Abstract
Background
The automatic substitution of bioequivalent generic for brand-name antiepileptic drugs (AEDs) has been linked by anecdotal report to loss of seizure control.
Objective
To evaluate studies comparing brand-name and generic AEDs and determine whether evidence exists of superiority of the brand-name version in maintaining seizure control.
Data Sources
English-language human studies identified in searches of MEDLINE, EMBASE, and International Pharmaceutical Abstracts (1984 to August 2009).
Study Selection
Randomized controlled trials (RCTs) and observational studies comparing seizure events or seizure-related outcomes between one brand-name AED and at least one alternate version produced by a distinct manufacturer.
Data Extraction
We identified 16 articles (9 RCTs, 1 prospective nonrandomized trial, 6 observational studies). We assessed characteristics of the studies and, for RCTs, extracted counts for patients whose seizures were characterized as “controlled” and “uncontrolled.”
Data Synthesis
Seven RCTs were included in the meta-analysis. The aggregate odds ratio (n=204) was 1.0 (95% confidence interval: 0.7–1.4), indicating no difference in the odds of uncontrolled seizure for patients on generic medications compared to patients on brand-name medications. In contrast, the observational studies identified trends in drug or health services utilization that the authors attributed to changes in seizure control.
Conclusions
Though most RCTs were short-term evaluations, the available evidence does not suggest an association between loss of seizure control and generic substitution of at least three types of AEDs. The observational study data may be explained by factors such as undue concern from patients or physicians about the effectiveness of generic AEDs after a recent switch. In the absence of better data, physicians may want to consider more intensive monitoring of high-risk patients taking AEDs when any switch occurs.
Keywords: Anti-epileptic drugs, generic drugs, systematic review
Introduction
Concerns about medication costs and drug safety have increased attention to the role and clinical equivalence of generic drugs. These products have the same active ingredient(s) as their brand-name counterparts, but may differ in peripheral features, such as pill color or shape, inert binders and fillers, and the specific manufacturing process.1 Generic drugs are frequently sold at prices far below their brand-name counterparts, and therefore can promote patient adherence to essential medications2, 3 and reduce health care spending.4 This can be particularly important for patients with limited income and public insurance programs with constrained budgets. Many countries permit or mandate pharmacists to substitute a generic version (if one is available) whenever a physician writes a prescription, a flexibility that is critical to encouraging appropriate use of generic products.
Since 1984, generic drugs have been approved by the Food and Drug Administration (FDA) on the basis of studies demonstrating that they are bioequivalent to the brand-name versions. Bioequivalency can be established on the basis of the maximum serum concentration of the drug (Cmax), the time until maximum concentration is reached, or the area under a curve defined by serum concentration as a function of time (AUC). The FDA definition of bioequivalence requires that the 90% confidence intervals for the ratio of brand-to-generic AUC and Cmax fall within an acceptance interval of 0.80–1.25 (known as the “-20%/+25% rule”).5 Studies and substantial clinical experience have supported this standard as a means of ensuring the safety of the vast majority of generic drugs,6 although anecdotal and media reports warning about the safety of generic drugs persist.7 In particular, some have expressed concern about using bioequivalency studies to approve generic versions of narrow therapeutic index (NTI) drugs, whose effective doses and toxic doses are separated by a small difference in plasma concentration.8
One such class of NTI drugs is those used in the management of epilepsy.9 Epilepsy is successfully treated with anti-epileptic drugs (AEDs) in approximately 70% to 80% of patients.10 The remainder tend to be medically-refractory, with seizures that can be very difficult to control. Some clinicians have stated that patients with epilepsy may be at higher risk of seizures when they are switched from brand-name to generic AEDs and have urged against generic substitution.11, 12 Occasional case reports have been invoked to support these concerns.13–16 If this were a real risk, it would have important clinical implications for patients and physicians, and important financial implications as well. Loss of seizure control can have substantial medical, financial, and social consequences for patients with epilepsy, especially for those who have been seizure-free on a particular medical regimen. For example, a patient who experiences a new seizure may to lose the ability to drive for 6 months.
The issue of the interchangeability of brand-name and generic AEDs has risen in prominence recently, as several frequently-prescribed brand-name AEDs have reached the end of their patent protection and generic versions have been approved for the market.17 In 2008, the Epilepsy Foundation of America requested the FDA issue a statement opposing mandatory switching of bioequivalent formulations of brand-name and generic AEDs. The FDA refused, finding no convincing evidence that switching led to loss of seizure control.18 Nonetheless, the topic has become politically contentious,19 and some US states have passed AED-specific legislation requiring informed consent from the prescriber and patient (Hawaii20, Tennessee21) or requiring notification of the prescriber (Utah22) before permitting generic substitution. Several other states have considered similar actions.23 This tension exists in Europe as well, where health authorities in certain countries, such as Sweden, have excluded AEDs from automatic generic substitution.24 Such so-called “carve-outs” are likely to be costly,25 and editorials and press reports raising concern about the safety of generic AEDs have cast doubts about the safety of generic drugs in general.26, 27
In a recent meta-analysis of cardiovascular medications, we found no evidence of superior clinical effects of the brand-name version of the anticoagulant warfarin (Coumadin), another NTI drug, or other drugs used to treat cardiac disease.28 To determine whether these findings extend to AEDs, we systematically evaluated trials and observational studies assessing seizure control with brand-name and generic AEDs. We reviewed studies published from 1984- 2009 and pooled all available results.
Methods
Sources of Data
We performed a systematic search of articles published in peer-reviewed health care-related journals between January 1984 and August 2009 using MEDLINE, EMBASE, and International Pharmaceutical Abstracts (IPA) with the help of Julie Whelan, a professional librarian.
We employed search terms in three categories: (1) terms describing study type (e.g., clinical study, cross-over, equivalen$, effect$, and outcome$); (2) terms describing the comparison of interest (e.g., brand-name, patented, and generic drugs); and (3) terms relating to epilepsy and AEDs, including the US and international generic and brand-names of all relevant therapeutic agents (see Table 1). AEDs were defined as drugs approved specifically for use in the treatment of epileptic seizures. In the third category, we also employed search terms describing the disease process (e.g., seizure, convulsion, and epilepsy) and therapeutic drug category (e.g., antiseizure, anticonvulsant, and antiepileptic). Articles containing at least one search term in each of the three main categories met criteria for the abstract review. MEDLINE and EMBASE searches employed relevant MeSH and EMTREE subject heading as well as text words, while IPA searches used only free text.
Table 1.
Antiepileptic drugs included in review search methodology*
| Amobarbital | Levetirecetam | Phenytoin |
| Carbamazepine | Mephenytoin | Pregabalin |
| Dimethadione | Mephobarbital | Primidone |
| Diphenylhydantoin | Metharbital | Sulthiamine |
| Divalproex | Methsuximide | Tiagibine |
| Ethosuximide | Oxcarbazepine | Topiramate |
| Ethotoin | Paramethadione | Trimethadione |
| Felbamate | Pentobarbital | Valproate |
| Fosphenytoin | Phenacemide | Valproic |
| Gabapentin | Phenobarbital | Vigabatrin |
| Lamotrigine | Phensuximide | Zonisamide |
Generic names listed here. All US and international trade names associated with these products were also included in the search.
Search terms and parameters were adjusted for each database, while maintaining a common overall architecture. Search results from MEDLINE and EMBASE were combined at the outset. Search results from IPA were handled separately because IPA cannot technically be searched at the same time as the other databases. Manual reference mining of a selection of relevant articles, letters, and commentaries supplemented the search results.
Study Selection
Studies were included if they reported a comparative evaluation of one brand-name drug and at least one alternate version produced by a distinct manufacturer. The evaluation had to include measurement of outcomes related to the number or severity of seizures. We included randomized controlled trials (RCTs) and observational studies. We excluded case studies as well as research in abstract form only, pharmacokinetic studies, qualitative analyses of effectiveness, pharmacoeconomic evaluations, and surveys. Studies were excluded if they were written in a language other than English or they were conducted in animals.
Data Extraction and Quality Assessment
Data were extracted (ASK, EJB, WHS) and checked (JJG), with disagreements resolved by consensus. We assessed a number of variables related to the organization and outcome of the studies: the study design, the setting (institution-level and country-level), the characteristics of the population studied, the number of participants, the mean age (or age range) of the participants, the clinical endpoints, and the self-identified source of funding (where listed). The methodological quality of the randomized clinical trials (RCTs) was assessed using the five-point scale developed by Jadad et al.29 The methodological quality of non-randomized trials was assessed using the 9-star Newcastle-Ottawa scale.30 This was done independently by two authors (ASK and WHS), with differences resolved by consensus.
Data Analysis
To conduct the meta-analysis, we identified RCTs where seizure outcomes were presented or could be derived from the published results. We extracted from each study the number of patients given generic and brand name medications and the number of patients in each arm whose seizures were characterized as “controlled” and “uncontrolled” in the manuscript. If only seizure numbers for individual patients were reported, we considered patients with no observed seizures during the study period to be “controlled” patients and patients with ≥ 1 seizure to be “uncontrolled.” For studies with a crossover design, we also obtained the overlapping outcomes between groups. Where we could not determine controlled and uncontrolled patients from the published data, we contacted the authors of studies to determine whether unpublished results were available (but none were).
We then compared the percentage of controlled and uncontrolled patients for the brand-name and generic drugs. To be conservative, if there was more than one generic version in the study we selected the drug with the most uncontrolled patients. Odds ratios (ORs) and confidence intervals for the cross-over trials were calculated according to the methods described by Elbourne et al.31 We applied a marginal approach, where the OR was computed assuming independent observations and the variance was adjusted for the between-period correlation. The summary estimate was a weighted average of ORs from the seven studies where the weights were the inverse of the variances of the ORs. A null result is therefore represented by an OR of 1.0. Estimates >1 indicate higher odds of poor control for generic medications compared to brand name medications. Conversely, estimates <1 indicate lower odds of poor control for generic medications compared to brand-name medications. All statistical analyses were conducted using SAS version 9.1 (SAS Institute Inc., Cary, North Carolina).
Results
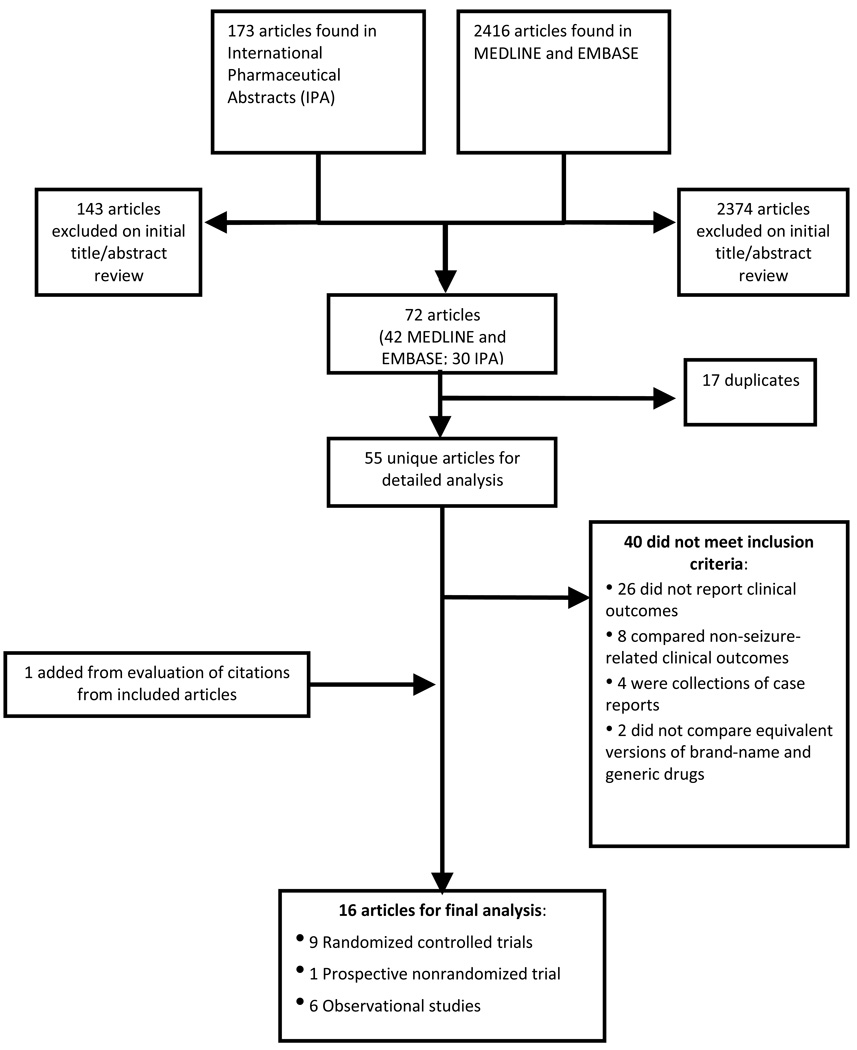
The search identified a combined 2,416 records from MEDLINE and EMBASE and 173 records from IPA. After applying our exclusion criteria, 55 articles from MEDLINE, EMBASE, and IPA were identified for further review. Fifteen studies met criteria for detailed analysis; 1 additional study was added from manual mining of references (Figure 1).
Figure 1. Study selection.
Randomized Controlled Trials
Nine studies compared brand-name and generic anti-epileptic drugs using RCTs (9/16, 56%). All were published between 1986 and 1997 (see Table 2).
Table 2.
Description of randomized controlled trials involving anti-epileptic drugs
| Source | Drugs studied | Population (setting) |
Study Design | Number of patients (age mean or range)/ Duration |
Jadad score* | Results | Conclusion | Listed source of funding |
|---|---|---|---|---|---|---|---|---|
| Hodges et al., 1986 | Dilantin (Parke-Davis) vs. 2 versions of phenytoin (Boots and Evans) | Newly diagnosed children with epilepsy (UK) | Randomized double-blind crossover trial | 19 (9.5 yrs)/12 wks (three 4-wk arms) | 2 | Dilantin: 43 seizures; phenytoin (Boots): 30 seizures; phenytoin (Evans): 60 seizures (not statistically significant) | One generic with plasma concentration differences, but no correlation with seizure outcomes | Brand-name mfr (Parke-Davis) |
| Kishore et al., 1986 | Dilantin (Parke-Davis) vs. epsolin (Cadila), eptoin (Boots), epileptin (Indian Drugs and Pharmaceuticals) | Newly diagnosed patients with epilepsy (India) | Randomized controlled trial | 60 (30.3 yrs)/3 mos | 2 | 4 uncontrolled patients in Dilantin group and 2 uncontrolled patients in each generic group (p>0.05) | No significant difference in clinical effects | Not listed |
| Jumao-as et al., 1989 | Tegretol (Geigy) vs. carbamazepine (Parke-Davis) | Epileptic patients uncontrolled on current med regimen, which included carbamazepine (US) | Randomized double-blind crossover trial | 10 (34–70 yrs)/10 wks (5 wks per arm) | 4 | 6 (out of 10) patients had seizures on brand and 6 (out of 10) had seizures on generic | No significant difference in mean seizure frequency | Medical center and government |
| Hartley et al., 1991 | Tegretol (Ciba-Geigy) vs. carbamazepine (Ethical Generics) | Children with history of epilepsy (10 stable on Tegretol, 13 new patients) (UK) | Randomized double-blind crossover trial | 23 (10.7)/12 wks (6 wks per arm) | 3 | 8 patients had seizures on brand and 8 patients had seizures on generic (p>0.32) | No significant difference in seizure frequency | Not listed (brand-name mfr [Ciba-Geigy] paid for lab measurements) |
| Soryal et al., 1992 | 2 versions of Dilantin (Parke-Davis) vs. 5 versions of phenytoin (Evans, APS, A H Cox, Thomas, Regent) | Epileptic patients on maintenance phenytoin (UK) | Randomized single-blind crossover trial | 14 (18–67)/28 wks (seven 4-wk arms) | 2 | No statistically significant difference when comparing incidence of seizure frequency | Plasma concentration differences, but no clinical differences | Not listed |
| Wolf et al., 1992 | 2 established versions of sustained-release carbamazepine vs. new sustained-release version (Sanofi Winthrop) | Epileptic patients stably treated on sustained-release carbamazepine monotherapy (Germany) | Randomized single-blind crossover trial | 10 (26)/9 days (three 3-day arms) | 2 | 3 patients with seizures on established brand #1, 3 on established brand #2, and 2 on the new version. | No indication of differences in therapeutic efficacy apart from 1 severely toxic patient | Sanofi Winthrop (mfr) |
| Oles et al., 1992 | Tegretol (Ciba-Geigy) vs. epitol (Lemmon) | 2 cohorts of epileptic patients: (1) stable on carbamazepine montherapy and (2) refractory (US) | Randomized double-blind crossover trial | (1) 15 (32); (2) 18 (40)/180 d (90 d per arm) | 4 | (1) 4/20 seizures on Tegretol vs. 2/20 seizures on epitol; (2) Avg seizure frequency 0.22 on Tegretol vs. Avg seizure frequency 0.25 on epitol (statistically similar, p=0.002) | Two drugs performed equally well in clinical efficacy | Lemmon (mfr) |
| Silpakit et al., 1997 | Tegretol (Ciba-Geigy) vs. carmapine [G1] (Central-poly), carzepine [G2] (Condrugs), panital [G3] Pharmaland) | Epileptic patients on stable dose of Tegretol (Thailand) | Randomized three-phase crossover trial | 18 (32.9)/12 wks (4 three-wk arms) | 4 | 2 patients had seizures on G1, 7 patients on G2, 3 patients on G3, 5 on brand Tegretol (p=0.08) | No significant difference in seizure frequency among brands | Hospital fund |
| Vadney et al., 1997 | Depakene (Abbott) vs. valproic acid [VPA] (Solvay) | Institutionalized patients stable on Depakene or VPA for seizures (US) | Randomized receiver-blinded crossover trial | 64 (39.6)/8 wks (4 wks per arm) | 2 | Mean number of seizures 50.89 for Depakene vs. 49.83 for VPA (p=0.89) | No significant differences in seizure control | Government |
Jadad score ranges 1–5, with 5 indicating highest quality.29
Three addressed seizure frequency in treatment with phenytoin (Dilantin). Kishore et al. conducted a non-crossover RCT in 60 newly-diagnosed epileptic patients, randomly assigning them to four equal-sized groups and assigning each one of the brand-name version and three generic versions available in India.32 Patients were followed for recurrent seizures for three months. Two patients in each of the three generic groups had recurrent seizures, as compared to five patients in the Dilantin group, which was not statistically significant (p>0.05). Hodges et al. enrolled newly diagnosed pediatric patients with epilepsy to a rotating schedule of three four-week blocks of Dilantin and two generic versions available in the United Kingdom (UK).33 Forty-three seizures were recorded among children in the Dilantin group, 30 in one generic group, and 60 in the other (not statistically significant). Paradoxically, the authors found that the generic version associated with the fewest recurrent seizures also had a significantly lower serum level. Soryal et al. randomized 14 patients on stable doses of phenytoin to seven four-week periods and serially switched them to two brand-name versions and five generic UK versions.34 Some variations in drug serum levels were noted, but there were no significant differences in seizure frequency.
Five RCTs addressed seizure frequency in treatment with regular and extended-release versions of carbamazepine (Tegretol). Oles et al. enrolled two cohorts of patients—a group on stable Tegretol monotherapy and another with refractory seizures—in a crossover study with three-month observational periods.35 Among the twenty previously controlled patients, 2 had seizures while receiving the generic drug and 4 had seizures while receiving the brand-name drug (p<0.05). Among the refractory patients with uncontrolled seizures, they found statistically similar “average seizure frequencies” (about 1 seizure every 4 days) in the brand-name and generic groups (p=0.002). They also identified the number of patients with a ≥50% difference in seizure frequency; of the eight patients who met this definition, all had a <20% difference in the pharmacokinetic AUC of the drug, irrespective of drug group. Jumao-as et al. enrolled ten patients who were uncontrolled on their current medical regimen to Tegretol and one generic version. Four patients had no seizures while on the brand-name, while four patients had no seizures with the generic. The number of seizures on average per month was 6.1 for the brand-name and 4.9 for the generic (not statistically significant).36
Among the other carbamazepine studies, Silpakit et al. randomized 18 patients on stable doses of Tegretol to the brand-name and three generic versions available in Thailand (named G1, G2, and G3).37 In the crossover trial, 7 patients had breakthrough seizures during the study: all 7 had seizures while on G2, 5 had seizures while on Tegretol, 3 had seizures on G3, and 2 had seizures on G1 (not statistically significant). Hartley et al. randomized pediatric patients to brand-name and generic versions of carbamazepine in the UK in a crossover-design trial.38 Eight patients had seizures during the brand-name drug period and another 8 had seizures during the generic period; 4 children appeared to have better seizure control with the generic and 3 had better seizure control with the brand-name (p>0.32). Finally, Wolf et al. compared two brand-name versions of extended-release carbamazepine in Germany to a new version that had been recently approved.39 Of the eight patients who had some seizures, three had them with all three preparations and four had them with only one preparation (one each with the two brand-name versions and two with the generic version). These rates were statistically indistinguishable.
The final trial addressed seizure frequency in treatment with valproic acid (Depakene). Vadney et al. enrolled 64 institutionalized patients on stable doses of Depakene or generic valproic acid and switched versions during two four-week observational periods.40 They found similar rates of seizure frequency and numbers of controlled and uncontrolled patients (p=0.89).
Aggregate Odds Ratios
Seven studies were included in the meta-analysis. Two were excluded because they did not report numbers of controlled/uncontrolled patients or provide individual-level data on patients with seizures. Six out of the seven had a cross-over design; the other was an RCT of new diagnoses without crossover. In two studies, we compared the percentage of uncontrolled patients receiving generic vs. brand name AEDs. In the remaining five studies, we compared the percentage of patients with at least one seizure for generic vs. brand name AEDs.
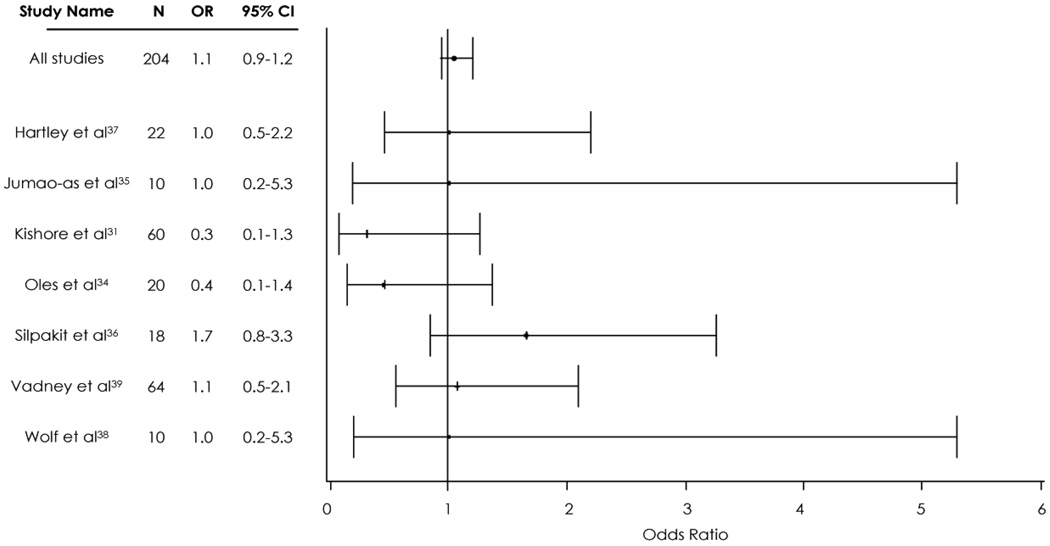
Overall, we found that brand-name AEDs were not shown to be better or worse than generic versions with regards to controlling patients’ seizures. As seen in Figure 2, the 95% confidence interval (CI) for each prospective study crossed one. The aggregate OR (n=204) was 1.0 (95% CI: 0.7–1.4), indicating no difference in the odds of uncontrolled seizure for patients on generic medications compared to patients on brand-name medications. We estimated a Cochrane’s Q-test statistic of 0.27, indicating that the studies are not significantly heterogeneous (p≫0.05).41
Figure 2. Meta-analysis of randomized controlled trials comparing generic and brand-name antiepileptic drugs.
Error bars represent 95% confidence intervals (CI). The Odds Ratio (OR) is odds of uncontrolled seizures after an AED switch. OR >1 suggests poor control for generic medications compared to brand name medications; OR <1 suggests lower odds of poor control for generic medications compared to brand-name medications. See Appendix for a breakdown of the number of patients who had uncontrolled seizures in the generic and brand-name groups.
Observational Studies
We identified one non-randomized prospective study and six other observational studies (see Table 3). The non-randomized prospective study was conducted in British Columbia. They enrolled ten institutionalized patients (aged 9–21 years) with epilepsy whose disease was stabilized on a generic version of carbamazepine. The patients’ seizure activity was then closely followed for a two-week period before they were switched to brand-name Tegretol and followed for another two-week period. There were no significant differences in seizure frequency.42
Table 3.
Description of non-randomized controlled trials involving anti-epileptic drugs
| Source | Drugs studied | Population (setting) |
Study Design | Number of patients (mean age for cases) |
Newcastle- Ottawa Score* |
Results | Conclusion | Source of funding |
|---|---|---|---|---|---|---|---|---|
| Christopher et al., 1993 | Tegretol (Ciba-Geigy) vs. Apo-carbamazepine (Apotex) | Institutionalized young patients with epilepsy on Apo-carbamazepine for > 2 mos (Canada) | Nonrandomized prospective switching study (2 four-wk phases) | 10 in each arm (16.6) | 6 | No significant differences in mean number of seizures (4.7 +/− 8.4 versus 4.4 +/− 6.4) | No effect on seizure rates | Not listed |
| Andermann et al., 2007 | Lamictal (GSK), Frisium (Sanofi-Aventis), and Depakene (Abbott) vs. 5 versions of lamotrigine, 6 versions of clobazam, and 15 version of valproic acid | Patients with claims codes for epilepsy using brand-name AEDs who were switched to generic versions in a public payer database (Canada) | (1) Drug-by-drug determination of switchbacks and comparison to non-AEDs; (2) Retrospective cohort study of switchbacks to Lamictal vs. patients staying with lamotrigine (“non-switchbacks”) | (1) 5,062 (all AEDs) vs. 163,370 (non-AEDs); (2) 149 (switchbacks) vs. 993 (non-switchbacks) (38.5) | 6 | (1) Switchbacks for AEDs (12.9–s20.9%) higher than non-AEDs (1.5–2.9%); (2) Non-switchbacks had significant average dosage increase of 6.2% over baseline (p<0.0001) (vs. 0.9% increase over baseline for switchbacks (p=0.69)) and a significant average increase over baseline in codispensed AEDs (13.4%, p<0.0001) and non-AEDs (19.3%, p<0.0001) (vs. 4.6% (p=0.13) and 8.8% (p=0.04) decrease from baseline for switchbacks | More switchbacks among AED users despite pre-auth hurdles and generic AED use associated with higher average dosage and use of other drugs | GSK (maker of Lamictal) |
| LeLorier et al., 2008 | Lamictal, Neurontin (Pfizer), Tegretol CR (Novartis), and Frisium vs. lamotrigine, gabapentin, carbamazepine-CR, and clobazam | Patients with claims codes for epilepsy using brand-name AEDs who were switched to generic versions in a public payer database (Canada) | (1) Aggregate determination of switchbacks and comparison to non-AEDs; (2) Retrospective cohort study of people staying with lamotrigine and switching back to Lamictal | (1) 2,784 (all AEDs) vs. 15,762 (non-AEDs); (2) 449 (Lamictal users) vs. 222 (lamotrigine users) (39) | 9 | (1) Patients receiving AEDs more likely to switchback than non-AEDs (HR 2.46; CI 1.93-3.14; p<0.0001); (2) During generic use period, other AEDs more often dispensed (RR 1.17; CI 1.14- 1.20; p<0.0001); non-AEDs more often dispensed (1.30; 1.27–1.33; p<0.0001); higher mean number outpatient visits (1.13; 1.09–1.18; p<0.0001). No difference in mean number of inpatient visits (1.14; 0.96- 1.35; p=0.13). | Higher healthcare utilization when switching from brand-name to generic AED | GSK (maker of Lamictal) |
| Zachry et al., 2008 | Any brand-name AED with generic version available from July 2006 – Dec 2006 | Patients with claims codes for epilepsy and on AED treatment in database of managed Medicare, Medicaid, and commercially insured patients (US) | Case-control study with controls matched 1:3 by seizure diagnosis type and age (within 5 yrs) | 416 cases vs. 1248 controls (37.4) | 8 | OR 1.81 (CI 1.25–2.63) for association between switch in AED formulation and seizure | Switching associated with seizure | Abbott (maker of Zonegran, for which generic version first available in July 2006) |
| Duh et al., 2009 | Topamax (Ortho-McNeil) vs. topiramate | Patients with claims codes for epilepsy using Topamax who were switched to a generic version in a public payer database (Canada) | (1) Aggregate determination of AED switching metrics, with comparison to non-AEDs; (2) Retrospective cohort study of brand and generic use periods for patients on Topamax and/or topiramate, stratified by receipt of single or multiple generic versions | (1) 3,667 (users of AEDs with generic entry on or after 2000) vs. 16,781 (users of AEDs with generic entry before 2000) vs. 9,806 (non-AEDs); (2) 948 Topamax or topiramate users (33.7- 37.5) | 5 | (1) Patients receiving older AEDs more likely to switchback than those receiving newer AEDs (19.2% vs. 14.7%), and both have higher switchback rates than non-AEDs (7.8%); (2) Multiple-generic periods associated with higher incidence of hospitalization relative to brand-only use (IRR 1.65, CI 1.3–2.1), but difference between single-generic period and brand-only use not significant (IRR 0.95, CI 0.95–1.02). No difference in mean number of outpatient visits. | Higher healthcare utilization and costs when switching from brand-name to generic AED | Ortho-McNeil Janssen Scientific Affairs (maker of Topamax) |
| Rascati et al., 2009 | Any brand-name AED with generic version available from April 2005 – Dec 2006 | Patients with claims codes for epilepsy, aged 12–64, and on AED treatment in database of over 55 million patients (US) | Case-control study with controls matched 1:3 by seizure diagnosis type (generalized, partial or other, and intractable or not), sex, and age (within 5 yrs) | 991 cases vs. 2973 controls (35.6) | 9 | OR 1.84 (CI 1.44–2.36) for association between switch in AED formulation and seizure. Adjusting for confounding due to age, sex, region of residence, diagnosis, and use of multiple antiepileptic drugs gave OR of 1.51 (1.17–1.96). | Switching associated with seizure | Abbott (unrestricted educational grant) |
| Hansen et al., 2009 | Any brand-name AED with generic version available in 2006 | Patients with claims codes for epilepsy, aged 12–64, and on AED treatment in database of commercially insured patients (US) | Case-control study with controls matched 1:3 by seizure diagnosis type (generalized, partial or other, and intractable or not) and age (within 5 yrs) | 757 cases vs. 2271 controls (36.9) | 8 | Adjusted OR 1.57 (CI 1.17 – 2.10) for association between switch in AED formulation and emergently-treated epilepsy-related event | Switching associated with seizure | Abbott (unrestricted educational grant) and Lilly |
RR = risk ratio, CI = confidence interval, OR = odds ratio, AED = anti-epileptic drug, IRR = incidence rate ratio
Newcastle-Ottawa score ranges from 1–9 stars, with 9 stars indicating highest quality.30
Two of the observational studies included very similar designs and were both conducted with sponsorship from AED manufacturers. The first, a cohort study by Andermann et al., used claims data from the Canadian province of Ontario to compare “switchback” rates of three brand-name AEDs (Lamictal, Frisium and Depakene) that occurred after a generic version was introduced and nearly all patients in the province were administratively switched to that version.43 They used a public-payer database of prescription drug dispensing claims. Switchback rates were higher for AEDs than for cholesterol-lowering and antidepressant drugs (range: 12.9% – 27.1% vs. 1.5% – 2.9%); the authors concluded that epilepsy patients were less satisfied with switching. They also found that the median dose of lamotrigine increased significantly after the switching period for patients remaining on the generic version. However, no seizure outcomes were measured; it is unclear if these switches were associated with adverse outcomes, as switching may have been driven by physician or patient perceptions of efficacy rather than meaningful clinical differences.
LeLorier et al. repeated this study design using Lamictal, Frisium, Tegretol CR, and Neurontin in another provincial health plan in Ontario.44 They found nearly 2.5-fold higher switchback rates (HR 2.46, CI 1.93–3.14) for AEDs compared with non-AEDs (a beta-blocker, ACE-inhibitor, and statin). These authors also evaluated the relation between switching from brand to generic and health services utilization, defined as any inpatient or outpatient physician visits. Among Lamictal users, after switching to generic lamotrigine, there were somewhat more outpatient physician visits (RR=1.13, CI 1.09–1.18), and no change in hospitalization rates (though there was a longer length of hospital stay (RR=1.48, CI not provided)). The authors concluded that switching was related to adverse health outcomes. However, the reason for the increased outpatient visits was not explored (only the actual number of visits was calculated). Many of the diagnoses associated with visits included in this analysis were for psychiatric conditions, and the results may have no relation with seizure activity.
Duh et al.,45 using a database similar to the prior two studies, found that switchback rates were higher among five AEDs with generic entry before 2000 (19.2% aggregate rate) than among three AEDs with generic entry in 2000 or later (14.7% aggregate rate for Topamax, Lamictal, and Neurontin). Both switchback rates were higher than for 4 selected non-AEDs (7.8% aggregate rate), although these results suffer from the same limitations as prior such studies. Duh et al. then focused on health care service utilization among patients who received either brand-name Topamax, a single generic version of topiramate, or more than one generic version. They found that multiple-generic use periods had higher hospitalization rates than brand-only periods (incidence rate ratio (IRR) 1.65, 95% CI 1.3–2.1), but single generic use periods did not (IRR 0.95, 95% CI 0.9–1.0). There was no difference between any of the groups in rate of outpatient visits. The authors also found a higher risk of hospitalization (and specifically claims for fracture or head injury) after generic-to-generic substitution (adjusted Hazard Ratio (HR) 1.6, 95% CI 1.1–2.5), but not after brand-to-generic substitution (adjusted HR 1.0, 95% CI 0.8–1.4). They concluded that these results suggested an association between generic switching of AEDs and increased health care utilization along with adverse clinical effects. The authors do not explain why they found differences in clinical outcomes among patients switched from generic-to-generic topiramate, but not brand-name to generic topiramate. Unmeasured confounders that differentiate patients receiving multiple generic versions may explain the results, because patients who use multiple generics may also use multiple pharmacies and have less stable living conditions or medical care.
The remaining three observational studies evaluated a potential association between substitution of generic AEDs and clinical outcomes. Zachry et al. and Rascati et al. were similarly-designed observational trials funded by Abbott Laboratories.46, 47 Each set of authors conducted a case-control study to assess the association between AED switching and hospital admissions and emergency medical encounters. They used pharmacy and medical claims data from a geographically diverse population aged 12–64 to identify cases with an inpatient hospitalization, emergency room visit, or ambulance service with a primary diagnosis of epilepsy and controls without such an epilepsy visit. The exposure of interest was any switch in A-rated formulations of AEDs in the 6 months prior to the index date. For Zachry et al., 47 cases out of 417 (11.3%) switched as compared to 81 controls out of 1,248 (6.5%) (OR 1.8, 95% CI 1.3–2.6). The investigators did not adjust for the number of AEDs prescribed to patients, which is a marker for epilepsy that is more difficult to manage and makes those patients more likely to switch. All of these factors could result in upwards confounding.
Rascati et al. found that 109 cases out of 991 (11.0%) switched in the six months prior to the index date as compared to 186 controls out of 2,973 (6.3%) (OR 1.8, 95% CI 1.4–2.4). However, cases were more than three times as likely to receive their insurance from Medicaid, suggesting that the populations may differ in other important characteristics. In a multivariable analysis which adjusted for potential confounding due to age, sex, region of residence, diagnosis, and use of multiple antiepileptic drugs, Rascati et al. found that the OR fell to 1.51 (95% CI, 1.17–1.96). While this study accounted for use of multiple antiepileptic drugs, residual confounding may persist because the authors defined this covariate dichotomously.
Hansen et al. conducted a matched case-control analysis with design and funding similar to Zachry et al. and Rascati et al., but also sought to account for multiple AED prescriptions.48 Using a large commercial database, Hansen et al. identified 84 cases out of 757 (11.1%) where a switch occurred in the six months prior to the index date, as compared to 147 out of 2,271 matched controls (6.5%) (OR 1.8, 95% CI 1.4–2.4). After controlling for the total number of AEDs filled (and gender), the authors found that the adjusted OR decreased to 1.6, which was still significant (95% CI 1.2 – 2.1). In Hansen et al. and the other observational studies, the investigators did not control for many unmeasured variables likely associated with switching, such as disease severity, use of other medications that may interact with AEDs, recent dose changes, sleep deprivation, or systemic illness. Investigators also did not control for important social characteristics, such as income, a stable living situation, or health-conscious behaviors.
Discussion
This study provides a comprehensive overview of the available evidence comparing seizure-related outcomes with use of brand-name and generic versions of anti-epileptic drugs. We evaluated 16 studies, including nine RCTs that involved over 200 patients. None of these studies found that the brand-name AED was superior or inferior to the generic in controlling seizures. On the other hand, the observational studies of patients with epilepsy who were switched from brand-name to generic formulations identified changes in drug or health services utilization that the authors concluded may suggest less adequate seizure control with generic products.
The RCTs involved three types of AEDs—phenytoin (Dilantin), carbamazepine (Tegretol), and valproic acid (Depakene); all but one of the studies used a cross-over design. None of the RCTs found any safety concerns or lack of efficacy with use of generic AEDs to treat patients with epilepsy. Many physicians have expressed concerns about the effect of switching a patient whose seizures have been well-controlled on a specific drug to a generic version of that product. The cross-over design addresses switching brand-name and generic forms of these drugs; these studies found no evidence of superior seizure control with brand-name medications.
Our study, however, has several limitations that reflect the underlying literature. Most trials identified by our search were short-term evaluations, included small populations, and were powered to assess differences in pharmacokinetic parameters, rather than clinical outcomes. For such trials, only large differences in clinical outcomes would have been statistically significant. Although our meta-analysis partly addresses the limitation of small sample size by pooling results across studies, we are still unable to rule out small differences in seizure rates between generic and branded AEDs. Furthermore, some clinical trial circumstances and patients were heterogeneous: studies included both uncontrolled subjects with epilepsy and controlled patients who were exposed to different formulations of the same active ingredient.
In addition to these limitations, the observational studies we identified came to conclusions at odds with the RCTs about the safety of brand/generic switches in this field. The observational studies by Andermann et al., LeLorier et al., and Duh et al. based their conclusions primarily on evidence of higher switchback rates among patients in one Canadian province switched to generic AEDs, as compared to non-AED drugs. While switchback rates can be a signal of adverse clinical outcomes with generic AEDs among patients with epilepsy, there are a number of alternative hypotheses that could account for this result. For example, neurologists may have been more likely than other physicians to accede to patient requests to support switchbacks even in the absence of new seizure outcomes because of concerns about generic AEDs stimulated by popular media reports or anecdotal experience.49, 50
In looking at clinical outcomes, LeLorier et al. found increased numbers of outpatient visits, although no change in hospitalization rates. Patients with epilepsy can experience significant anxiety with any change to their AEDs. A brand-to-generic switch is likely to cause increased anxiety and worry for many of these patients,51 which may be a reason for the increased clinic visits. Additionally, switchback rates may indicate high levels of vigilance and concern in light of the perceived switching problem, even in the absence of a true clinical problem. The prescribing physician may have requested more frequent visits in the initial post-switch period, as many epilepsy specialists do, in order to carefully monitor for adverse events or symptoms. Notably, Duh et al. found no change in outpatient visits, but an increase in hospitalizations. Associations found in an observational study design do not prove causation, and such inconsistencies raise further questions about whether practicing physicians should rely on these results.
The observational studies by Zachry et al. and Rascati et al. based their conclusion that generic AED substitution may be dangerous on more convincing evidence of increased health services utilization (inpatient hospitalization, emergency room visit or ambulance service) following a switch between any A-rated AED alternatives. While an association between switching and adverse outcomes was seen, the authors themselves emphasized that these results may be impacted by confounders unmeasured in the claims data used. Without controlling for these and other factors, along with the added concern of potential coding errors, the associations seen with switching and seizures may have been artificially magnified. For example, when Hansen et al. included one important potential confounder in their study—co-prescription of multiple AEDs—the adjusted odds ratio fell from 1.8 to 1.6.
While this meta-analysis supports the conclusion that at least three brand-name AEDs are not superior to generic versions in maintaining seizure control, case study reports and the observational data should be carefully considered, given the substantial medical and social cost of loss of seizure control. It is also important to note that the RCTs involved three older AEDs, while the observational studies involved a wide range of AEDs, including some newer products. Additional prospective studies in this class of NTI drugs may help clarify whether there are high-risk patients in whom switching between versions of a particular AED may be dangerous. If possible, such studies should examine the effect of brand name/generic switches, as well as seemingly minor alterations in a brand-name company’s manufacturing processes or switches between products originating from a company’s different factories. As we await better prospective trial data, observational studies properly adjusted for potential confounders could help to identify whether certain sub-populations may be at greater risk for loss of seizure control.
Medication substitution without prior knowledge may cause undue concern for patients with epilepsy, leading to increased health care system utilization (including phone calls and clinic visits) and switchback requests. Close monitoring of high-risk patients taking AEDs is appropriate when any change occurs, whether it is a dose alteration or a switch from generic to brand name, brand name-to-generic, or generic-to-generic. When switching AEDs is temporally associated with a seizure, physicians should consider whether other factors—such as new co-prescriptions or patient co-morbidities—may have affected reduced seizure control. If the medication change is determined be the primary contributor, policymakers and insurers ought to be flexible with coverage decisions, and provide patients with the ability to switch back to their original AED medication regimen.
Conclusion
A systematic review and meta-analysis of trials comparing seizure outcomes from use of brand-name and generic AEDs shows no association between loss of seizure control and generic substitution for at least three types of AEDs. Observational study data suggest that brand name-to-generic AED switching may be associated with “switchbacks” and increased rates of health services utilization, but these studies are limited by unmeasured confounders and other factors in their design. Though physicians may want to consider more intensive monitoring of high-risk patients taking AEDs when any medication change occurs, in the absence of better data, there is little evidenced-based rationale to challenge the implementation of generic substitution for AEDs in most cases.
Acknowledgements
The authors would like to thank our colleagues Edward Bromfield, M.D. (now deceased) and Jong Woo Lee, M.D., Ph.D. for their comments on a previous version.
Footnotes
None of the authors has any conflict of interest to disclose. There was no funding for this study.
References
- 1.Strom BL. Generic drug substitution revisited. N Engl J Med. 1987 Jun 4;316(23):1456–1462. doi: 10.1056/NEJM198706043162306. [DOI] [PubMed] [Google Scholar]
- 2.Shrank WH, Hoang T, Ettner SL, et al. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med. 2006 Feb 13;166(3):332–337. doi: 10.1001/archinte.166.3.332. [DOI] [PubMed] [Google Scholar]
- 3.Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA. 2007 Jul 4;298(1):61–69. doi: 10.1001/jama.298.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kesselheim A, Fischer M, Avorn J. Extensions of intellectual property rights and delayed adoption of generic drugs: effects on Medicaid spending. Health Affairs. 2006;25:1637–1647. doi: 10.1377/hlthaff.25.6.1637. [DOI] [PubMed] [Google Scholar]
- 5.Food and Drug Administration Center for Drug Evaluation and Research. [Accessed 2010 Jan 21];Guidance for industry: bioavailability and bioequivalence studies for orally-administered drug products - general considerations. 2003 http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070124.pdf.
- 6.Food and Drug Administration. [Accessed 2010 Jan 21];Therapeutic equivalence of generic drugs: letter to health practitioners. 1998 http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/ucm073182.htm.
- 7.Beck M. Inexact copies: how generics differ from brand names. Wall Street Journal. 2008 April 22; [Google Scholar]
- 8.Meredith P. Bioequivalence and other unresolved issues in generic drug substitution. Clin Ther. 2003 Nov;25(11):2875–2890. doi: 10.1016/s0149-2918(03)80340-5. [DOI] [PubMed] [Google Scholar]
- 9.Browne TR, Holmes GL. Epilepsy. N Engl J Med. 2001 Apr 12;344(15):1145–1151. doi: 10.1056/NEJM200104123441507. [DOI] [PubMed] [Google Scholar]
- 10.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000 Feb 3;342(5):314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 11.Perucca E, Albani F, Capovilla G, Bernardina BD, Michelucci R, Zaccara G. Recommendations of the Italian League against Epilepsy working group on generic products of antiepileptic drugs. Epilepsia. 2006;47 Suppl 5:16–20. doi: 10.1111/j.1528-1167.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 12.Jobst BC, Holmes GL. Prescribing antiepileptic drugs: should patients be switched on the basis of cost? CNS Drugs. 2004;18(10):617–628. doi: 10.2165/00023210-200418100-00001. [DOI] [PubMed] [Google Scholar]
- 13.Makus KG, McCormick J. Identification of adverse reactions that can occur on substitution of generic for branded lamotrigine in patients with epilepsy. Clin Ther. 2007 Feb;29(2):334–341. doi: 10.1016/j.clinthera.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Welty TE, Pickering PR, Hale BC, Arazi R. Loss of seizure control associated with generic substitution of carbamazepine. Ann Pharmacother. 1992 Jun;26(6):775–777. doi: 10.1177/106002809202600605. [DOI] [PubMed] [Google Scholar]
- 15.Wyllie E, Pippenger CE, Rothner AD. Increased seizure frequency with generic primidone. JAMA. 1987 Sep 4;258(9):1216–1217. [PubMed] [Google Scholar]
- 16.Berg MJ, Gross RA, Tomaszewski KJ, Zingaro WM, Haskins LS. Generic substitution in the treatment of epilepsy: case evidence of breakthrough seizures. Neurology. 2008 Aug 12;71(7):525–530. doi: 10.1212/01.wnl.0000319958.37502.8e. [DOI] [PubMed] [Google Scholar]
- 17.Rubenstein S. Industry fights switch to generics for epilepsy. Wall Street Journal. 2007 Jul 13; [Google Scholar]
- 18.Woodcock J. Letter to Eric R. Hargis, President and CEO, Epilepsy Foundation of America. 2008 [Google Scholar]
- 19.Sipkoff M. Mandatory generic substitution continues to be questioned. Drug Topics. 2008;152(4):45–46. [Google Scholar]
- 20.Hawaii Revised Statutes. §328-92(3)(c) 2008 [Google Scholar]
- 21.Tennessee Code Annotated. §53-10-210. 2009 [Google Scholar]
- 22.Utah Code Annotated. §58-17b-605. 2008 [Google Scholar]
- 23.National Conference of State Legislatures. Condition-specific drug substitution legislation: epilepsy; [Accessed 2010 Jan 21]. http://www.ncsl.org/programs/health/rx-substitution08.htm. [Google Scholar]
- 24.Kramer G, Biraben A, Carreno M, et al. Current approaches to the use of generic antiepileptic drugs. Epilepsy Behav. 2007 Aug;11(1):46–52. doi: 10.1016/j.yebeh.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Pharmaceutical Care Management Association. [Accessed 2010 Jan 21];Undermining generic drug substitution: the cost of generic carve-out legislation. 2008 http://www.pcmanet.org/wp-content/uploads/2008/11/generic-carve-out-final1.pdf.
- 26.Sipkoff M. The epilepsy battle in the war between brands and generics. Manag Care. 2008 Mar;17(3):24–27. [PubMed] [Google Scholar]
- 27.Eban K. Are generic drugs a bad bargain? Self. 2009 June [Google Scholar]
- 28.Kesselheim AS, Misono AS, Lee JL, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008 Dec 3;300(21):2514–2526. doi: 10.1001/jama.2008.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled clinical trials. 1996 Feb;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 30.Deeks JJ, Dinnes J, D'Amico R, et al. Evaluating non-randomised intervention studies. In: Wells G, Shay B, editors. Data extraction for nonrandomized systematic reviews. Vol 7. Ottawa: University of Ottawa; 2003. [Google Scholar]
- 31.Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002 Feb;31(1):140–149. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- 32.Kishore K, Jailakhani BL, Sharma JN, Ahuja GK. Serum phenytoin levels with different brands. Indian journal of physiology and pharmacology. 1986 Apr-Jun;30(2):171–176. [PubMed] [Google Scholar]
- 33.Hodges S, Forsythe WI, et al. Bio-availability and dissolution of three phenytoin preparations for children. Developmental Medicine and Child Neurology. 1986;28(6):708–712. doi: 10.1111/j.1469-8749.1986.tb03921.x. [DOI] [PubMed] [Google Scholar]
- 34.Soryal I, Richens A. Bioavailability and dissolution of proprietary and generic formulations of phneytoin. J Neurol Neurosurg Psychiatry. 1992;55(8):688–691. doi: 10.1136/jnnp.55.8.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oles KS, Penry JK, Smith LD, Anderson RL, Dean JC, Riela AR. Therapeutic bioequivalency study of brand name versus generic carbamazepine. Neurology. 1992 Jun;42(6):1147–1153. doi: 10.1212/wnl.42.6.1147. [DOI] [PubMed] [Google Scholar]
- 36.Jumao-as A, Bella I, Craig B, Lowe J, Dasheiff RM. Comparison of steady-state blood levels of two carbamazepine formulations. Epilepsia. 1989 Jan-Feb;30(1):67–70. doi: 10.1111/j.1528-1157.1989.tb05283.x. [DOI] [PubMed] [Google Scholar]
- 37.Silpakit O, Amornpichetkoon M, Kaojarern S. Comparative study of bioavailability and clinical efficacy of carbamazepine in epileptic patients. Ann Pharmacother. 1997 May;31(5):548–552. doi: 10.1177/106002809703100504. [DOI] [PubMed] [Google Scholar]
- 38.Hartley R, Aleksandrowicz J, Bowmer CJ, Cawood A, Forsythe WI. Dissolution and relative bioavailability of two carbamazepine preparations for children with epilepsy. The Journal of pharmacy and pharmacology. 1991 Feb;43(2):117–119. doi: 10.1111/j.2042-7158.1991.tb06644.x. [DOI] [PubMed] [Google Scholar]
- 39.Wolf P, May T, Tiska G, Schreiber G. Steady state concentrations and diurnal fluctuations of carbamazepine in patients after different slow release formulations. Arzneimittel-Forschung. 1992 Mar;42(3):284–288. [PubMed] [Google Scholar]
- 40.Vadney VJ, Kraushaar KW. Effects of switching from Depakene to generic valproic acid on individuals with mental retardation. Mental retardation. 1997 Dec;35(6):468–472. doi: 10.1352/0047-6765(1997)035<0468:EOSFDT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 41.Thompson SG. Why sources of heterogeneity in meta-analysis should be investigated. BMJ. 1994 Nov 19;309(6965):1351–1355. doi: 10.1136/bmj.309.6965.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christopher A, Levine M, et al. A comparison of two brands of carbamazepine in young patients with epilepsy. Canadian Journal of Hospital Pharmacy. 1993;46(2):62–71. [Google Scholar]
- 43.Andermann F, Duh MS, Gosselin A, Paradis PE. Compulsory generic switching of antiepileptic drugs: high switchback rates to branded compounds compared with other drug classes. Epilepsia. 2007 Mar;48(3):464–469. doi: 10.1111/j.1528-1167.2007.01007.x. [DOI] [PubMed] [Google Scholar]
- 44.LeLorier J, Duh MS, Paradis PE, et al. Clinical consequences of generic substitution of lamotrigine for patients with epilepsy. Neurology. 2008 May 27;70(22 Pt 2):2179–2186. doi: 10.1212/01.wnl.0000313154.55518.25. [DOI] [PubMed] [Google Scholar]
- 45.Duh MS, Paradis PE, Latremouille-Viau D, et al. The risks and costs of multiple-generic substitution of topiramate. Neurology. 2009 Jun 16;72(24):2122–2129. doi: 10.1212/WNL.0b013e3181aa5300. [DOI] [PubMed] [Google Scholar]
- 46.Rascati KL, Richards KM, Johnsrud MT, Mann TA. Effects of antiepileptic drug substitutions on epileptic events requiring acute care. Pharmacotherapy. 2009 Jul;29(7):769–774. doi: 10.1592/phco.29.7.769. [DOI] [PubMed] [Google Scholar]
- 47.Zachry WM, Doan QD, et al. Case-control analysis of ambulance, emergency room, or inpatient hospital events for epilepsy and antiepileptic drug formulation changes. Epilepsia. 2009;50(3):493–500. doi: 10.1111/j.1528-1167.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- 48.Hansen RN, Campbell JD, Sullivan SD. Association between antiepileptic drug switching and epilepsy-related events. Epilepsy Behav. 2009 Aug;15(4):481–485. doi: 10.1016/j.yebeh.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 49.Berg MJ, Gross RA, Haskins LS, Zingaro WM, Tomaszewski KJ. Generic substitution in the treatment of epilepsy: patient and physician perceptions. Epilepsy Behav. 2008 Nov;13(4):693–699. doi: 10.1016/j.yebeh.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Wilner AN. Therapeutic equivalency of generic antiepileptic drugs: results of a survey. Epilepsy Behav. 2004 Dec;5(6):995–998. doi: 10.1016/j.yebeh.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 51.Papsdorf TB, Ablah E, Ram S, Sadler T, Liow K. Patient perception of generic antiepileptic drugs in the Midwestern United States. Epilepsy Behav. 2009 Jan;14(1):150–153. doi: 10.1016/j.yebeh.2008.09.009. [DOI] [PubMed] [Google Scholar]