Abstract
Mitochondria are cytoplasmic organelles responsible for life and death. Extensive evidence from animal and clinical studies suggests that mitochondria play a critical role in aging, cancer, diabetes and neurodegenerative diseases, such as Alzheimer's disease, Parkinson's disease, and Huntington's disease. Several lines of research suggest that mitochondrial oxidative damage is an important cellular change in most late-onset neurodegenerative diseases. Further, emerging evidence suggests that structural changes in mitochondria, including increased mitochondrial fragmentation and decreased mitochondrial fusion, are critical factors associated with mitochondrial dysfunction and cell death in aging and age-related diseases. In addition, epigenetic factors and lifestyle activities may contribute to selective disease susceptibility for each of these diseases. This paper discusses research that has elucidated features of mitochondria that are associated with cellular dysfunction in aging and neurodegenerative diseases. This paper also discusses mitochondrial abnormalities and potential mitochondrial therapeutics in AD.
Introduction
A growing body of evidence suggests that mitochondrial abnormalities are involved in aging and in age-related neurodegenerative diseases, cancer, diabetes, and several other diseases known to be affected by mitochondria (Lin and Beal 2006, Reddy 2008, DiMauro and Schon 2008). Causal factors for most age-related neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS) and Freidriech ataxia (FRDA), are largely unknown. Genetic defects are reported to cause a small number of neurodegenerative diseases (see Table 1), but cellular, molecular, and pathological mechanisms of disease progression and selective neuronal cell death are not understood fully in these diseases. However, based on several cellular, molecular, and animal model studies of AD, PD, ALS, FRDA, cancer, and diabetes – aging may play a large role in cell death, in these diseases (Beal 2005, Manczak et al 2005). Age-dependent, mitochondrially generated reactive oxygen species (ROS) has been identified as an important factor that is responsible for disease progression and cell death, particularly in late-onset diseases, in which genetic mutations are not causal factors (Swerdlow and Khan 2004, Beal 2005 Lin and Beal 2006, Reddy and Beal, 2008, Reddy 2008). This article discusses the role of mitochondria in the progression of age-related neurodegenerative diseases, the connection between mitochondrial abnormalities and AD progression, and drugs targeted to mitochondria in AD and other neurodegenerative diseases.
Table 1.
Common, Age-related Diseases That Involve Mitochondrial Abnormalities
| Age-related Diseases |
Prevalence | Mitochondrial Abnormalities |
|---|---|---|
| Aging | Aging is critical in several diseases that involve mitochondrial dysfunction. | DNA defects in the mitochondrial genome are responsible for aging and senescence. Accumulation of DNA defects is responsible for increased ROS production and oxidative damage in aged tissues. |
| Cancer | Most common in the aged (over 60 years of age). | Tumor cells excessively accumulate mitochondrial DNA defects and decrease mitochondrial respiration and ATP synthesis. Cancer cells up-regulate enzymes of glycolysis and adapt to decreased oxygen tension, a characteristic of most solid tumors. |
| Diabetes | Worldwide, 8% of the population suffers from diabetes. | Hyperglycemia causes pathological features of type 1 and 2 diabetes. Increased free radical production is found in hyperglycemia and has been found to disrupt glucose-stimulated insulin secretion by pancreatic beta cells. |
| AD | Worldwide, 5% of persons 65 years of age, and 50% of persons 85 years of age and older suffer from AD. 2% of the total number of AD patients have genetic mutations in APP, PS1, and PS2 genes. | Amyloid precursor proteins and amyloid beta are found in mitochondrial membranes and in the matrix of neurons affected by AD. Mitochondria APP and amyloid beta induce free radicals, decrease cytochrome oxidase activity, and inhibit ATP production. N-terminal ApoE4 is associated with mitochondria and causes mitochondrial oxidative damage. |
| PD | Worldwide, 0.5 to 1% of persons 65 to 69 years of age suffer from AD. | Both wild-type and mutant α-synuclein are found in the mitochondrial membranes and are determined to cause mitochondrial dysfunction. DJ1 is a redox sensor protein, localized to mitochondrial inter-membrane space and matrix. PINK 1 is a nuclear-encoded, mitochondrial kinase protein. Overexpression of PINK1 causes reduced mitochondrial membrane potential. Parkin is a gene product of Ubiquitin E3 ligase and is associated with the outer mitochondrial membrane. Parkin induces free radical production. LRRK2 is associated with the outer mitochondrial membrane and may induce free radicals. OMI/HTRA2 are pro-apoptotic serine proteases is a pro-apoptotic serine proteases found in the mitochondrial inter-membrane space. |
| HD | 4–10 persons per 100,000 (mainly Caucasians) suffer from HD. | Mutant huntingtin binds to the outer mitochondrial membrane and induces free radical production. This induction is interrupted with calcium uptake. Mitochondrial movement is interrupted in mitochondria from HD neurons. |
| ALS | 1–2 persons per 100,000 (variety of ethnic groups) suffer from ALS. 10% of the total number of ALS patients have genetic defects. |
SOD1 is a cytosolic ROS scavenging enzyme. Mutant SOD1 aggregates have been found in the outer mitochondrial membrane, the inner mitochondrial membrane space, and the matrix. Mutant SOD1 induces free radicals and mitochondrial dysfunction in ALS patients. |
| Friedreich Ataxia | Worldwide, 1–2 persons per 100,000 (variety of ethnic groups). | Frataxin, the gene product of Friedreich ataxia, is a mitochondrial protein that is responsible for heme biosynthesis and the formation of iron-sulfur clusters. Mutations in frataxin facilitate the accumulation of iron in mitochondria, induce free radicals, and cause mitochondrial dysfunction. |
Why are mitochondria important in neurodegenerative diseases?
Mitochondria are cytoplasmic organelles essential for life and death. They perform several cellular functions, including: 1) intracellular calcium regulation, 2) ATP production, 3) the release of proteins that activate the caspase family of proteases, 4) alteration of the reduction-oxidation potential of cells, and 5) free-radical scavenging (Reddy 2007). Mitochondria are compartmentalized into 2 lipid membranes: the outer mitochondrial membrane and the inner mitochondrial membrane. The outer membrane is porous and allows the passage of low molecular-weight substances between the cytosol and the inter-membrane space. The inner membrane provides a highly efficient barrier to ionic flow, houses the mitochondrial respiratory chain or electron transport chain (ETC), and covers the mitochondrial matrix, which contains tricarboxylic acid (TCA) and beta-oxidation (see Fig. 1). Mitochondria are transmitted maternally. However, in rare situations, paternal inheritance and a recombination of mitochondrial DNA (mtDNA) have been reported (reviewed in Reddy and Beal 2005).
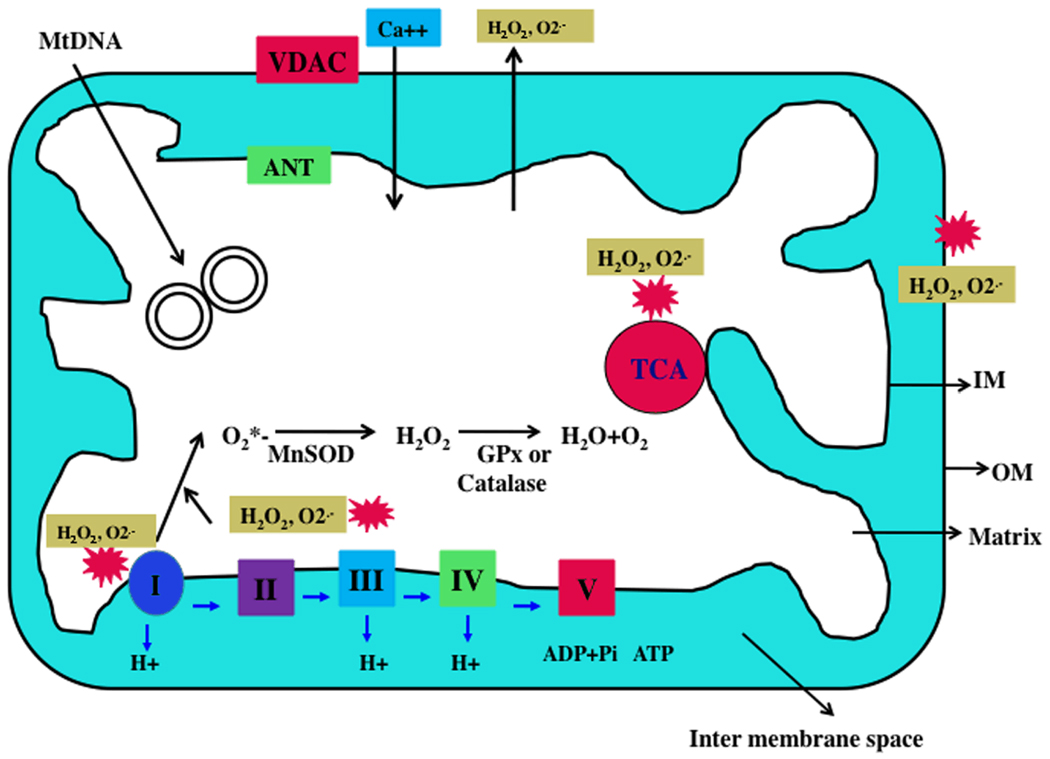
Figure 1. Structure of mitochondria.
A mitochondrion is compartmentalized with two lipid membranes: the inner mitochondrial membrane and the outer mitochondrial membrane. The inner mitochondrial membrane houses the mitochondrial respiratory chain and provides a highly efficient barrier to ionic flow. In the ETC, complexes I and III leak electrons to oxygen, producing primarily superoxide radicals. Superoxide radicals are dismutated by manganese superoxide dismuase and produce H2O2. In addition, ETC involves H2O2 reducing to H2O and O2 by catalase or glutathione peroxidase accepting electrons donated by NADH and FADH2 and then yielding energy to generate ATP from adenosine diphosphate and inorganic phosphate. Free radicals are also generated by tricarboxylic acid in the matrix.
Mitochondria are controlled by both nuclear and mitochondrial genomes. mtDNA consists of a 16,571 base-pair, double-stranded, circular DNA molecule (Anderson et al 1981). A mitochondrion contains 2–10 copies of mtDNA (Reddy 2008). mtDNA contains 13 polypeptide genes that encode essential components of the ETC. mtDNA also encodes the 12S and 16S rRNA genes and the 22 tRNA genes required for mitochondrial protein synthesis (Reddy and Beal, 2005). Nuclear genes encode the remaining mitochondrial proteins, metabolic enzymes, DNA and RNA polymerases, ribosomal proteins, and mtDNA regulatory factors, such as mitochondrial transcription factor A. Nuclear mitochondrial proteins are synthesized in the cytoplasm and are subsequently transported into mitochondria.
Sites of Free Radical Production in the Mitochondria
Mitochondrial ATP is generated via oxidative phosohorylation (OXPHOS) within the inner mitochondrial membrane (Fig. 1). Free radicals are generated as a byproduct of OXPHOS. In the respiratory chain, complexes I and III leak electrons to oxygen, producing primarily superoxide radicals. The superoxide radicals are dismutated by manganese superoxide dismutase (Mn-SOD), generating H2O2 and oxygen. Complex I generates only toward the mitochondrial matrix, but complex III generates toward both the inter-membrane space and the matrix. In addition, the components of tricarboxylic acid, including α-ketoglutarate dehydrogenase, generate superoxide radicals in the matrix. These mitochondrially generated free radicals and superoxide radicals are carried to the cytoplasm via voltage-dependent anion channels and participate in lipid peroxidation, and protein and DNA oxidation. Mitochondria are critical in the metabolism of all mammalian cells, including brain neurons, and abnormalities in mitochondrial structure and function may lead to age-related neurodegenerative diseases.
Mutant Proteins Associated with Mitochondria
Several recent molecular, cellular, biochemical, and animal model studies of inherited neurodegenerative diseases revealed that mutant proteins – such as amyloid beta in AD, mutant huntingtin in HD, mutant SOD1 in ALS, mutant parkin, mutant DJ1 and mutant α-synuclein in PD, and frataxin in FRDA – are localized to mitochondrial membranes, leading to an increased production of free radicals, a low production of cellular ATP, and ultimately cell death (Beal 2005, Reddy 2008) (Fig. 2). Increasing evidence suggests that mitochondrial dysfunction is a common cellular change in the disease progression of several neurodegenerative diseases, cancer and diabetes (listed in Table 1).
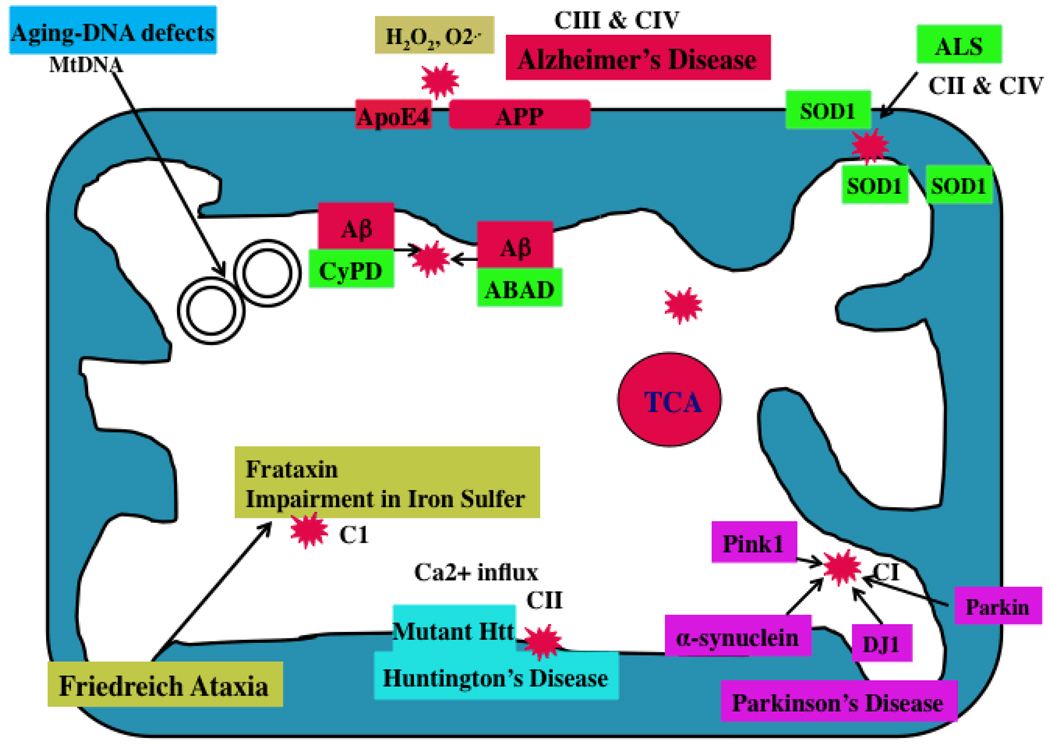
Figure 2. Mutant proteins and mitochondria.
In AD, Aβ peptides enter mitochondria and interact with mitochondrial proteins, induce free radicals, decrease cytochrome oxidase activity, and inhibit ATP generation. In AD brains, APP is transported to outer mitochondrial membranes, blocks the import of nuclear cytochrome oxidase proteins to mitochondria, and may be responsible for decreased cytochrome oxidase activity. In HD neurons, mutant Htt binds to the outer mitochondrial membrane and induces free radical production. Free radicals may interrupt with calcium uptake. In PD neurons, mutant proteins of α-synuclein, parkin, PINK1, and DJ1 are associated with mitochondria and cause mitochondrial dysfunction. Complex I activity is inhibited in PD neurons. In ALS, mutant SOD1 is localized to the inner and outer mitochondrial membranes and matrix, and induces free radical production and oxidative damage. Impairment of complexes II and IV are associated with ALS. Frataxin is a mitochondrial protein responsible for heme biosynthesis and the formation of iron-sulfur clusters. In Friedriech ataxia, mutant frataxin facilitates the accumulation of iron in mitochondria and induces free radicals.
Role of Aging and Epigenetic Factors in Neurodegenerative Diseases
In AD and PD, there are no differences pathologically between early onset familial patients and late onset patients. Only difference is that in late-onset patients, pathological changes occur later than in the early-onset patients. In early-onset cases, genetic mutations accelerate the disease process. In late-onset patients, in the absence of genetic mutations, age-related cellular changes control disease progression, which is why late-onset AD and PD patients take more time to exhibit pathological features. As described above, age-related mitochondrial abnormalities contribute to disease progression in late-onset AD and PD. However, if age-dependent mitochondrial abnormalities are likely factors affecting the development of AD and PD (and possibly even affecting cancer and diabetes in aged individuals), it is still unclear what makes some aged persons susceptible to PD development and others, to AD. Epigenetic factors and lifestyle activities may contribute to age-dependent susceptibility to these diseases (see Table 1).
In HD, mutant huntingtin, in association with outer mitochondrial membrane, may block mitochondrial pores and disrupt the transport of nuclear-encoded mitochondrial proteins to mitochondria and may induce free radical production and calcium influx. These events occur more frequently in neurons affected by HD than in neurons unaffected by HD (Reddy et al 2009).
Alzheimer’s Disease and Mitochondrial Dysfunction
AD is a late-onset, progressive, age-dependent neurodegenerative disease, characterized by the progressive decline of memory, cognitive functions, and changes in behavior and personality (Selkoe 2001, Mattson 2004, Reddy and Beal 2008). Two major pathological features have been observed in postmortem brains from AD patients: 1) intracellular neurofibrillary tangles and 2) extracellular amyloid beta (Aβ) deposits in the regions of the brain that are responsible for learning and memory. AD is also associated with the loss of synapses, synaptic function, mitochondrial abnormalities, inflammatory responses, and neuronal loss. Genetic mutations in APP, PS1, and PS2 genes cause about 2% of all AD cases; however, causal factors are still unknown for a vast majority of AD patients. Several factors – including lifestyle, diet, environmental exposure, Apolipoprotein allele E4, and a genetic variant in sortilin-related receptor 1 gene – may contribute to late-onset AD (Reddy and Beal 2008).
The prevalence of AD is high among aged persons with all brain diseases: 5% of individuals 65 years old and 50% of individuals 85 years of age and older (Reddy and McWeeney 2006). These numbers translate to extremely high health care costs.
Although AD pathogenesis involves a large number of molecular and cellular events, 2 events that occur early in AD development are: 1) synaptic damage and 2) mitochondrial dysfunction (Selkoe 2002, Nunomura et al 2001, Reddy and Beal 2008). These 2 events are likely caused by mutant APP/Aβ and aging. It is generally accepted that an age-dependent accumulation of Aβ at synapses and synaptic mitochondria interfere with synaptic activities, including the release of synaptic vesicles and neurotransmitters, and the production of ATP at synapses. For normal communication across neurons, and normal cognitive and memory functions, it is critical that synaptic activities and ATP supply are normal. However, it is these events that are interrupted more and more frequently in elderly individuals and in AD patients. In addition, mitochondrial trafficking is largely interrupted in neurons affected by AD. In normal, healthy neurons, mitochondria move from cell body to axons, dendrites, and synapses by an anterograde mechanism, supplying ATP at nerve terminals. Mitochondria then travel back to the cell body from synapses through a retrograde mechanism. In AD neurons, these mechanisms are abnormal primarily due to defective or functionally inactive mitochondria (Reddy 2009) (Fig. 3). Recently, mitochondrial abnormalities have been identified: changes in mitochondrial DNA, decreased mitochondrial enzyme activities, abnormal mitochondrial gene expressions, increased mitochondrial fragmentation, and decreased mitochondrial fusion (Reddy 2009). These events occur very early in the development and progression of AD, and are described below.
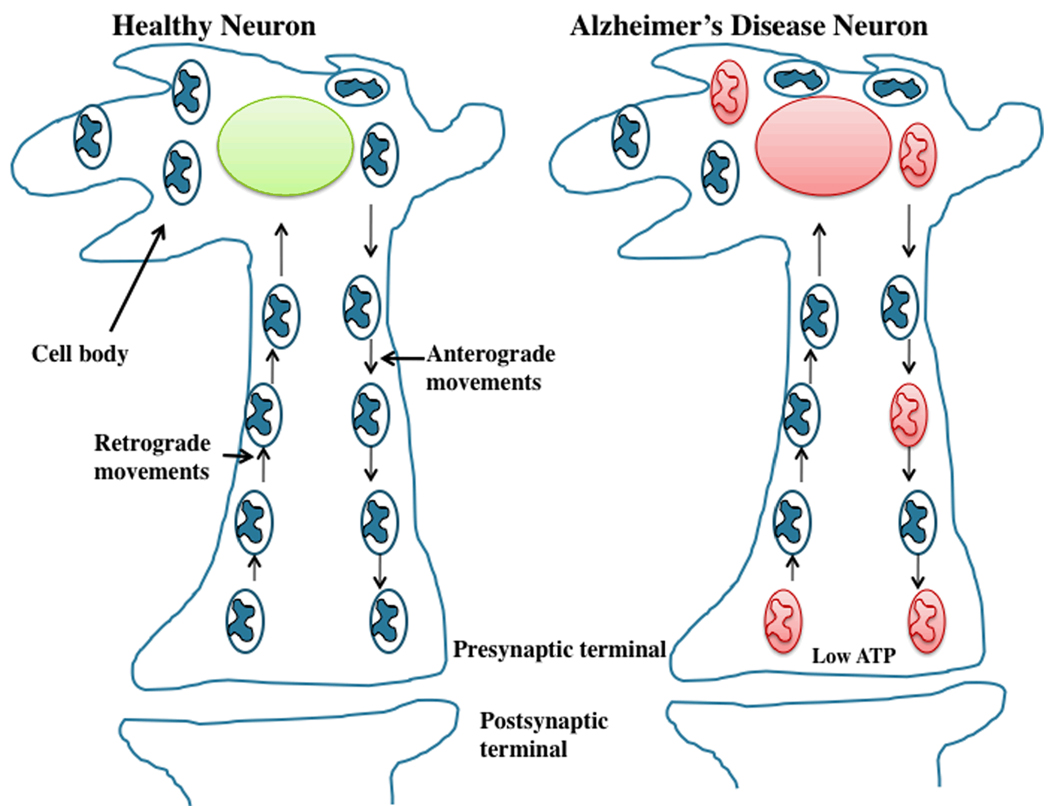
Figure 3. Abnormal mitochondrial movements in AD neurons.
In normal, healthy neurons, mitochondria move from cell body to axons, dendrites, and synapses by an anterograde mechanism, supplying ATP at nerve terminals. Mitochondria then travel back to the cell body from synapses through a retrograde mechanism. In AD neurons, these mechanisms are abnormal primarily due to defective or functionally inactive mitochondria. Mitochondria associated with mutant APP and Aβ are largely dysfunctional and not able to move from body to synapses and supply ATP to nerve terminals (shown in red color). Decreased ATP levels at synapses contribute to synaptic degeneration, synaptic loss and ultimate neuronal death and cognitive impairments.
Mutant APP and Aβ Associated with Mitochondria
Several lines of evidence from studies of neurons in postmortem brain specimens from AD patients and from AD mice suggest that APP derivatives, including Aβ monomers and oligomers, are associated with mitochondrial membranes (Manczak et al 2006, Crouch et al 2005, Caspersen et al 2006, Devi et al 2006). Recently studies also found that Aβ is transported into mitochondria via the translocase of the outer membrane machinery (Hanson Peterson et al 2008).
Several recent in vitro and in vivo studies found APP in mitochondrial membranes, in neurons affected by AD (Anandatheerthavarada et al 2003, Devi et al 2006, Kiel et al 2004, Park et al 2006). Interestingly, these studies also found that mitochondrial APP in the N-terminal inside the mitochondria and C-terminal of the protein faces the cytosolic side (Anandatheerthavarada et al 2003, Park et al 2006).
Devi and colleagues found that full-length APP and C-terminal truncated (lacking Aβ domain) APP species accumulate in mitochondria, in patients with mild, moderate, and severe AD, but not in the age-matched healthy subjects (Devi et al 2006). They also found that APP increasingly accumulate in mitochondrial membranes as AD progresses, suggesting that APP is associated with mitochondria and is critically involved in AD progression.
Mitochondrial Abnormalities in Alzheimer’s Disease
Mitochondrial dysfunction has been observed in AD postmortem brains, in platelets from AD patients, in AD transgenic mice, and in cell lines that express mutant APP and/or cells treated with Aβ (Reddy 2009). Several lines of evidence suggest that mitochondrial abnormalities play a large role in AD pathogenesis:
Abnormal Mitochondrial Enzyme Activities
Studies of mitochondrial enzyme activities found decreased levels of cytochrome oxidase activity, pyruvate dehydrogenase, and α-ketodehydrogenase in fibroblasts, lymphoblasts, and postmortem brains from AD patients, compared to neurons, fibroblasts, and lymphoblasts from age-matched healthy subjects (reviewed in Reddy and Beal 2008).
Defective Mitochondrial Function
Several studies found increased free radical production, lipid peroxidation, oxidative DNA damage, oxidative protein damage, decreased ATP production, and decreased cell viability in postmortem AD brains compared to brains from age-matched healthy subjects (Gibson et al 1998, Parker et al, 1990, Maurer et al 2000, Smith et al 1996, Devi et al 2006).
Mitochondrial DNA Defects
Mitochondrial DNA changes were found increased in postmortem brain tissue from AD patients and aged-matched healthy subjects, compared to DNA changes in postmortem brain tissue from young, healthy subjects, suggesting that the accumulation of mitochondrial DNA in AD pathogenesis is age-related (Lin et al 2002, Coskun et al 2004).
Abnormal Mitochondrial Gene Expression
Several groups investigated mitochondrial gene expressions in postmortem AD brains and in brain specimens from AD transgenic mice (Chandrasekharan et al 1994, Reddy et al 2004, Manczak et al 2005). They found mitochondrially encoded genes abnormally expressed in the brains AD patients and AD mice. A recent, time-course global gene expression study in Tg2576 mice and age-matched non-transgenic littermates revealed an up-regulation of mitochondrial genes in the Tg2576 mice, suggesting that mitochondrial metabolism is impaired by mutant APP/Aβ and that the up-regulation of mitochondrial genes may be a compensatory response to mutant APP/Aβ (Reddy et al 2004). Further, Manczak et al. found an abnormal expression of mitochondrially encoded genes in postmortem AD brains compared to the brains of healthy subjects (Manczak et al 2004), suggesting impaired mitochondrial metabolism in AD.
Mitochondrial Structural Studies
Recent studies of mitochondrial structure in brain tissue from AD patients and neuronal cells expressing mutant APP found that Aβ fragments mitochondria and causes structural changes in neurons from AD patients (Hirai et al 2001, Wang et al 2008, Reddy and Manczak 2008).
Mitochondrial Therapeutics
As mentioned above, synaptic damage and mitochondrial dysfunction have been reported in early pathogenic events associated with aging, neurodegenerative diseases, cancer, and diabetes. It may be possible to treat these pathogenic events by: 1) developing molecules that treat mitochondria (by targeting ROS). These molecules decrease free radical production and oxidative damage, and boost overall mitochondrial function, which ultimately increases synaptic branching of neurons, and 2) therapeutically boosting ATP levels in mitochondria that ultimately increases synaptic outgrowth and neuronal connectivity.
Given the huge involvement of mitochondrial dysfunction in aging and neurodegenerative diseases, it is reasonable to treat or supplement diet with antioxidants in patients with neurodegenerative diseases such as AD, PD and HD. However, recent intake of natural antioxidants to AD patients gave mixed results. Some epidemiologic studies suggest that the increased intake of antioxidant vitamins (including vitamin E, vitamin C, and beta carotene) might reduce the risk of developing AD or PD, while other studies did not (Reddy and Beal 2008). Currently, available antioxidant approaches are not effective in treating neurodegenerative diseases because naturally occurring antioxidants, such as vitamins E and C, do not cross the blood-brain barrier and so cannot reach the relevant sites of free radical generation. To overcome these problems and to better assess whether antioxidant approaches may be valuable therapeutic treatments, improved delivery of antioxidants to the brains of patients with neurodegenerative diseases is needed.
In the last decade, considerable progress has been made in developing mitochondrially targeted antioxidants. To increase the delivery of antioxidants into mitochondria, several antioxidants have been developed: the triphenylphosphonium-based antioxidants (MitoQ, MitoVitE, and MitoPBN), the cell-permeable, small peptide-based antioxidant SS31, and mitochondrial permeability transition pore inhibitors such as Dimebon (Szeto 2008, Murphy and Smith, 2007, Reddy 2008, Doody et al 2008). The application of these mitochondrially targeted agents to neurodegenerative diseases is at its early stages and is focused on animal models of AD, PD and ALS. Recent clinical studies of AD treatments conducted in Russia found that Dimebon significantly improved the clinical course of mild-to-moderate AD and may be safe and well-tolerated (Doody et al 2008).
Recently, Wu and colleagues found that Dimebon acts as an inhibitor of NMDA receptors and voltage-gated calcium channels in neurons from wild-type mice and in HD YAC128 transgenic mice (Wu et al 2008). They also found that 50 µM Dimebon stabilized glutamate-induced Ca2+ signals in a YAC128 medium of spiny neurons and protected the neurons from glutamate-induced apoptosis. Findings from this study suggest that Dimebon may beneficially affect HD neurons through its capacity to alter NMDA receptors and voltage-gated calcium channels. Currently, several laboratories across the world are investigating the mode of neuroprotective action of Dimebon in neurodegenerative diseases by investigating cell and mouse models of neurodegenerative diseases, including AD and HD.
Conclusions and Future Directions
Increasing evidence suggests that mitochondrial dysfunction is involved in aging, cancer, diabetes, and neurodegenerative diseases. Age-dependent accumulation of mitochondrial abnormalities and mutant proteins lead to both structural and functional changes in neuronal function and to cell death. In addition to aging, epigenetic factors and lifestyle activities may likely contribute to neurodegeneration and cell death. Further, in AD, mutant APP and Aβ enter mitochondria and/or block the transport of nuclear-encoded mitochondrial proteins to mitochondria, in turn inducing free radicals, increasing oxidative damage, and causing cell death.
Through studies that are elucidating the role of mitochondria in disease onset and development, investigators have begun focusing research efforts on developing therapies, such as molecules that target and protect mitochondria and neurons from the toxicity of aging and mutant proteins. Tremendous progress is being made in this research, and optimism running high, that significant steps can continue, taking us much closer to future time when such devastating diseases as AD, PD, cancer, and diabetes, can be controlled.
Acknowledgements
The research presented in this article was supported by grants from American Federation For Aging Research and National Institutes of Health (AG020872 and AG025061).
References
- Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer's amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol. 2003;161:41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran K, Giordano T, Brady DR, Stoll J, Martin LJ, Rapoport SI. Impairment in mitochondrial cytochrome oxidase gene expression in Alzheimer disease. Brain Res Mol Brain Res. 1994;24:336–340. doi: 10.1016/0169-328x(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Coskun PE, Beal MF, Wallace DC. Alzheimer's brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci U S A. 2004;101:10726–10731. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li QX, Barnham KJ, Curtain CC, Cherny RA, Cappai R, Dyrks T, Masters CL, Trounce IA. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1-42. J Neurosci. 2005;25:672–679. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer's disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMauro S, Schon EA. Mitochondrial disorders in the nervous system. Annu Rev Neurosci. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, Seely L, Hung D dimebon investigators. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer's disease: a randomised, double-blind, placebo-controlled study. Lancet. 2008;372:207–215. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]
- Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Sheu KF, Blass JP. Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm. 1998;105:855–870. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- Hansson Petersen CA, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, Leinonen V, Ito A, Winblad B, Glaser E, Ankarcrona M. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci U S A. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil U, Bonert A, Marques CA, Scherping I, Weyermann J, Strosznajder JB, Müller-Spahn F, Haass C, Czech C, Pradier L, Müller WE, Eckert A. Amyloid beta-induced changes in nitric oxide production and mitochondrial activity lead to apoptosis. J Biol Chem. 2004;279:50310–50320. doi: 10.1074/jbc.M405600200. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lin MT, Simon DK, Ahn CH, Kim LM, Beal MF. High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer's disease brain. Hum Mol Genet. 2002;11:133–145. doi: 10.1093/hmg/11.2.133. [DOI] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- Manczak M, Jung Y, Park BS, Partovi D, Reddy PH. Time-course of mitochondrial gene expressions in mice brains: implications for mitochondrial dysfunction, oxidative damage, and cytochrome c in aging. J Neurochem. 2005;92:494–504. doi: 10.1111/j.1471-4159.2004.02884.x. [DOI] [PubMed] [Google Scholar]
- Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer's disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer I, Zierz S, Möller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim SS, Seong YM, Kim KH, Goo HG, Yoon EJ, Min do S, Kang S, Rhim H. Beta-amyloid precursor protein is a direct cleavage target of HtrA2 serine protease. Implications for the physiological function of HtrA2 in the mitochondria. J Biol Chem. 2006;281:34277–34287. doi: 10.1074/jbc.M603443200. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer's disease. Neurology. 1990;40:1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial dysfunction in aging and Alzheimer's disease: strategies to protect neurons. Antioxid Redox Signal. 2007;9:1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular Med. 2008;10:291–315. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer's disease. Exp Neurol. 2009;218:286–292. doi: 10.1016/j.expneurol.2009.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Are mitochondria critical in the pathogenesis of Alzheimer's disease? Brain Res Brain Res Rev. 2005;49:618–632. doi: 10.1016/j.brainresrev.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med. 2008 Feb;14(2):45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W, Jr, Kaye J, Manczak M. Differential loss of synaptic proteins in Alzheimer's disease: implications for synaptic dysfunction. J Alzheimers Dis. 2005 Apr;7(2):103–117. doi: 10.3233/jad-2005-7203. discussion 173–180. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Manczak M. Neuroprotection of mitochondrial-targeted antioxidants in Alzheimer’ disease. Paper presented at the Society for Neuroscience 2008 meeting held in Washington DC; November 15–19, 2008; 2008. Abstract #704.7. [Google Scholar]
- Reddy PH, Mao P, Manczak M. Mitochondrial structural and functional dynamics in Huntington's disease. Brain Res Rev. 2009;61:33–48. doi: 10.1016/j.brainresrev.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, McWeeney S. Mapping cellular transcriptosomes in autopsied Alzheimer's disease subjects and relevant animal models. Neurobiol Aging. 2006;27:1060–1077. doi: 10.1016/j.neurobiolaging.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Reddy PH, McWeeney S, Park BS, Manczak M, Gutala RV, Partovi D, Jung Y, Yau V, Searles R, Mori M, Quinn J. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer's disease. Hum Mol Genet. 2004;13:1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer's. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. A "mitochondrial cascade hypothesis" for sporadic Alzheimer's disease. Med Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Szeto HH. Development of mitochondria-targeted aromatic-cationic peptides for neurodegenerative diseases. Ann N Y Acad Sci. 2008;1147:112–121. doi: 10.1196/annals.1427.013. [DOI] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Li Q, Bezprozvanny I. Evaluation of Dimebon in cellular model of Huntington's disease. Mol Neurodegener. 2008;3:15. doi: 10.1186/1750-1326-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]