Abstract
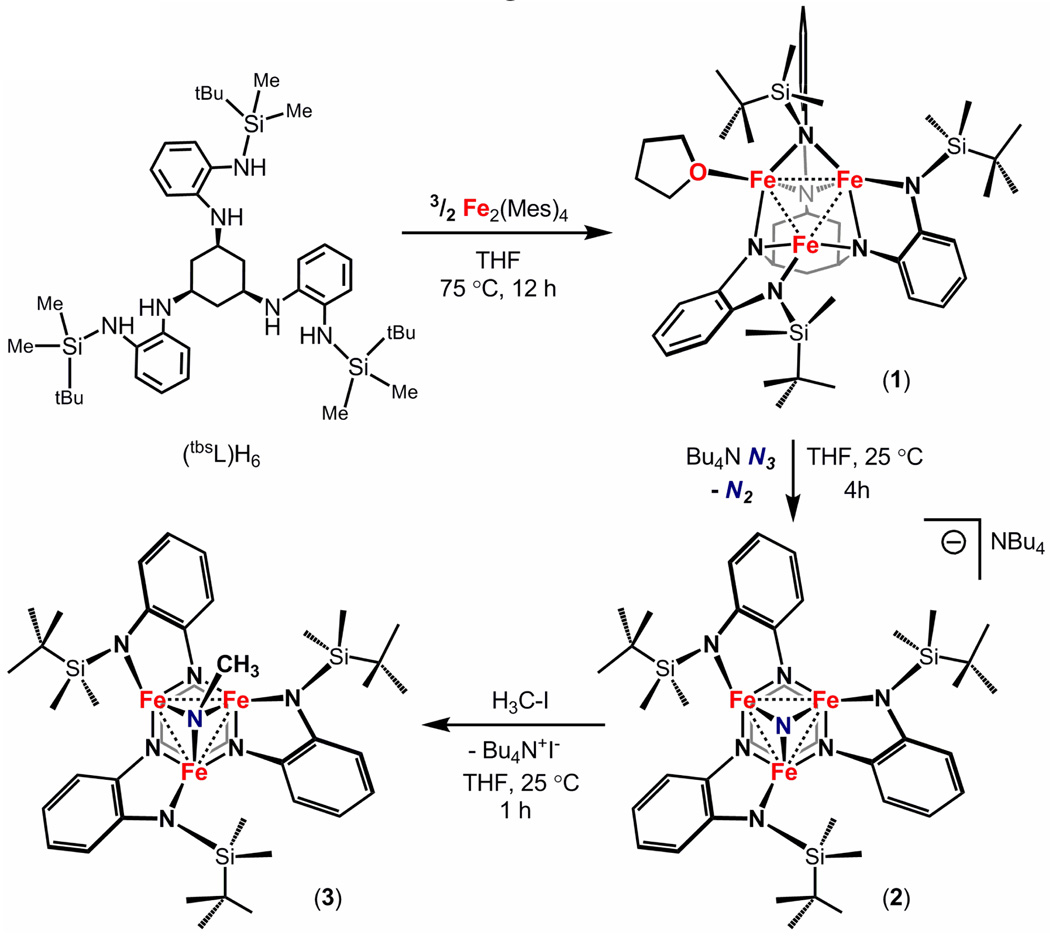
Utilizing a hexadentate ligand platform, a high-spin trinuclear iron complex of the type (tbsL)Fe3(thf) was synthesized and characterized ([tbsL]6− = [1,3,5-C6H9(NPh-o-NSitBuMe2)3]6−). The silyl-amide groups only permit ligation of one solvent molecule to the tri-iron core, resulting in an asymmetric core wherein each iron ion exhibits a distinct local coordination environment. The triiron complex (tbsL)Fe3(thf) rapidly consumes inorganic azide ([N3]NBu4) to afford an anionic, trinuclear nitride complex [(tbsL)Fe3(μ3-N)]NBu4. The nearly C3-symmetric complex exhibits a highly pyramidalized nitride ligand that resides 1.205(3) Å above the mean triiron plane with short Fe–N (1.871(3) Å) distances and Fe–Fe separation (2.480(1) Å). The nucleophilic nitride can be readily alkylated via reaction with methyl iodide to afford the neutral, trinuclear methylimide complex (tbsL)Fe3(μ3-NCH3). Alkylation of the nitride maintains the approximate C3-symmetry in the imide complex, where the imide ligand resides 1.265(9) Å above the mean triiron plane featuring lengthened Fe–Nimide bond distances (1.892(3) Å) with nearly equal Fe–Fe separation (2.483(1) Å).
In our lab we are currently investigating the synthesis and reactivity of polynuclear metal complexes. Recent interest in such systems stems from their potential to cooperatively bind and activate small molecules, utilizing the expanded redox reservoir afforded by the polynuclear core.1 This design strategy may prove effective as functional surrogates for polynuclear reaction sites found in heterogeneous catalysts (i.e., Fe(111) face in Haber-Bosch dinitrogen reduction2) or in metalloenzyme cofactors (e.g., FeMo-cofactor in nitrogenase3). The polynuclear reaction sites in the cited examples likely facilitate binding, activation and chemical breakdown of substrate. New methods for generating polynuclear reaction sites featuring coordinatively unsaturated metal ions are required before the cooperative reactivity can be assessed. Herein we report the synthesis of a reactive triiron complex that exhibits the ability to mediate multi-electron reaction chemistry within the trinuclear core, as demonstrated by activation of inorganic azide to produce a nucleophilic triiron μ3-nitrido complex.
Previously we have shown the utility of using hexa-amide based ligand platforms to proximally coordinate three divalent metal ions, supporting trinuclear cores with a wide span of metal ion separations (2.274(1)–2.847(1) Å).4 To probe the cooperative reaction chemistry of a triiron scaffold, we targeted a ligand platform that would restrict exogenous ligands from binding to the metal ions. Following a similar protocol for the preparation of tame-based scaffolds (tame = 1,1,1-tris(aminomethyl)ethane),4 the tris-amine α-α-α-1,3,5-trisaminocyclohexane hydrobromic acid (tach·3HBr)5 was derivatized with o-fluoronitrobenzene via nucleophilic aromatic substitution (6 eq. Cs2CO3, ACN 120°C for 72 h). Subsequent reduction of the bright orange tri-nitro species using Zn dust (15 equiv) and saturated NH4Cl in tetrahydrofuran afforded the hexamine platform 1,3,5-C6H9(NHPh-o-NH2)3 in good overall yield (80%). Deprotonation of 1,3,5-C6H9(NHPh-o-NH2)3 with three equivalents of n-butyl lithium followed by reaction with t-butyldimethylsilylchloride afforded the target ligand 1,3,5-C6H9(NHPh-o-NHSiMe2tBu)3 (tbsLH6) in good isolated yield (45%) on a multigram scale.
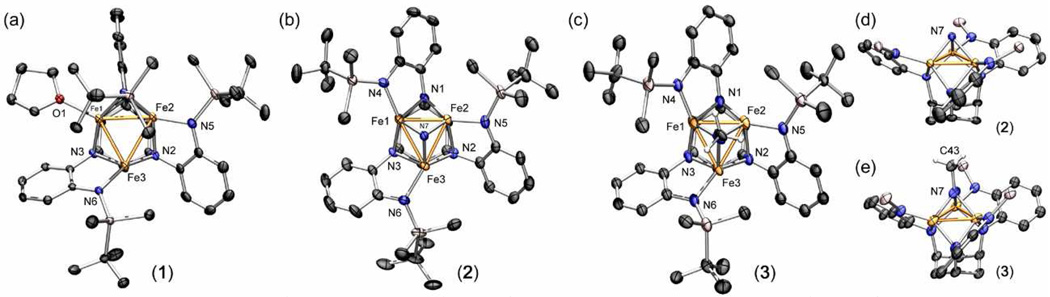
Metallation of the ligand platform was effected by heating tbsLH6 with Fe2(mes)4 (1.5 equivalents) to 75 °C for 12 hours in tetrahydrofuran. Evolution of mesitylene and consumption of ligand was determined by 1H NMR, though the reaction product is 1H NMR silent. Storing the brown oil in cold hexanes (−33 °C) precipitated the triiron complex (tbsL)Fe3(thf) (1) in good overall yield (80%, Scheme 1). Crystallographic analysis of single crystals of 1 provided the composition consisting of three iron ions and an asymmetrically bound hexa-amide ligand (Figure 1a). Unlike the trinuclear complexes we have previously reported, the large silyl substituents on the ligand platform prevent two of the three apical amide groups from bridging adjacent metal ions. Only a single silylamide bridges Fe1 and Fe2, while the other two silylamides are terminally bound to Fe2 and Fe3, giving each iron ion a distinct coordination environment. The average Fe–Fe separation is 2.577(6) Å, which is comparable to previously reported complexes.4 The zero-field 57Fe Mössbauer spectrum of 1 reflects its asymmetry as the 110 K spectrum can only be modeled using three quadrupole doublets (δ, |ΔEQ| (mm/s)): component 1 0.89, 1.68; component 2 0.49, 1.57; component 3 0.50, 1.92). The 1H NMR silent material is consistent with the large solution magnetic moment for paramagnetic 1 (12.0(2) μB) determined by the Evans' method.
Scheme 1.
Figure 1.
Solid-state structures for (a) (tbsL)Fe3(thf) (1), (b) [(tbsL)Fe3(μ3-N)][NBu4] (2) (side view in d), and (c) (tbsL)Fe3(μ3-NMe) (3) (side view in e) with the thermal ellipsoids set at the 50% probability level (hydrogen atoms, solvent molecules, and Bu4N cation in 2 omitted for clarity; Fe orange, C black, H white, N blue, O red, Si Pink). Bond lengths (Å) for 1: Fe1-Fe2, 2.6129(5); Fe1-Fe3, 2.5061(5); Fe2-Fe3, 2.6118(5); Fe-Nbase, 2.047(2); Fe-Ntbs, 1.950(2); Fe-O, 2.1162(18); for 2: Fe1-Fe2, 2.4212(7); Fe1-Fe3, 2.5444(7); Fe2-Fe3, 2.4737(7); Fe-N7avg, 1.871(3); Fe-Nbase, 2.030(3); Fe-Ntbs, 1.950(3); for 3: Fe1-Fe2, 2.449(3); Fe1-Fe3, 2.487(2); Fe2-Fe3, 2.513(2); Fe-N7avg, 1.892(3); N7-C43, 1.457(14); Fe-Nbase, 1.996(10); Fe-Ntbs, 1.904(10).
Reaction of complex 1 with tetrabutylammonium azide at room temperature results in the dissociation of thf from 1 and consumption of the azide as judged by the absence of the azide stretch (νN3) in the IR spectrum. Storing the reaction product in diethyl ether at −33 °C deposited crystals of the reaction product suitable for X-ray diffraction analysis. The solid-state molecular structure for the product, shown in Figure 1b, confirmed the breakdown of the azide to produce the nearly C3-symmetric nitride product [(tbsL)Fe3(μ3-N)]NBu4 (2) (Scheme 1). While formation of iron-nitride complexes proceeding via thermal or photolytic decomposition of iron azides embedded in tetraazamacrocylic ligand environments is well precedented,7 most polynuclear (nuclearity exceeding two) iron-nitride species form via reduction of nitrosyl ligands8 or via metathetical routes using N(SnMe3)3.9 However, these routes give rise to unpredictable nuclearity and cluster geometries. Formation of the anionic nitride 2 proceeds via the two electron oxidation of 1 where the overall complex geometry is dictated by the trinucleating ligand (tbsL).
Complex 2 features three iron centers that bind the central μ3-nitride ligand with average Fe–N and Fe–Fe bond lengths of 1.871(3) and 2.480(1) Å, respectively. Each of the iron ions sit in distorted tetrahedral, nearly cis-divacant octahedral, sites, bridged by two ligand internal amide residues (average Fe-Nint 2.030(3) Å) and capped by one terminal silyl-amide (average Fe-NSi 1.950(3) Å). The ligand reorganization accommodates binding of the monoatomic nitride ligand. The nitride is heavily pyramidalized (Σ(Fe-N7-Fe) = 248.94(14)°, NH3 is 319.8°), sitting 1.205(3) Å above the triiron basal plane. The solution magnetic moment for paramagnetic 2 is 7.3(2) μB, while the zero-field 57Fe Mössbauer spectrum of 2 obtained at 120 K shows two quadrupole doublets (δ, |ΔEQ| (mm/s)): component 1 0.37, 1.78 (48%); component 2 0.39, 1.23 (51%)). The lower isomer shift of 2 compared to 1 reflects the increased oxidation of the trinuclear core.4
In contrast to the electrophilic nature of terminal Fe-nitride complexes,6 the nucleophilicity of the nitride in complex 2 can be demonstrated by its rapid reaction with methyl iodide to afford a hexane-soluble methyl imido complex (tbsL)Fe3(μ3-NCH3) (3) with generation of Bu4NI. Previous examples of triiron imido complexes were synthesized via reaction of iron carbonyl precursors with silylazide, nitroarene, or alkyldiazene reagents.10 Storing complex 3 in hexanes at −33 °C deposited crystals suitable for X-ray diffraction analysis. The solid-state molecular structure for 3 is shown in Figure 1c. Structurally similar to 2, complex 3 features a central μ3-imide ligand with average Fe–N and Fe–Fe bond lengths of 1.892(3) and 2.483(3) Å, respectively. The imide ligand sits 1.265(9) Å above the triiron basal plane, slightly extended from the nitride (see core highlights shown in Figure 1d and e). Like 2, the zero field 57Fe Mössbauer spectrum of paramagnetic 3 (5.3(2) μB) obtained at 110 K shows two quadrupole doublets with identical isomer shifts (δ, |ΔEQ| (mm/s)): component 1 0.36, 1.67 (65%); component 2 0.37, 0.94 (35%)). Obtaining a spectrum at 180 K causes the two quadrupole doublets to coalesce (δ, |ΔEQ| (mm/s)): 0.34, 1.44), suggesting the Fe nuclei relax faster than the Mössbauer time scale (10−7 s).
In summary, the silyl-substituted ligand platform [tbsL]6− supports triiron complex formation, maintaining a degree of coordinative unsaturation at the iron ions. Despite the asymmetry of the triiron thf-bound complex, the compound rapidly reacts with a single equivalent of inorganic azide to produce a nearly C3-symmetric, μ3-nitride complex. The observed nucleophilic reactivity of the anionic nitride demonstrates the utility of embedding a polynuclear reaction site within a single ligand manifold. This strategy permits control of the nuclearity of the resultant complex and elaboration of a cooperatively bound substrate, as demonstrated by alkylation of the nitride to afford a bridging imido complex. Work is currently underway to understand the electronic structure of these complexes and canvass the scope of reactivity of this and related trinuclear platforms with small molecule substrates.
Supplementary Material
Acknowledgments
The authors thank Harvard University for financial support, Prof. R. H. Holm for the generous use of his Mössbauer spectrometer, Novartis for a predoctoral fellowship for TMP, and the NIH for an NIH Ruth L. Kirschstein NRSA fellowship for ARF.
Footnotes
Supporting Information Available: Experimental procedures and spectral data for 1, 2, and 3; Mössbauer spectrum and parameters for 1, 2, and 3; selected crystallographic data and bond lengths for 1, 2, and 3; CIF file for 1, 2, and 3. This material is available free of charge via the internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Adams RD. J. Organometallic Chem. 2000;600:1–6. [Google Scholar]; (b) Suzuki H. Eur. J. Inorg. Chem. 2002:1009–1023. [Google Scholar]; (c) Dyson PJ. Coord. Chem. Rev. 2004;248:2443–2458. [Google Scholar]; (d) Pap JS, DeBeer George S, Berry JF. Angew. Chem. Int. Ed. 2008;47:10102–10105. doi: 10.1002/anie.200804397. [DOI] [PubMed] [Google Scholar]
- 2.(a) Bozso F, Ertl G, Grunze M, Weiss M. J. Cat. 1977;49:18–41. [Google Scholar]; (b) Ertl G. In: Catalytic Ammonia Synthesis. Jennings JR, editor. New York: Plenum Press; 1991. pp. 109–131. [Google Scholar]
- 3.(a) Burgess BK, Lowe DJ. Chem. Rev. 1996;96:2983–3012. doi: 10.1021/cr950055x. [DOI] [PubMed] [Google Scholar]; (b) Dos Santos PC, Igarashi RY, Lee H-I, Hoffman BM, Seefeldt LC, Dean DR. Acc. Chem. Res. 2005;38:208–214. doi: 10.1021/ar040050z. [DOI] [PubMed] [Google Scholar]; (c) Hoffman BM, Dean DR, Seefeldt LC. Acc. Chem. Res. 2009;42:609–619. doi: 10.1021/ar8002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Q, Betley TA. Angew. Chem. Int. Ed. 2011;50:709–712. doi: 10.1002/anie.201005198. [DOI] [PubMed] [Google Scholar]
- 5.Bowen T, Planalp RP, Brechbiel MW. Bioorg. & Med. Chem. Lett. 1996;6:807–810. [Google Scholar]
- 6.(a) Betley TA, Peters JC. J. Am. Chem. Soc. 2004;126:6252–6254. doi: 10.1021/ja048713v. [DOI] [PubMed] [Google Scholar]; (b) Vogel C, Heinemann FW, Sutter J, Anthon C, Meyer K. Angew. Chem., Int. Ed. 2008;47:2681–2683. doi: 10.1002/anie.200800600. [DOI] [PubMed] [Google Scholar]; (c) Scepaniak JJ, Fulton MD, Bontchev RP, Duesler EN, Kirk ML, Smith JM. J. Am. Chem. Soc. 2008;130:10515–10517. doi: 10.1021/ja8027372. [DOI] [PubMed] [Google Scholar]
- 7.(a) Summerville DA, Cohen IA. J. Am. Chem. Soc. 1976;98:1747–1752. doi: 10.1021/ja00423a019. [DOI] [PubMed] [Google Scholar]; (b) Wagner W-D, Nakamoto K. J. Am. Chem. Soc. 1988;110:4044–4045. [Google Scholar]; (c) Meyer K, Bill E, Mienert B, Weyhermüller T, Wieghardt K. J. Am. Chem. Soc. 1999;121:4859–4876. [Google Scholar]
- 8.(a) Fjare DE, Gladfelter WL. J. Am. Chem. Soc. 1981;103:1572–1574. [Google Scholar]; (b) Hourihane R, Spalding TR, Ferguson G, Deeney T, Zanello P. J. Chem. Soc., Dalton Trans. 1993:43–46. [Google Scholar]; (c) Della Pergola R, Bandini C, Demartin F, Diana E, Garlaschelli L, Stanghellini PL, Zanello P. J. Chem. Soc., Dalton Trans. 1996:747–754. [Google Scholar]
- 9.Bennett MV, Stoian S, Bominaar EL, Munck E, Holm RH. J. Am. Chem. Soc. 2005;127:12378–12386. doi: 10.1021/ja052150l. [DOI] [PubMed] [Google Scholar]
- 10.Silylazide: Barnett BL, Kruger C. Angew. Chem., Int. Ed. 1971;10:910–911.. Nitroarene: Landesberg JM, Katz L, Olsen C. J. Org. Chem. 1972;37:930. Ragaini F, Song J-S, Ramage DL, Geoffroy GL, Yap GAP, Rheingold AL. Organometallics. 1995;14:387–400.. Alkyldiazene: Wucherer EJ, Tasi M, Hansert B, Powell AK, Garland M-T, Halet J-F, Saillard J-Y, Vahrenkamp H. Inorg. Chem. 1989;28:3564–3572.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.