Abstract
Histone acetylation plays a critical role during long-term memory formation. Several studies have demonstrated that the histone acetyltransferase (HAT) CBP is required during long-term memory formation, but the involvement of other HAT proteins has not been extensively investigated. The HATs CBP and p300 have at least 400 described interacting proteins including transcription factors known to play a role in long-term memory formation. Thus, CBP and p300 constitute likely candidates for transcriptional coactivators in memory formation. In this study, we took a loss-of-function approach to evaluate the role of p300 in long-term memory formation. We used conditional knock-out mice in which the deletion of p300 is restricted to the postnatal phase and to subregions of the forebrain. We found that p300 is required for the formation of long-term recognition memory and long-term contextual fear memory in the CA1 area of the hippocampus and cortical areas.
It is now well established that long-term memory formation requires activation of transcription (Alberini 2009). Recently, the focus of many studies has been on the role of epigenetic mechanisms that control chromatin remodeling and regulate gene expression in synaptic plasticity and memory formation (Duman and Newton 2007; Sweatt 2009). In particular, histone acetylation is thought to play a critical role in this process (Barrett and Wood 2008; Sharma 2010). Protein acetylation is catalyzed by histone acetyltransferase (HAT) proteins and involves the addition of an acetyl group to lysines of histone N-terminal tails and other proteins. For histones, acetylation is modeled to facilitate transcription by reducing the repression imposed by chromatin structure and by influencing the binding of transcriptional proteins recruited to chromatin. Studies have shown that memory consolidation correlates with increases in histone acetylation (Levenson et al. 2004; Fontan-Lozano et al. 2008) and that inhibition of histone deacetylases (HDACs) facilitates learning and synaptic plasticity (Fischer et al. 2007; Vecsey et al. 2007; Stefanko et al. 2009; Roozendaal et al. 2010). Consistent with these findings, genetically modified mice expressing mutant HAT proteins have memory impairments. There are four main families of mammalian HATs: GCN5 and PCAF, the MYST family, the nuclear receptor coactivator family, and the CBP and p300 family (Allis et al. 2007). Several studies have demonstrated that impaired CBP activity leads to memory impairments (Alarcon et al. 2004; Korzus et al. 2004; Wood et al. 2005, 2006), but the involvement of other HAT proteins has not been extensively investigated. The HATs CBP and p300 have at least 400 described interacting proteins (Kasper et al. 2006; Bedford et al. 2010) including transcription factors known to play a role in long-term memory formation. Thus, CBP and p300 act as pivotal molecules in gene regulation and constitute primordial candidates as transcriptional coactivators in memory formation.
The first suggestion for a role for CBP in cognitive function came from a study in which mutations in the CBP gene (CREBBP) were identified in patients with Rubinstein-Taybi Syndrome (RTS) (Petrij et al. 1995). Later, studies with animal models for RTS carrying mutations in the Cbp gene showed cognitive impairments (Oike et al. 1999). However a clear demonstration for a role for CBP in memory formation was only achieved when the cognitive function of CBP conditional mutants, allowing restriction of CBP mutations to the forebrain and post-developmental phases, was evaluated (Korzus et al. 2004; Wood et al. 2005, 2006). Recently, Viosca and colleagues evaluated the cognitive function of p300 heterozygous knock-out mice and reported largely normal performance in learning and memory tasks (Viosca et al. 2010). However, the developmental defects exhibited by those mice may confound the interpretation of the behavioral phenotypes and the study of a role for p300 in memory formation in the adult brain. Moreover, p300 heterozygous knock-out mice still have 50% of p300 protein levels.
We have recently demonstrated that transgenic mice expressing a truncated form of p300 postnatally exhibit long-term memory deficits (Oliveira et al. 2007). Transgenic approaches involve the overexpression of wild type or mutated forms of a gene. The risks associated with overexpression studies include nonspecific interactions, gene dosage effects, and compensatory alterations. CBP has been extensively implicated in long-term memory formation; therefore, one concern is that the dominant negative form of p300 (p300Δ1) (Oliveira et al. 2007) might interact with molecules that normally recruit CBP, due to the high degree of homology between CBP and p300. In a loss-of-function approach, non-specific interactions are not a concern. Thus, in this study a loss-of-function approach was taken to study the role of p300 in long-term memory formation. Conditional knock-out mice were generated using the Cre/LoxP system to allow for regional control of the deletion of the p300 gene. Cre recombinase expression was driven by the calcium/calmodulin-dependent protein kinase II α (CaMKIIα) promoter, which drives expression postnatally in excitatory neurons within the hippocampus, striatum, amygdala, and cortex (Burgin et al. 1990; Mayford et al. 1996). The postnatal expression of Cre recombinase restricts the deletion of the p300 gene to adulthood allowing us to evaluate the role of p300 in memory formation without potential developmental confounds.
The p300 conditional knock-out mice used in this study lack p300 expression mainly in superficial layers of the cortex and CA1. Behaviorally, our mutant mice showed impairments in long-term recognition memory and long-term contextual fear memory. These results indicate that p300 function in the subregions targeted in our mutant mice plays a role in the formation of object recognition and contextual fear memories.
Results
In this study, we used p300 conditional knock-out mice to investigate the role of p300 in long-term memory formation. These mice were created using the Cre/LoxP system, which allows spatial and temporal control of the deletion of genes. This approach avoids potential developmental defects and allows deletion to be restricted to brain areas and cell types known to be important for learning and memory. Furthermore, a loss of function approach was chosen because in this type of approach nonspecific interactions of a mutant transgenic protein are not a concern.
To inactivate p300 in specific brain regions, we used p300 flox/flox mice (Kasper et al. 2006), carrying a CaMKIIα-Cre transgene (R4ag#11, Dragatsis and Zeitlin 2000). The CaMKIIα promoter is only activated postnatally in excitatory neurons of the forebrain (Burgin et al. 1990; Mayford et al. 1996), thus restricting the deletion of the p300 flox gene to adulthood and to excitatory neurons of the amygdala, hippocampus, and cortex (Fig. 1A). The CaMKIIα-Cre transgenic mice (Dragatsis and Zeitlin 2000) were crossed to the p300 flox/flox mice (Kasper et al. 2006) to generate Cre+;p300 flox/flox and Cre−;p300 flox/flox mice. Cre−; p300 flox/flox mice were used as control animals (see Materials and Methods for mating details). Cre+;p300 flox/flox mice do not show gross morphological abnormalities or reduced size compared to control littermates, and the expected Mendelian ratio of genotypes was observed suggesting no developmental defects in these mice (data not shown). Importantly, it has previously been shown that mice expressing Cre recombinase in the forebrain do not exhibit learning and memory deficits (Chen et al. 2006).
Figure 1.
p300 conditional knock-out mice show reduced expression of p300 in the forebrain. (A) Immunohistochemical labeling of p300 in brain coronal sections from control and p300 conditional knock-out mice. (B) Western blot analysis of the levels of p300 in the cerebellum, hippocampus, and cortex of p300 conditional knock-out mice and control littermates (n = 5 per group); *P < 0.05. Picture shows a representative blot from hippocampal tissue. (C) Immunohistochemical labeling of CBP in brain coronal sections from control and p300 conditional knock-out mice. (D) Western blot analysis of CBP protein expression in the cerebellum, hippocampus, and cortex of p300 conditional knock-out mice and control littermates (n = 5 per group). Picture shows a representative blot from hippocampal tissue. Abbreviations: cKO, p300 conditional knock-out; Ct, control littermate; PRh, Perirhinal cortex; Ent, Entorhinal cortex; I, Insular cortex; CA1, CA1 area of the hippocampus; CA3, CA3 area of the hippocampus; DG, dentate gyrus area of the hippocampus; Amy, amygdala; Hipp, Hippocampus; Cb, Cerebelum; Cx, Cortex.
We next characterized the p300 deletion pattern in p300 conditional knock-out mice. Using immunohistochemistry and Western blot analysis (Fig. 1A,B), we observed a reduction in p300 expression in CA1, the dentate gyrus, and superficial layers of the cortex. Cells lacking p300 expression were also observed within the amygdala (Fig. 1A). In Cre−;p300 flox/flox control mice, p300 is broadly expressed, reflecting the wild type expression of p300 (Oliveira et al. 2007), which demonstrates that the loxP sites do not interfere with gene function as found by Kasper et al. (2006). The Western blot analysis showed that levels of p300 in the hippocampus and cortex of p300 conditional knock-out mice are significantly reduced compared to their control littermates (hippocampus: t(6) = −4.12, P < 0.05; cortex: t(7) = −3.12, P < 0.05) (Fig. 1B). Levels of p300 in the cerebellum, where Cre recombinase is not expressed, are not significantly different between p300 conditional knock-out mice and control animals (Fig. 1B). We also examined CBP expression pattern and levels in the same brain structures (Fig. 1C,D). No significant differences between knock-out mice and control mice were observed, suggesting that CBP is not being upregulated to compensate for the reduction of p300 and that CBP protein levels are normal in neurons lacking p300.
p300 is a histone acetyltransferase; thus we hypothesized that histone acetylation would be changed in cells lacking p300. We assessed the global acetylation levels of histones H3 and H4 by immunohistochemistry in the brain regions where p300 deletion is clear (CA1 and the medial temporal cortex) (Fig. 2). Interestingly, we observed a selective reduction in histone H3 acetylation in the caudal perirhinal cortex (Fig. 2A,B). No changes were observed in the levels of acetylation of histone H4 in the brain regions analyzed or histone H3 in CA1 or the rostral perirhinal cortex.
Figure 2.
Global histone acetylation levels in p300 conditional knock-out mice in CA1 and cortex. (A) Quantification of the global level of acetylation of histones H3 and H4 in the CA1 and perirhinal cortex of p300 conditional knock-out mice (n = 5) and control littermates (n = 7) assessed by immunohistochemistry. Reduced AcH3 is observed in caudal perirhinal cortex of p300 conditional knock-out mice; *P < 0.05. (B) Representative images of the rostral and caudal perirhinal cortex of p300 conditional knock-out mice and control littermates. Coronal brain sections were stained with an antibody against the acetylated form of histone H3. cKO, p300 conditional knock-out; Ct, control littermate; PRh, Perirhinal cortex; CA1, CA1 area of the hippocampus.
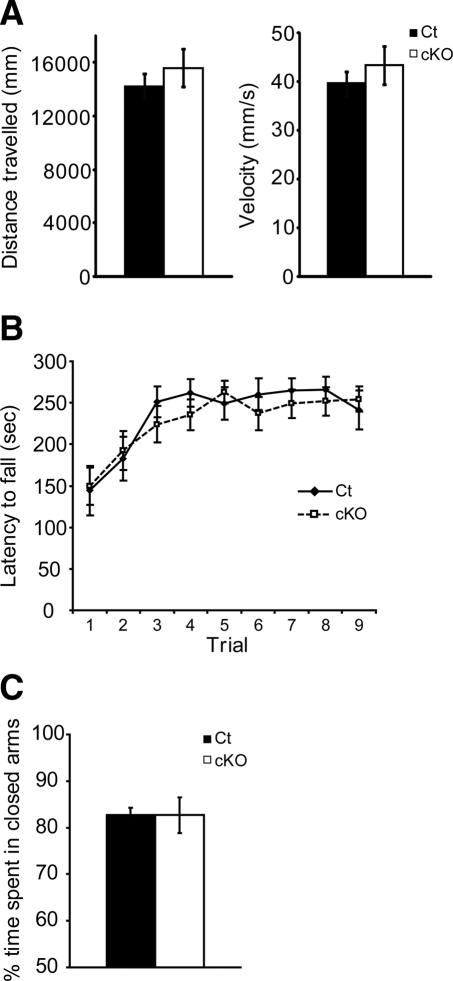
We next investigated whether p300 conditional knock-out mice exhibit locomotor or anxiety-like phenotypes. Such phenotypes could potentially confound the interpretation of the animals' performance in memory tasks. We observed that p300 conditional knock-out mice exhibit normal locomotor activity, motor skill learning, and anxiety-related responses (Fig. 3). We tested locomotor activity and motor skill learning in the open field and accelerating rotarod, respectively (Fig. 3A,B). Spontaneous activity, expressed as total distance traveled and velocity in an open field, is not different between p300 conditional knock-out mice and control littermates (Fig. 3A). In the rotarod, both p300 conditional knock-out mice and control littermates exhibit a significant improvement in time spent on the rotating rod across trials (effect of trial F(8,192) = 11.64, P < 0.0001). No effect of genotype (F(1,192) = 0.145, P = NS) or genotype × trial interaction (F(8,192) = 0.59, P = NS) was observed (Fig. 3B). The identical performance between control littermates and p300 conditional knock-out mice in the first trials and similar improvement across trials indicates that p300 conditional knock-out mice have no impairment in locomotor activity or motor skill learning, respectively.
Figure 3.
p300 conditional knock-out mice show normal spontaneous activity, motor coordination, motor skill learning, and anxiety-related behavior. (A) p300 conditional knock-out mice (n = 5) and control littermates (n = 5) show similar distance traveled and velocity in an open field. (B) p300 conditional knock-out mice (n = 13) and control littermates (n = 13) show similar time on the rotarod across trials. (C) p300 conditional knock-out mice (n = 6) and control littermates (n = 6) spent similar time in the closed arms of the elevated zero-maze. cKO, p300 conditional knock-out; Ct, control littermate.
We assessed anxiety-like behavior in the elevated zero-maze. In this task, mice face the conflict between their tendency to explore new environments and the innate aversion to open and bright spaces. Knock-out and control mice spent similar time in the closed arms when tested in the elevated zero-maze (Fig. 3C), showing that p300 conditional knock-out mice exhibit normal anxiety-like behavior.
To assess spatial memory, p300 conditional knock-out mice and control littermates were tested in the hidden platform version of the Morris water maze task (Fig. 4). The ability to acquire this task has been shown to depend on an intact hippocampus (Morris et al. 1982; Logue et al. 1997). Knock‐out mice and control littermates showed a significant improvement across the acquisition phase (effect of training day F(7,154) = 11.12, P < 0.0001), showing that both groups acquire the task. No effect of genotype (F(1,22) = 3.39, P = NS) or genotype × day interaction (F(7,154) = 1.20, P = NS) was observed. Overall, p300 conditional knock-out mice exhibit similar latencies to find the hidden platform as their control littermates (Fig. 4A). To assess spatial memory, two probe trials were performed: the first, one day after session four, and the second, one day after day eight of training. No differences were observed between p300 conditional knock-out mice and control littermates in the time spent in the target quadrant or number of crossings over the area where the platform was located during training (Fig. 4B,C). Swim speed, thigmotaxis, and total swimming distance were not different between the two groups (data not shown). We have also tested the performance of p300 conditional knock-out mice in a reversal learning task. In this task, the mice learn a new platform location to assess learning flexibility. No differences were found between p300 conditional knock-out mice and control littermates (data not shown).
Figure 4.
p300 conditional knock-out mice show normal spatial memory when tested in the Morris water maze. (A) Control (n = 12) and p300 conditional knock-out (n = 12) mice do not show a significant difference in the latency to find the hidden platform during the acquisition phase of the task. (B) During the probe trial performed after session 8, p300 conditional knock-out mice and control littermates showed similar percentage of time spent swimming in the target quadrant. (C) During the probe trial performed after session 8, the number of crossings over the area where the platform was located during acquisition was not different between control and p300 conditional knock-out mice. cKO, p300 conditional knock-out; Ct, control littermate.
We next tested the performance of p300 conditional knock-out mice in a novel object recognition task (Fig. 5), a task that is based on the animals' natural tendency to explore novel environments and objects. We observed that p300 conditional knock-out mice had significantly lower preference for a novel object when tested 24 h after training (t(10) = 4.49, P < 0.05). In contrast, object recognition memory tested 30 min after training was not different between p300 conditional knock-out mice and control littermates. Different batches of animals were used to perform short-term and long-term memory experiments. These results indicate that p300 conditional knock-out mice display normal short-term recognition memory but impaired long-term recognition memory. Importantly, the total time spent exploring both objects during training and testing phases among the different conditions tested was not different between the two genotypes (Table 1).
Figure 5.

p300 conditional knock-out mice show impaired long-term recognition memory. p300 conditional knock-out mice (n = 7) and control littermates (n = 7) show similar preference for the novel object when tested 30 min after training. However, when tested 24 h after training, p300 conditional knock-out mice (n = 6) show a significant lower preference for the novel object than their control littermates (n = 6); *P < 0.05. cKO, p300 conditional knock-out; Ct, control littermate.
Table 1.
Time exploring objects (sec) during the training session of novel object recognition tests (Fig. 5)
| Short-term memory | Long-term memory | |
|---|---|---|
| Wild type | 38.62 ± 4.33 | 51.64 ± 4.93 |
| p300 conditional knock-out | 39.78 ± 3.96 | 44.93 ± 2.50 |
Values correspond to average ± standard error of the mean (SEM).
Finally, we assessed the performance of p300 conditional knock-out mice in an associative form of memory using fear conditioning (Fig. 6). In contextual fear conditioning, mice learn the association between a specific environmental context and a foot shock. This task is known to require the function of the hippocampus and the amygdala (Phillips and LeDoux 1992; Maren and Quirk 2004). When p300 conditional knock-out mice were re-exposed to the same context 24 h after training, they showed significantly lower freezing compared with their control littermates (t(20) = 2.21, P < 0.05) (Fig. 6B). However, when testing occurred 1 h after training, no significant difference was observed between control and p300 conditional knock-out mice, suggesting that short-term memory is not affected in these mice (Fig. 6A). In cued fear conditioning, mice learn the association between a tone and a foot shock. This memory depends on the function of the amygdala but not the hippocampus (Phillips and LeDoux 1992; Maren and Quirk 2004). p300 conditional knock-out mice showed normal long-term cued fear memory as assessed by re-exposing the mice to the same cue (tone) 24 h after training in a novel context (Fig. 6C).
Figure 6.
p300 conditional knock-out mice show impaired long-term contextual fear memory and normal cued fear memory. (A) p300 conditional knock-out mice (n = 10) show identical percentage of freezing compared to their control littermates (n = 10) when tested 1 h after training in the cued fear conditioning. (B) p300 conditional knock-out mice (n = 12) show a significant lower percentage of freezing compared to their control littermates (n = 12) when tested 24 h after training in the contextual fear conditioning; *P < 0.05. (C) p300 conditional knock-out mice (n = 8) show an identical percentage of freezing compared to their control littermates (n = 10) when tested 24 h after training in the cued fear conditioning. cKO, p300 conditional knock-out; Ct, control littermate.
Discussion
In this study, we demonstrate that p300 is required for long-term memory formation. We used a loss of function approach to show that p300 conditional knock-out mice exhibit impairments in long-term recognition memory and long-term memory for contextual fear conditioning, with no deficits in spatial memory tested in the Morris water maze and motor skill learning. Our previous study, in which we used transgenic mice expressing a truncated form of p300, already suggested a role for p300 in long-term memory (Oliveira et al. 2007). In the present study, we confirmed that p300 plays a critical role during memory formation, and the restriction of the p300 gene deletion to subregions of the hippocampus and cortex allowed us to begin to define the cells in which p300 activity is required. Importantly, p300 conditional knock-out mice exhibited reduced acetylation of histone H3 specifically in the caudal perirhinal cortex, suggesting that p300 activity is critical in this brain region.
Recently, Viosca and colleagues studied the cognitive function of p300 heterozygous knock-out mice and found no impairments in memory tests; however, the mice exhibited reduced size and increased anxiety (Viosca et al. 2010). Reduced weight and size is consistent with developmental defects in p300 knock-out mice which could potentially confound the interpretation of memory deficits due to potential compensatory mechanisms. Furthermore, only heterozygous mice were used in that study which opens the possibility that monoallelic expression of p300 is sufficient for memory formation.
p300 conditional knock-out mice show normal anxiety and locomotor activity suggesting that basal behavioral responses are not altered in these animals. Furthermore, the deletion of the p300 gene should be restricted to the postnatal phase, due to the fact that the expression of Cre recombinase is under the control of the CaMKIIα promoter. This minimizes the likelihood of developmental defects. In the case of the study of the role of p300 in memory formation, this is a critical aspect as p300 is known to be involved in embryonic development (Yao et al. 1998). Furthermore, deletion is restricted to neurons, assuring that the phenotypes observed are due to the loss of activity of p300 in this cell type rather than in glial cells. Importantly, the levels of CBP in the brain of p300 conditional knock-out mice were not changed, suggesting no compensatory upregulation of the p300 homologue, CBP.
We consistently observed that p300 mutant mice exhibit normal spatial memory in the Morris water maze (this study and Oliveira et al. 2007). Both p300Δ1 transgenic mice and p300 conditional knock-out mice lack p300 function in the hippocampus, and it is known that the function of the hippocampus is critical for spatial memory formation (Morris et al. 1982). Thus, our results suggest that p300 may not play a role in spatial memory formation. In contrast, this study shows that p300 plays a role in contextual fear memory, another hippocampus-dependent task. Other studies have also observed a molecular dissociation between distinct types of hippocampus-dependent long-term memory. In particular, differences in the molecular mechanisms underlying spatial and contextual memory formation (for review, see Mizuno and Giese 2005). Studies in which the role of CBP in spatial memory was addressed showed that CBP function is required for the Morris water maze task performance (Korzus et al. 2004; Wood et al. 2005). It is a possibility that CBP and p300 can function interchangeably during the formation of spatial memory, or that only the CBP function is required. Indeed, evidence for distinct functions for CBP and p300 has been shown (Tanaka et al. 1997; Yao et al. 1998; Kasper et al. 2002, 2006; Oliveira et al. 2006). However, these hypotheses remain to be tested.
p300 conditional knock-out mice show impairments in long-term contextual fear conditioning but normal cued fear conditioning. Both contextual and cued fear conditioning require the activity of the amygdala, but only contextual fear conditioning requires the hippocampus (Maren and Quirk 2004). Therefore, the impairment in contextual fear conditioning suggests that the activity of p300 in the hippocampus is important for associative memory formation. In contrast, normal cued fear conditioning memory was observed in p300 conditional knock-out mice. This may indicate that the amygdala neurons in which the expression of p300 remains intact can support the formation of cued fear memory, or that p300 activity in the amygdala may not be required for this type of memory. Interestingly, p300Δ1 transgenic mice also show normal long-term cued fear memory (Oliveira et al. 2007). Thus, two distinct mouse models in which the activity of p300 is compromised exhibit unaltered cued fear conditioning, supporting the view that the activity of p300 in the amygdala is not necessary for this form of memory. The involvement of cortical areas, in particular, the perirhinal cortex, in contextual fear memory consolidation has been demonstrated (Sacchetti et al. 1999). Therefore, we should not exclude the possibility that the absence of p300 in cortical areas contributes to this phenotype.
The role of the hippocampus in object recognition memory has been a matter of debate, whereas cortical regions have consistently been shown to be involved in recognition memory (for review, see Dere et al. 2007). Studies have suggested that deficits in object recognition memory are only evident after near complete hippocampus lesions. Another prevalent hypothesis is that the hippocampus is only required for object recognition memory when spatial or contextual cues are relevant during object encoding. We have recently demonstrated that the novel object recognition memory test established in our laboratory is hippocampus-independent (Oliveira et al. 2010). Thus, the impairment exhibited by p300 conditional knock-out mice in long-term recognition memory is likely due to p300 inactivation in cortical regions. However, it remains to be investigated whether full deletion of p300 in the hippocampus induces long-term object recognition memory deficits. Cortical regions such as the perirhinal and insular cortices have been implicated in the consolidation of object recognition memory (Bermudez-Rattoni et al. 2005; Winters and Bussey 2005; Barker et al. 2007; Balderas et al. 2008; Winters et al. 2008; Roozendaal et al. 2010) and p300 conditional knock-out mice lack p300 in cells within these regions. Importantly, acetylation of histone H3 is reduced in the caudal perirhinal cortex in p300 conditional knock-out mice, and lesions of this region have been correlated with recognition memory deficits (Albasser et al. 2009). Furthermore, recent studies have implicated histone acetylation as an important mechanism during recognition memory consolidation (Fontan-Lozano et al. 2008; Roozendaal et al. 2010). Thus, our study strongly suggests that p300 is a key player in this mechanism. Fontan-Lozano and colleagues showed that the levels of acetylated H3 are significantly increased in the perirhinal cortex after training in the novel object recognition task (Fontan-Lozano et al. 2008). Another study showed that infusion of a HDAC inhibitor into the insular cortex leads to enhanced novel object recognition (Roozendaal et al. 2010). Interestingly, Roozendaal and colleagues demonstrated a double dissociation between post-training HDAC inhibitor infusion into the insular cortex and the hippocampus on the enhancement of object recognition and object location memory respectively. This suggests that histone acetylation in the insular cortex, but not in the hippocampus, plays an important role during novel object memory formation.
Both in the novel object recognition task and contextual fear conditioning, the memory impairment exhibited by p300 conditional knock-out mice was restricted to the 24-h delay test (long-term memory), whereas short-term memory was normal. This suggests that p300 plays a role in memory consolidation as a transcription regulator. p300 is likely to be recruited during the activation of gene expression induced by learning and to modulate transcription through its histone acetyltransferase (HAT) activity or by stabilizing multiprotein complexes at the promoter region of target genes. Besides participating in acetylation of histones, p300 can also acetylate non-histone transcription regulators (Kouzarides 2000). p300 and CBP have at least 400 described interacting proteins (Kasper et al. 2006; Bedford et al. 2010) including transcription factors known to play a role in long-term memory formation (e.g., CREB and family members, c-Fos, Elk-1, C/EBP, NF-κB). Thus, CBP and p300 act as critical molecules in gene regulation, and involvement in memory formation should be highly expected. In p300 conditional knock-out mice, transcription regulators that require p300 activity will have their function compromised. These factors should include not only transcription factors that recruit p300 HAT activity to initiate transcription but also transcription factors that require acetylation for activation. One extensively studied example is the transcription factor p65, a member of the NF-κB family. Acetylation of p65 is required for p65-dependent activation of transcription (Chen and Greene 2004). Interestingly, p300 was shown to acetylate p65, and this acetylation resulted in activation of p65 and consequently enhanced expression of the NMDA receptor subunits, NR2A and NR2B (Tai et al. 2009), critical for synaptic plasticity and memory formation.
At this point, it is becoming clear that p300 plays a critical role in long-term memory formation. To further understand the role of p300 in this process, it will be critical to identify the molecules that recruit p300 during learning-induced transcription activation and the genes targeted by p300-containing complexes.
Materials and Methods
Mice
All mice were 2–4 mo old and had free access to food and water in their home cages. Lights were maintained on a 12-h light–dark cycle, with all behavioral experiments carried out during the light portion of the cycle. CaMKIIα-Cre (line R4ag#11) mice were a kind gift from Dr. Ioannis Dragatsis (University of Tennessee). CaMKIIα-Cre (line R4ag#11) and p300flox were generated as previously described (Dragatsis and Zeitlin 2000, Kasper et al. 2006). CaMKIIα-Cre and p300flox were backcrossed to C57BL/6J mice at least 5 generations.
To obtain p300 conditional knock-out mice, we first crossed p300flox/flox mice with mice that contained one transgenic allele expressing Cre recombinase under the control of the CaMKIIα promoter (Cre+). Cre+;p300flox/+ mice were crossed with p300flox/flox to obtain mice that contain the CaMKIIα-Cre transgene and are p300flox/flox homozygous (Cre+;p300flox/flox). Cre−;p300flox/flox littermates were used as control mice.
Genotyping was performed as previously described (Dietrich et al. 2000; Kasper et al. 2006). All experiments were carried out in accordance with National Institutes of Health Guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Western blotting
Nuclear extracts from cortex, hippocampus, and cerebellum were obtained. The tissue from one mouse was homogenized in solution A (10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) pH 7.9, 1 mM ethylenediamine tetraacetic acid (EDTA), 1.5 mM MgCl2, 10 mM KCl, 1 mM dithiothreitol (DTT), 1% protease inhibitor cocktail (Sigma)). The nuclear pellet was resuspended in solution C (20 mM HEPES pH 7.9, 0.2 mM EDTA, 1.5 mM MgCl2, 420 mM KCl, 25% glycerol, 1 mM DTT, 1% protease cocktail inhibitor (Sigma)) and the supernatant was used as nuclear extract. The protein quantification was done using the Bradford reagent (BioRad). Samples were loaded in a 4%–12% gradient acrylamide gel (Invitrogen) and blotted onto a nitrocellulose membrane (BioRad). The membrane was blocked in Tris-buffered saline (TBS) and 5% nonfat dry milk and probed with actin (1:2500, Chemicon), CBP and p300 (1:1000 and 1:500, respectively, Santa Cruz Biotechnology) antibodies diluted in TBS containing 0.05% Tween 20 (TBST) and 2% milk. Blots were then incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse secondary antibodies (1:5000 dilution in TBST and 2% milk, Chemicon). Finally, blots were incubated in enhanced chemiluminescence (ECL) plus substrate (Amersham) and exposed to BioMax MR film (Kodak).
Immunohistochemistry
Mice were anesthetized with isoflurane and transcardially perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in PBS using a peristaltic perfusion pump. Fixed brains were dissected, post-fixed overnight, and then cryoprotected for 48 h in 30% sucrose at 4°C. Brains were flash frozen in 2-methylbutane on dry ice and mounted on cryostat chucks using Tissue Freezing Medium (Electron Microscopy Sciences, Hatfield, PA). Coronal sections were cut at a thickness of 30 µm and collected in PBS containing 0.02% sodium azide and stored at 4°C until further processing. The procedure used for immunohistochemistry was executed as described previously (Havekes et al. 2006; Isiegas et al. 2008). Floating sections were washed with PBS (5 min), incubated with 0.1% H2O2 in PBS (30 min) followed by rinsing in PBS (3 times, 5 min). Sections were pre-incubated in 8% normal goat serum (Jackson ImmunoResearch Laboratories) in PBS containing 0.3% Triton X-100 for 1 h at room temperature. After pre-incubation, sections were incubated with either p300, CBP, AcH3 or AcH4 antibodies (p300: C-20 and N-15, 1:500 each, Santa Cruz Biotechnology, CBP: A-22 and C-20, 1:1000 each, Santa Cruz Biotechnology, AcH3: 06-599, 1:1000, Millipore, AcH4: 06-866, 1:1000, Millipore) overnight at 4°C in 2% normal goat serum, 0.3% Triton X-100 in PBS. Sections were then rinsed in PBS (3 times, 5 min) and incubated for 2 h with biotinylated goat anti-rabbit IgG (1:500; Jackson ImmunoResearch Laboratories) in PBS containing 0.3% Triton X-100 and 2% normal goat serum at 4°C. After rinsing with PBS (3 times, 5 min), sections were incubated with the avidin-biotin-horseradish peroxidase complex (A and B components of ABC kit, 1:1000 each, Vector Laboratories) in PBS containing 0.3% Triton X-100 for 2 h at room temperature. Finally, after rinsing in PBS (2 times, 5 min, then overnight) sections were processed with diaminobenzidine (0.02% in PBS) with 0.003% H2O2 as a reaction initiator. After 7 min (p300), 5.5 min (CBP, AcH3) or 4.5 min (AcH4), the reaction was stopped by washing the sections with PBS containing 0.02% sodium azide. Sections were mounted on Superfrost Plus microscope slides (Fisher Scientific) and dried for 24 h. The mounted sections were then dehydrated using ethanol and xylenes, coverslipped with Permount (Fisher Scientific), and dried for another 24 h. Images were taken using a Fisher Micromaster microscope with camera.
Immunohistochemistry quantification
Perirhinal cortex was identified in rostral (−1.70 Bregma) and caudal (−2.80 Bregma) sections. Quantification of signal was performed with Image J. The area of interest was identified, and the total background intensity was subtracted from total intensity to obtain the total intensity of the signal (Isignal = Itotal– Ibackground). The mean intensity of the signal was calculated by dividing the total intensity of the signal by the area of the signal (msignal = Isignal/Asignal). To calculate the signal value, the mean intensity of the signal was inverted in the 255 scale and multiplied by the fraction of the area that the signal occupies (signal = −[ms- 255] × [Asignal/Atotal]). Quantification of the CA1 area of the hippocampus was performed by selecting the area of interest, and the value was normalized with the signal of adjacent thalamic areas.
Behavioral experiments
Open-field
The experimental apparatus consisted of a gray rectangular open field (38.5 cm × 30.5 cm × 26 cm). The mice were allowed to explore the open field for 6 min. Exploratory activity in the experimental arena was measured with the use of “TopScan” (Clever Systems Inc.).
Accelerating rotarod
The rotarod apparatus (Ugo Basile, Stoelting Co.) has a 3-cm-diameter rotating rod raised 16 cm above a platform and divided into five sections for testing multiple mice simultaneously. The rotarod gradually increases its rotation speed from 4 to 40 rpm over the course of 5 min. Latency to fall was recorded. Three trials a day were given during three consecutive days with an inter-trial interval of 1 h. Each trial started at the same time every day and ended when mice fell off the rod, or when mice ran for 300 sec.
Elevated zero-maze
The zero-maze apparatus consists of a raised circular track divided into two open and two closed quadrants. The track had an internal diameter of 40.5 cm and a width of 5.1 cm and was elevated off the floor at a height of 40 cm. The closed quadrants had walls 11-cm-high. Mice were placed at the entrance of a closed quadrant and observed for 5 min. The time spent in the closed and open quadrants was scored.
Water maze
The hidden platform experiment was performed using the method previously described (Wood et al. 2005; Oliveira et al. 2007). On the first day, mice were trained to sit on a submerged platform in a bucket for two 30-sec trials. Then mice were placed in the pool twice a day where a submerged platform was located in a fixed position (approximately 5- min inter-trial interval) for 4 d. Next, mice were given a probe trail on the fifth day and then four more days of training followed. A second probe trial was given on the tenth day. Each training trial lasted a maximum of 60 sec, and when mice did not find the platform, they were placed on it and allowed to sit for 20 sec. Each probe trial lasted 60 sec. For reversal training, the mice had four training trials per day, with an inter-trial interval of approximately 40 min. Also for reversal training, each training trial lasted a maximum of 120 sec. Mice were trained for 7 d in the original platform position, followed by 7 d in a new platform position in the opposite quadrant. The path of the mouse was recorded using a video tracking system (HVS Image, Water2020 version 1/2001).
Fear conditioning
The fear conditioning experiments were performed using the methods previously described (Wood et al. 2005; Oliveira et al. 2007). For contextual fear conditioning, mice were placed into the conditioning chamber and received a 2-sec 1.5-mA scrambled footshock 2.5 min after placement into the chamber. Mice were removed from the chamber after 3 min. During testing, mice received one 5-min exposure to the same context in the absence of footshock 1 h or 24 h after conditioning. Different sets of animals were used for 1 h and 24 h tests. For cued fear conditioning, mice were placed into the chamber and the cue (white noise) was activated from 2–2.5 min after placement into the chamber with a 1.5-mA footshock delivered in the last 2 sec of the cue. Mice were in the chamber for a total of 3 min. On testing day, mice were exposed to a novel context for a total of 5 min (a different conditioning chamber with smooth flat floor, altered dimensions, and a novel odorant), the onset of the cue was 2 min after mice were placed in the chamber, and lasted 3 min. Freezing was scored at 5-sec intervals and the percentage of those intervals in which the mouse froze was calculated. Different sets of mice were used for contextual and cued conditioning experiments.
Novel object recognition
The novel object recognition experiments were performed using the methods described (Wood et al. 2006; Oliveira et al. 2007). The experimental apparatus consisted of a white rectangular open field (60 cm × 50 cm × 26 cm). Prior to training, mice were handled for 1 min a day for 2 d and habituated to the experimental apparatus for 5 min in the absence of objects. During the training phase, mice were placed in the experimental apparatus in the presence of two identical objects and allowed to explore for 15 min. After a retention interval of 30 min or 24 h, mice were placed again in the apparatus where, this time, one of the objects was replaced by a novel one. Mice were allowed to explore for 15 min. Objects were rinsed with ethanol between trials and before the first trial. All testing and training sessions were videotaped and analyzed by an experimenter blind to the genotype of the animals. It was considered exploration of the objects when mice were facing an object and/or touching it. Preference for the novel object was expressed as the percent time spent exploring the novel object relative to the total time spent exploring both objects. The identity of the objects—which was novel or familiar—as well as their spatial locations were balanced between subjects.
Statistical analyses
Statistical analyses were performed using Student's t-test, with the exception of the Morris water maze and rotarod experiments. In the Morris water maze and rotarod experiments, two-way repeated-measures ANOVA were performed using Statistica. To compare groups, we used the Student-Newman-Keuls multiple comparisons post-hoc test. All experiments were done by an individual blind to genotype.
Acknowledgments
We thank Dr. Ioannis Dragatsis (University of Tennessee) for kindly providing the CaMKIIα-Cre transgenic mice (line R4ag#11). This work was supported by grants from the Human Frontiers Science Program (to T.A.), National Institutes of Health (to T.A.), PENN-PORT (to M.A.E.), NIH Pre-doctoral Training Program in Genetics (to J.D.H.), and Foundation for Science and Technology, Portugal (to A.M.M.O.).
References
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A 2004. Chromatin acetylation, memory, and LTP are impaired in CBP+/- mice: A model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 42: 947–959 [DOI] [PubMed] [Google Scholar]
- Albasser MM, Davies M, Futter JE, Aggleton JP 2009. Magnitude of the object recognition deficit associated with perirhinal cortex damage in rats: Effects of varying the lesion extent and the duration of the sample period. Behav Neurosci 123: 115–124 [DOI] [PubMed] [Google Scholar]
- Alberini CM 2009. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev 89: 121–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, Pillus L, Reinberg D, Shi Y, Shiekhattar R, et al. 2007. New nomenclature for chromatin-modifying enzymes. Cell 131: 633–636 [DOI] [PubMed] [Google Scholar]
- Balderas I, Rodriguez-Ortiz CJ, Salgado-Tonda P, Chavez-Hurtado J, McGaugh JL, Bermudez-Rattoni F 2008. The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learn Mem 15: 618–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Bird F, Alexander V, Warburton EC 2007. Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci 27: 2948–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett RM, Wood MA 2008. Beyond transcription factors: The role of chromatin modifying enzymes in regulating transcription required for memory. Learn Mem 15: 460–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford DC, Kasper LH, Fukuyama T, Brindle PK 2010. Target gene context influences the transcriptional requirement for the KAT3 family of CBP and p300 histone acetyltransferases. Epigenetics 5: 9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez-Rattoni F, Okuda S, Roozendaal B, McGaugh JL 2005. Insular cortex is involved in consolidation of object recognition memory. Learn Mem 12: 447–449 [DOI] [PubMed] [Google Scholar]
- Burgin KE, Waxham MN, Rickling S, Westgate SA, Mobley WC, Kelly PT 1990. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J Neurosci 10: 1788–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LF, Greene WC 2004. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol 5: 392–401 [DOI] [PubMed] [Google Scholar]
- Chen AP, Ohno M, Giese KP, Kuhn R, Chen RL, Silva AJ 2006. Forebrain-specific knockout of B-raf kinase leads to deficits in hippocampal long-term potentiation, learning, and memory. J Neurosci Res 83: 28–38 [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA 2007. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev 31: 673–704 [DOI] [PubMed] [Google Scholar]
- Dietrich P, Dragatsis I, Xuan S, Zeitlin S, Efstratiadis A 2000. Conditional mutagenesis in mice with heat shock promoter-driven cre transgenes. Mamm Genome 11: 196–205 [DOI] [PubMed] [Google Scholar]
- Dragatsis I, Zeitlin S 2000. CaMKIIalpha-Cre transgene expression and recombination patterns in the mouse brain. Genesis 26: 133–135 [DOI] [PubMed] [Google Scholar]
- Duman RS, Newton SS 2007. Epigenetic marking and neuronal plasticity. Biol Psychiatry 62: 1–3 [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH 2007. Recovery of learning and memory is associated with chromatin remodelling. Nature 447: 178–182 [DOI] [PubMed] [Google Scholar]
- Fontan-Lozano A, Romero-Granados R, Troncoso J, Munera A, Delgado-Garcia JM, Carrion AM 2008. Histone deacetylase inhibitors improve learning consolidation in young and in KA-induced-neurodegeneration and SAMP-8-mutant mice. Mol Cell Neurosci 39: 193–201 [DOI] [PubMed] [Google Scholar]
- Havekes R, Nijholt IM, Luiten PG, Van der Zee EA 2006. Differential involvement of hippocampal calcineurin during learning and reversal learning in a Y-maze task. Learn Mem 13: 753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isiegas C, McDonough C, Huang T, Havekes R, Fabian S, Wu LJ, Xu H, Zhao MG, Kim JI, Lee YS, et al. 2008. A novel conditional genetic system reveals that increasing neuronal cAMP enhances memory and retrieval. J Neurosci 28: 6220–6230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper LH, Boussouar F, Ney PA, Jackson CW, Rehg J, van Deursen JM, Brindle PK 2002. A transcription-factor-binding surface of coactivator p300 is required for haematopoiesis. Nature 419: 738–743 [DOI] [PubMed] [Google Scholar]
- Kasper LH, Fukuyama T, Biesen MA, Boussouar F, Tong C, de Pauw A, Murray PJ, van Deursen JM, Brindle PK 2006. Conditional knockout mice reveal distinct functions for the global transcriptional coactivators CBP and p300 in T-cell development. Mol Cell Biol 26: 789–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M 2004. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron 42: 961–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T 2000. Acetylation: A regulatory modification to rival phosphorylation? Embo J 19: 1176–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD 2004. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem 279: 40545–40559 [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM 1997. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci 111: 104–113 [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ 2004. Neuronal signalling of fear memory. Nat Rev 5: 844–852 [DOI] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER 1996. Control of memory formation through regulated expression of a CaMKII transgene. Science 274: 1678–1683 [DOI] [PubMed] [Google Scholar]
- Mizuno K, Giese KP 2005. Hippocampus-dependent memory formation: Do memory type-specific mechanisms exist? J Pharmacol Sci 98: 191–197 [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J 1982. Place navigation impaired in rats with hippocampal lesions. Nature 297: 681–683 [DOI] [PubMed] [Google Scholar]
- Oike Y, Hata A, Mamiya T, Kaname T, Noda Y, Suzuki M, Yasue H, Nabeshima T, Araki K, Yamamura K 1999. Truncated CBP protein leads to classical Rubinstein-Taybi syndrome phenotypes in mice: Implications for a dominant-negative mechanism. Hum Mol Genet 8: 387–396 [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Abel T, Brindle PK, Wood MA 2006. Differential role for CBP and p300 CREB-binding domain in motor skill learning. Behav Neurosci 120: 724–729 [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Wood MA, McDonough CB, Abel T 2007. Transgenic mice expressing an inhibitory truncated form of p300 exhibit long-term memory deficits. Learn Mem 14: 564–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AM, Hawk JD, Abel T, Havekes R 2010. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem 17: 155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrij F, Giles RH, Dauwerse HG, Saris JJ, Hennekam RC, Masuno M, Tommerup N, van Ommen GJ, Goodman RH, Peters DJ, et al. 1995. Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature 376: 348–351 [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE 1992. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106: 274–285 [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, Haettig J, Wood MA 2010. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J Neurosci 30: 5037–5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Lorenzini CA, Baldi E, Tassoni G, Bucherelli C 1999. Auditory thalamus, dorsal hippocampus, basolateral amygdala, and perirhinal cortex role in the consolidation of conditioned freezing to context and to acoustic conditioned stimulus in the rat. J Neurosci 19: 9570–9578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK 2010. Protein acetylation in synaptic plasticity and memory. Neurosci Biobehav Rev 34: 1234–1240 [DOI] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA 2009. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci 106: 9447–9452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD 2009. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry 65: 191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai DJ, Su CC, Ma YL, Lee EH 2009. SGK1 phosphorylation of IkappaB Kinase alpha and p300 up-regulates NF-kappaB activity and increases N-Methyl-D-aspartate receptor NR2A and NR2B expression. J Biol Chem 284: 4073–4089 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Naruse I, Maekawa T, Masuya H, Shiroishi T, Ishii S 1997. Abnormal skeletal patterning in embryos lacking a single Cbp allele: A partial similarity with Rubinstein-Taybi syndrome. Proc Natl Acad Sci 94: 10215–10220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, et al. 2007. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci 27: 6128–6140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viosca J, Lopez-Atalaya JP, Olivares R, Eckner R, Barco A 2010. Syndromic features and mild cognitive impairment in mice with genetic reduction on p300 activity: Differential contribution of p300 and CBP to Rubinstein-Taybi syndrome etiology. Neurobiol Dis 37: 186–194 [DOI] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ 2005. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J Neurosci 25: 52–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ 2008. Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev 32: 1055–1070 [DOI] [PubMed] [Google Scholar]
- Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AM, Lombardi TL, Abel T 2005. Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn Mem 12: 111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MA, Attner MA, Oliveira AM, Brindle PK, Abel T 2006. A transcription factor-binding domain of the coactivator CBP is essential for long-term memory and the expression of specific target genes. Learn Mem 13: 609–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao TP, Oh SP, Fuchs M, Zhou ND, Ch'ng LE, Newsome D, Bronson RT, Li E, Livingston DM, Eckner R 1998. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell 93: 361–372 [DOI] [PubMed] [Google Scholar]