Abstract
As a social species, humans have a fundamental need to belong that encourages behaviors consistent with being a good group member. Being a good group member requires the capacity for self-regulation, which allows people to alter or inhibit behaviors that would place them at risk for group exclusion. Self-regulation requires four psychological components. First, people need to be aware of their behavior so as to gauge it against societal norms. Second, people need to understand how others are reacting to their behavior so as to predict how others will respond to them. This necessitates a third mechanism, which detects threat, especially in complex social situations. Finally, there needs to be a mechanism for resolving discrepancies between self-knowledge and social expectations or norms, thereby motivating behavior to resolve any conflict that exists. This article reviews recent social neuroscience research on the psychological components that support the human capacity for self-regulation.
Keywords: self-awareness, theory of mind, need to belong, social neuroscience, neuroimaging, addiction
Introduction
Many of the adaptive challenges facing our earliest ancestors were social in nature, such as differentiating friends from foes, identifying and evaluating potential mates, understanding the nature and structure of group relations, and so on. Those ancestors who were able to solve survival problems and adapt to their social environments were most likely to reproduce and pass along their genes. As such, humans have evolved a fundamental need to belong that encourages behaviors consistent with being a good group member (Baumeister & Leary 1995). Belonging to a good group had considerable value, including access to shared resources, security from various threats, and even assistance with daily chores. Hence, the human brain has adapted within a complex social environment and is likely to have evolved dedicated neural mechanisms that are acutely sensitive to social context, especially for any signs that group membership is imperiled (Heatherton & Wheatley 2010, Mitchell & Heatherton 2009).
The Need for Inhibition
Being a good group member is not always easy, however. There is an inherent conflict between what is enjoyable for the individual and what is best for the group. From an individual perspective, basic motivational reward processes encourage behaviors that bring pleasure. Left to our own devices and without fear of social evaluation, we might indulge our appetites without restraint: eat as much fattening tasty food as our stomachs can hold, ingest chemical substances that activate dopamine receptors, and generally follow the hedonistic rule of doing whatever feels good. But eating more than a fair share of food or otherwise monopolizing group resources comes with a cost to other group members and thus can threaten our status in the group. Inhibitions are therefore important for harmonious social relations, and evolution has undoubtedly favored those who could control undesirable impulses.
Inhibition is a core feature of self-regulation, which refers to the process by which people initiate, adjust, interrupt, stop, or otherwise change thoughts, feelings, or actions in order to effect realization of personal goals or plans or to maintain current standards (Baumeister et al. 1994a, Baumeister & Heatherton 1996, Carver & Scheier 1998). At the broadest level, self-regulation refers to intentional or purposeful acts that are directed from within the person (Bandura 1989). From this perspective, learning, physiology, and culture predispose certain behaviors, thoughts, or emotions in specific circumstances, but self-regulation allows people to change or overcome them. Although all humans have an impressive capacity for self-regulation, failures are common, and people lose control of their behavior in a wide variety of circumstances (Baumeister & Heatherton 1996, Baumeister et al. 1994a). Such failures are an important cause of several contemporary societal problems—obesity, sexual predation, addiction, and sexual infidelity, to name but a few. That even revered figures, including Catholic priests, celebrity/sports role models, and respected political leaders, have been publicly castigated for their spectacular failures of self-control is testament to the difficulties inherent in trying to control the self. This article discusses the neural bases of fundamental components of the social brain, focusing on how having a “self” serves the basic social skills necessary for maintaining effective relations with group members.
There are, of course, other important features of self-regulation, such as initiating self-regulatory efforts in order to achieve personal goals (Shah 2005). For example, Higgins (1997) distinguished self-regulatory efforts aimed at achieving desirable outcomes from those aimed at avoiding undesirable outcomes. Promotion goals are those in which people approach ideal goals with aspiration and a sense of accomplishment, focusing on potential gains. By contrast, prevention goals are those in which people try to avoid losses by playing it safe or doing what they ought to do. This framework has proven useful for understanding a great deal of social behavior, from how people behave in intergroup contexts (Shah et al. 2004) to how they respond to awkward interracial interactions (Trawalter & Richeson 2006). Although understanding how people initiate behavior to attain personal goals is clearly important for many aspects of human behavior, particularly health behavior (Bandura 1991, Carver & Scheier 1998, Rothman et al. 2004), there is not yet a substantial body of neuroscience research addressing this aspect of self-regulation (for exceptions, see Cunningham et al. 2005, Eddington et al. 2007). Accordingly, much of the focus of this article is on regulation and control of ongoing psychological activity.
Components of the Social Brain
Controlling oneself to be a good group member involves an awareness of how one is thinking, feeling, or behaving and the ability to alter any of these to satisfy the standards or expectations of the group. This implies the need for at least four psychological components, the failure of any of which can lead to poor outcomes and censure from the group (Heatherton 2010, Krendl & Heatherton 2009, Mitchell & Heatherton 2009, Wagner & Heatherton 2010b).
Self-Awareness
First, people need self-awareness to reflect on their behaviors, including their emotional displays, so as to judge them against group norms. An empirical understanding of the self has a long history in psychology (see Baumeister 1998), dating back to William James' important distinction between the self as the knower (“I”) and the self as the object that is known (“me”). In the sense of the knower, the self is the subject doing the thinking, feeling, and acting. In the sense of the objectified self, the self consists of the knowledge that people hold about themselves, as when they contemplate their best and worst qualities. The experience of self as the object of attention is the psychological state known as self-awareness, which encourages people to reflect on their actions and understand the extent to which those actions match both personal values and beliefs as well as group standards (Carver & Scheier 1981, Duval & Wicklund 1972). Whether certain aspects of the self, such as self-serving biases and motivations, truly are adaptive is open to some debate (Leary 2004), although there is considerable evidence that a symbolically representational self provided considerable advantages to humans over the course of evolution, such as facilitating communication and cooperation with group members (Sedikides & Skowronski 1997).
Mentalizing
Understanding that violating social norms is problematic requires people to appreciate that they are the objects of social evaluation, which in turn necessitates knowing that others are capable of making such evaluations. That is, people need the ability to infer the mental states of others to predict their actions, a skill referred to as mentalizing or having “theory of mind” (Amodio & Frith 2006, Gallagher & Frith 2003, Mitchell 2006). Mentalizing allows people to be aware that other people have thoughts and also attempt to understand the content of those thoughts. Ultimately, this allows people to empathize with observers to be able to predict their judgments or behaviors.
Threat Detection
The ability to mentalize is crucial for the third mechanism, threat detection, which monitors the environment for any cues or other evidence of possible group exclusion. If humans have a fundamental need to belong, then there needs to be a mechanism for detecting inclusionary status (Leary et al. 1995, Macdonald & Leary 2005). Indeed, feeling socially anxious or worrying about potential rejection should lead to heightened social sensitivity, and research has demonstrated that people who worry most about social evaluation (i.e., the shy and lonely) show enhanced memory for social information, are more empathetically accurate, and show heightened abilities to decode social information (Gardner et al. 2000, 2005; Pickett et al. 2004).
Self-Regulation
Once people are aware that their actions have violated group standards and that others are evaluating them negatively (i.e., threat has been detected), they need the ability to rectify the situation to re-establish good relations with other group members. Doing so requires the executive aspects of the self (the “I” as knower) that allow people to change according to social context, including altering their thoughts, actions, and emotions. Thus, people need to inhibit their impulses, stifle their desires, resist temptations, undertake difficult or unpleasant activities, banish unwanted and intrusive thoughts, and control their emotional displays, all of which are difficult to do but are necessary for staying in the good graces of others (Heatherton & Vohs 1998). Of course, people also need to regulate behavior proactively, such as avoiding appearing prejudiced or making a good impression. As mentioned, people also self-regulate in order to promote positive goals (Higgins 1997). Thus, people initiate diets in order to lose weight, and they save money to allow themselves to live more prosperously in the future. Self-regulation involves both the initiation and maintenance of behavioral change in addition to inhibiting undesired behaviors or responding to situational demands.
A Social Neuroscience Approach
From a neuroscience perspective, it is likely that the brain has evolved distinct mechanisms for knowing ourselves, knowing how others respond to us, detecting threats from within the social group, and regulating actions in order to avoid being excluded from those groups (Krendl & Heatherton 2009). Within social psychology, efforts to understand bodily involvement in social phenomena also have a long history, from the use of skin-conductance measures to indicate whether experimental conditions produce arousal (e.g., Lanzetta & Kleck 1970), to the assessment of activity in facial muscles to identify emotional expression (e.g., Cacioppo & Petty 1981), to patient studies that examine the effects of brain injury on social behavior and personality (Klein & Kihlstrom 1998). More recently, there has been enthusiasm for using brain-imaging techniques that allow researchers to watch the working mind in action (Adolphs 2009, Lieberman 2009, Macrae et al. 2004a, Ochsner 2007, Ochsner & Lieberman 2001). The advent of imaging has led to an explosion of research on social neuroscience, and several recent literature reviews have appeared (Amodio & Frith 2006, Cacioppo et al. 2007, Heatherton & Wheatley 2010, Lieberman 2009, Mitchell & Heatherton 2009, Ochsner 2007) as well as methodological critiques raising concerns about the value of imaging for elucidating psychological processes (Adolphs 2010, Cacioppo et al. 2003, Vul et al. 2009). The remainder of this article examines the contributions of a neuroscience approach to understanding the components of the social brain, focusing mainly on studies of self-awareness/knowledge and self-regulation (Figure 1, see color insert).
Figure 1.
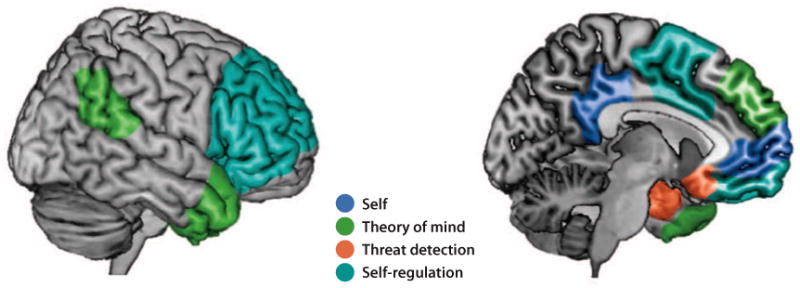
The components of the social brain. Brain regions that are commonly activated for studies of self, theory of mind, threat detection, and self-regulation.
Self-Awareness and Self-Knowledge
Humans possess an impressive degree of self-awareness. Not only are we able to identify ourselves as distinct from others, but we are able to think critically about what makes us unique and develop a sense of self that includes our background and superficially distinguishing characteristics such as name, hometown, and occupation as well as an even deeper sense of “who we are,” including personality traits, our core beliefs and attitudes, what we like and don't like about ourselves, and therefore what we might like to change. The remarkable extent of our self-awareness can be a mixed blessing; too much self-directed thinking can be maladaptive (Leary 2004) and is associated with depressive disorders (Ingram 1990) and the tendency to ruminate over negative events (Donaldson et al. 2007, Joormann 2006, Siegle et al. 2002). Without such capacities for self-recognition and self-knowledge, however, the social world as we know it could not exist.
Is Self Special?
The centrality of the self-concept to social functioning gives rise to the question of whether the self is somehow “special” as a cognitive structure or whether information about the self is processed in the same way as everything else is processed, an issue that engendered considerable debate among social and cognitive psychologists in the late 1970s into the 1980s (Bower & Gilligan 1979, Greenwald & Banaji 1989, Klein & Kihlstrom 1986, Maki & McCaul 1985, Rogers et al. 1977). As discussed by Higgins & Bargh (1987), the gist of the debate was whether the superior memory performance that resulted from encoding information with reference to self was due to a unique cognitive structure (i.e., self) or whether it obtained from standard psychological mechanisms that would apply to any memory context. Macrae et al. (2004a) noted that a frustrating feature of this debate was that all theories made the same behavioral prediction (e.g., superior memory for material encoded with reference to self), and therefore the scientific question was difficult to resolve (see also Gillihan & Farah 2005). One line of support for the idea that memory for self is somehow special can be found in studies of patients with conditions such as Alzheimer's disease and severe amnesia. Although these patients' conditions profoundly affect their ability to recall various important details of their lives, they can often accurately report whether particular trait adjectives describe them (Klein 2004), suggesting that one's sense of self is not easily extinguished.
With the advent of neuroimaging, scientists had new methods to address longstanding questions, such as whether the self was somehow special as a memory structure. Beginning with studies using positron emission tomography (PET) (Craik et al. 1999) and functional magnetic resonance imaging (fMRI) (Kelley et al. 2002), numerous subsequent studies have examined brain regions that are involved in processing information about self compared to those associated with processing semantic information more generally or processing information about other people, with the vast majority finding heightened activity in medial prefrontal cortex (MPFC), posterior cingulate cortex, and precuneus (for reviews, see Heatherton et al. 2007, Moran et al. 2010, Northoff et al. 2006). An important study by Macrae and colleagues (2004b) demonstrated that activity in MPFC predicted subsequent memory for information processed with reference to self, thereby establishing the role of MPFC in self-referential memory enhancement.
Studies using other tasks to examine different aspects of self have revealed similar patterns of brain activity. Heightened MPFC activity has also been observed when subjects engage in free-form refection on their selves as compared to when they engage in free-form reflection of another individual (D'Argembeau et al. 2005, Farb et al. 2007, Johnson et al. 2006, Kjaer et al. 2002) and when they are instructed to attend to their personal preferences relative to nonreflective control tasks (Goldberg et al. 2006, Gusnard et al. 2001, Johnson et al. 2005, Ochsner et al. 2004). Cabeza et al. (2004) found heightened MPFC activation for episodic memory retrieval of autobiographical events. In their study, participants were presented with photographs that either they had taken around campus or that someone else had taken. The participants showed heightened MPFC activity for photographs they themselves had taken. Even the passive viewing of self-relevant words (such as one's name or street address) during an unrelated task results in heightened MPFC activity (Moran et al. 2009). Other findings indicate that MPFC activity may be part of the default neural network engaged during free-form thinking in the absence of an explicit task (D'Argembeau et al. 2005, Gusnard et al. 2001, Wicker et al. 2003), suggesting that the mind spontaneously turns to the self when allowed to wander (Mason et al. 2007). Indeed, a heightened level of MPFC activity has been linked to trait self-consciousness, which is the degree to which people are generally aware of their behavior (Eisenberger et al. 2005). Finally, mindfulness meditation practices, aimed at disciplining one's stream-of-consciousness type of musings by effectively reducing explicit self-related thoughts in exchange for an overall basic sense of self-awareness, have been shown to decrease MPFC activity (Farb et al. 2007).
Studies of patients with brain injury provide additional evidence for the importance of prefrontal areas such as the MPFC to self-awareness and self-knowledge. Patients with frontal lobe lesions show significant impairment in their ability to engage in self-reflection and introspection (Beer et al. 2003, Stuss & Benson 1986, Wheeler et al. 1997). Patients with MPFC lesions specifically have shown deficiencies in their ability to recall personal preferences, with their answers to questions soliciting their attitudes on various stimuli varying widely between sessions (Fellows & Farah 2007).
Social and Cultural Context
The ubiquity of the MPFC findings for any task that involves the self has provided researchers with opportunities to test various psychological theories related to the self. For instance, some theories suggest an intimate other may become incorporated into one's self-concept (Aron & Aron 1996). If this theory is correct, one might expect to see that same MPFC activation when individuals reflect on these intimate others as when they reflect on their self. Unfortunately, attempts to test this hypothesis using neuroimaging have yielded mixed results: Some studies have reported MPFC activation for intimate others as well as the self (Ochsner et al. 2005, Schmitz et al. 2004, Seger et al. 2004), and others have found such activity for the self only (Heatherton et al. 2006). It is possible that methodological issues lie at the heart of these disparate findings, as the studies used different targets and imaging designs, but for now, more research is needed to resolve this issue.
A new twist on this idea is reflected in the cultural psychology notion that whereas individualist, Western cultures construe the self as a unique identity considerably independent from others, collectivist, Eastern cultures construe a self that is fluid, contextual, and defined in a large part by its relations to others (Markus & Kitayama 1991). To investigate whether such a difference in self-construal is also observed on the neural level, Zhu and colleagues (2007) asked Chinese and Western participants questions about themselves and their mothers while using fMRI. Whereas Chinese participants showed heightened levels of MPFC activation while reflecting on both themselves and their mothers, Western subjects showed heightened activity only when thinking about themselves. Likewise, Zhang et al. (2006) showed across two experiments that when Chinese participants reflect on themselves relative to another, MPFC is more engaged for self when the other is not close, but it is equally engaged for self and mother. In another study, Chiao and colleagues (2009b) found that activity in MPFC in response to self-relevance judgments of traits in both general and specific contexts predicted the extent to which subjects endorsed individualist or collectivist values, respectively. Similarly, bicultural participants, whose backgrounds reflected both collectivist and individualist values, showed heightened MPFC activation toward general trait judgments relative to contextual judgments when primed with individualist values, and participants showed the opposite pattern when primed with collectivist values (Chiao et al. 2009a). These studies provide converging evidence to suggest that culture can have an impact on how the self is construed on a neural level.
Age-Related Changes
Because of age-related structural changes in MPFC, one might expect that self-referential processing would also change with age. Adolescence has long been known to be associated with heightened self-focus (Enright et al. 1980). Therefore, it is not surprising that Pfeifer et al. (2007) found greater MPFC activity for children than for adults when contrasting ratings for self with ratings for a well-known fictional character (i.e., Harry Potter). Likewise, in line with the theory that adolescence is marked by a heightened preoccupation with others' opinions about oneself and that these perceived opinions help inform the adolescent's self-concept (Harter 1999, Harter et al. 1998), Pfeifer and colleagues (2009) found that, relative to mature adults, adolescents engage brain areas related to social cognition (see Theory of Mind section below for a description of these regions) during self-reflection in addition to MPFC and posterior cingulate cortex. Replicating their prior finding (Pfeifer et al. 2007), brain activity was once again greater in self-relevant regions for adolescents than for adults. By contrast, although aging in later adulthood is associated with a number of changes in memory processes, it appears that the self-referent enhancement of memory remains intact and that there is a similar pattern of MPFC activity associated with this effect for younger and older adults (Glisky & Marquine 2009, Gutchess et al. 2007, Mueller et al. 1986).
The Affective Self and Psychopathology
Another important psychological process relevant to self is emotion. One critical aspect of the sense of self is that it produces affect—evaluations of the self inevitably lead to emotional reactions that influence subsequent thoughts and actions. But, focusing too much on the self can be associated with psychopathology, as with the tendencies of depressed patients to ruminate about negative self-relevant information and make negative attributions to themselves (Grunebaum et al. 2005, Ingram 1990, Northoff 2007, Rimes & Watkins 2005). Recent imaging studies have identified abnormalities in many cortical and subcortical midline structures associated with depression (Grimm et al. 2009, Lemogne et al. 2009). For example, Johnson and colleagues (2009) observed that depressed individuals showed sustained activity in areas involved in self-reflection during nonreflective distraction tasks as opposed to controls, suggesting a relative difficulty in disengaging from self-reflective processes. Moran and colleagues (2006) found that whereas MPFC was responsive to the personal relevance of information (i.e., whether the trait is self-descriptive or not), an adjacent region, the ventral anterior cingulate cortex (vACC, sometimes referred to as subgenual anterior cingulate), was responsive to the emotional valence of this material but only for traits that were judged to be self-descriptive. This suggests that these adjacent prefrontal regions subserve cognitive and emotional aspects of self-reflection, respectively. Specifically, activity in vACC is attenuated when unfavorable information is considered self-descriptive. This finding dovetails nicely with research showing that vACC is implicated in emotional disorders such as depression and posttraumatic stress disorder (Drevets et al. 1997). For instance, researchers have observed differential activation of vACC to emotional facial expressions between depressed and control participants (Gotlib et al. 2005). Research on vACC has promising translational value. In a particularly striking study, Mayberg and colleagues (2005) demonstrated that deep brain stimulation in vACC was effective in alleviating depression in treatment-resistant patients.
There have also been recent attempts to examine the neural basis of self-referential processing among those with other mental health disorders. Studies performed on patients with schizophrenia or other psychoses indicate dysfunctional MPFC activity among such populations (Paradiso et al. 2003, Taylor et al. 2007, Williams et al. 2004), such as hypoactivity in MPFC during explicit self-referential tasks (Blackwood et al. 2004). Disturbances to one's sense of self observed as a result of such disorders are manifested in a number of ways, including an impairment in self-insight that results in an unawareness of one's illness (Amador & David 2004, Cooney & Gazzaniga 2003) and the inability to distinguish self-generated stimuli from externally generated stimuli that is theorized to be responsible for reports of sensory disturbances and auditory hallucinations (Ditman & Kuperberg 2005, Seal et al. 2004). Because MPFC is implicated both in self-reflection and the task of differentiating endogenously and exogenously generated stimuli (Simons et al. 2006, Turner et al. 2008), it is conceivable that abnormal activity in MPFC contributes to such symptoms, though more research is necessary to better understand the link between brain activity, self-referential processing deficits, and psychopathology (Nelson et al. 2009, van der Meer et al. 2010).
Is MPFC the Self?
Although research has consistently demonstrated increased MPFC for conditions that involve some aspect of self, this is not to suggest that the MPFC reflects the physical location of the “self” or that other areas are not vital for the phenomenological experiences associated with the self. Rather, the experience of the self involves various sensory, affective, and motor processes contributed by disparate brain regions outside the cortical midline area (Turk et al. 2003). Indeed, some have argued that the most important psychological processes that produce activation of MPFC involve inferential processing, whether about the self or anything else (Legrand & Ruby 2009). More recently, Jason Mitchell (2009) proposed that any type of social cognition that involves internally generated “fuzzy” representations that are inexact and subject to revision, such as judging attitudes about self or others, or even objects in general, activates MPFC. At the same time, the preponderance of evidence indicates that the conditions most robustly producing MPFC activity typically feature extensive self-involvement (Moran et al. 2010). Given the importance of MPFC to social brain functioning, there are likely to be many more theories of its functioning as well as studies to test them.
Theory of Mind
One of the most important attributes of the social brain is the ability to infer the mental states of others in order to predict their actions (Amodio & Frith 2006, Gallagher & Frith 2003, Mitchell 2006). In addition to recognizing our own mental states, living harmoniously in social groups requires that we be able to interpret the emotional and mental states of others (Heatherton & Krendl 2009). For example, social emotions require that we be able to draw inferences about the emotional states of others (even if those inferences are inaccurate). For instance, to feel guilty about hurting a loved one, people need to understand that other people have feelings (Baumeister et al. 1994b). Similarly, interpersonal distress results from knowing that people are evaluating you (thereby giving rise to emotions such as embarrassment), which at its core means recognizing that other people make evaluative judgments. The ability to infer the mental states of others is commonly referred to as mentalizing or having the capacity for theory of mind (ToM). ToM enables individuals to empathize and cooperate with others, accurately interpret other people's behavior, and even deceive others when necessary. Neuroimaging research on mentalizing has consistently implicated a small number of regions in making inferences about the mental characteristics of other people: MPFC, temporoparietal junction (TPJ), temporal poles, and medial parietal cortex (Amodio & Frith 2006, Gallagher & Frith 2003, Mitchell 2006, Saxe 2006, Saxe et al. 2004).
Using Self as a Template
Neuroimaging research has demonstrated that the ability to mentalize relies heavily on similar neural networks engaged in processing self-relevant information, notably the MPFC. The area of greatest activity in the MPFC tends to be more dorsal in theory-of-mind studies than in self-reference studies. Sometimes overlap between ventral and dorsal MPFC is observed when perceivers are asked to infer the mental states of targets—other people—who are most similar to them (Mitchell et al. 2005). This finding suggests the possibility that mental simulation is engaged during theory-of-mind tasks, posing the question “What would I do if I were that person?” Of course, using the self to simulate others would work only if they are reasonably likely to respond in the same way in a given situation (Mitchell & Heatherton 2009). Mitchell and colleagues found support for this idea in an interesting series of neuroimaging studies. In these studies, as perceivers mentalized about the preferences and opinions of a similar other (e.g., someone who shared the same social and political attitudes), a region of ventral MPFC was engaged, which was the same region that was active when subjects considered their own preferences. In contrast, a more dorsal region of MPFC was preferentially engaged when mentalizing about dissimilar others (Jenkins et al. 2008; Mitchell et al. 2005, 2006). These results suggest that people may draw on their own knowledge about self to understand the mental states of others who are similar to them.
Mentalizing the Outgroup
To the extent that group members are likely to be perceived as more similar to the self than those from other groups, it seems likely that people will mentalize more about members of the ingroup than members of the outgroup. After all, the evaluations made of us by members of our own groups are likely to have a much greater impact on our lives than similar judgments made by those from other groups. Indeed, Harris & Fiske (2006) found reduced activity in dorsal MPFC when people made judgments about extreme outgroups, such as homeless people and drug addicts. Likewise, Freeman et al. (2010) found that individuating members of the ingroup (i.e., same race) produced activity in dorsal MPFC whereas it did not do so for members of an outgroup (i.e., different race), although Harris & Fiske (2007) found that the processing of individuating information did increase activity in dorsal MPFC for some outgroup members (e.g., drug addicts). Although research on this topic is in its infancy, understanding how people mentalize about members of ingroups and outgroups has important ramifications for understanding group relations. What is most relevant to this discussion is the idea that people are aware that others are capable of mentalizing and therefore of making judgments about them.
Detection of Threat
One value of having theory of mind is that it supports a third mechanism, which is threat detection, a process particularly useful in complex situations. A wide variety of research indicates that the amygdala plays a special role in responding to stimuli that are threatening (Feldman Barrett & Wager 2006, LeDoux 1996). Affective processing in the amygdala is a hard-wired circuit that has developed over the course of evolution to protect animals from danger. For example, much data supports the notion that the amygdala is robustly activated in response to primary biologically relevant stimuli (e.g., faces, odors, tastes) even when these stimuli remain below the subjects' level of reported awareness (e.g., Whalen et al. 1998). The role of the amygdala in processing social emotions has emerged from patient and neuroimaging research. For instance, Adolphs et al. (2002) presented facial expressions of social emotions (arrogance, guilt, admiration, flirtatiousness) to patients with amygdala damage. Patients with unilateral or bilateral amygdala damage were impaired when recognizing those specific emotions; moreover, they were more impaired at recognizing social emotions than basic emotions. Ruby & Decety (2004) conducted a PET study in which participants were asked to choose the appropriate reaction (from varying perspectives) to sentences that represented different social emotions (embarrassment, pride, shame, guilt, admiration, irritation) or nonsocial emotions and nonemotional sentences. Results revealed heightened amygdala activation during the processing of all social emotions, regardless of the perspective taken during the task. Indeed, the amygdala has been shown to robustly respond to situations in which social norms are violated (Berthoz et al. 2006).
Adaptive Social Emotions
Social emotions facilitate successful social relationships through two primary pathways: they provide incentives to engage in social interactions (e.g., affection, love, feelings of pride or admiration for those with whom we interact), and they increase the likelihood that people will adhere to societal norms that are necessary for group living. When such norms are violated, people experience negative social emotions (e.g., feelings of guilt, embarrassment, or shame) that subsequently encourage them to act within the bounds of socially acceptable conduct, thereby reducing the risk of social exclusion and promoting positive social interactions. Moreover, long-lasting social emotions (such as remembering an embarrassing moment from adolescence) reduce the likelihood of repeat violations. As might be expected, processing information about social emotions also is associated with activity in ACC and dorsal MPFC (for reviews, see Heatherton & Krendl 2009, Krendl & Heatherton 2009).
Social Rejection and Interpersonal Distress
Feeling guilty or ashamed may lead people to obsess about potential expulsion from the group. Social psychologists have documented the pernicious effects of interpersonal rejection on mood, behavior, and cognition (Smart & Leary 2009). A recent series of neuroimaging studies has examined social rejection. Most prominent is the study by Naomi Eisenberger and her colleagues (2003), who found that the dorsal region of the ACC (dACC) was responsive during a video game designed to elicit feelings of social rejection when virtual interaction partners suddenly and surprisingly stopped cooperating with the research participant.
Since this initial study, other studies have also implicated ACC, although there is open debate about whether ventral or dorsal regions of ACC are more crucial. For instance, one study found that social feedback about acceptance or rejection was associated with differential activity in the vACC (Somerville et al. 2006), and another found vACC activity for rejected adolescents (Masten et al. 2009). One interesting study using paintings portraying rejection imagery observed a somewhat different pattern than found in either of the previous studies (Kross et al. 2007). Although these authors also found dACC to be responsive to rejection imagery, the response was in a different area of dACC from that found by Eisenberger et al. (2003), and the relation between feelings of rejection and activity in this area was opposite that reported by Eisenberger et al. Another recent study (Burklund et al. 2007) found a relationship between both dACC and vACC activity and rejection sensitivity during emotional processing. Clarifying the roles of dACC and vACC in social feedback is clearly one goal for research on interpersonal rejection.
Finally, Somerville et al. (2010) found that it was primarily individuals with low self-esteem who show enhanced activity in vACC for social feedback. This latter study is consistent with the ideas behind sociometer theory (Leary et al. 1995), which proposes that changes in the self-esteem of individuals may facilitate motivation to engage in behaviors to preserve their status as group members. Indeed, Leary and colleagues suggest that those with low self-esteem are more sensitive to social feedback and are more concerned about possible group exclusion than are those with high self-esteem.
Stereotype Threat
Stereotype threat is the apprehension or fear that some people might experience if they believe that their performance on tests might confirm negative stereotypes about their racial group (Steele & Aronson 1995). It causes distraction and anxiety, interfering with performance by reducing the capacity of short-term memory and undermining confidence and motivation (Schmader 2010). The knowledge that social evaluation threat is associated with vACC activity has provided an interesting opportunity to examine whether stereotype threat effects on performance are due primarily to evaluation apprehension or to interference produced by cognitive load. Krendl et al. (2008) conducted an fMRI study in which women were reminded of gender stereotypes about math ability while they were completing difficult math problems. Women showed an increase in vACC activity while performing difficult math problems after a social threat was induced (reminding them of gender stereotypes), whereas in the absence of social threat, women instead showed heightened activation over time in regions associated with math learning (i.e., angular gyrus, left parietal and prefrontal cortex) and no change in vACC activation. Not surprisingly, women who were threatened exhibited a decrease in math performance over time, whereas women who were not threatened improved in performance over time. Given the above findings, it is reasonable to conclude that the vACC is engaged in social evaluative threat.
Self-Regulation
The fourth component necessary for successful functioning in the social world is self-regulation. Without it people could be impulsive, emotional wrecks, lashing out upon the smallest provocation, blurting out the first thing that comes to mind, and engaging in whatever behavior feels good at the time. However, threat detection and social emotions that arise from perceived social evaluation serve as guides for subsequent behavior, which is what makes something like feeling guilty adaptive (Baumeister et al. 1994b). Feeling socially excluded, which threatens the need to belong, motivates behavior to repair social relationships; feeling ashamed about considering cheating on our partner helps reign in temptations. Put another way, social emotions promote self-regulation, which allows people to change their behaviors so as to prevent being rejected.
Cognitive Neuroscience of Self-Regulation
Various cortical regions have been implicated in self-regulation (for reviews, see Banfield et al. 2004, Krendl & Heatherton 2009), with the prefrontal cortex most notable for the executive functions that support the various cognitive processes that are involved in self-regulation (Curtis & D'Esposito 2003, Goldberg 2001, Miller & Cohen 2001). Much of what is known about the neural substrates of self-regulation comes from neuropsychological case studies (see Wagner et al. 2010, Wagner & Heatherton 2010b). Beginning with the famous case of Phineas Gauge, the railroad foreman who suffered a tamping iron through the head in a work-related accident, numerous instances have been told of dramatic personality changes following damage to PFC. In most cases these changes were marked by disinhibited and often inappropriate behavior and, sometimes, severe loss of motivation in the absence of any observed cognitive impairment. The three main areas of PFC particularly important to self-regulatory functioning are ventromedial PFC (vMPFC) including orbitofrontal cortex, lateral PFC, and ACC.
Case after case of vMPFC damage, from the late-nineteenth century up through today, remark on various ways in which patients appear unable to regulate their social, affective, or appetitive behaviors (Anderson et al. 1999, Beer et al. 2006, Grafman et al. 1996; for review, see Wagner & Heatherton 2010b). Such patients might become aggressive, antisocial, or inappropriately jocular; exhibit hypersexuality; or engage in excessive overeating. Damage to this region of the brain often results in a deficiency in incorporating feedback from others (and social norms) to make appropriate behavioral choices in social contexts, resulting in social disinhibition and inappropriate approach behavior toward other individuals (Beer et al. 2003, 2006). Given the breadth of social norms violated by vMPFC-damaged patients, one might be tempted to imagine that vMPFC is somehow responsible for storing the knowledge of such norms and that damage to it therefore results in a lack of awareness of social norms. However, most patients appear to be fully aware of the impropriety of their actions, yet are unable to control their bad behavior nonetheless (Saver & Damasio 1991). What emerges from all these cases is that vMPFC damage involves a general dysregulation of social behavior along with difficulty controlling primary physiological drives.
A considerable amount of research has also implicated lateral regions of PFC in self-regulatory processes. Unlike those suffering from injuries affecting vMPFC function, patients with lateral PFC damage are quite capable of following social norms, understanding emotional cues, and inhibiting inappropriate behaviors. Their struggle, instead, revolves around planning and initiating behaviors, especially complex behaviors requiring the maintenance of multiple goals. One commonly observed symptom can be described as a kind of apathetic listlessness coupled with a loss of motivational drive, even when it comes to things as important as finding employment or mustering the interest necessary to stay in school (Stuss & Benson 1986). A striking example of these symptoms is the difficulty these patients demonstrate when asked to complete relatively simple real-world tasks such as following a shopping list (Barceló & Knight 2002, Shallice & Burgess 1991).
Another frontal region known to be crucial for self-regulation is the ACC. Most of our knowledge of ACC function comes not from neuropsychology but instead from neuroimaging and electrophysiological studies implicating this region in conflict monitoring (Carter et al. 1998, Gehring & Knight 2000, MacDonald et al. 2000) and in signaling the need for cognitive control (Kerns et al. 2004). In the few studies that do exist of focal damage to ACC, a common symptom is of a general apathy along with impoverished affect and difficulty in carrying out goal-directed behaviors (Cohen et al. 1999). Some have thus theorized a role for ACC in detecting and signaling the need for increased cognitive control to bolster self-regulatory efforts, such as may be necessary to overcome temptation (Botvinick et al. 2001, Kerns et al. 2004, Paus 2001, Peterson et al. 1999).
Emotion Regulation
People need to be able to regulate their emotions to function in society. Failure to do so can lead to aggression, violence, and others forms of antisocial behavior. Emotion regulation is also vitally important for overall psychological well-being. Disorders of emotion regulation involve not only aggressive disorders such as antisocial personality disorder, but also encompass debilitating mood disorders such as posttraumatic stress disorder and major depressive disorder. Depression, in particular, poses a large burden on society and is easily the most prevalent (Kessler et al. 2005) and most costly (Stewart et al. 2003) mental health disorder.
Over the past decade, a number of studies have focused on discovering the neural correlates of emotion regulation (see Ochsner & Gross 2005). Taken together, such research supports a model of top-down regulation of the amygdala, a brain region vitally important for affective processing, by the PFC (Davidson et al. 2000, Ochsner et al. 2004, Ochsner & Gross 2005). Typically, in neuroimaging studies of emotion regulation, participants view negatively valenced images and are asked to engage in specific emotion-regulation strategies, such as suppressing their affective response or engaging in cognitive reappraisal of the negative events depicted in the image (such as converting them from their apparent negativity into something more benign). Studies of this kind have revealed a consistent pattern of results whereby regions of the PFC (e.g., vMPFC and lateral PFC) show increased activity when participants are actively regulating their emotions. Conversely, the amygdala shows reduced activity during suppression of affective responses. Importantly, activity in these two regions is inversely correlated, a finding that is interpreted as evidence of downregulation of amygdala activity by the PFC (Ochsner et al. 2002). The precise region of the PFC responsible for this effect is somewhat in contention, with some studies implicating the vMPFC (Johnstone et al. 2007) and others the lateral PFC (Ochsner et al. 2002, Hariri et al. 2003). Whatever influences the lateral PFC exerts, however, must be indirect because this area has no direct connections of its own to the amygdala. In fact, Johnstone and colleagues (2007) found support for the proposition that the vMPFC mediates the influence of the lateral PFC over the amygdala, which might help explain the disparate findings of previous studies.
Research focusing on clinical populations provides further evidence of the importance of this amygdala-PFC circuit to emotion regulation. Johnstone and colleagues (2007) showed that when patients with major depressive disorder were asked to regulate their emotions, activation of vMPFC failed to inversely correlate to amygdala activity. Rather, both vMPFC and amygdala activation were exaggeratedly high, suggesting a breakdown in normal modulatory influence of vMPFC over the amygdala. Studies performed on patients with borderline personality disorder have shown similarly exaggerated activation of the amygdala in response to emotional stimuli (Donegan et al. 2003), further supporting the notion of a breakdown in the VMPFC-amygdala circuit among these populations. Patients suffering from posttraumatic stress disorder, too, show interesting patterns of prefrontal and limbic activity in response to emotional stimuli. Shin and colleagues (2005) demonstrated that the exaggerated amygdala activation exhibited by such patients in response to reminders of their traumatic event actually generalizes to unrelated negative emotional stimuli as well. Taken together, these findings of dysfunctional amygdala-prefrontal circuitry in mood disorders highlight the importance of emotion regulation for psychological well-being.
Regulation of Thought
One often-observed effect of damage to the prefrontal cortex is the frequency of expression of offensive, vulgar, or profane language (Damasio et al. 1990) even as these patients recognize the impropriety of their actions (Saver & Damasio 1991). Having undesirable thoughts rise to mind is a universal human experience, such as finding someone's cooking, hairstyle, or newborn repulsive. As Wegner (2009) notes, such unwanted thoughts are likely to emerge at the most inopportune times. Fortunately, most people are able to keep their offending thoughts to themselves.
Although cognitive neuroscientists have a long history of studying response inhibition, there is considerably less work on the neural mechanisms underlying thought suppression (Anderson & Levy 2009). Wyland and colleagues (2003) had participants engage in a thought-suppression task during imaging with fMRI. Compared to blocks of unrestrained thought, suppression of a specific thought recruited ACC, whereas attempts to clear the mind of any thoughts recruited not only ACC but also lateral PFC and insula. In this particular case, it may be that ACC activity was indexing failures to suppress thoughts or was instead signaling an increased need for cognitive control. Because subjects were not required to notify the experimenters if and when, despite their efforts at suppression, an unwanted thought nonetheless slipped into their consciousness, it remained unclear whether this ACC activation signified the thought-suppression process or rather the intrusion of the thoughts that were to be suppressed. As noted, the ACC is thought to be involved with monitoring for errors (Carter et al. 1998, Gehring & Knight 2000, MacDonald et al. 2000) and signaling the need for additional cognitive control (Kerns et al. 2004), increasing the plausibility of the latter possibility. To test these two hypotheses, Mitchell and colleagues (2007) performed a similar study in which they asked subjects to notify them, via button press, each time a specific unwanted thought entered their awareness. Critically, the authors employed a state-item design (Visscher et al. 2003) that allowed for separation of regions showing a sustained response during active thought suppression from regions demonstrating transient responses to thought intrusions. Results from this experiment showed that the right lateral PFC demonstrated greater sustained activity during thought suppression compared to epochs of unrestrained thought. The ACC, however, demonstrated transient activity to intrusions of a forbidden thought during periods of thought suppression compared to when that same thought was permissible (e.g., during unrestrained thought epochs). These findings are interpreted as demonstrating that the ACC monitors for conflict and signals the need for additional control, while the lateral PFC is involved in implementing and maintaining cognitive control over the duration of thought suppression periods and is insensitive to temporary failures in thought suppressions (Mitchell et al. 2007). In a related finding, Anderson and colleagues (2004) found evidence for lateral PFC involvement in suppressing the expression of learned word pairs.
Another category of undesirable thoughts in need of routine suppression are those associated with stereotypes and bias. Over the past 20 years, a wealth of social psychological research has demonstrated that racial bias and stereotypes can be automatically activated and that individuals differ in their motivation to engage in deliberate control in suppressing these prejudices (Devine 1989, Devine et al. 2002, Fiske 1998, Greenwald et al. 1998, Payne 2001). Neuroimaging research on prejudice and race bias has mainly focused on the relative involvement of amygdala and PFC regions, the former being implicated in the automatic component of stereotyping (Phelps et al. 2000), whereas the PFC is involved in top-down control of attitudes (Lieberman et al. 2005). The role of the amygdala in the evaluation of racial ingroup and outgroup members is not simply a story of greater amygdala activity for outgroup members (see Hart et al. 2000). Rather, the response in the amygdala to racial outgroup members is more nuanced, reflecting individual differences in automatic negative evaluations of blacks as measured by the implicit association test (IAT) (Cunningham et al. 2004, Phelps et al. 2000), the opportunity to engage in top-down control (Cunningham et al. 2004, Richeson et al. 2003), and perceiver's evaluative goals (Wheeler & Fiske 2005).
Cunningham and colleagues (2004) attempted to separate the roles of amygdala and PFC in race evaluations by capitalizing on the fact the amygdala responds rapidly to subliminal presentation of affective stimuli (Whalen et al. 1998). Thus, by presenting black and white faces both implicitly (30 ms) and explicitly (525 ms), the investigators were able to separately assess conditions in which participants were unlikely to engage in cognitive control (implicit presentation) compared to when participants had the opportunity to regulate their responses (explicit presentation). Their findings demonstrated that the amygdala showed greater activity to black faces when participants were unaware that any faces had been presented. However, when participants were given sufficient time to engage in self-regulation, activity in the amygdala did not differentiate between black and white faces; instead, Cunningham et al. (2004) found increased recruitment of lateral PFC regions during the explicit presentation of black compared to white faces, indicating active regulation.
Richeson and colleagues (2003) directly tested of the involvement of PFC regions in controlling prejudice by relating neural activity in the PFC to the amount of cognitive depletion participants experienced after an interracial interaction. After interacting with an African American confederate on a racially charged political issue (e.g., racial profiling), Caucasian participants completed the Stroop task. As found in previous research (Richeson & Shelton 2003), participants with greater automatic negative evaluations of blacks showed increased interference on the Stroop task, indicating that they expended more self-regulatory resources during the interracial interaction, leaving them depleted and less able to inhibit their responses during the Stroop task (see Baumeister & Heatherton 1996). Importantly, these same participants later completed an ostensibly unrelated fMRI experiment in which they viewed images of black and white faces. As with the experiment by Cunningham et al. (2004), participants engaged lateral PFC and ACC regions in response to the black compared to white faces. However, Richeson and colleagues were then able to relate the magnitude of PFC activity to the degree to which participants exhibited increased Stroop interference in the previous interracial interaction experiment. Activity in both the lateral PFC and ACC was positively correlated with both increased Stroop interference and with a measure of implicit racial stereotyping (Richeson et al. 2003). That is, participants who exhibited greater self-regulatory depletion following a face-to-face interracial interaction were also more likely to recruit regions of the PFC involved in cognitive control when viewing black faces. The notion of cognitive depletion stems from the theory that cognitive resources are finite; hence, actions that overexert these resources (i.e., restraining impulses, forcing oneself to perform a tedious task) deplete them (Baumeister & Heatherton 1996). Thus, Richeson & Shelton's (2003) finding that inhibiting prejudice appears to deplete cognitive resources suggests that the act of controlling prejudice requires cognitive control.
Regulation of Behaviors
The modern world is filled with temptations. Every day, people have to resist the lure of sugar-filled desserts, cigarettes, alcohol, drugs, sex, sleep when they should be awake, browsing the Internet when they should be working—the list goes on ad infinitum. Psychologists have made considerable progress in identifying the individual and situational factors that encourage or impair self-control (Baumeister et al. 1994, Mischel et al. 1996, Posner & Rothbart 1998). Failure to self-regulate is implicated in a variety of negative behaviors, including substance abuse, prejudice, and criminal behavior (see Baumeister & Heatherton 1996). Conversely, those who are better able to self-regulate demonstrate improved relationships, increased job success, and better mental health (Duckworth & Seligman 2005, Tangney et al. 2004). In spite of the numerous studies of executive function and inhibition, relatively few neuroimaging studies have directly examined social psychological models of self-regulation (Wagner & Heatherton 2010b).
Although there are many causes of self-regulation failure, a common process involves latent motivations and activating stimuli (Baumeister & Heatherton 1996). That is, a hungry person may not decide to act on his or her hunger until seeing an advertisement for tasty fast food. For others, seeing the fast food commercial reminds the person that he or she is trying to avoid excess calories and so the urge to eat is overridden. Controlling behavior in this circumstance is difficult because neural mechanisms of reward, namely the mesolimbic dopamine system, encourage us to engage in activities that activate dopamine neurons in the nucleus accumbens (NAcc). A common feature of all rewards, including drugs of abuse, is that they activate dopamine receptors in the NAcc (Carelli et al. 2000, Kelley & Berridge 2002, Koob & Le Moal 1997). In neuroimaging research there is convergent evidence in the form of increased activation of the NAcc region in response to the ingestions of food (O'Doherty et al. 2003) and of drugs of abuse (Breiter et al. 1997, Zubieta et al. 2005). The involvement of these regions in reward processing and expectation has been well established by numerous neuroimaging studies (Cloutier et al. 2008, Delgado et al. 2000, Knutson et al. 2005).
Moreover, simply viewing images of primary rewards, such as erotic images (Karama et al. 2002) or images of drugs (David et al. 2007, Garavan et al. 2000), can lead to activation of mesolimbic reward systems. This “cue-reactivity” paradigm has been instrumental in research on obesity and drug addiction, which has repeatedly demonstrated that obese individuals (Rothemund et al. 2007, Stoeckel et al. 2008), smokers (David et al. 2007, Due et al. 2002), and drug addicts (Childress et al. 1999, Garavan et al. 2000, Maas et al. 1998, Wexler et al. 2001) exhibit greater cue reactivity than do control participants. Importantly, this cue-related activity predicts self-reported cravings for food or drug items (McClernon et al. 2005, Myrick et al. 2008, Wang et al. 2004).
Activation of reward systems, whether in the face of real objects or their visual representations, poses a challenge to persons trying not to engage in the putatively rewarding activity. As might be anticipated by the discussion above, various PFC regions are important for resisting temptation. For example, Beauregard and colleagues found that subjects recruited lateral PFC and ACC when asked to inhibit arousal in response to erotic images (Beauregard et al. 2001), and when Brody and colleagues asked smokers to suppress their cravings, they observed heightened ACC activation compared to when they were asked to increase cravings (Brody et al. 2007). An example of the importance of these regions for the regulation of appetitive behaviors comes from a study of successful and nonsuccessful dieters. In response to food consumption, successful dieters show increased activity in lateral PFC (i.e., dorsal lateral prefrontal cortex), suggesting that they spontaneously engage self-regulatory strategies in order to curtail food-seeking behavior (DelParigi et al. 2007).
Among the common patterns of self-regulatory failure identified by Baumeister & Heatherton (1996) were lapse-activated causes, in which people responded to an initial indulgence in a forbidden substance (e.g., alcohol, food, or tobacco) by consuming more of it; “just one cigarette” quickly turns into half a pack, have “just one drink” and before you know it the whole bottle is gone. For example, in a laboratory study, Herman & Mack (1975) forced chronic dieters to break their diets by drinking a large calorie-dense milkshake and found that they subsequently overate, as compared to controls, in a supposed taste test. Once the diet is broken for the day, dieters appear to give up control, perhaps anticipating starting their diets anew the next day. Similar findings have been obtained in many subsequent studies (see review by Heatherton & Baumeister 1991).
Theories of drug addiction posit that hypersensitivity of the reward areas to drug cues (Stoeckel et al. 2008) along with a failure of the normal top-down prefrontal regulation of such regions (Bechara 2005; Koob & Le Moal 1997, 2008) combine to result in the failure of addicts to control behavior. This theory was put to the test in a study examining food-cue reactivity in the nucleus accumbens in chronic dieters and nondieters (Demos et al. 2010). Using a milkshake preload similar to that of Herman & Mack (1975), half of the participants had their diet broken prior to viewing food cues. Dieters who drank the milkshake, which presumably broke their diets, showed increased NAcc food-cue reactivity compared to both the nondieters and the chronic dieters whose diet had not been broken (Demos et al. 2010). Interestingly, chronic dieters demonstrated increased recruitment of lateral PFC in response to food cues compared to nondieters. But there was no effect of the diet-breaking preload on lateral PFC activity, suggesting that dieters who drank the milkshake were still engaged in self-regulation but were nevertheless failing to inhibit cue-related activity in reward systems. This phenomenon is mimicked in obese individuals who show enhanced activity in brain reward systems to images of food in comparison with matched controls (Stoeckel et al. 2008). Taken together, these findings paint a picture of a dysregulated reward system whereby NAcc, no longer under the influence of top-down control from the PFC, demonstrates an exaggerated response to food cues, leading to eventual collapse of self-control.
Self-Regulation as a Limited Resource
Although the self-regulation of emotion, thought, and behavior can be considered separately, it is likely that similar processes are common across all domains of self-regulation. Baumeister & Heatherton (1996) proposed a strength model of self-regulation in which a general resource is exhausted by repeated attempts at self-regulation (Muraven & Baumeister 2000, Vohs & Heatherton 2000). For instance, regulating emotions impairs dieters' abilities to restrain themselves from eating and to maintain diet standards (Hofmann et al. 2007). Putting participants under high cognitive load has also been shown to impair self-regulation, causing dieters to exhibit unrestrained eating in comparison with participants under low cognitive load (Ward & Mann 2000). Similarly, Muraven and colleagues showed that participants who engaged in an effortful thought-suppression manipulation subsequently showed impaired impulse control and drank more alcohol than did control participants (Muraven et al. 2002). Also, as mentioned, if one is high on implicit bias toward the members of another race, interacting with one of them can significantly interfere with one's ability to complete tasks involving response inhibition, such as the Stroop task (Richeson & Shelton 2003).
Evidence from neuroimaging studies echoes these findings. In one study, subjects who completed a difficult attention-control task showed reduced recruitment of lateral PFC and became less adept at regulating emotion (Wagner & Heatherton 2010a). In a study of chronic dieters, emotion-regulation tasks had the same effect of reducing lateral PFC activation as well as increasing NAcc activity in response to food cues (Heatherton et al. 2010). As discussed above in this section, lateral PFC appears to be recruited as a means of top-down control of emotional and appetitive impulses. A failure to fully recruit its assistance in regulating such impulses, therefore, can undoubtedly help explain the failures in self-regulation exhibited by subjects in behavioral studies.
Conclusion
As members of a highly complex social species, humans have evolved a fundamental need to belong that encourages them to be good group members and avoid actions that would have them expelled. Four basic components allow people to modify their actions so as to avoid expulsion, namely, self-awareness, theory of mind, threat detection, and self-regulation. Recent research in social neuroscience has provided insights into the cognitive bases of these components, such as how material processed with reference to self might have special status in human cognition, how people might use themselves as templates to predict the actions of others, how outgroup members may not be imbued with theory of mind, how evaluation apprehension might underlie stereotype threats, and how frontal inhibitory mechanisms may be challenged by cues that activate brain reward regions. It is likely that the nature of these processes changes as the other components are considered, such as threat detection differing as a function of whether the threat is from an ingroup or outgroup source and differential self-processing underlying self-regulatory success or failure. The methods and theories of social and cognitive neuroscience are likely to continue to grow increasingly sophisticated, furthering our understanding of the social brain.
Acknowledgments
Thanks are due to Anne Krendl, Dylan Wagner, and Jane Tucker for their assistance with this review, which is also supported in part by grants from NIDA (22582) and NIMH (59282).
- Self-regulation
the process by which people change thoughts, feelings, or actions in order to satisfy personal and society goals and standards
- Social neuroscience
an emerging scientific field concerned with identifying the brain mechanisms underlying social behavior
- PET
positron emission tomography
- fMRI
functional magnetic resonance imaging
- MPFC
medial prefrontal cortex
- vACC
ventral anterior cingulate cortex
- Social emotions
complex subjective experiences (e.g., pride, admiration, jealousy, envy, embarrassment, and guilt) that promote long-term relationships and group stability
- Theory of mind (ToM)
the ability to explain and predict other people's behavior as a result of recognizing their mental state
- TPJ
temporoparietal junction
- dACC
dorsal anterior cingulate cortex
- Stereotype threat
the apprehension or fear that some people might experience if they believe that their performance on tests might confirm negative stereotypes about their racial group
- vMPFC
ventromedial prefrontal cortex
- Emotion regulation
initiation or alteration of ongoing emotional responses through cognitive processes
- Thought suppression
efforts to restrain or ignore unwanted or undesirable thoughts
- Cognitive depletion
a reduction in self-regulatory resources that makes people less able to inhibit or control thoughts, emotions, or behavior
- NAcc
nucleus accumbens
- Strength model of self-regulation
a model that proposes that the capacity for regulating behavior relies on a general resource that can be depleted by situational demands
Footnotes
DISCLOSURE STATEMENT: The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Literature Cited
- Adolphs R. The social brain: neural basis of social knowledge. Annu Rev Psychol. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. Conceptual challenges and directions for social neuroscience. Neuron. 2010;65:752–67. doi: 10.1016/j.neuron.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci. 2002;14:1264–74. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- Amador XF, David AS. Insight and Psychosis: Awareness of Illness in Schizophrenia and Related Disorders. 2nd London: Oxford Univ. Press; 2004. p. 416. [Google Scholar]
- Amodio DM, Frith CD. Meeting of the minds: the medial frontal cortex and social cognition. Nature. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Levy BJ. Suppressing unwanted memories. Curr Dir Psychol Sci. 2009;18:189–94. doi: 10.1177/0963721417689881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MC, Ochsner K, Kuhl B, Cooper J, Robertson E, et al. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–35. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032–37. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Aron A, Aron EN. Self and self-expansion in relationships. In: Fletcher G, Fitness J, editors. Knowledge Structures in Close Relationships: A Social Psychological Approach. Hillsdale, NJ: Erlbaum; 1996. pp. 325–44.pp. 441 [Google Scholar]
- Bandura A. Human agency in social cognitive theory. Am Psychol. 1989;44:1175–84. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social cognitive theory of self-regulation. Organ Behav Hum Decis Process. 1991;50:248–87. [Google Scholar]
- Banfield J, Wyland CL, Macrae CN, Munte TF, Heatherton TF. The cognitive neuroscience of self-regulation. In: Baumeister RF, Vohs KD, editors. The Handbook of Self-Regulation. New York: Guilford; 2004. pp. 62–83. [Google Scholar]
- Barceló F, Knight RT. Both random and perseverative errors underlie WCST deficits in prefrontal patients. Neuropsychologia. 2002;40:349–56. doi: 10.1016/s0028-3932(01)00110-5. [DOI] [PubMed] [Google Scholar]
- Baumeister RF. The self. In: Gilbert DT, Fiske ST, Lindzey G, editors. The Handbook of Social Psychology. 4th Boston, MA: McGraw-Hill; 1998. pp. 680–740. [Google Scholar]
- Baumeister RF, Heatherton TF. Self-regulation failure: an overview. Psychol Inq. 1996;7:1–15. [Google Scholar]
- Baumeister RF, Heatherton TF, Tice DM. Losing Control: How and Why People Fail at Self-Regulation. San Diego, CA: Academic; 1994a. p. 307. [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychol Bull. 1995;117:497–529. [PubMed] [Google Scholar]
- Baumeister RF, Stillwell AM, Heatherton TF. Guilt: an interpersonal approach. Psychol Bull. 1994b;115:243–67. doi: 10.1037/0033-2909.115.2.243. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–63. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: insights from patients with orbitofrontal damage. J Personal Soc Psychol. 2003;85:594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- Beer JS, John OP, Scabini D, Knight RT. Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. J Cogn Neurosci. 2006;18:871–80. doi: 10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Grezes J, Armony JL, Passingham RE, Dolan RJ. Affective response to one's own moral violations. Neuroimage. 2006;31:945–50. doi: 10.1016/j.neuroimage.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Blackwood NJ, Bentall RP, Ffytche DH, Simmons A, Murray RM, Howard RJ. Persecutory delusions and the determination of self-relevance: an fMRI investigation. Psychol Med. 2004;34:591–96. doi: 10.1017/S0033291703008997. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bower GH, Gilligan SG. Remembering information related to one's self. J Res Personal. 1979;13:420–32. [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, et al. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–51. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burklund LJ, Eisenberger NI, Lieberman MD. The face of rejection: rejection sensitivity moderates dorsal anterior cingulate activity to disapproving facial expressions. Soc Neurosci. 2007;2:238–53. doi: 10.1080/17470910701391711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, et al. Brain activity during episodic retrieval of autobiographical and laboratory events: an fMRI study using a novel photo paradigm. J Cogn Neurosci. 2004;16:1583–94. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Amaral DG, Blanchard JJ, Cameron JL, Carter CS, et al. Social neuroscience: progress and implications for mental health. Perspect Psychol Sci. 2007;2:99–123. doi: 10.1111/j.1745-6916.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Lorig TS, Norris CJ, Rickett E, et al. Just because you're imaging the brain doesn't mean you can stop using your head: a primer and set of first principles. J Personal Soc Psychol. 2003;85:650–61. doi: 10.1037/0022-3514.85.4.650. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Petty RE. Electromyograms as measures of extent and affectivity of information processing. Am Psychol. 1981;36:441–56. doi: 10.1037//0003-066x.36.5.441. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J Neurosci. 2000;20:4255–66. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, et al. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–49. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carver C, Scheier M. Attention and Self-Regulation. New York: Springer-Verlag; 1981. [Google Scholar]
- Carver CS, Scheier MF, editors. On the Self-Regulation of Behaviour. New York: Cambridge Univ. Press; 1998. p. 433. [Google Scholar]
- Chiao JY, Harada T, Komeda H, Li Z, Mano Y, et al. Dynamic cultural influences on neural representations of the self. J Cogn Neurosci. 2009a;22:1–11. doi: 10.1162/jocn.2009.21192. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Komeda H, Li Z, Mano Y, et al. Neural basis of individualistic and collectivistic views of self. Hum Brain Mapp. 2009b;30:2813–20. doi: 10.1002/hbm.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier J, Heatherton TF, Whalen PJ, Kelley WM. Are attractive people rewarding? Sex differences in the neural substrates of facial attractiveness. J Cogn Neurosci. 2008;20:941–51. doi: 10.1162/jocn.2008.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Kaplan RF, Moser DJ, Jenkins MA, Wilkinson H. Impairments of attention after cingulotomy. Neurology. 1999;53:819–24. doi: 10.1212/wnl.53.4.819. [DOI] [PubMed] [Google Scholar]
- Cooney J, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7:161–65. doi: 10.1016/s1364-6613(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, et al. In search of the self: a positron emission tomography study. Psychol Sci. 1999;10:26–34. [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Gatenby JC, Gore JC, et al. Separable neural components in the processing of black and white faces. Psychol Sci. 2004;15:806–13. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Neural correlates of evaluation associated with promotion and prevention regulatory focus. Cogn Affect Behav Neurosci. 2005;5:202–11. doi: 10.3758/cabn.5.2.202. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Success and failure suppressing reflexive behavior. J Cogn Neurosci. 2003;15:409–18. doi: 10.1162/089892903321593126. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Collette F, Van der Linden M, Laureus S, Del Fiore G, et al. Self-referential reflective activity and its relationship with rest: a PET study. Neuroimage. 2005;25:616–24. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- David SP, Munafo MR, Johansen-Berg H, Mackillop J, Sweet LH, et al. Effects of acute nicotine abstinence on cue-elicited ventral striatum/nucleus accumbens activation in female cigarette smokers: a functional magnetic resonance imaging study. Brain Imaging Behav. 2007;1:43–57. doi: 10.1007/s11682-007-9004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science. 2000;289:591–94. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–77. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond) 2007;31:440–48. doi: 10.1038/sj.ijo.0803431. [DOI] [PubMed] [Google Scholar]
- Demos KE, Kelley WM, Heatherton TF. Dietary restraint violations influence reward processes in nucleus accumbens and amygdala. J Cogn Neurosci. 2010 doi: 10.1162/jocn.2010.21568. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine PG. Stereotypes and prejudice—their automatic and controlled components. J Personal Soc Psychol. 1989;56:5–18. [Google Scholar]
- Devine PG, Plant EA, Amodio DM, Harmon-Jones E, Vance SL. The regulation of explicit and implicit race bias: the role of motivations to respond without prejudice. J Personal Soc Psychol. 2002;82:835–48. [PubMed] [Google Scholar]
- Ditman T, Kuperberg GR. A source-monitoring account of auditory verbal hallucinations in patients with schizophrenia. Harvard Rev Psychiatry. 2005;13:280–99. doi: 10.1080/10673220500326391. [DOI] [PubMed] [Google Scholar]
- Donaldson C, Lam D, Mathews A. Rumination and attention in major depression. Behav Res Ther. 2007;45:2664–78. doi: 10.1016/j.brat.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, et al. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol Psychiatry. 2003;54:1284–93. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–27. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Duckworth AL, Seligman MEP. Self-discipline outdoes IQ in predicting academic performance of adolescents. Psychol Sci. 2005;16:939–44. doi: 10.1111/j.1467-9280.2005.01641.x. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–60. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Duval S, Wicklund R. A Theory of Objective Self-Awareness. New York: Academic; 1972. p. 238. [Google Scholar]
- Eddington KM, Dolcos F, Cabeza R, Krishnan KRR, Strauman TJ. Neural correlates of promotion and prevention goal activation: an fMRI study using an idiographic approach. J Cogn Neurosci. 2007;19:1–11. doi: 10.1162/jocn.2007.19.7.1152. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Satpute AB. Personality from a controlled processing perspective: an fMRI study of neuroticism, extraversion and self-consciousness. Cogn Affect Behav Neurosci. 2005;5:169–81. doi: 10.3758/cabn.5.2.169. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–92. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Enright R, Shukla D, Lapsley D. Adolescent egocentrism and self-consciousness. J Youth Adolesc. 1980;9:101–16. doi: 10.1007/BF02087929. [DOI] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Mayberg H, Bean J, McKeon D, et al. Attending to the present: Mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci. 2007;2:313–22. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman Barrett L, Wager TD. The structure of emotion: evidence from neuroimaging studies. Curr Dir Psychol Sci. 2006;15:79–83. [Google Scholar]
- Fellows LK, Farah MJ. The role of ventromedial prefrontal cortex in decision making: judgment under uncertainty or judgment per se? Cereb Cortex. 2007;17:2669–74. doi: 10.1093/cercor/bhl176. [DOI] [PubMed] [Google Scholar]
- Fiske ST. Stereotyping, prejudice, and discrimination. In: Gilbert DT, Fiske ST, Lindzey G, editors. Handbook of Social Psychology. Vol. 2. Hoboken, NJ: Wiley; 1998. pp. 357–411. [Google Scholar]
- Freeman JB, Schiller D, Rule NO, Ambady N. The neural origins of superficial and individuated judgments about ingroup and outgroup members. Hum Brain Mapp. 2010;31:150–59. doi: 10.1002/hbm.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of “theory of mind”. Trends Cogn Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–98. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Gardner WL, Pickett CL, Brewer MB. Social exclusion and selective memory: how the need to belong influences memory for social events. Personal Soc Psychol Bull. 2000;26:486–96. [Google Scholar]
- Gardner WL, Pickett CL, Jefferis V, Knowles M. On the outside looking in: loneliness and social monitoring. Personal Soc Psychol Bull. 2005;31:1549–60. doi: 10.1177/0146167205277208. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3:516–20. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Gillihan SJ, Farah MJ. Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychol Bull. 2005;13:76–97. doi: 10.1037/0033-2909.131.1.76. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Marquine MJ. Semantic and self-referential processing of positive and negative trait adjectives in older adults. Memory. 2009;17:144–57. doi: 10.1080/09658210802077405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg E. The Executive Brain: The Frontal Lobes and the Civilized Mind. New York: Oxford Univ. Press; 2001. [Google Scholar]
- Goldberg II, Harel M, Malach R. When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron. 2006;50:329–39. doi: 10.1016/j.neuron.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Sivers H, Gabrieli JD, Whitfield-Gabrieli S, Goldin P, et al. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 2005;16:1731–34. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, et al. Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology. 1996;46:1231–38. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Banaji MR. The self as a memory system: powerful, but ordinary. J Personal Soc Psychol. 1989;57:41–54. [Google Scholar]
- Greenwald AG, McGhee DE, Schwartz JL. Measuring individual differences in implicit cognition: the Implicit Association Test. J Personal Soc Psychol. 1998;74:1464–80. doi: 10.1037//0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- Grimm S, Jutta E, Boesiger P, Schuepbach D, Hell D, et al. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Hum Brain Mapp. 2009;30:2617–27. doi: 10.1002/hbm.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunebaum MF, Keilp J, Li S, Ellis SP, Burke AK, et al. Symptom components of standard depression scales and past suicidal behavior. J Affect Disord. 2005;87:73–82. doi: 10.1016/j.jad.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Yoon C, Schacter DL. Ageing and the self-reference effect in memory. Memory. 2007;15:822–37. doi: 10.1080/09658210701701394. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Harris LT, Fiske ST. Dehumanizing the lowest of the low: neuroimaging responses to extreme outgroups. Psychol Sci. 2006;17:847–53. doi: 10.1111/j.1467-9280.2006.01793.x. [DOI] [PubMed] [Google Scholar]
- Harris LT, Fiske ST. Social groups that elicit disgust are differentially processed in mPFC. Soc Cogn Affect Neurosci. 2007;2:45–51. doi: 10.1093/scan/nsl037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AJ, Whalen PJ, Shin LM, McInerney SC, Fischer H, et al. Differential response in the human amygdala to racial outgroup versus ingroup face stimuli. Neuroreports. 2000;11:2351–55. doi: 10.1097/00001756-200008030-00004. [DOI] [PubMed] [Google Scholar]
- Harter S. The Construction of the Self A Developmental Perspective. New York: Guilford; 1999. p. 434. [Google Scholar]
- Harter S, Waters P, Whitesell NR. Relational self-worth: differences in perceived worth as a person across interpersonal contexts. Child Dev. 1998;69:756–66. [PubMed] [Google Scholar]
- Heatherton TF. Building a social brain. In: Todorov A, Fiske ST, Prentice D, editors. Social Neuroscience: Toward Understanding the Underpinnings of the Social Mind. New York: Oxford Univ. Press; 2010. In press. [Google Scholar]
- Heatherton TF, Baumeister RF. Binge eating as an escape from self-awareness. Psychol Bull. 1991;110:86–108. doi: 10.1037/0033-2909.110.1.86. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Demos KE, Amble C, Wagner DD, Kelley WM. An fMRI study of the effects of glucose on self-regulatory depletion. 2010 Manuscript in preparation. [Google Scholar]
- Heatherton TF, Krendl AC. Social emotions: neuroimaging. In: Squire L, editor. Encyclopedia of Neuroscience. Vol. 9. London: Academic; 2009. pp. 35–39. [Google Scholar]
- Heatherton TF, Krendl AC, Macrae CN, Kelley WM. A social brain sciences approach to understanding the self. In: Sedikides C, Spencer S, editors. Frontiers in Social Psychology: The Self. New York: Psychol Press; 2007. pp. 3–20. [Google Scholar]
- Heatherton TF, Vohs K. Why is it so difficult to inhibit behavior? Psychol Inq. 1998;9:212–16. [Google Scholar]
- Heatherton TF, Wheatley T. Social neuroscience. In: Baumeister RF, Finkel E, editors. Advanced Social Psychology. New York: Oxford Univ. Press; 2010. pp. 575–612. [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Soc Affect Cogn Neurosci. 2006;1:18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman CP, Mack D. Restrained and unrestrained eating. J Personal. 1975;43:647–60. doi: 10.1111/j.1467-6494.1975.tb00727.x. [DOI] [PubMed] [Google Scholar]
- Higgins ET. Beyond pleasure and pain. Am Psychol. 1997;52:1280–300. doi: 10.1037//0003-066x.52.12.1280. [DOI] [PubMed] [Google Scholar]
- Higgins ET, Bargh JA. Social cognition and social perception. Annu Rev Psychol. 1987;38:369–425. doi: 10.1146/annurev.ps.38.020187.002101. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Rauch W, Gawronski B. And deplete us not into temptation: automatic attitudes, dietary restraint, and self-regulatory resources as determinants of eating behavior. J Exp Soc Psychol. 2007;43:497–504. [Google Scholar]
- Ingram RE. Self-focused attention in clinical disorders: review and conceptual model. Psychol Bull. 1990;107:156–76. doi: 10.1037/0033-2909.107.2.156. [DOI] [PubMed] [Google Scholar]
- Jenkins A, Macrae CN, Mitchell JP. Repetition suppression of ventromedial prefrontal activity during judgments of self and others. Proc Natl Acad Sci USA. 2008;105:4507–12. doi: 10.1073/pnas.0708785105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Nolen-Hoeksema S, Mitchell KJ, Levin Y. Medial cortex activity, self-reflection and depression. Soc Cogn Affect Neurosci. 2009;4:313–27. doi: 10.1093/scan/nsp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Green EJ. Dissociating medial frontal and posterior cingulated activity during self-reflection. Soc Affect Cogn Neurosci. 2006;1:56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Kawahara-Baccus TN, Rowley HA, Alexander AL, et al. The cerebral response during subjective choice with and without self-reference. J Cogn Neurosci. 2005;17:1897–906. doi: 10.1162/089892905775008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J. Differential effects of rumination and dysphoria on the inhibition of irrelevant emotional material: evidence from a negative priming task. Cogn Ther Res. 2006;30:149–60. [Google Scholar]
- Karama S, Lecours AR, Leroux JM, Bourgouin P, Beaudoin G, et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–11. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, et al. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, et al. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–26. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. NeuroImage. 2002;17:1080–86. [PubMed] [Google Scholar]
- Klein SB. The cognitive neuroscience of knowing one's self. In: Gazzaniga MS, editor. The Cognitive Neurosciences. Vol. 3. Cambridge, MA: MIT Press; 2004. pp. 1077–90.pp. 1,385 [Google Scholar]
- Klein SB, Kihlstrom JF. Elaboration, organization, and the self-reference effect in memory. J Exp Psychol: Gen. 1986;115:26–38. doi: 10.1037//0096-3445.115.1.26. [DOI] [PubMed] [Google Scholar]
- Klein SB, Kihlstrom JF. On bridging the gap between social-personality psychology and neuropsychology. Personal Soc Psychol Rev. 1998;2:228–42. doi: 10.1207/s15327957pspr0204_1. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25:4806–12. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Krendl AC, Richeson JA, Kelley WM, Heatherton TF. The negative consequences of threat: an fMRI investigation of the neural mechanisms underlying women's underperformance in math. Psychol Sci. 2008;19:68–175. doi: 10.1111/j.1467-9280.2008.02063.x. [DOI] [PubMed] [Google Scholar]
- Krendl AK, Heatherton TF. Self versus others/self-regulation. In: Berntson GG, Cacioppo JT, editors. Handbook of Neuroscience for the Behavioral Sciences. Hoboken, NJ: Wiley; 2009. pp. 859–78. [Google Scholar]
- Kross E, Egner T, Ochsner K, Hirsch J, Downey G. Neural dynamics of rejection sensitivity. J Cogn Neurosci. 2007;19:945–56. doi: 10.1162/jocn.2007.19.6.945. [DOI] [PubMed] [Google Scholar]
- Lanzetta JT, Kleck RE. Encoding and decoding of nonverbal affect in humans. J Personal Soc Psychol. 1970;16:12–19. doi: 10.1037/h0029850. [DOI] [PubMed] [Google Scholar]
- Leary MR. The Curse of the Self: Self-Awareness, Egotism, and the Quality of Human Life. New York: Oxford Univ. Press; 2004. [Google Scholar]
- Leary MR, Tambor ES, Terdal SK, Downs DL. Self-esteem as an interpersonal monitor: the sociometer hypothesis. J Personal Soc Psychol. 1995;68:518–30. [Google Scholar]
- LeDoux JE. The Emotional Brain. New York: Simon & Schuster; 1996. [Google Scholar]
- Legrand D, Ruby P. What is self-specific? Theoretical investigation and critical review of neuroimaging results. Psychol Rev. 2009;116:252–82. doi: 10.1037/a0014172. [DOI] [PubMed] [Google Scholar]
- Lemogne C, le Bastard G, Mayberg H, Volle E, Bergouignan L, et al. In search of the depressive self: extended medial prefrontal network during self-referential processing in major depression. Soc Cogn Affect Neurosci. 2009;4:305–12. doi: 10.1093/scan/nsp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience. In: Fiske ST, Gilbert DT, Lindzey G, editors. The Handbook of Social Psychology. 5th New York: Wiley; 2009. p. 848. [Google Scholar]
- Lieberman MD, Hariri A, Jarcho JM, Eisenberger NI, Bookheimer SY. An fMRI investigation of race-related amygdala activity in African-American and Caucasian-American individuals. Nat Neurosci. 2005;8:720–72. doi: 10.1038/nn1465. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, et al. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–26. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–38. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Macdonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychol Bull. 2005;131:202–23. doi: 10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Heatherton TF, Kelley WM. A self less ordinary: the medial prefrontal cortex and you. In: Gazzaniga MS, editor. The Cognitive Neurosciences. 3rd Cambridge, MA: MIT Press; 2004a. pp. 1067–77.pp. 1385 [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cereb Cortex. 2004b;14:647–54. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Maki RH, McCaul KD. The effects of self-reference versus other reference on the recall of traits and nouns. Bull Psychon Soc. 1985;23:169–72. [Google Scholar]
- Markus HR, Kitayama S. Culture of the self: implications for cognition, emotion, and motivation. Psychol Rev. 1991;98:224–53. [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus independent thought. Science. 2007;315:393–95. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc Cogn Affect Neurosci. 2009;4:143–57. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related fMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–47. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mischel W, Cantor N, Feldman S. Principles of self-regulation: the nature of willpower and self-control. In: Higgins ET, Kruglanski AW, editors. Social Psychology: Handbook of Basic Principles. New York: Guilford; 1996. pp. 329–60.pp. 948 [Google Scholar]
- Mitchell JP. Mentalizing and Marr: an information processing approach to the study of social cognition. Brain Res. 2006;1079:66–75. doi: 10.1016/j.brainres.2005.12.113. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Social psychology as a natural kind. Trends Cogn Sci. 2009;13:246–51. doi: 10.1016/j.tics.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. General and specific contributions of the medial prefrontal cortex to knowledge about mental states. NeuroImage. 2005;28:757–62. doi: 10.1016/j.neuroimage.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF. Components of a social brain. In: Gazzaniga MS, editor. The Cognitive Neurosciences. 4th Cambridge, MA: MIT Press; 2009. pp. 951–58.pp. 1385 [Google Scholar]
- Mitchell JP, Heatherton TF, Kelley WM, Wyland CL, Wegner DM, et al. Separating sustained from transient aspects of cognitive control during thought suppression. Psychol Sci. 2007;18:292–97. doi: 10.1111/j.1467-9280.2007.01891.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. J Cogn Neurosci. 2006;18:1586–94. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Moran JM, Heatherton TF, Kelley WM. Modulation of cortical midline structure by implicit and explicit self-relevance evaluation. Soc Neurosci. 2009;4:197–211. doi: 10.1080/17470910802250519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Kelley WM, Heatherton TF. Self-knowledge. In: Ochsner KN, Kosslyn S, editors. Oxford Handbook of Cognitive Neuroscience. New York: Oxford Univ. Press; 2010. In press. [Google Scholar]
- Mueller JH, Wonderlich S, Dugan K. Self-referent processing of age-specific material. Psychol Ageing. 1986;4:293–99. doi: 10.1037//0882-7974.1.4.293. [DOI] [PubMed] [Google Scholar]
- Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychol Bull. 2000;126:247–59. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- Muraven M, Collins RL, Nienhaus K. Self-control and alcohol restraint: an initial application of the self-control strength model. Psychol Addict Behav. 2002;16:113–20. doi: 10.1037//0893-164x.16.2.113. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, et al. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–75. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N, Fornito A, Harrison BJ, Yücel M, Sass LA, et al. A disturbed sense of self in the psychosis prodrome: linking phenomenology and neurobiology. Neurosci Behav Rev. 2009;33:807–17. doi: 10.1016/j.neubiorev.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Northoff G. Psychopathology and pathophysiology of the self in depression—neuropsychiatry hypothesis. J Affect Disord. 2007;104:1–14. doi: 10.1016/j.jad.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H. Self-referential processing in our brain. NeuroImage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN. Social cognitive neuroscience: historical development, core principles, and future promise. In: Kruglanski A, Higgins ET, editors. Social Psychology: A Handbook of Basic Principles. 2nd New York: Guilford; 2007. pp. 39–66.pp. 948 [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, et al. The neural correlates of direct and reflected self-knowledge. NeuroImage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–49. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Haneline J, Ramachandran T, et al. Reflecting upon feelings: systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Lieberman MD. The emergence of social cognitive neuroscience. Am Psychol. 2001;56:717–34. [PubMed] [Google Scholar]
- O'Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–37. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Andreasen NC, Crespo-Facorro B, O'Leary DS, Watkins GL, et al. Emotions in unmedicated patients with schizophrenia during evaluation with positron emission tomography. Am J Psychiatry. 2003;160:1775–83. doi: 10.1176/appi.ajp.160.10.1775. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–24. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Payne BK. Prejudice and perception: the role of automatic and controlled processes in misperceiving a weapon. J Personal Soc Psychol. 2001;81:181–92. doi: 10.1037//0022-3514.81.2.181. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, et al. An fMRI study of Stroop word-color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry. 1999;45:1237–58. doi: 10.1016/s0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M. I know you are but what am I? Neural bases of self and social knowledge retrieval in children and adults. J Cogn Neurosci. 2007;19:1323–37. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Borofsky LA, Dapretto M, Fuligni AJ, et al. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Dev. 2009;80:1016–38. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Cunningham WA, Funayama ES, Gatenby JC. Performance on indirect measures of race evaluation predicts amygdala activation. J Cogn Neurosci. 2000;12:729–38. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Pickett CL, Gardner WL, Knowles M. Getting a cue: the need to belong and enhanced sensitivity to social cues. Personal Soc Psychol Bull. 2004;30:1095–107. doi: 10.1177/0146167203262085. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353:1915–27. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeson JA, Baird AA, Gordon HL, Heatherton TF, Wyland CL, et al. An fMRI investigation of the impact of interracial contact on executive function. Nat Neurosci. 2003;6:1323–28. doi: 10.1038/nn1156. [DOI] [PubMed] [Google Scholar]
- Richeson JA, Shelton JN. When prejudice does not pay: effects of interracial contact on executive function. Psychol Sci. 2003;14:287–90. doi: 10.1111/1467-9280.03437. [DOI] [PubMed] [Google Scholar]
- Rimes KA, Watkins E. The effects of self-focused rumination on global negative self-judgments in depression. Behav Res Ther. 2005;43:1673–81. doi: 10.1016/j.brat.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. J Personal Soc Psychol. 1977;35:677–88. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37:410–21. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Rothman AJ, Baldwin A, Hertel A. Self-regulation and behavior change: disentangling behavioral initiation and behavioral maintenance. In: Vohs K, Baumeister R, editors. The Handbook of Self-Regulation. New York: Guilford; 2004. pp. 130–48. [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. J Cogn Neurosci. 2004;16:988–99. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Saver JL, Damasio AR. Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia. 1991;29:1241–49. doi: 10.1016/0028-3932(91)90037-9. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Curr Opin Neurobiol. 2006;16:235–39. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Saxe R, Carey S, Kanwisher N. Understanding other minds: linking developmental psychology and functional neuroimaging. Annu Rev Psychol. 2004;55:87–124. doi: 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- Schmader T. Stereotype threat deconstructed. Curr Dir Psychol Sci. 2010;19:14–18. [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. NeuroImage. 2004;22:941–47. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Seal ML, Aleman A, Maguire PK. Compelling imagery, unanticipated speech and deceptive memory: neurocognitive models of auditory verbal hallucinations in schizophrenia. Cogn Neuropsychiatry. 2004;9:43–72. doi: 10.1080/13546800344000156. [DOI] [PubMed] [Google Scholar]
- Sedikides C, Skowronski JJ. The symbolic self in evolutionary context. Personal Soc Psychol Rev. 1997;1:80–102. doi: 10.1207/s15327957pspr0101_6. [DOI] [PubMed] [Google Scholar]
- Seger CA, Stone M, Keenan JP. Cortical activations during judgments about the self and an other person. Neuropsychologia. 2004;42:1168–77. doi: 10.1016/j.neuropsychologia.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Shah JY. The automatic pursuit and management of goals. Curr Dir Psychol Sci. 2005;14:10–13. [Google Scholar]
- Shah JY, Brazy PB, Higgins ET. Promoting us or preventing them: regulatory focus and the nature of ingroup bias. Personal Soc Psychol Bull. 2004;30:433–46. doi: 10.1177/0146167203261888. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727–41. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin KA, et al. An fMRI study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–81. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Simons JS, Davis SW, Gilbert SJ, Frith CD, Burgess PW. Discriminating imagined from perceived information engages brain areas implicated in schizophrenia. NeuroImage. 2006;32:696–703. doi: 10.1016/j.neuroimage.2006.04.209. [DOI] [PubMed] [Google Scholar]
- Smart Richman L, Leary MR. Reactions to discrimination, stigmatization, ostracism, and other forms of interpersonal rejection: a multimotive model. Psychol Rev. 2009;116:365–83. doi: 10.1037/a0015250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Disambiguating anterior cingulate cortex function: differential response to experiences of expectancy violation and social rejection. Nat Neurosci. 2006;9:1007–8. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Kelley WM, Heatherton TF. Self-esteem modulates medial prefrontal cortical responses to evaluative social feedback. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq049. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CM, Aronson J. Stereotype threat and the intellectual test performance of African Americans. J Personal Soc Psychol. 1995;69:797–811. doi: 10.1037//0022-3514.69.5.797. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of lost productive work time among US workers with depression. JAMA. 2003;289:3135–44. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41:636–47. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Benson DF. The Frontal Lobes. New York: Raven; 1986. p. 320. [Google Scholar]
- Tangney JP, Baumeister RF, Boone AL. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. J Personal. 2004;72:271–324. doi: 10.1111/j.0022-3506.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Welsh RC, Chen AC, Velander AJ, Liberzon I. Medial frontal hyperactivity in reality distortion. Biol Psychiatry. 2007;61:1171–78. doi: 10.1016/j.biopsych.2006.11.029. [DOI] [PubMed] [Google Scholar]
- Trawalter S, Richeson JA. Regulatory focus and executive function after interracial interactions. J Exp Soc Psychol. 2006;42:406–12. [Google Scholar]
- Turk DJ, Heatherton TF, Macrae CN, Kelley WM, Gazzaniga MS. Out of contact, out of mind: the distributed nature of the self. Ann N Y Acad Sci. 2003;1001:65–78. doi: 10.1196/annals.1279.005. [DOI] [PubMed] [Google Scholar]
- Turner MS, Simons JS, Gilbert SJ, Frith CD, Burgess PW. Distinct roles for lateral and medial rostral prefrontal cortex in source monitoring of perceived and imagined events. Neuropsychologia. 2008;46:1442–53. doi: 10.1016/j.neuropsychologia.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David A. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Behav Rev. 2010;34:935–46. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, et al. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. NeuroImage. 2003;19:1694–708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Vohs KD, Heatherton TF. Self-regulatory failure: a resource-depletion approach. Psychol Sci. 2000;11:249–54. doi: 10.1111/1467-9280.00250. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci. 2009;4:274–90. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Wagner DD, Demos K, Heatherton TF. Staying in control: the neural basis of self-regulation and its failure. In: Cacioppo JC, Decety J, editors. Handbook of Social Neuroscience. New York: Oxford Univ. Press; 2010. In press. [Google Scholar]
- Wagner DD, Heatherton TF. Expending cognitive effort leads to emotion dysregulation. 2010a Manuscript submitted. [Google Scholar]
- Wagner DD, Heatherton TF. Giving in to temptation: the emerging cognitive neuroscience of self-regulatory failure. In: Baumeister RF, Vohs KD, editors. The Handbook of Self-Regulation. 2nd. New York: Guilford; 2010b. In press. [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, et al. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21:1790–97. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Ward A, Mann T. Don't mind if I do: disinhibited eating under cognitive load. J Personal Soc Psychol. 2000;78:753–63. doi: 10.1037//0022-3514.78.4.753. [DOI] [PubMed] [Google Scholar]
- Wegner DM. How to think, say, or do precisely the worst thing for any occasion. Science. 2009;325:48–50. doi: 10.1126/science.1167346. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, et al. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–18. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: the frontal lobes and autonoetic consciousness. Psychol Bull. 1997;121:331–54. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Fiske ST. Controlling racial prejudice: social-cognitive goals affect amygdala and stereotype activation. Psychol Sci. 2005;16:56–63. doi: 10.1111/j.0956-7976.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- Wicker B, Ruby P, Royet JP, Fonlupt P. A relation between rest and the self in the brain? Brain Res Rev. 2003;43:224–30. doi: 10.1016/j.brainresrev.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Williams LM, Das P, Harris AW, Liddell BB, Brammer MJ, et al. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am J Psychiatry. 2004;161:480–89. doi: 10.1176/appi.ajp.161.3.480. [DOI] [PubMed] [Google Scholar]
- Wyland CL, Kelley WM, Macrae CN, Gordon HL, Heatherton TF. Neural correlates of thought suppression. Neuropsychologia. 2003;41:1863–67. doi: 10.1016/j.neuropsychologia.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou T, Zhang J, Liu Z, Fan J, Zhu Y. In search of the Chinese self: an fMRI study. J China C Life Sci. 2006;49:89–96. doi: 10.1007/s11427-004-5105-x. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Fan L, Han S. Neural basis of cultural influence on self-representation. NeuroImage. 2007;34:1310–16. doi: 10.1016/j.neuroimage.2006.08.047. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Xu Y, Koeppe RA, Ni L, et al. Regional cerebral blood flow responses to smoking in tobacco smokers after overnight abstinence. Am J Psychiatry. 2005;162:567–77. doi: 10.1176/appi.ajp.162.3.567. [DOI] [PubMed] [Google Scholar]


