment (p=0.002) were found among the 3 diagnostic groups. Specifically, subjects with rhinitis alone had higher income and less unemployment due to health reasons.
Table 2.
Subject characteristics by condition.
| Characteristics | All subjects (n=545) | Asthma + Rhinitis (n=450) | Asthma only (n=75) | Rhinitis only (n=20) | p |
|---|---|---|---|---|---|
| Age in years, mean ± SD | 52.5 ± 9 | 52.3 ± 9 | 54.4 ± 9 | 49.8 ± 9 | 0.06 |
| Female, n (%) | 405 (74) | 339 (75) | 53 (71) | 13 (65) | 0.40 |
| White, non-Hispanic, n (%) | 325 (60) | 269 (60) | 41 (55) | 15 (75) | 0.26 |
| Education, high school or less, n (%) | 102 (19) | 81 (18) | 20 (27) | 1 (5) | 0.07 |
| Annual household income <$40,000, n (%)* | 158 (29) | 123 (28) | 32 (43) | 3 (15) | 0.01 |
| Unemployed for health reasons, n (%) | 157 (29) | 130 (29) | 27 (36) | 0 (0) | 0.002 |
| Smoking status, n (%) | 0.55 | ||||
| Current | 51 (9) | 42 (9) | 8 (11) | 1 (5) | |
| Former | 185 (34) | 147 (33) | 31 (41) | 7 (35) |
missing data on income for 3 subjects
Cluster analysis and item reduction
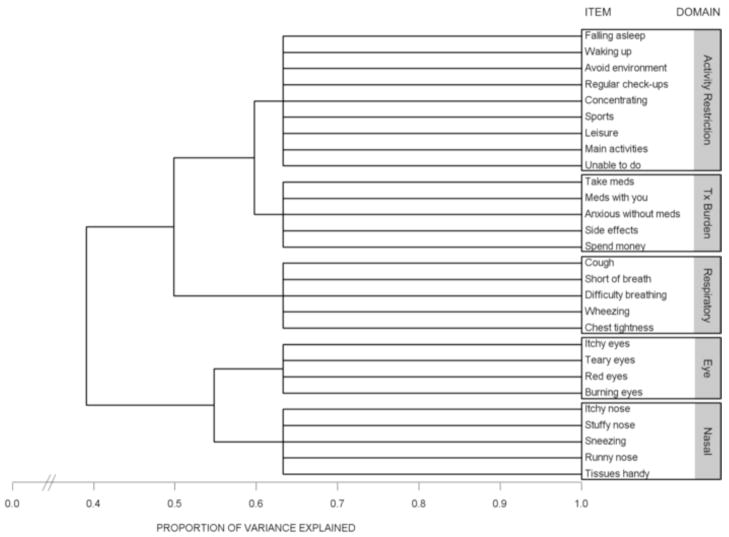
Variable cluster analysis of the original 30-item Rhinasthma yielded 5 discrete item clusters. We eliminated 2 items, ‘Loss of smell’ and ‘Clearing your throat,’ that correlated poorly with other items within the same cluster. Repeat variable cluster analysis, excluding these 2 items, produced the same 5-cluster solution, explaining 63.3% of the total variance (Figure 2). Item content within each cluster demonstrated good face validity: 5 items related to nasal symptoms; 4 to eye symptoms; 5 to lower respiratory symptoms; 9 to activity restriction; and 5 to treatment burden (see Appendix).
Figure 2.
Dendrogram (or “Tree” diagram) illustrating the iterative splitting technique utilized by variable cluster analysis. Successive splits are shown from left to right. The distance of each vertical branch from the origin at the left represents the proportion of variance explained for each cluster solution. The total variance explained by the 5-cluster solution was 63.3%.
Known groups validity
Summary statistics for the 28-item Rhinasthma (Rhinasthma-28) and its 5 domains identified by cluster analysis are shown in Table 3. Total Rhinasthma-28 scores were significantly higher (greater impairment) for subjects with concomitant asthma and rhinitis compared to subjects with either asthma or rhinitis alone (Tukey’s HSD test, p<0.05). Subjects with both conditions also scored higher in the activity restriction and treatment burden domains. Total Rhinasthma-28 scores were not significantly different between subjects with asthma only and rhinitis only (p=0.51). However, subjects with asthma (with or without rhinitis) scored higher in the respiratory domain compared to subjects with rhinitis alone (p<0.001). Conversely, subjects with rhinitis (with or without asthma) scored higher in the nasal and eye domains compared to subjects with asthma alone (p<0.001 in both cases).
Table 3.
Summary statistics of Rhinasthma-28 and other health status measures.
| All subjects (n=545) | Asthma + Rhinitis (n=450) | Asthma only (n=75) | Rhinitis only (n=20) | ||
|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Mean ± SD | Mean ± SD | |
| Rhinasthma-28* | |||||
| Total | 31 ± 21 | (0–98) | 34 ± 21 | 21 ± 18* | 18 ± 16* |
| Nasal | 33 ± 25 | (0–100) | 37 ± 24 | 11 ± 16* | 33 ± 22† |
| Eye | 23 ± 26 | (0–100) | 26 ± 26 | 6 ± 14* | 26 ± 26† |
| Respiratory | 31 ± 27 | (0–100) | 32 ± 27 | 29 ± 29 | 9 ± 12*† |
| Activity restriction | 32 ± 26 | (0–100) | 33 ± 26 | 26 ± 26 | 12 ± 17* |
| Treatment burden | 36 ± 30 | (0–100) | 39 ± 30 | 26 ± 26* | 17 ± 19* |
| SF-12 PCS | 40 ± 12 | (12–63) | 40 ± 12 | 40 ± 13 | 51 ± 7*† |
| EQ-5D utility index | 0.77 ± 0.23 | (−0.11–1.00) | 0.76 ± 0.23 | 0.76 ± 0.25 | 0.92 ± 0.10*† |
| Marks Asthma QoL‡ | 20 ± 16 | (0–60) | 20 ± 16 | 18 ± 17 | NA |
p<0.05 compared to Asthma + Rhinitis group
p<0.05 compared to Asthma only group
Marks Asthma QoL missing for 5 subjects with asthma + rhinitis and not administered to 20 subjects with rhinitis only
The SF-12 PCS, EQ-5D, and Marks AQoL scores were not significantly different between subjects with concomitant asthma and rhinitis compared to those with asthma alone. Subjects with rhinitis only, however, had higher SF-12 PCS, and EQ-5D scores (better general health status) than subjects with both asthma and rhinitis or those with asthma alone.
Internal consistency
Cronbach’s alpha for the Rhinasthma-28 and its 5 domains identified by cluster analysis are shown in Table 4. Internal consistency of the entire 28-item instrument was very high (α=0.94) for all subjects, as well as within each of the 3 diagnostic groups (α=0.93 to 0.96). Internal consistency was also high within each of the individual domains. Internal consistency of the individual domains remained strong within each of the 3 diagnostic groups being the lowest for the nasal and treatment burden domains among subjects with asthma alone (α=0.78 and 0.77, respectively) and for the respiratory domain among subjects with rhinitis alone (α=0.73).
Table 4.
Internal consistency (Cronbach’s α) of the Rhinasthma-28 and individual subscales.
| All subjects (n=521) | Asthma + Rhinitis (n=431) | Asthma only (n=70) | Rhinitis only (n=20) | |
|---|---|---|---|---|
| Rhinasthma-28 | ||||
| Total (28 items) | 0.94 | 0.94 | 0.93 | 0.96 |
| Nasal (5 items) | 0.83 | 0.81 | 0.78 | 0.85 |
| Eye (4 items) | 0.87 | 0.86 | 0.89 | 0.92 |
| Respiratory (5 items) | 0.91 | 0.90 | 0.92 | 0.73 |
| Activity restriction (9 items) | 0.90 | 0.90 | 0.91 | 0.94 |
| Treatment burden (5 items) | 0.83 | 0.83 | 0.77 | 0.84 |
Convergent validity
Correlations of the Rhinasthma-28 with other validated health status measures are shown in Table 5. Rhinasthma-28 scores correlated well with both the SF-12 PCS and EQ-5D in the expected direction. Correlations were robust across the 3 diagnostic groups, but were slightly stronger for subjects with asthma (with or without concomitant rhinitis) than for those with rhinitis alone. Among subjects with asthma, the Rhinasthma-28 correlated strongly with the asthma-specific HRQL Marks AQoL, as well as with disease severity quantified by the SAS.
Table 5.
Correlation of the Rhinasthma-28 with other health status measures.
| All subjects (n=545) | Asthma + Rhinitis (n=450) | Asthma only (n=75) | Rhinitis only (n=20) | |
|---|---|---|---|---|
| SF-12 PCS | −0.56 | −0.54 | −0.67 | −0.45 |
| EQ-5D utility index | −0.52 | −0.51 | −0.60 | −0.41† |
| Marks Asthma QoL | 0.78* | 0.77 | 0.93 | NA |
| Severity of Asthma Score | 0.50* | 0.52 | 0.52 | NA |
excluding 20 subjects with rhinitis only
p=0.07; all others p values <0.05.
Spearman correlation coefficients reported.
Higher scores for SF-12 and EQ-5D represent better health status.
Higher scores for RhinAsthma-28, Mark’s Asthma Quality of Life, and Severity-of-Asthma Score represent worse health status.
The domains of the Rhinasthma-28 also converged with conceptually related measures in the expected direction (data not in table). The SF-12 PCS, a generic measure of physical health status, correlated most strongly with the activity restriction domain of the Rhinasthma-28 (r=−0.62, p<0.001, n=545). Among 525 subjects with asthma, the breathlessness component of the Marks AQoL, a measure of asthma-specific HRQL, correlated most strongly with the respiratory domain of the Rhinasthma-28 (r=0.84, p<0.001; 5 subjects with missing data). Moreover, the medication component of the SAS, reflecting number of asthma medications used, converged well with the respiratory (r=0.56, p<0.001) and treatment burden domains of the Rhinasthma-28 (r=0.51, p<0.001) in the anticipated fashion.
Logistic regression analysis
Results of multiple logistic regression are shown in Table 6. The Rhinasthma-28 was strongly associated with increased odds of asthma- and of rhinitis-related disability, even after taking into account physical health status as measured by the SF-12 PCS. In contrast, the SF-12 PCS was strongly associated with disability due to any health problem, but was a weaker risk factor for asthma-related disability and was not associated with rhinitis-related disability. Models adjusted for age, sex, race, and education (data not provided) yielded comparable results.
Table 6.
Rhinasthma-28 and SF-12 PCS as independent predictors of disability.
| Disability due to* |
|||
|---|---|---|---|
| Any health problem | Asthma | Rhinitis | |
| SF-12 PCS | 3.30 (2.50–4.34) | 1.96 (1.51–2.53) | 0.92 (0.69–1.23) |
| Rhinasthma-28 | 2.68 (2.00–3.58) | 3.94 (2.92–5.33) | 3.45 (2.54–4.70) |
Defined as 1 or more days of restricted activity during the past month
Odds ratios (95% confidence interval) reported per 1 standard deviation worsening in score.
Multiple logistic regression models include SF-12 PCS and Rhinasthma-28 simultaneously.
DISCUSSION
Although other measures have been developed for assessing HRQL in either asthma or rhinitis, the Rhinasthma is the only combined instrument designed to assess HRQL where both conditions may coexist.(17)(18, 19) In this study, we thoroughly and systematically assessed the psychometric performance of an English-language version of the Rhinasthma within a large, well-described population with mixed upper and lower airway disease. Our results suggest that this version of the Rhinasthma was psychometrically sound and a valid measure of the construct. First, employing a variable cluster analysis approach we identified 5 clinically relevant domains into which 28 of the 31 original Rhinasthma items fell. In particular, our analysis resolves overlapping constructs associated with the 3 factor solution originally reported.(17) Second, the refined Rhinasthma-28 performed in the anticipated manner for the discrete condition groups of asthma plus rhinitis and for either condition alone. Third, we found high internal consistency and good convergence with measures of general health status, asthma-specific HRQL, and asthma severity. Finally, the Rhinasthma-28 was independently associated with disability over and above the explanatory power of general health status measured by the SF-12 PCS. Taken together, these attributes demonstrate the potential value of using the Rhinasthma-28 when studying HRQL outcomes in asthma and rhinitis.
Despite the potential utility of the Rhinasthma, its construct validity and performance characteristics have not previously been studied in depth. The instrument was originally developed in Italy with data from 148 subjects (49 with concomitant asthma and rhinitis). In that study, its construct validity was assessed by an exploratory factor analysis and a focused comparison with the SF-36.(17) To date, only 2 other studies have published data employing the Rhinasthma. In the first of those studies, the questionnaire was adapted for use in German-speaking subjects, and its validity assessed among 85 patients with allergic rhinitis and/or asthma. In the second study, the Rhinasthma was translated into Finnish and Swedish and used to assess the burden of illness in 119 patients with occupational rhinitis (but without asthma). The SF-36 was also co-administered in that second study, but no direct comparisons between the instruments were reported.(18, 19) To our knowledge, a study validating an English language adaptation of the Rhinasthma has never been reported.
As a disease-specific instrument that covers a broad range of allergic airway symptoms, the Rhinasthma possesses key advantages over other types of measures. Generic measures, such as the SF-12 PCS or the EQ5-D, may be less sensitive to changes in health status driven by disease-specific phenomena that may result in significant HRQL impairment without necessarily impacting overall mobility or physical functioning. The Rhinasthma also has advantages over more highly disease-specific instruments, such as those that focus solely on either asthma or rhinitis alone. For example, treatment of rhinitis may have the secondary benefit of improving a patient’s asthma control.(34) Conversely, therapies developed for asthma, such as montelukast, have been shown to be efficacious for allergic rhinitis.(35) Thus, use of a narrowly-focused measure may underestimate the actual benefit of a therapeutic intervention. A major advantage of using a combined asthma-rhinitis HRQL instrument, such as the Rhinasthma, is its ability to capture a broader range of impacts of allergic airway disease using a single measure, while still allowing for the determination of domain-specific effects (e.g., nasal and eye versus respiratory, as observed in our analysis).
Although this study cohort represents the largest in which the Rhinasthma has been administered, certain limitations should be considered in the interpretation of our data. Most notably, the majority of subjects in our analysis had both asthma and rhinitis. Only 20 subjects had rhinitis alone, making it difficult to draw definitive conclusions regarding this particular subgroup. Furthermore, subjects with rhinitis alone had higher income and less unemployment, covariates that may have contributed in part to some of the differences observed. With these considerations in mind, we stratified each of our analyses by diagnostic group, in addition to conducting analyses on the cohort as a whole. In most cases, the performance of the adapted Rhinasthma-28 appeared to be robust; however, further validation may be required among persons with either asthma or rhinitis alone. In our analyses, we used a variation of principal components analysis, called variable cluster analysis, to identify domains with discrete, non-overlapping item content. It is possible that we would have found an alternative statistical solution by utilizing other factor rotation methods. Our approach, however, yielded results that demonstrated good face validity and are intuitively appealing, considerations that are central—albeit often underappreciated—in the conceptual development of any questionnaire-based instrument. Finally, the subjects for this analysis were originally recruited as part of separate study cohorts. Differences in recruitment methods and the exclusive recruitment of subjects from the northern California region may affect the generalizability of our results.
In summary, our English language Rhinasthma-28 performs well in assessing HRQL in a large population with mixed airway disease. The Rhinasthma-28 is particularly well-suited instrument for studying patients with allergic airway disease that could potentially manifest as either asthma- or rhinitis-related HRQL impairment, or for whom diagnostic heterogeneity may exist.
Acknowledgments
Funding source: Funding provided by NIH grant R01-ES10906 and NIH grant K23-HL86585 (H.C.)
Footnotes
Author contribution: All 8 authors contributed substantially to the analysis of data, interpretation of results, writing of the manuscript, and critical revisions. The authors certify that 100% of the manuscript was collectively written by the authors listed (e.g. no ghost writers/authors, no honorary authorship). Data analysis was personally performed by H.C. and M.C. First draft of the manuscript was written by H.C. Conception and design of study was conceived by P.B.
Copyright transfer: This data has not been previously published and is not under review at another journal.
Conflicts of interest: None of the authors have any conflicts of interests to report. This study and its authors are funded by the NIH. None of the authors listed are government employees.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leynaert B, Neukirch F, Demoly P, Bousquet J. Epidemiologic evidence for asthma and rhinitis comorbidity. J Allergy Clin Immunol. 2000 Nov;106(5 Suppl):S201–5. doi: 10.1067/mai.2000.110151. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen PA, Weeke ER. Asthma and allergic rhinitis in the same patients. Allergy. 1983 Jan;38(1):25–9. doi: 10.1111/j.1398-9995.1983.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 3.Greisner WA, 3rd, Settipane RJ, Settipane GA. Co-existence of asthma and allergic rhinitis: a 23-year follow-up study of college students. Allergy Asthma Proc. 1998 Jul–Aug;19(4):185–8. doi: 10.2500/108854198778557836. [DOI] [PubMed] [Google Scholar]
- 4.Linneberg A, Henrik Nielsen N, Frolund L, Madsen F, Dirksen A, Jorgensen T. The link between allergic rhinitis and allergic asthma: a prospective population-based study. The Copenhagen Allergy Study. Allergy. 2002 Nov;57(11):1048–52. doi: 10.1034/j.1398-9995.2002.23664.x. [DOI] [PubMed] [Google Scholar]
- 5.Settipane RJ, Hagy GW, Settipane GA. Long-term risk factors for developing asthma and allergic rhinitis: a 23-year follow-up study of college students. Allergy Proc. 1994 Jan–Feb;15(1):21–5. doi: 10.2500/108854194778816634. [DOI] [PubMed] [Google Scholar]
- 6.Guerra S, Sherrill DL, Martinez FD, Barbee RA. Rhinitis as an independent risk factor for adult-onset asthma. J Allergy Clin Immunol. 2002 Mar;109(3):419–25. doi: 10.1067/mai.2002.121701. [DOI] [PubMed] [Google Scholar]
- 7.Leynaert B, Bousquet J, Neukirch C, Liard R, Neukirch F. Perennial rhinitis: An independent risk factor for asthma in nonatopic subjects: results from the European Community Respiratory Health Survey. J Allergy Clin Immunol. 1999 Aug;104(2 Pt 1):301–4. doi: 10.1016/s0091-6749(99)70370-2. [DOI] [PubMed] [Google Scholar]
- 8.Togias A. Systemic cross-talk between the lung and the nose. Am J Respir Crit Care Med. 2001 Sep 1;164(5):726–7. doi: 10.1164/ajrccm.164.5.2106122b. [DOI] [PubMed] [Google Scholar]
- 9.Gaga M, Lambrou P, Papageorgiou N, Koulouris NG, Kosmas E, Fragakis S, et al. Eosinophils are a feature of upper and lower airway pathology in non-atopic asthma, irrespective of the presence of rhinitis. Clin Exp Allergy. 2000 May;30(5):663–9. doi: 10.1046/j.1365-2222.2000.00804.x. [DOI] [PubMed] [Google Scholar]
- 10.Azzawi M, Bradley B, Jeffery PK, Frew AJ, Wardlaw AJ, Knowles G, et al. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1407–13. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- 11.Djukanovic R, Lai CK, Wilson JW, Britten KM, Wilson SJ, Roche WR, et al. Bronchial mucosal manifestations of atopy: a comparison of markers of inflammation between atopic asthmatics, atopic nonasthmatics and healthy controls. Eur Respir J. 1992 May;5(5):538–44. [PubMed] [Google Scholar]
- 12.Leynaert B, Neukirch C, Liard R, Bousquet J, Neukirch F. Quality of life in allergic rhinitis and asthma. A population-based study of young adults. Am J Respir Crit Care Med. 2000 Oct;162(4 Pt 1):1391–6. doi: 10.1164/ajrccm.162.4.9912033. [DOI] [PubMed] [Google Scholar]
- 13.Leynaert B, Soussan D. Monitoring the quality-of-life in allergic disorders. Curr Opin Allergy Clin Immunol. 2003 Jun;3(3):177–83. doi: 10.1097/00130832-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Hartge P, Cahill J. Field methods in epidemiology: questionnaires and respondent burden. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3. Lippincott Williams & Wilkins; 2008. pp. 503–4. [Google Scholar]
- 15.Ulrich CM, Wallen GR, Feister A, Grady C. Respondent burden in clinical research: when are we asking too much of subjects? IRB. 2005 Jul–Aug;27(4):17–20. [PubMed] [Google Scholar]
- 16.Sharp LM, Frankel J. Respondent Burden: A Test of Some Common Assumptions. Public Opin Q. 1983;47(1):36–53. [Google Scholar]
- 17.Baiardini I, Pasquali M, Giardini A, Specchia C, Passalacqua G, Venturi S, et al. Rhinasthma: a new specific QoL questionnaire for patients with rhinitis and asthma. Allergy. 2003 Apr;58(4):289–94. doi: 10.1034/j.1398-9995.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 18.Airaksinen LK, Luukkonen RA, Lindstrom I, Lauerma AI, Toskala EM. Long-term exposure and health-related quality of life among patients with occupational rhinitis. J Occup Environ Med. 2009 Nov;51(11):1288–97. doi: 10.1097/JOM.0b013e3181b9b242. [DOI] [PubMed] [Google Scholar]
- 19.Mosges R, Schmalz P, Koberlein J, Kaciran M, Baiardini I. The RHINASTHMA-Quality of Life Scale German Adapted Version: validation of a new disease specific quality of life scale for patients suffering from allergic rhinitis and bronchial hyperreactivity. HNO. 2007 May;55(5):357–64. doi: 10.1007/s00106-006-1453-0. [DOI] [PubMed] [Google Scholar]
- 20.Blanc PD, Cisternas M, Smith S, Yelin EH. Asthma, employment status, and disability among adults treated by pulmonary and allergy specialists. Chest. 1996 Mar;109(3):688–96. doi: 10.1378/chest.109.3.688. [DOI] [PubMed] [Google Scholar]
- 21.Blanc PD, Katz PP, Henke J, Smith S, Yelin EH. Pulmonary and allergy subspecialty care in adults with asthma. Treatment, use of services, and health outcomes. West J Med. 1997 Dec;167(6):398–407. [PMC free article] [PubMed] [Google Scholar]
- 22.Eisner MD, Katz PP, Yelin EH, Henke J, Smith S, Blanc PD. Assessment of asthma severity in adults with asthma treated by family practitioners, allergists, and pulmonologists. Med Care. 1998 Nov;36(11):1567–77. doi: 10.1097/00005650-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Blanc PD, Trupin L, Eisner M, Earnest G, Katz PP, Israel L, et al. The work impact of asthma and rhinitis: findings from a population-based survey. J Clin Epidemiol. 2001 Jun;54(6):610–8. doi: 10.1016/s0895-4356(00)00349-8. [DOI] [PubMed] [Google Scholar]
- 24.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. Jama. 2002 May 15;287(19):2519–27. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 25.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992 May;82(5):703–10. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisner MD, Klein J, Hammond SK, Koren G, Lactao G, Iribarren C. Directly measured second hand smoke exposure and asthma health outcomes. Thorax. 2005 Oct;60(10):814–21. doi: 10.1136/thx.2004.037283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erickson S, Tolstykh I, Selby JV, Mendoza G, Iribarren C, Eisner MD. The impact of allergy and pulmonary specialist care on emergency asthma utilization in a large managed care organization. Health Serv Res. 2005 Oct;40(5 Pt 1):1443–65. doi: 10.1111/j.1475-6773.2005.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marks GB, Dunn SM, Woolcock AJ. A scale for the measurement of quality of life in adults with asthma. J Clin Epidemiol. 1992 May;45(5):461–72. doi: 10.1016/0895-4356(92)90095-5. [DOI] [PubMed] [Google Scholar]
- 29.Blanc PD, Cisternas M, Smith S, Yelin EH. Asthma, employment status, and disability among adults treated by pulmonary and allergy specialists. Chest. 1996 Mar;109(3):688–96. doi: 10.1378/chest.109.3.688. Erratum 2000;118:564. [DOI] [PubMed] [Google Scholar]
- 30.Blanc PD, Jones M, Besson C, Katz P, Yelin E. Work disability among adults with asthma. Chest. 1993 Nov;104(5):1371–7. doi: 10.1378/chest.104.5.1371. [DOI] [PubMed] [Google Scholar]
- 31.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996 Mar;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997 Nov;35(11):1095–108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Pasta DJ, Suhr D. SUGI 29 Proceedings. 2004. Creating Scales from Questionnaires: PROC VARCLUS vs. Factor Analysis; pp. 1–18. Paper 205–29. [Google Scholar]
- 34.Nelson HS. Prospects for antihistamines in the treatment of asthma. J Allergy Clin Immunol. 2003 Oct;112(4 Suppl):S96–100. doi: 10.1016/s0091-6749(03)01883-9. [DOI] [PubMed] [Google Scholar]
- 35.Meltzer EO. Clinical evidence for antileukotriene therapy in the management of allergic rhinitis. Ann Allergy Asthma Immunol. 2002 Apr;88(4 Suppl 1):23–9. doi: 10.1016/s1081-1206(10)62025-x. [DOI] [PubMed] [Google Scholar]