Abstract
Background
Methamphetamine use disorders are pervasive global social problems that produce large medical and public health burdens. Abnormalities in pituitary hormonal regulation have been observed in preclinical models of substance abuse and in human substance abusers. They have not been studied before, however, in methamphetamine-dependent human subjects.
Objectives
To determine if methamphetamine-dependent research volunteers differ from healthy control subjects in plasma levels of adrenocorticotropic hormone (ACTH), cortisol, or prolactin, or in pituitary dopamine D2 receptor availability during early abstinence from methamphetamine.
Methods
Methamphetamine-dependent subjects (N=31), who were not seeking treatment, resided on an inpatient ward for up to 5 weeks. Abstinence was confirmed by daily urine drug screening. Venous blood was sampled for plasma hormone levels, and positron emission tomography with [18F]fallypride was performed to determine dopamine D2 receptor availability during the first week of abstinence. Venous blood was sampled again for hormone levels during the fourth week of abstinence. Matched healthy volunteers (N=23) participated as a comparison group.
Results
Methamphetamine-dependent and healthy comparison subjects did not differ in plasma ACTH or cortisol levels, but had an elevated plasma prolactin at both the first week and fourth week of abstinence. There was no group difference in pituitary dopamine D2 receptor availability.
Conclusion
Methamphetamine-dependent individuals have abnormalities in prolactin regulation, which is not likely due to alterations in pituitary dopamine D2 receptor availability.
Scientific Significance
Methamphetamine dependence is associated with elevated prolactin levels, which may contribute to medical co-morbidity in afflicted individuals.
Keywords: methamphetamine, ACTH, cortisol, prolactin, pituitary, PET, fallypride, dopamine
INTRODUCTION
Exposure to environmental stress and concomitant abnormalities in hormonal markers of stress regulation are considered to be risk factors for the development of substance use disorders (1). Conversely, substance abuse itself may cause alterations in hormonal stress regulation (1). Despite extensive literature demonstrating abnormalities of pituitary hormonal regulation associated with substance abuse (including cocaine; reviewed in [2]), little is known about the effects of chronic methamphetamine (MA) abuse on pituitary hormonal regulation.
Neural circuits in the tuberoinfundibular tract regulate production of pituitary hormones, including adrenocorticotropin (ACTH) and prolactin (3). In response to stress, cortisol release is stimulated by ACTH, and provides negatively feedback on ACTH production as part of the hypothalamic-pituitary-adrenal axis (2). Unlike the secretion of other anterior pituitary hormones, prolactin secretion is tonically suppressed by the hypothalamus via adenohypophyseal dopaminergic projections (3). Dopamine inhibits the synthesis and secretion of prolactin through interactions at pituitary dopamine D2 receptors (3; 4).
Plasma levels of ACTH increase following acute cocaine administration in animals and humans, but basal ACTH levels remain unchanged with chronic administration (5; 6). By contrast, cortisol levels are elevated during early abstinence from cocaine in humans (7; 8). Elevated plasma prolactin has been demonstrated in preclinical and human studies of cocaine dependence (5; 9; 10; 7; 8). However, to date, there have been no reports on plasma levels of ACTH, cortisol, or prolactin associated with MA dependence. We therefore tested whether plasma concentrations of these hormones are abnormal in MA-dependent participants, thereby possibly contributing to the medical co-morbidity associated with MA dependence. MA dependence is associated with abnormalities in striatal dopamine D2/D3 receptor binding potential [11]. Since adenohypophyseal dopaminergic projections onto pituitary dopamine D2 receptors inhibit prolactin secretion, we also tested whether pituitary dopamine D2 receptor availability differs in abstinent MA-dependent participants compared to a matched healthy comparison group.
METHODS
Participants
Thirty-one MA-dependent participants (MA subjects) and 23 healthy comparison subjects participated in this study after providing written informed consent, as approved by the UCLA Institutional Review Board. Participants in both groups were excluded if they had any major medical illness as determined by medical history, physical examination, and laboratory studies. MA subjects met criteria for current MA dependence, were not dependent on other substances (besides nicotine) and had a positive MA urine drug screen at study entry. Comparison subjects had no history of substance use disorders (besides nicotine dependence), and their urine drug screens tested negative throughout the study. Subjects were excluded in both groups if they met criteria for any other current Axis I diagnoses based on SCID-IV, or any current psychotropic medication use. Pregnant or breastfeeding women were excluded from the study. MA subjects resided at the UCLA inpatient General Clinical Research Center (GCRC) for the duration of the studies (either 2 or 5 weeks), and abstinence was confirmed via daily urine and alcohol breath testing. Comparison subjects were studied on an outpatient basis. Most of the MA subjects identified smoking as the primary route of MA administration. All subjects were compensated for their participation.
Hormone Assays
Blood samples were collected at a standard time (2:30 PM), on days 2 and 30 of supervised abstinence for the MA subjects, and for the comparison subjects at study entry and 1 month later. Blood samples (5 ml each) were collected in heparinized tubes (cortisol, prolactin) and EDTA-containing tubes (ACTH), centrifuged, and the plasma transferred to cryovials. Plasma samples were stored at −70°C prior to assay. Hormone assays on thawed plasma samples were conducted on a Siemens Medical Solution Diagnostic IMMULITE 1000 Chemiluminescent Immunoassay instrument (Siemens USA, Deerfield, IL). Intra- and inter-assay variability was less than 10%.
Positron Emission Tomography (PET)
MA subjects underwent [18F]fallypride PET scanning during days 4–8 of inpatient abstinence, whereas comparison subjects were scanned within 2 weeks of study entry. Details of our methods and procedures for PET analysis of dopamine D2/D3 receptor availability with [18F]fallypride have been previously described (11). Dopamine D3 receptor expression has been shown to be absent in the pituitary gland (12); therefore, pituitary [18F]fallypride PET signal is due to D2 receptor binding. Details of PET acquisition and image processing have been previously described [11]. Regions of Interest (ROIs) for the pituitary gland were directly defined via a 12-mm diameter by 15-mm long cylinder placed on the pituitary using a spatially aligned mean PET scan image (reoriented in the axial plane) for each subject, after Moresco et al. (13). Details for the generation of both pituitary and cerebellar ROIs and subject-specific K2’ values have been previously described (11). Time-activity curves (TACs) were extracted from ROIs in the realigned PET frames as described (11). Both a simplified reference-tissue model (against bilateral cerebellar ROIs) and Logan non-invasive model plot (14; using subject-specific K2’ values previously generated (see [11] for details)) were used to calculate binding potentials (BPNDs)from the TACs using PMOD 3.0 (PMOD Technologies, Zurich, Switzerland).
Data Analysis
Demographic variables were analyzed by one-way analysis of variance (ANOVA) comparisons (continuous variables) or Pearson’s Chi-square (categorical variables). The main effects of group (MA, comparison subjects), time, and the interaction of group x time for hormone levels at both time-points and dopamine D2 receptor availability were done using ANOVA. Correlations were performed using Pearson’s moment correlation. Data for continuous variables are reported as mean ± standard deviation. Demographic and behavioral variables that differed between study groups (smoking, education) were used as covariates to adjust for potential bias. Repeated-measures ANOVA was conducted with post hoc Bonferroni correction. All statistics were performed using SPSS software (v. 17.0; Chicago, IL).
RESULTS
Characteristics of participants (Table 1)
Table 1.
Demographics of Study Participants
| Comparison | MA | ||||
|---|---|---|---|---|---|
| # of subjects | N=23 | N=31 | |||
| F | df | p | |||
| Age | 34.1(8.7) | 34.6(10.1) | 0.04 | 53 | 0.842 |
| Years Education: | 14.2(2.5) | 12.5(1.2) | 11.33 | 53 | 0.001** |
| Years Maternal Education: | 13.4(4.0) | 12.5(2.6) | 0.98 | 52 | 0.33 |
| χ2 | df | p | |||
| Smokers: | 13 (56%) | 26 (84%) | 4.9 | 1 | 0.027* |
| Gender: | |||||
| Male | 12 (58%) | 18 (52%) | 0.557 | 1 | 0.456 |
| Ethnicity: |
overall ethnicity |
6.0 | 5 | 0.299 | |
| White | 13 (63%) | 19 (56%) | |||
|
Substance Use |
|||||
| Years of MA use | n.a. | 12.2(7.4) | |||
| Days of Use/30 days | n.a. | 19.1(9.3) | |||
Continuous variables are listed as mean of each group with standard deviation in parentheses. Categorical variables are listed as the total of each group with percentage in parentheses. P values reaching statistical significance are highlighted:
significant group difference p<0.05;
group difference significance p<0.01.
A greater percentage of MA subjects (84%) than comparison subjects (56%) were tobacco smokers (1 df, p=0.027), and MA subjects had fewer years of education (12.5±1.2 vs 14.2±2.5; F(1,53)=11.33, p=0.001). The groups did not differ in age, gender, overall ethnicity, or maternal educational level. MA subjects reported using MA for 12.2±7.4 years, and using 19.1±9.3 days out of the last 30 prior to study entry.
Hormone Levels
There was no significant difference between groups for either ACTH (Fig. 1A; MA: N=16; control: N=18, F(1,64)=0.546, p=0.463) or cortisol (Fig. 1B; MA: N=16; control: N=17, F(1,62)=1.365, p=0.247). However, plasma prolactin levels tested at day 2 of abstinence were higher for MA subjects (Fig. 1C; N=31; 11.3±4.2 ng/ml) than levels obtained from a control comparison group at study entry (Fig. 1C; N=23; 8.1±4.4 ng/ml; F(4,53)=4.679, p=0.003). A significant effect of group was also found for a subset of subjects re-tested after 4 weeks of abstinence (Fig 1C; (N=14 MA, N=17 control subjects; F(2,58)=4.7, p=0.013)). There was no group x time interaction or main effect of time for prolactin levels (Fig 1C; F(1,58)=0.080, p=0.778).
Figure 1.
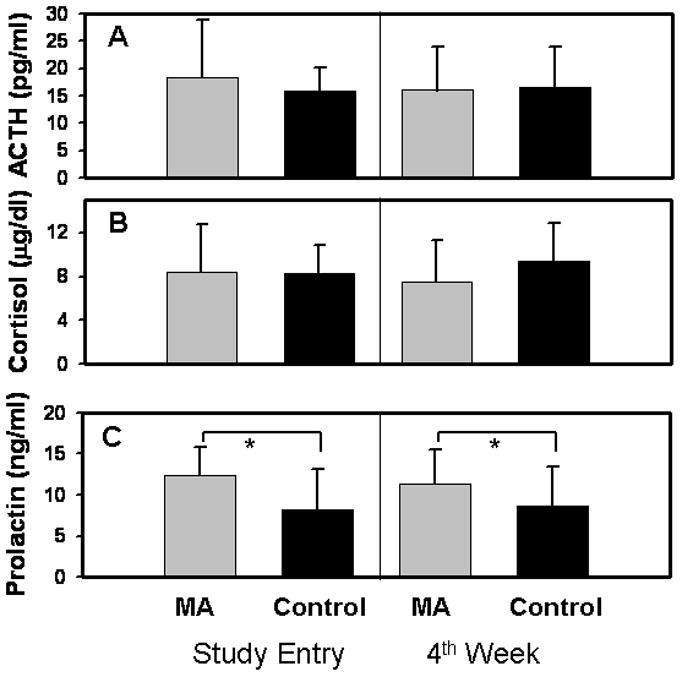
Plasma levels of ACTH, cortisol, and prolactin in MA subjects and comparison subjects (control). Data represent mean plasma levels of each hormone at each time-point; error bars represent standard deviation of each value. There were no group differences in ACTH (fig 1A) or cortisol (fig 1B). Plasma ACTH (fig 1A, N=16) and cortisol (fig 1B, N=18) in MA subjects (gray bars) at day 2 and 30 of abstinence, compared to control subjects (black bars).
Fig 1C: Prolactin was significantly higher in MA subjects (N=14) at both day 2 and day 30 of abstinence than in comparison subjects assessed at study entry and 1 month later (N=17). Comparisons of hormone levels among MA and control subjects demonstrated no effect of time, but a significant effect of group. *: p<0.05 for the group difference.
Prolactin levels differed by gender, with lower values for males than females for both control subjects (males 6.6±3.3 ng/ml, n=9; females 9.8±5.2 ng/ml, n=11) and MA subjects (males 10.5±4.0 ng/ml, n=18; females 12.3±4.5 ng/ml, n=13), and the effect of gender was statistically significant (F(1,50)=4.11, p=0.05).
Among MA subjects, there was no correlation between prolactin level at day 2 of abstinence and either years of MA use (r=−0.23, p=0.21) or frequency of MA use in the last 30 days (r=−0.02, p=0.91) among MA subjects.
Pituitary D2 receptor binding potential in abstinent MA subjects
D2 receptor availability in the pituitary glands of the MA subjects (N=14) was found to be: for simplified reference tissue model (SRTM), BPND 3.68±1.21; for Logan plot, BPND 3.58±1.29. We compared these values to the similarly calculated pituitary binding potentials for the comparison subjects (N=11), which yielded BPNDs of 4.02±1.10 (SRTM) and 4.00±1.09 (Logan plot), and found no significant differences between groups for either model (SRTM: F(1,23)=0.51, p=0.48; Logan plot: F(1,23)=0.72, p=0.41). There was no group difference in average pituitary volume (MA: 917.6±267.7 mm3; control: 945.8±244.9 mm3; F(1,23)=0.07, p=0.79).
DISCUSSION
Plasma ACTH and Cortisol
To our knowledge, this is the first assessment of ACTH and cortisol levels in MA-abusing subjects, showing no difference from those measured in a healthy comparison group. These findings stand in contrast to reports of higher cortisol levels in recently abstinent abusers of alcohol, opioids, and cocaine (7; 15; 16; 17). In a study of cocaine-dependent subjects, afternoon cortisol levels were elevated early in treatment, then returned to a baseline, similar to values in a control group, by the third week of abstinence (7).
ACTH is elevated in response to acute cocaine administration to humans, but basal ACTH levels are normal in cocaine-dependent individuals (reviewed in [18]). Therefore, our finding of normal basal ACTH levels in abstinent MA subjects is consistent with previously published studies of stimulant dependence.
Plasma Prolactin
Our findings of elevated plasma prolactin at days 2 and 30 in abstinent MA subjects have not been previously described in the literature. Elevated prolactin has been reported in the first week of abstinence from chronic cocaine abuse (N=21), with gradual normalization over 3 weeks (7). In a larger study of 141 treatment-seeking cocaine dependent outpatients, prolactin levels were elevated at study entry compared to a control group, and were also correlated with amount (g/day) of cocaine use (8). Conversely, prolactin was not elevated by acute MA administration in humans (19).
When combined with the results in MA subjects (this manuscript), these data provide evidence that chronic stimulant exposure can induce deficits in prolactin regulation during early abstinence.
Pituitary dopamine D2 receptor binding potential
It is well-established that MA dependence is associated with a reduction in striatal dopamine D2/D3 receptor binding potential (20), which recovers only partially, even after several years of abstinence (21). Our data on the relative preservation of dopamine D2 receptor binding in the pituitary in MA subjects therefore adds to data from an overlapping subject pool, which demonstrated that caudate and putamen dopamine D2/D3 receptor binding was lower in MA-dependent subjects than in healthy control subjects, with no group difference in D2/D3 binding potential in the nucleus accumbens (11).
In a postmortem study of a mixed population of 76 MA-abusers and individuals with other drug use histories, there were more adenohypophyseal clusterin-containing follicles in the drug users than in a non-drug using control group (22). This finding was thought to indicate dysfunctional dopaminergic neurotransmission originating in the hypothalamus (22). Taken together, our findings of intact dopamine D2 receptor binding in the pituitary in MA subjects, together with data suggesting that MA abuse is associated with abnormalities in hypophyseal dopaminergic projections implicate dysfunctional dopaminergic release at the adenohypophyseal nerve terminals as responsible for impaired pituitary prolactin regulation in stimulant dependence.
Limitations
The research participants in this study were screened to exclude any subjects with co-morbid medical or psychiatric illness. Therefore, the results of this study may not be generalizable to treatment-seeking MA-abusing patients. MA subjects were studied as inpatients, whereas our healthy comparison subjects were studied as outpatients. The sample size was relatively small, which limits the power to detect modest differences in either hormone levels or pituitary dopamine D2 receptor binding potential between study groups. Finally, our study sampled hormonal levels at only 1 afternoon time-point, which may not provide for adequate time resolution to detect subtle diurnal changes in hormone levels (e.g., [7]).
Implications for the medical burden of MA dependence
Our finding of elevated prolactin in human MA-dependent subjects adds to the clinical literature on the toxicity of MA. Aside from its actions on lactation and reproductive processes, prolactin plays a role in the immune system and the stress response (23). Plasma concentrations of prolactin follow a circadianrhythm and peak during sleep (4). Hyperprolactinemia (prolactin levels >2 times normal range) is associated with antipsychotic treatment, pregnancy, and endocrine tumors and may result in gynecomastia, galactorrhea, infertility, and sexual dysfunction and increased long term risk of breast cancer and osteoporosis (reviewed in [23]). The medical consequences of prolonged hyperprolatinemia include reproductive problems including amenorrhea and compromised immune function [18]. Our findings of a roughly 40% higher level of basal prolactin in abstaining MA subjects compared to control subjects suggests that some MA abusers are at risk for developing symptoms of hyperprolactinemia. Symptoms of MA withdrawal (including subjective sleep disturbance and depressive symptoms) typically resolve within several weeks of abstinence (24; 25), and therefore do not correlate with our finding of continuously elevated prolactin over 1 month of abstinence. The clinical significance of elevated prolactin levels in abstinence from MA is unclear at present, but could be an area for future investigation.
Supplementary Material
Acknowledgments
Supported by: NIH Grants DA022539, DA020726, DA15179 (EDL), MO1 RR 00865 (UCLA GCRC), an endowment from the Katherine K. and Thomas P. Pike Chair in Addiction Studies, and by a gift from the Marjorie M. Greene Trust.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest with the work described here.
References
- 1.Uhart M, Wand GS. Stress, alcohol and drug interaction: an update of human research. Addict Biol. 2009;14(1):43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–30. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durham RA, Eaton MJ, Moore KE, Lookingland KJ. Effects of selective activation of dopamine D2 and D3 receptors on prolactin secretion and the activity of tuberoinfundibular dopamine neurons. Eur J Pharmacol. 1997;335(1):37–42. doi: 10.1016/s0014-2999(97)01179-5. [DOI] [PubMed] [Google Scholar]
- 4.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure function and regulation of secretion. Physiol Rev. 2000;(4):1523–631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 5.Pilotte NS, Sharpe LG, Dax EM. Multiple, but not acute, infusions of cocaine alter the release of prolactin in male rats. Brain Res. 1990;512(1):107–12. doi: 10.1016/0006-8993(90)91177-i. [DOI] [PubMed] [Google Scholar]
- 6.Mello NK, Mendelson JH. Cocaine’s effects on neuroendocrine systems: clinical and preclinical studies. Pharmacol Biochem Behav. 1997;57(3):571–99. doi: 10.1016/s0091-3057(96)00433-9. [DOI] [PubMed] [Google Scholar]
- 7.Contoreggi C, Herning RI, Koeppl B, Simpson PM, Negro PJ, Jr, Fortner-Burton C, Hess J. Treatment-seeking inpatient cocaine abusers show hypothalamic dysregulation of both basal prolactin and cortisol secretion. Neuroendocrinology. 2003;78(3):154–62. doi: 10.1159/000072797. [DOI] [PubMed] [Google Scholar]
- 8.Patkar AA, Mannelli P, Certa KM, Peindl K, Murray H, Vergare MJ, Berrettini WH. Relationship of serum prolactin with severity of drug use and treatment outcome in cocaine dependence. Psychopharmacology (Berl) 2004;176(1):74–81. doi: 10.1007/s00213-004-1856-0. [DOI] [PubMed] [Google Scholar]
- 9.Farré M, de la Torre R, González ML, Terán MT, Roset PN, Menoyo E, Camí J. Cocaine and alcohol interactions in humans: neuroendocrine effects and cocaethylene metabolism. J Pharmacol Exp Ther. 1997;283(1):164–76. [PubMed] [Google Scholar]
- 10.Mendelson JH, Teoh SK, Lange U, Mello NK, Weiss R, Skupny A, Ellingboe J. Anterior pituitary, adrenal, and gonadal hormones during cocaine withdrawal. Am J Psychiatry. 1988;145(9):1094–8. doi: 10.1176/ajp.145.9.1094. [DOI] [PubMed] [Google Scholar]
- 11.Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29(47):14734–40. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neto LV, Machado Ede O, Luque RM, Taboada GF, Marcondes JB, Chimelli LM, Quintella LP, Niemeyer P, Jr, de Carvalho DP, Kineman RD, Gadelha MR. Expression analysis of dopamine receptor subtypes in normal human pituitaries, nonfunctioning pituitary adenomas and somatotropinomas, and the association between dopamine and somatostatin receptors with clinical response to octreotide-LAR in acromegaly. J Clin Endocrinol Metab. 2009;94(6):1931–7. doi: 10.1210/jc.2008-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moresco RM, Cavallaro R, Messa C, Bravi D, Gobbo C, Galli L, Lucignani G, Colombo C, Rizzo G, Veloná I, Smeraldi E, Fazio F. Cerebral D2 and 5-HT2 receptor occupancy in Schizophrenic patients treated with olanzapine or clozapine. J Psychopharmacol. 2004;18(3):355–65. doi: 10.1177/026988110401800306. [DOI] [PubMed] [Google Scholar]
- 14.Logan J, Fowler JS, Volkow ND, Wang G-J, Ding Y-S, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005;29(7):1351–5. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovallo WR. Cortisol secretion patterns in addiction and addiction risk. Int J Psychophysiol. 2006;59(3):195–202. doi: 10.1016/j.ijpsycho.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li SX, Li J, Epstein DH, Zhang XY, Kosten TR, Lu L. Serum cortisol secretion during heroin abstinence is elevated only nocturnally. Am J Drug Alcohol Abuse. 2008;34(3):321–8. doi: 10.1080/00952990802013664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mello NK, Mendelson JH. Cocaine’s effects on neuroendocrine systems: clinical and preclinical studies. Pharmacol Biochem Behav. 1997;57(3):571–99. doi: 10.1016/s0091-3057(96)00433-9. [DOI] [PubMed] [Google Scholar]
- 19.Gouzoulis-Mayfrank E, Thelen B, Habermeyer E, Kunert HJ, Kovar KA, Lindenblatt H, Hermle L, Spitzer M, Sass H. Psychopathological, neuroendocrine and autonomic effects of 3,4-methylenedioxyethylamphetamine (MDE), psilocybin and d-methamphetamine in healthy volunteers. Results of an experimental double-blind placebo-controlled study. Psychopharmacology (Berl) 1999;142(1):41–50. doi: 10.1007/s002130050860. [DOI] [PubMed] [Google Scholar]
- 20.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158(12):2015–21. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 21.Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21(23):9414–8. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishikawa T, Zhu BL, Miyaishi S, Ishizu H, Maeda H. Increase in clusterin-containing follicles in the adenohypophysis of drug abusers. Int J Legal Med. 2007;121(5):395–402. doi: 10.1007/s00414-006-0138-2. [DOI] [PubMed] [Google Scholar]
- 23.Bostwick JR, Guthrie SK, Ellingrod VL. Antipsychotic-induced hyperprolactinemia. Pharmacotherapy. 2009;29(1):64–73. doi: 10.1592/phco.29.1.64. [DOI] [PubMed] [Google Scholar]
- 24.Newton TF, Kalechstein AD, Duran S, Vansluis N, Ling W. Methamphetamine abstinence syndrome: preliminary findings. Am J Addict. 2004;13(3):248–55. doi: 10.1080/10550490490459915. [DOI] [PubMed] [Google Scholar]
- 25.McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100(9):1320–9. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


