Abstract
This article reviews recent studies on: (1) the synthesis of novel calcium phosphate and calcium fluoride nanoparticles and their incorporation into dental resins to develop nanocomposites; (2) the effects of key microstructural parameters on Ca, PO4, and F ion release from nanocomposites, including the effects of nanofiller volume fraction, particle size, and silanization; and (3) mechanical properties of nanocomposites, including water-aging effects, flexural strength, fracture toughness, and three-body wear. This article demonstrates that a major advantage of using the new nanoparticles is that high levels of Ca, PO4, and F release can be achieved at low filler levels in the resin, because of the high surface areas of the nanoparticles. This leaves room in the resin for substantial reinforcement fillers. The combination of releasing nanofillers with stable and strong reinforcing fillers is promising to yield a nanocomposite with both stress-bearing and caries-inhibiting capabilities, a combination not yet available in current materials.
Keywords: dental nanocomposite, nanoparticles, strength, Ca and PO4 ion release, fluoride release, tooth caries inhibition
Introduction
Resin composites are being increasingly used in dentistry for tooth cavity restorations. They are usually composed of reinforcing fillers in an acrylic monomer matrix that is polymerized to form a solid restoration (Ferracane, 1995; Bayne et al., 1998; Drummond and Bapna, 2003; Imazato, 2003; Lu et al., 2005; Drummond, 2008; Wan et al., 2008). Previous efforts have improved the resin compositions and cure conditions, and reduced poly-merization shrinkage (Stansbury, 1990; Eick et al., 1993; Ferracane and Mitchem, 1994; Loza-Herrero et al., 1998; Dauvillier et al., 2000; Watts et al., 2003; Lu et al., 2005; Krämer et al., 2006). Fracture resistance and wear resistance of the composites have also been improved (Tyas, 1990; Ferracane and Berge, 1995; Ruddell et al., 2002; Drummond, 2008; Watts et al., 2008).
However, recent reports show that secondary caries and restoration fracture remain the two main challenges (Sarrett, 2005). Although composites are generally satisfactory for small restorations, they are not recommended for large, stress-bearing restorations (Sakaguchi, 2005). Secondary caries refers to the recurrence of tooth decay after the initial restoration, and is cited as the most frequent reason for the replacement of existing restorations (Mjör et al., 2000). More than half of the restorations placed annually are replacements, and replacement dentistry costs about $5 billion/year in the US alone (Jokstad et al., 2001).
The sustained release of fluoride ions (F) could be a substantial benefit for a dental restoration, because the fluoride could enrich neighboring enamel or dentin to combat secondary caries (Hsu et al., 1998; Hicks et al., 2003; Weigand et al., 2007). F-releasing restorative materials include glass ionomers, resin-modified glass ionomers, compomers, and resin composites (Glasspoole et al., 2001; Asmussen and Peutzfeldt, 2002; Xu and Burgess, 2003; Itota et al., 2004; Anusavice et al., 2005). These materials have received much attention due to their significant release of fluoride, the uptake of fluoride into cavity walls and plaque, and the enhanced reprecipitation of calcium and phosphate promoted by the fluoride release (Hicks et al., 2003; Weigand et al., 2007). However, the inferior mechanical properties of glass-ionomer and resin-modified glass-ionomer materials have limited their use (Wilson and McLean, 1988; Sidhu et al., 1997; Ellakuria et al., 2003). It was correctly predicted that “the most intractable problem is likely to be lack of strength and toughness” (Wilson and McLean, 1988). The addition of a resin to the matrix did not reduce the problems of glass ionomers (Sidhu et al., 1997). When traditional and resin-modified glass ionomers were immersed in water for 12 months (Ellakuria et al., 2003), the addition of resins to glass ionomer did not improve the microhardness. Therefore, extensive studies have been undertaken to understand and improve the performance of F-releasing restoratives (Ten Cate, 1997; Xu et al., 2000; Glasspoole et al., 2001; Tyas and Burrow, 2002; Carey et al., 2003; Anusavice et al., 2005; Weigand et al., 2007; Ling et al., 2009).
Another approach to combating caries was the development of composites with the release of calcium (Ca) and phosphate (PO4) ions, which can form hydroxyapatite [Ca10(PO4)6(OH)2], the putative mineral in natural teeth (Skrtic et al., 1996a; Dickens et al., 2003). These composites remineralized the decayed enamel and dentin in vitro by increasing the mineral content in the lesions (Skrtic et al., 1996a; Dickens et al., 2003). However, like traditional and resin-modified glass ionomers, the Ca-PO4 composites had relatively low mechanical strengths, which were “inadequate to make these composites acceptable as bulk restoratives” (Skrtic et al., 2000). Accordingly, it was recommended that the Ca-PO4 composites “could serve as a restoration-supporting lining materials” (Dickens et al., 2003), and the amorphous calcium phosphate (ACP) composites could be “useful as pit and fissure sealants” (Skrtic et al., 2000).
Currently available posterior composites and hybrid composites can be used as restorations in functional stress-bearing areas, but they generally do not release Ca, PO4, or F ions. In contrast, restoratives that do release Ca, PO4, or F ions are relatively weak and cannot be used in large stress-bearing restorations. Therefore, there is a need to develop new composites that are as strong and wear-resistant as a posterior composite, while at the same time having sustained release of Ca, PO4, and F ions to inhibit caries. This article reviews recent studies on a new class of nanocomposites that may have the potential to meet this need.
Regarding literature search criteria, our search used the PubMED database, and we screened the publications individually to focus on peer-reviewed dental and biomedical journals. The literature search focused on dental nanocomposites, nanoparticle-filled resin-based dental composites, nanocomposites with fluoride ion release, and nanocomposites with calcium and phosphate ion release. A review such as this one cannot cover the vast amount of meritorious publications on traditional glass-ionomer cements, resin-modified glass ionomers, and compomers that are not described as nanostructured. The reader is referred to recent comprehensive review articles on these materials (Hicks et al., 2003; Burke et al., 2006; Weigand et al., 2007).
Non-Releasing Nanofiller-Resin Composites
Several in-depth review articles exist on dental resin composites in general (Sakaguchi, 2005; Sarrett, 2005), fillers, resins, and coupling agents (Ferracane, 1995), monomer systems for composites (Peutzfeldt, 1997), antibacterial properties of resins (Imazato, 2003), polymerization shrinkage stresses (Braga et al., 2005; Ferracane, 2008), hygroscopic and hydrolytic properties (Ferracane, 2006), and the degradation and fatigue failure of composites (Drummond, 2008). These articles reviewed important topics on dental composites, but did not specifically review nanocomposites. Hence the review of these topics will not be repeated here. Instead, the present review focuses on dental nanofiller-resin composites.
Nanoscale science and technology involve materials on the scale of typically 1 to 100 nanometers (nm). In the biomedical research field, sizes of several hundred nm have also been referred to as nano-sized (e.g., nanofibers with diameters of about 300–500 nm; Moioli et al., 2007). Clusters of small numbers of atoms or molecules in nanostructured materials often have properties (such as strength, electrical resistivity and conductivity, and optical properties) that are significantly different from the properties of the same matter at the bulk scale. In the case of nanoparticle-filled dental resin composites, the most interesting and potentially useful attributes are the small particle size, high surface area, and optical properties of the resulting composite. The types of nanofillers in dental composites included silica (Wilson et al., 2005; Chen et al., 2006), tantalum ethoxide (Furman et al., 2000), zirconia-silica (Mitra et al., 2003), alumina (Wang et al., 2007), nano-fibrillar silicate (Tian et al., 2008), ordered colloidal particles (Wan et al., 2008), and titanium oxide (Xia et al., 2008). Nanoparticles were used either as the sole filler of the composite (Wilson et al., 2005), or in combination with other types of fillers (Condon and Ferracane, 2002; Xu et al., 2004a; Garoushi et al., 2008). These nanocomposites did not release Ca, PO4, or F ions to combat tooth caries.
The reported mechanical properties of several nanocomposites were as good as those of universal hybrid composites (Beun et al., 2007; Rodrigues et al., 2008); hence these nanocomposites were recommended for posterior as well as anterior restorations, due to their high esthetics (Beun et al., 2007). Compressive strength, flexural strengths, and wear resistance of two nanocomposites were similar to those of hybrid composites (Mitra et al., 2003). However, the worn surfaces of the nanocomposites were smoother, and the gloss retention after tooth brushstrokes was higher, compared with those of hybrid composites. Furthermore, the translucency of these nanocomposites was higher than that of the hybrid composite control (Mitra et al., 2003).
Key challenges that still remain in the development of dental nanocomposites include: the dispersion of nanoparticles in the resin to avoid agglomeration; achieving high nanofiller levels to reduce polymerization shrinkage while maintaining good handling characteristics; and manufacturing cost. In addition, further studies are needed to improve our understanding of the effects of nanofiller size, morphology, composition, and filler hybridization on composite properties, as well as long-term durability in vivo. Furthermore, the aforementioned nanocomposites do not release Ca, PO4, or F ions and do not meet the caries-inhibition need. Hence another aspect in nanocomposite development is designing nanocomposites with ion-releasing and stress-bearing capabilities, reviewed in the following sections.
Fluoride-Releasing Nanocomposite
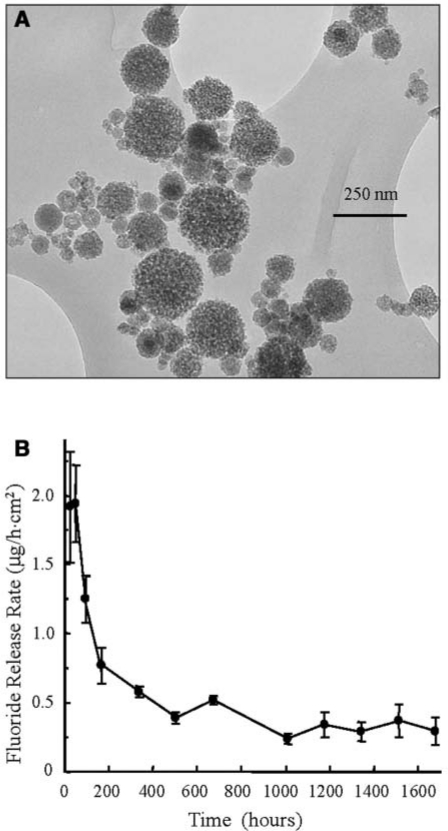
In recent studies, for the first time calcium fluoride (CaF2) nanoparticles were prepared by means of a spray-drying system (Sun and Chow, 2008; Xu et al., 2008a). A typical transmission electron microscopy (TEM) micrograph of the CaF2 nanoparticles is shown in Fig. 1A. The specific surface area of these nanoparticles was measured to be A = 35.5 m2/g. With the density of CaF2, ρ = 3.18 g/cm3, the CaF2 particle diameter was calculated to be d = 6/(Aρ) = 53 nm.
Figure 1.
CaF2 nanocomposite (A) TEM micrograph of CaF2 nanoparticles. BET measurement yielded a specific surface area, A = 35.5 m2/g. With the density of CaF2, ρ = 3.18 g/cm3, the CaF2 nanoparticle diameter, d = 6/(Aρ)= 53 nm. (B) Fluoride release from CaF2 nanocomposite. Fluoride ion (F) release was calculated as the release rate per hour per composite specimen surface area vs. immersion time. (Adapted from Xu et al., 2008a, with permission.)
In the fabrication of a resin composite, a monomer consisting of 48.975% Bis-GMA (bisphenol glycidyl methacrylate), 48.975% TEGDMA (triethylene glycol dimethacrylate), 0.05% 2,6-di-tert-butyl-4-methylphenol, and 2% BPO (benzoyl peroxide) was used to form part I, the initiator, of a two-part chemically activated resin. Part II, the accelerator resin, consisted of 49.5% Bis-GMA, 49.5% TEGDMA, and 1% N,N-dihydroxyethyl-p-toluidine (Xu et al., 2008a).
To combat the two challenges of secondary caries and restoration fracture, two types of fillers were used in the resin: CaF2 nanofillers to release F ions, and reinforcing fillers for stress-bearing capability. Silica nanoparticles were fused onto ceramic whiskers and used as reinforcing fillers (Xu et al., 2008a). The fusion facilitated silanization, minimized whisker entanglement, and enhanced filler retention in the resin by roughening the filler surfaces (Xu et al., 2002). The whisker composites demonstrated flexural strength and fracture toughness nearly two-fold those of current dental composites. They showed superior performance in thermal cycling for 105 cycles (Xu et al., 2002), long-term water aging for 2 years (Xu, 2003), and three-body wear (Xu et al., 2004a). An in vitro study showed that the whisker composites were non-cytotoxic and supported osteoblastic cell attachment and proliferation (Xu et al., 2004b).
In a recent study (Xu et al., 2008a), the F release was measured for a nanocomposite containing 25% whiskers, 20% CaF2 nanopowder, and 20% dicalcium phosphate anhydrous nanopowder, with a total filler mass fraction of 65%, to form a flowable paste. F release rate per hour per specimen surface area is shown in Fig. 1B. The initial F release rate was 1.94 µg/(hr·cm2). The release rate decreased to about 0.5 µg/(hr·cm2) after 500 hours; it further decreased to 0.29 µg/(hr·cm2) after 1680 hours (10 wks).
Another study measured the F release for traditional and resin-modified glass ionomers (Glasspoole et al., 2001). The initial F release rate was approximately 2.9 µg/(hr·cm2) for a glass ionomer (Ketac-Fil), 0.4 µg/(hr·cm2) for another glass ionomer (Fuji II), and 0.4 µg/(hr·cm2) for a resin-modified glass ionomer (Vitremer) (estimated from Fig. 1 of Glasspoole et al., 2001). A separate study examined the F release of a resin filled with a commercial CaF2 powder which was ground to yield particle sizes of 0.04–3.0 µm (Anusavice et al., 2005). At a CaF2 filler mass fraction of 23%, the initial F release rate was about 0.6 µg/(hr·cm2) at a pH of 6 (Anusavice et al., 2005). In comparison, the nanocomposite used a slightly lower filler mass fraction of 20% of CaF2 nanopowder (Xu et al., 2008a), and achieved a higher initial F release of 1.94 µg/(hr·cm2).
The sustained (or longer-term) F release, at 50 days, was approximately 0.1 µg/(hr·cm2) for Ketac-Fil, 0.03 µg/(hr·cm2) for Fuji II, and 0.04 µg/(hr·cm2) for Vitremer (calculated from Table 4 in Glasspoole et al., 2001). In another study (Anusavice et al., 2005), for the resin filled with 23% of a CaF2 powder, the F release rate at 70 days was similar to that at 83 days; both were approximately 0.05 µg/(hr·cm2). In comparison, the nanocomposite (Xu et al., 2008a) had a higher F release rate of approximately 0.29 µg/(hr·cm2) after 70 days.
Regarding mechanical properties, the CaF2 nanocomposite (Xu et al., 2008a) had a flexural strength of 100 ± 7 MPa, similar to the 108 ± 19 MPa of a commercial hybrid composite (TPH, Caulk/Dentsply). Both were higher than the 60 ± 6 MPa of a resin-modified glass ionomer (Vitremer, 3M). In comparison, another study reported a diametral tensile strength of 15 MPa for Ketac and 40 MPa for Vitremer (Glasspoole et al., 2001). The nanocomposite (Xu et al., 2008a) had an elastic modulus of 14.6 ± 1.2 GPa, similar to the 11.6 ± 2.6 GPa of the hybrid composite (TPH), and 11.8 ± 1.4 GPa of a resin-modified glass ionomer (Vitremer).
Therefore, the advantage of the CaF2 nanocomposite was that its F release matched or exceeded that of resin-modified glass ionomer, while being as mechanically strong as a non-releasing hybrid composite. This was likely because of the small size and high surface area of the nanoparticles, capable of releasing high levels of ions at a low filler level, thereby enabling the incorporation of reinforcing (but non-releasing) fillers in the same resin matrix. The weakness of the CaF2 nanocomposite was that, due to the refractive index mismatch between the resin and the fillers, the paste was opaque and was chemically cured with a two-part resin. Further study is needed to improve the esthetics of this F-releasing, high-strength nanocomposite.
Ca-PO4 Nanofiller-Resin Composite
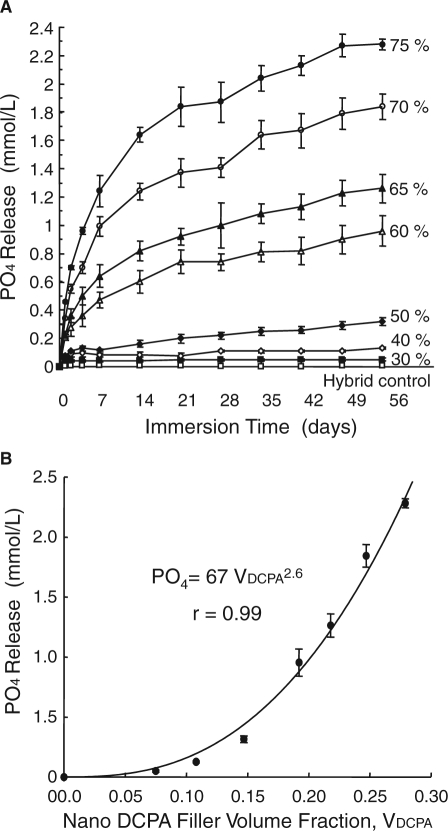
Besides CaF2 nanoparticles, calcium phosphate nanoparticles were also synthesized recently and used as fillers in resins (Chow et al., 2004; Xu et al., 2006). One calcium phosphate compound, dicalcium phosphate anhydrous (DCPA, CaHPO4), was used because it was used in calcium phosphate bone cements (Chow, 2000), and in Ca- and PO4-releasing dental materials (Dickens et al., 2003). In a recent study, two types of fillers were combined in the resin: DCPA nanofillers and reinforcing fillers (Xu et al., 2006). The PO4 ion release from the composite vs. filler level is shown in Fig. 2. The release increased rapidly with time before reaching a plateau. The Ca release showed a similar trend (Xu et al., 2006, 2007a). Increasing the filler level at a DCPA:whisker ratio of 1:1 significantly increased the amount of ion release.
Figure 2.
Effect of releasing filler level. (A) PO4 release from the nanocomposite vs. filler mass fraction. Ca release had a similar trend (not shown). Increasing the filler level increased the ion release. (B) Effect of DCPA volume fraction in the resin, VDCPA. The released PO4 concentration at 56 days was related to VDCPA by: PO4 = 67VDCPA2.6, with a correlation coefficient r = 0.99. The Ca concentration was related to VDCPA: Ca = 4.46VDCPA1.6 (not included). (Adapted from Xu et al., 2007a, with permission.)
There appear to be three main factors that influence the Ca and PO4 ion release from the composite. (1) A higher volume fraction of DCPA in the composite (VDCPA) increased the source of ions in the resin. (2) A higher VDCPA also increased the filler-matrix inter-facial area, which served as a relatively easier path for water and ion diffusion. (3) The resin matrix might have a slightly lower polymerization conversion as VDCPA increased. Increasing the filler level usually decreases the polymerization conversion (Xu et al., 2007b), because a higher concentration of air in the heavily filled composite may adversely affect the conversion. In addition, the fillers may partially absorb the heat of polymerization, thereby moderating the exotherm of polymerization. Therefore, with higher VDCPA in the composite, there was not only more DCPA for ion release, but the diffusion of water and ions through the resin might also be somewhat enhanced, due to the increased interfacial area and the decreased polymerization conversion. If only factor (1) were operative, the relationship between VDCPA and Ca and PO4 release might be simply linear. However, these three factors might all be operative. Hence, the ion release would likely increase with increasing VDCPA at a rate greater than a linear rate. Based on these reasons, the following empirical relationships were proposed:
| (1) |
| (2) |
where Ca and PO4 are the released ion concentrations (mmol/L), and k, α, and β are coefficients. Investigators used the filler and resin masses and the density values to calculate the volume fraction of DCPA in the composite, VDCPA (Xu et al., 2007a). Fitting the above equations to the measured data at 56 days yielded: PO4 = 67 VDCPC2.6 (Fig. 2B). Similarly, Ca = 4.46 VDCPA1.6. These equations showed that as VDCPA was increased, the ion release increased at a rate faster than being linearly proportional to VDCPA. These equations provide an understanding of the effect of Ca-PO4 nanoparticle content in the resin on the amount of ion release, and a basis for tailoring the volume fraction to achieve a desired level of ion release.
Previous Ca-PO4 composites, when measured by a similar approach (Skrtic et al., 1996b; Dickens et al., 2003), released PO4 to concentrations of 0.1-0.7 mmol/L, and Ca to 0.3-1.0 mmol/L. These composites effectively remineralized tooth lesions in vitro (Skrtic et al., 1996a; Dickens et al., 2003). The DCPA-whisker nanocomposites released PO4 with concentrations of up to 2 mmol/L, and Ca up to 0.7 mmol/L, even when half of the fillers were non-releasing whiskers (Xu et al., 2007a). This was likely because the DCPA nanoparticles had a high surface area of 18.6 m2/g. In a previous study (Dickens et al., 2003), the DCPA particle size was 1.1 µm and the TTCP (tetracalcium phosphate) particle size was 16 µm, corresponding to a surface area of 1.9 m2/g for DCPA, and 0.12 m2/g for TTCP. Therefore, their surface areas were 1-2 orders of magnitude less than those of the nanopowder. As a result, these traditional composites needed to be fully filled with Ca-PO4 fillers to have significant ion release. Replacing part of their Ca-PO4 fillers with reinforcing (but non-releasing) fillers would substantially reduce the ion release. For example, based on Fig. 1 of Skrtic et al. (1996b), if only 10% of the ACP fillers had been replaced by reinforcing fillers, the ion release would have been decreased from about 0.75 mmol/L to only 0.1 mmol/L. Therefore, there was little room left in traditional Ca-PO4 composites for reinforcement fillers without diminishing the ion release.
Previous studies (Skrtic et al., 1996a; Dickens et al., 2003) showed that when the Ca and PO4 were released from the composite restoration, they re-precipitated to form hydroxyapatite outside the composite and inside the tooth lesions, significantly increasing the mineral content of the lesion. The fact that the Ca and PO4 released from the nanocomposite matched or exceeded that of the previous composites (Xu et al., 2006, 2007a) suggests that the nanocomposite may also be an effective remineralizer. Hence, the synergistic use of releasing nanofillers and reinforcing fillers yielded nanocomposite with the potential of having both stress-bearing and caries-inhibiting capabilities, a combination not available in current dental materials. However, the Ca-PO4 nanocomposite was relatively opaque. Further studies should use esthetic glass fillers as reinforcement to develop a photo-activated, ion-releasing, stress-bearing nanocomposite.
Effects of Ca-PO4 Particle Size And Silanization
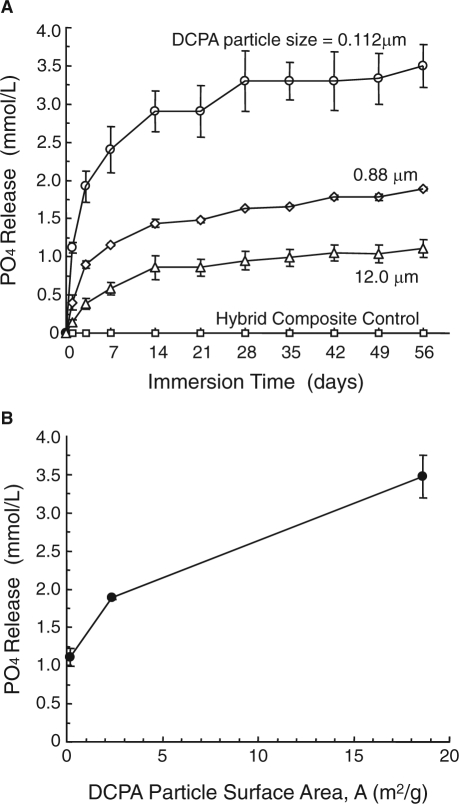
A recent study investigated the effect of Ca-PO4 particle size on Ca and PO4 release (Xu et al., 2007b). Three different DCPA fillers were used. The first was the DCPA nanopowder with a particle size of 112 nm. The second was a commercial DCPA powder (J.T. Baker Chemical, Phillipsburg, NJ, USA), with a median particle diameter of 12.0 µm. For a third powder, the as-received DCPA was ball-milled for 24 hrs, which reduced the median diameter to 0.88 µm. The nanocomposite filled with the nano-DCPA released significantly more ions than did the other composites (Fig. 3A). Decreasing the particle size, which increased the specific surface area of the powder, increased the ion release (Fig. 3B). Ca release (not shown) also increased with decreasing DCPA particle size and increasing surface area (Xu et al., 2007b).
Figure 3.
Effect of Ca-PO4 particle size. (A) PO4 release from composite vs. DCPA particle size. (B) PO4 concentration at 56-day vs. DCPA particle surface area. Increasing the particle surface area increased the PO4 release from the composite. Ca ion release (not shown) also increased with decreasing DCPA particle size and increasing particle surface area. (Adapted from Xu et al., 2007b, with permission.)
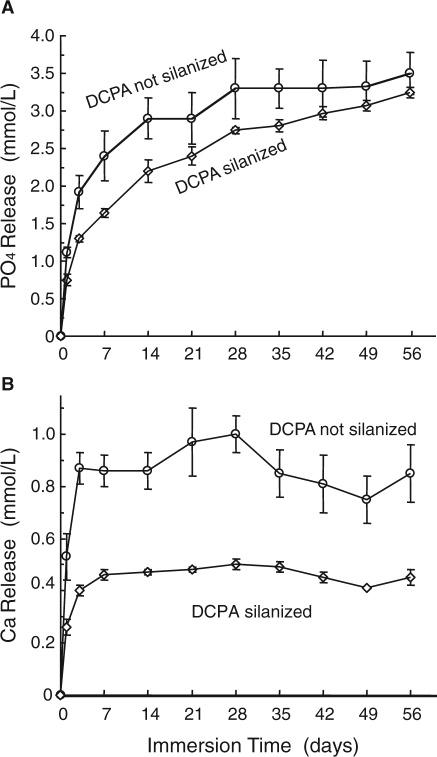
The recent study also examined the effect of filler silanization on ion release (Xu et al., 2007b). Previous studies on Ca-PO4 composites used unsilanized Ca-PO4 fillers and had not investigated the effect of silanization (Skrtic et al., 2000; Dickens et al., 2003). The silane coupling agent is a bifunctional coating on the filler that would enhance the bonding between the filler and the resin matrix, as well as improve the mixing and handling of the paste. The DCPA nanopowder was silanized with 4% by mass of 3-methacryloxypropyltrimethoxysilane and 2% n-propylamine (Xu et al., 2002). The effect of silanization on ion release from the composite is shown in Fig. 4. The composite containing unsilanized DCPA released significantly more ions than did the composite containing silanized DCPA. This was likely because the silane coupling agent hindered the diffusion of water into, and ions out of, the filler particles in the resin. Therefore, a more feasible approach to improving the stress-bearing capability of the composite would be to use a strong reinforcing co-filler that is silanized (e.g., silanized whiskers or glass fillers) to impart strengthening to the composite. Strengthening the composite via silanization of the Ca-PO4 fillers did not appear to be desirable, because it reduced the ion release.
Figure 4.
Effect of Ca-PO4 filler silanization on ion release. (A) PO4 and (B) Ca ion release from nanocomposite containing 65% of DCPA nanopowder. The composite containing unsilanized DCPA released more ions than did the composite containing silanized DCPA. (Adapted from Xu et al., 2007b, with permission.)
Mechanical Properties
Composite Strength
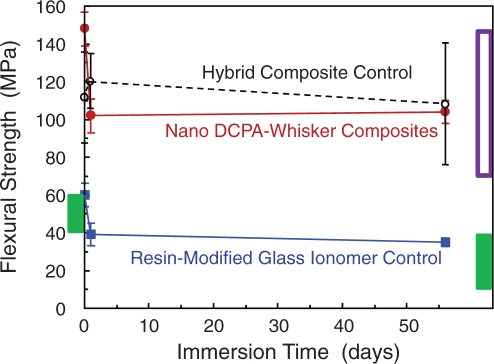
Calcium phosphate nanocomposites generally had strengths matching those of currently available hybrid composites (Xu et al., 2006, 2007a,b). For example, the flexural strength of a DCPA-whisker nanocomposite is shown in Fig. 5, along with a commercial hybrid composite (TPH) and a resin-modified glass ionomer (Vitremer), after water immersion for 1 day and 56 days. The nanocomposite had strengths that matched those of the hybrid composite; both were about two-fold that of the resin-modified glass ionomer.
Figure 5.
Flexural strength of composites. Specimens were tested either without immersion, or with immersion in water at 37°C for 1 day or 56 days. The ion-releasing nanocomposite matched the strengths of a commercial non-releasing hybrid composite. Both composites had strengths about two-fold that of a resin-modified glass ionomer. The box at the left axis indicates the reported strengths of previous Ca-PO4 composites before immersion. The filled box at the right axis indicates reported strengths of previous Ca-PO4 composites after immersion. The unfilled box at the right axis indicates strengths for non-releasing, stress-bearing composites (Xu et al., 2008b).
In another study, amorphous calcium phosphate (ACP) fillers were incorporated into a remineralizing composite (Skrtic et al., 1996b). Using dry specimens without immersion, the ACP composite had a flexural strength of 47 ± 5 MPa using unmilled ACP, and 56 ± 16 MPa using milled ACP (O’Donnell et al., 2005). It was concluded that “all the amorphous calcium phosphate fillers yielded polymerized materials weaker than unfilled polymers” (Skrtic et al., 1996b). Another composite, using micron-sized DCPA, had flexural strengths of 40–50 MPa (Dickens et al., 2004), consistent with the ACP composite. In Fig. 5, the box at the left axis indicates the reported strengths of these traditional Ca-PO4 composites before immersion in water. In comparison, the nanocomposite with Ca and PO4 release (at a DCPA:whisker mass ratio of 1:2) had a higher flexural strength of about 150 MPa before immersion.
After 56 days of immersion in water, the flexural strength of the Ca-PO4 nanocomposite decreased to 104 MPa (Fig. 5). In comparison, a previous composite with micron-sized DCPA had a biaxial flexural strength of 40–50 MPa before immersion; the strength decreased to 10–20 MPa after 90 days of immersion (Dickens et al., 2004). The strength of the ACP composite decreased to 40 MPa after 11 days of immersion (Skrtic et al., 1996b). In Fig. 5, the filled rectangular box at the right axis indicates the reported strengths of the traditional Ca-PO4 composites after immersion. It shows that the traditional Ca-PO4 composites had strengths similar to that of the resin-modified glass ionomer, while the new Ca-PO4 nanocomposite had strength similar to that of the non-releasing, hybrid composite.
For composites without ion release, flexural strengths of about 90-150 MPa after 1-day immersion and 70-110 MPa after 6 months of immersion have been reported (Ferracane et al., 1998). In a round-robin study, the flexural strengths of several posterior composites after 1-day immersion ranged from about 70 to 130 MPa (Ferracane and Mitchem, 1994). Recently, a nanocomposite without ion release was reported to have a flexural strength of about 90 MPa after 1-day immersion (Wilson et al., 2005). These values are indicated in Fig. 5 by the unfilled box (upper box) at the right axis. After 2 months of immersion, the nancomposite with Ca-PO4 release had strength matching those of non-releasing composites, and exceeding those of releasing restoratives (Fig. 5). Further study is needed to investigate longer-term water-aging and durability of the Ca-PO4 nanocomposite.
Fracture Toughness (KIC)
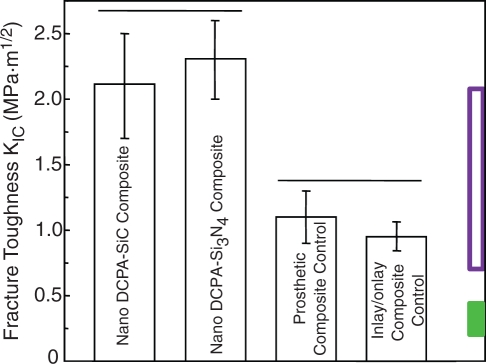
KIC was measured for DCPA nanocomposites at a DCPA:whisker mass ratio of 1:2, with silicon nitride and silicon carbide whiskers having a total filler mass fraction of 74%. The composite specimens were heat-cured at 120°C for 30 min for indirect restorations. The KIC values are shown in Fig. 6. The Ca-PO4 nanocomposites had significantly higher KIC than did the commercial control composites without ion release.
Figure 6.
Fracture toughness (KIC) via a single-edge-notched-beam approach. Horizontal line indicates similar values (p > 0.1). The two control composites are: indirect inlay/onlay composite (Concept, Ivoclar, Amherst, NY, USA) and prosthetic composite (Artglass, Heraeus Kulzer, Germany). The unfilled box at the right axis indicates reported KIC for non-releasing, stress-bearing composites; the filled box indicates reported KIC of glass ionomer and resin-modified glass ionomer (Xu et al., 2008b).
A literature search did not find fracture toughness data for the traditional Ca-PO4 dental composites. For glass-ionomer and resin-modified glass-ionomer materials, Mathis and Ferracane (1989) reported KIC of 0.23-0.29 MPa·m1/2 at 24 hrs. Kao et al. (1996) measured the KIC of experimental ionomer materials as well as Fuji II, which ranged 0.20-0.39 MPa·m1/2. These values are indicated by the filled box (lower box) at the right axis in Fig. 6.
For composites without ion release, Lloyd and Iannetta (1982) reported KIC of 0.8-1.1 MPa·m1/2. Indrani et al. (1995) measured KIC of 0.7-1.4 MPa·m1/2 for dental composites. Ferracane and Berge (1995) showed a slightly wider range for KIC of 0.7-2 MPa·m1/2 for several resin composites. In addition, Ferracane et al. (1995) reported KIC for heat-cured composites of 1.3-2.1 MPa·m1/2. More recently, Rodrigues et al. (2008) reported KIC of 1.3-1.5 MPa·m1/2 for hybrid and nanofill composites. Drummond (2008) reviewed the literature and summarized a KIC range of about 1.2-1.6 MPa·m1/2 for dental resin composites. These reported values of KIC are indicated in Fig. 6 by the unfilled box (upper box) at the right axis. Hence, the heat-cured nanocomposite with Ca and PO4 ion release matched or exceeded the KIC of previous stress-bearing composites without ion release. Further studies are needed to measure the KIC of photo-activated nanocomposites with Ca, PO4, and F release vs. immersion time.
Three-body Wear
For the Ca-PO4 nanocomposite, the wear resistance was recently tested in a four-station wear apparatus (Caulk/Dentsply, Milford, DE, USA) for three-body occlusal wear. The nanocomposite had a DCPA:whisker mass ratio of 1:2, at a filler level of 74%, and was heat-cured at 120°C for 30 min for indirect restorations. A commercial indirect prosthetic composite (Artglass) was used as a control. After 400,000 wear cycles, the “dimple-like” wear scar was measured by profilometry (Xu et al., 2004a). The wear scar depth and diameter for the Ca-PO4 nanocomposite were similar to those of the non-releasing prosthetic composite (Fig. 7).
Figure 7.
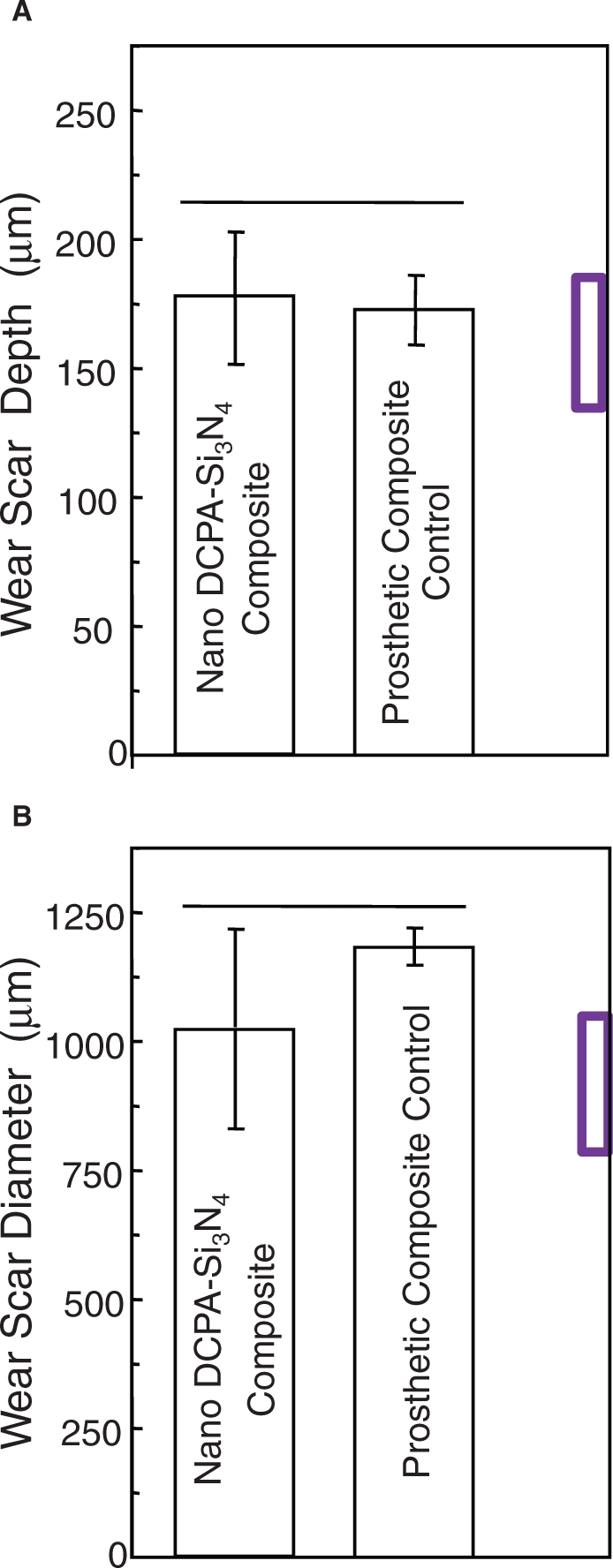
Three-body wear. (A) Wear scar depth and (B) diameter. Horizontal line indicates values not significantly different (p > 0.1). Nanocomposite with Ca-PO4 release matched the wear of a commercial indirect composite without ion release. Box at right axis indicates wear of amalgam measured in the same way (Xu et al., 2008b).
Comparison should be made with dental amalgam, which is known for its resistance to occlusal wear and is taken as the standard by which newer restorative materials are judged. A previous study using the same equipment by the same operator subjected amalgam (Dispersalloy, Dentsply, Milford, DE, USA) to 400,000 cycles of wear, and measured a wear scar depth of 134 ± 54 µm and a diameter of 778 ± 270 µm (Xu et al., 1999). These values are indicated by the box at the right axis in Fig. 7, and they are not significantly different from those of the DCPA nanocomposite (p > 0.1). Regarding the correlation between these in vitro wear values and clinical wear, a previous study (Leinfelder and Suzuki, 1999) used the same type of wear machine and compared the results with in vivo data. They found that the 400,000-cycle in vitro wear agreed with the in vivo wear values over a three-year period. This is consistent with a separate study (DeLong et al., 1985) showing that a wear depth of 100-160 µm occurred for amalgam in 2-3 years. Therefore, a heat-cured nanocomposite with Ca and PO4 ion release matched the wear resistance of a commercial indirect stress-bearing composite without ion release as well as a dental amalgam. Further study should investigate the wear behavior of photo-cured nanocomposites with Ca, PO4, and F release.
Potential Applications And Future Research
The processing of Ca, PO4, and F ion-releasing nanocomposites occurs by this approach: Nanocomposite = ion-releasing nanofillers + reinforcing (non-releasing) fillers + matrix resin. This approach can be applied to other materials by the use of different ion-releasing fillers (e.g., various Ca-PO4 fillers or fluoride-releasing fillers) together with different reinforcing fillers (e.g., whiskers, glass particles, or glass fibers). The primary goal of this nanocomposite development strategy is to address the two major challenges: secondary caries and restoration fracture. Especially in large posterior restorations, currently available ion-releasing and caries-inhibiting restoratives have inadequate stress-bearing capabilities. Thus, a potential application for the new, mechanically strong nanocomposites with Ca, PO4, and F release would be posterior restorations where amalgam and hybrid composites are currently used. For posterior restorations, esthetics may not be as important as for anterior restorations. The silicon nitride whisker-reinforced nanocomposite is relatively opaque with a whitish color due to a refractive index mismatch between the whiskers and the resin, which may be useful in large posterior restorations due to strength and toughness as well as ion release. Studies are under way to develop more esthetic nanocomposites with Ca, PO4, and F release.
Additional potential applications for the Ca-, PO4-, and F-releasing nanocomposites may be in treatments where complete removal of caries tissue is contra-indicated, where caries lesions are beginning or are likely to occur, and for individuals at high risk for dental caries (e.g., receiving radiation treatments or certain medications, or with dry mouth). This may be especially applicable to persons of certain ethnicity and poverty levels, with high incidence of untreated caries, for whom the atraumatic restorative treatment (ART) can be widely and relatively easily performed (Mandari et al., 2002; Frencken et al., 2004). Many areas of developing countries do not have electricity. ART does not require electricity; hence, the two-part chemically cured nanocomposites with Ca, PO4, and F release may be useful. ART requires neither running water nor anesthesia, but may not completely remove the carious tissue (Mandari et al., 2002; Frencken et al., 2004). Hence, a Ca-, PO4-, and F-releasing nanocomposite may be beneficial in remineralization of caries remnants and inhibition of future caries, while the opacity of a whitish composite may be less of a concern for these individuals. However, the opacity of the composite will hinder uses where a high level of esthetics is desired. Hence, further studies should develop esthetic nanocomposites with stress-bearing and Ca-, PO4-, and F-releasing capabilities. Studies are also needed to investigate the caries-inhibition efficacy of the new nanocomposites in human teeth.
Acknowledgments
We are grateful to Drs. Jack L. Ferracane, Franklin Garcia-Godoy, Sabine H. Dickens, Drago Skrtic, and Gary E. Schumacher for fruitful discussions. We thank Anthony Giuseppetti, Janet Quinn, Max Peltz, Chiara Ferraris, and Bernard Hockey for assistance. We are grateful to Editor-in-Chief Dr. Anthony Smith, and to Dr. Dana Graves, editor of the Critical Reviews in Oral Biology & Medicine section, for inviting us to write this review. Thanks are also due to the two anonymous reviewers who provided invaluable comments.
Footnotes
We gratefully acknowledge the following support: NIH R01 grants DE14190 (HX), DE17974 (HX), and DE16416 (LC), Maryland Nano-Biotechnology Initiative Award (HX), the University of Maryland Dental School in Baltimore, NIST, and ADAF. This is an official contribution of the National Institute of Standards and Technology (NIST); not subject to copyright in the United States.
References
- Anusavice KJ, Zhang NZ, Shen C. (2005). Effect of CaF2 content on rate of fluoride release from filled resins. J Dent Res 84:440-444 [DOI] [PubMed] [Google Scholar]
- Asmussen E, Peutzfeldt A. (2002). Long-term fluoride release from a glass ionomer cement, a compomer, and from experimental resin composites. Acta Odontol Scand 60:93-97 [DOI] [PubMed] [Google Scholar]
- Bayne SC, Thompson JY, Swift EJ, Jr, Stamatiades P, Wilkerson M. (1998). A characterization of first-generation flowable composites. J Am Dent Assoc 129:567-577 [DOI] [PubMed] [Google Scholar]
- Beun S, Glorieux T, Devaux J, Vreven J, Leloup G. (2007). Characterization of nanofilled compared to universal and microfilled composites. Dent Mater 23:51-59 [DOI] [PubMed] [Google Scholar]
- Braga RR, Ballester RY, Ferracane JL. (2005). Factors involved in the development of polymerization shrinkage stress in resin-composites: a systematic review. Dent Mater 21:962-970 [DOI] [PubMed] [Google Scholar]
- Burke FM, Ray NJ, McConnell RJ. (2006). Fluoride-containing restorative materials. Int Dent J 56:33-43 [DOI] [PubMed] [Google Scholar]
- Carey CM, Spencer M, Gove RJ, Eichmiller FC. (2003). Fluoride release from a resin-modified glass-ionomer cement in a continuous-flow system: effect of pH. J Dent Res 82:829-832 [DOI] [PubMed] [Google Scholar]
- Chen MH, Chen CR, Hsu SH, Sun SP, Su WF. (2006). Low shrinkage light curable nanocomposite for dental restorative material. Dent Mater 22:138-145 [DOI] [PubMed] [Google Scholar]
- Chow LC. (2000). Calcium phosphate cements: chemistry, properties, and applications. Mat Res Soc Symp Proc 599:27-37 [Google Scholar]
- Chow LC, Sun L, Hockey B. (2004). Properties of nanostructured hydroxyapatite prepared by a spray drying technique. J Res Natl Inst Stand Technol 109:543-551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon JR, Ferracane JL. (2002). Reduced polymerization stress through non-bonded nanofiller particles. Biomaterials 23:3807-3815 [DOI] [PubMed] [Google Scholar]
- Dauvillier BS, Feilzer AJ, De Gee AJ, Davidson CL. (2000). Visco-elastic parameters of dental restorative materials during setting. J Dent Res 79:818-823 [DOI] [PubMed] [Google Scholar]
- DeLong R, Sakaguchi RL, Douglas WH, Pintado MR. (1985). The wear of dental amalgam in an artificial mouth: a clinical correlation. Dent Mater 1:238-242 [DOI] [PubMed] [Google Scholar]
- Dickens SH, Flaim GM, Takagi S. (2003).Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dent Mater 19:558-566 [DOI] [PubMed] [Google Scholar]
- Dickens SH, Flaim GM, Floyd CJE. (2004). Effect of resin composition on mechanical and physical properties of calcium phosphate filled bonding systems. Polymer Preprints 45:329-330 [Google Scholar]
- Drummond JL. (2008). Degradation, fatigue, and failure of resin dental composite materials. J Dent Res 87:710-719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond JL, Bapna MS. (2003). Static and cyclic loading of fiber-reinforced dental resin. Dent Mater 19:226-231 [DOI] [PubMed] [Google Scholar]
- Eick JD, Byerley TJ, Chappell RP, Chen GR, Bowles CQ, Chappelow CC. (1993). Properties of expanding SOC/epoxy copolymers for dental use in dental composites. Dent Mater 9:123-127 [DOI] [PubMed] [Google Scholar]
- Ellakuria J, Triana R, Minguez N, Soler I, Ibaseta G, García-Godoy F, et al. (2003). Effect of one-year water storage on the surface microhardness of resin-modified versus conventional glass-ionomer cements. Dent Mater 19:286-290 [DOI] [PubMed] [Google Scholar]
- Ferracane JL. (1995). Current trends in dental composites. Crit Rev Oral Biol Med 6:302-318 [DOI] [PubMed] [Google Scholar]
- Ferracane JL. (2006). Hygroscopic and hydrolytic effects on dental polymer networks. Dent Mater 22:211-222 [DOI] [PubMed] [Google Scholar]
- Ferracane JL. (2008). Buonocore Lecture. Placing dental composites—a stressful experience. Oper Dent 33:247-257 [DOI] [PubMed] [Google Scholar]
- Ferracane JL, Berge HX. (1995). Fracture toughness of experimental dental composites aged in ethanol. J Dent Res 74:1418-1423 [DOI] [PubMed] [Google Scholar]
- Ferracane JL, Mitchem JC. (1994). Properties of posterior composites: results of round robin testing for a specification. Dent Mater 10:92-99 [DOI] [PubMed] [Google Scholar]
- Ferracane JL, Hopkin JK, Condon JR. (1995). Properties of heat-treated composites after aging in water. Dent Mater 11:354-358 [DOI] [PubMed] [Google Scholar]
- Ferracane JL, Berge HX, Condon JR. (1998). In vitro aging of dental composites in water—effect of degree of conversion, filler volume, and filler/matrix coupling. J Biomed Mater Res 42:465-472 [DOI] [PubMed] [Google Scholar]
- Frencken JE, van’t Hof MA, van Amerongen WE, Holmgren CJ. (2004). Effectiveness of single-surface ART restorations in the permanent dentition: a meta-analysis. J Dent Res 83:120-123 [DOI] [PubMed] [Google Scholar]
- Furman B, Rawls HR, Wellinghoff S, Dixon H, Lankford J, Nicolella D. (2000). Metal-oxide nanoparticles for the reinforcement of dental restorative resins. Crit Rev Biomed Eng 28:439-443 [DOI] [PubMed] [Google Scholar]
- Garoushi S, Vallittu PK, Watts DC, Lassila LV. (2008). Effect of nanofiller fractions and temperature on polymerization shrinkage on glass fiber reinforced filling material. Dent Mater 24:606-610 [DOI] [PubMed] [Google Scholar]
- Glasspoole EA, Erickson RL, Davidson CL. (2001). A fluoride-releasing composite for dental applications. Dent Mater 17:127-133 [DOI] [PubMed] [Google Scholar]
- Hicks J, Garcia-Godoy F, Donly K, Flaitz C. (2003). Fluoride-releasing restorative materials and secondary caries. J CA Dent Assoc 31:229-245 [PubMed] [Google Scholar]
- Hsu CYS, Donly KJ, Drake DR, Wefel JS. (1998). Effects of aged fluoride-containing restorative materials on recurrent root caries. J Dent Res 77:418-425 [DOI] [PubMed] [Google Scholar]
- Imazato S. (2003). Review: Antibacterial properties of resin composites and dentin bonding systems. Dent Mater 19:449-457 [DOI] [PubMed] [Google Scholar]
- Indrani DJ, Cook WD, Televantos F, Tyas MJ, Harcourt JK.(1995). Fracture toughness of water-aged resin composite restorative materials. Dent Mater 11:201-207 [DOI] [PubMed] [Google Scholar]
- Itota T, Carrick TE, Yoshiyama M, McCabe JF. (2004). Fluoride release and recharge in glass giomer, compomer and resin composite. Dent Mater 20:789-795 [DOI] [PubMed] [Google Scholar]
- Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. (2001). Quality of dental restorations. FDI Commission Projects 2-95. Int Dent J 51:117-158 [DOI] [PubMed] [Google Scholar]
- Kao EC, Culbertson BM, Xie D. (1996). Preparation of glass ionomer cement using N-acryloyl-substituted amino acid monomers—evaluation of physical properties. Dent Mater 12:44-51 [DOI] [PubMed] [Google Scholar]
- Krämer N, García-Godoy F, Reinelt C, Frankenberger R. (2006). Clinical performance of posterior compomer restorations over 4 years. Am J Dent 19:61-66 [PubMed] [Google Scholar]
- Leinfelder KF, Suzuki S. (1999). In vitro wear device for determining posterior composite wear. J Am Dent Assoc 130:1347-1353 [DOI] [PubMed] [Google Scholar]
- Ling L, Xu X, Choi GY, Bilodeaux D, Guo G, Diwan RM. (2009). Novel F-releasing composites with improved mechanical properties. J Dent Res 88:83-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CH, Iannetta RV. (1982). The fracture toughness of dental composites. Part I: The development of strength and fracture toughness J Oral Rehabil 9:55-66 [DOI] [PubMed] [Google Scholar]
- Loza-Herrero MA, Rueggeberg FA, Caughman WF, Schuster GS, Lefebvre CA, Gardner FM. (1998). Effect of heating delay on conversion and strength of a post-cured resin composite. J Dent Res 77:426-431 [DOI] [PubMed] [Google Scholar]
- Lu H, Stansbury JW, Bowman CN. (2005). Impact of curing protocol on conversion and shrinkage stress. J Dent Res 84:822-826 [DOI] [PubMed] [Google Scholar]
- Mandari GJ, Frencken JE, van’t Hof MA. (2002). Six-year success rates of occlusal amalgam and glass-ionomer restorations placed using three minimally intervention approaches. Caries Res 37:246-253 [DOI] [PubMed] [Google Scholar]
- Mathis RS, Ferracane JL. (1989). Properties of a glass-ionomer/resin-composite hybrid material. Dent Mater 5:355-358 [DOI] [PubMed] [Google Scholar]
- Mitra SB, Wu D, Holmes BN. (2003). An application of nanotechnology in advanced dental materials. J Am Dent Assoc 134:1382-1390 [DOI] [PubMed] [Google Scholar]
- Mjör IA, Moorhead JE, Dahl JE. (2000). Reasons for replacement of restorations in permanent teeth in general dental practice. Int Dent J 50:361-366 [DOI] [PubMed] [Google Scholar]
- Moioli EK, Clark PA, Xin X, Lal S, Mao JJ. (2007). Matrices and scaffolds for drug delivery in dental, oral and craniofacial tissue engineering. Adv Drug Deliv Rev 59:308-324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell JNR, Antonucci JM, Skrtic D. (2005). Mechanical properties of amorphous calcium phosphate composites (abstract). J Dent Res 84 (Spec Iss A):Abstract No. 586 http://iadr.confex.com/iadr/2005Balt/techprogram/abstract_60928.htm [Google Scholar]
- Peutzfeldt A. (1997). Resin composites in dentistry: the monomer systems. Eur J Oral Sci 105:97-116 [DOI] [PubMed] [Google Scholar]
- Rodrigues SA, Jr, Scherrer SS, Ferracane JL, Della Bona A. (2008). Microstructural characterization and fracture behavior of a microhybrid and a nanofill composite. Dent Mater 24:1281-1288 [DOI] [PubMed] [Google Scholar]
- Ruddell DE, Maloney MM, Thompson JY. (2002). Effect of novel filler particles on the mechanical and wear properties of dental composites. Dent Mater 18:72-80 [DOI] [PubMed] [Google Scholar]
- Sakaguchi RL. (2005). Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Dent Mater 21:3-6 [DOI] [PubMed] [Google Scholar]
- Sarrett DC. (2005). Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater 21:9-20 [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Sherriff M, Watson TF. (1997). The effects of maturity and dehydration shrinkage on resin-modified glass-ionomer restorations. J Dent Res 76:1495-1501 [DOI] [PubMed] [Google Scholar]
- Skrtic D, Hailer AW, Takagi S, Antonucci JM, Eanes ED. (1996a). Quantitative assessment of the efficacy of amorphous calcium phosphate/methacrylate composites in remineralizing caries-like lesions artificially produced in bovine enamel. J Dent Res 75:1679-1686 [DOI] [PubMed] [Google Scholar]
- Skrtic D, Antonucci JM, Eanes ED. (1996b). Improved properties of amorphous calcium phosphate fillers in remineralizing resin composites. Dent Mater 12:295-301 [DOI] [PubMed] [Google Scholar]
- Skrtic D, Antonucci JM, Eanes ED, Eichmiller FC, Schumacher GE. (2000). Physicochemical evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. J Biomed Mater Res 53:381-391 [DOI] [PubMed] [Google Scholar]
- Stansbury JW. (1990). Cyclopolymerizable monomers for use in dental resin composites. J Dent Res 69:844-848 [DOI] [PubMed] [Google Scholar]
- Sun L, Chow LC. (2008). Preparation and properties of nano-sized calcium fluoride for dental applications. Dent Mater 24:111-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Gao Y, Liu Y, Liao Y, Hedin NE, Fong H. (2008). Fabrication and evaluation of Bis-GMA/TEGDMA dental resins/composites containing nano fibrillar silicate. Dent Mater 24:235-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Cate JM. (1997). Review on fluoride, with special emphasis on calcium fluoride mechanisms in caries prevention. Eur J Oral Sci 105:461-465 [DOI] [PubMed] [Google Scholar]
- Tyas M. (1990). Correlation between fracture properties and clinical performance of composite resins in Class IV cavities. Aust Dent J 35:46-49 [DOI] [PubMed] [Google Scholar]
- Tyas MJ, Burrow MF. (2002). Clinical evaluation of a resin-modified glass ionomer adhesive system—results at 5 years. Oper Dent 27:438-441 [PubMed] [Google Scholar]
- Wan Q, Sheffield J, McCool J, Baran G. (2008). Light curable dental composites designed with colloidal crystal reinforcement. Dent Mater 24:1694-1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lee JJ, Lloyd IK, Wilson OC, Jr, Rosenblum M, Thompson V. (2007). High modulus nanopowder reinforced dimethacrylate matrix composites for dental cement applications. J Biomed Mater Res A 82:651-657 [DOI] [PubMed] [Google Scholar]
- Watts DC, Marouf AS, Al-Hindi AM. (2003). Photo-polymerization shrinkage-stress kinetics in resin-composites: methods development. Dent Mater 19:1-11 [DOI] [PubMed] [Google Scholar]
- Watts DC, Issa M, Ibrahim A, Wakiaga J, Al-Samadani K, Al-Azraqi M, et al. (2008). Edge strength of resin-composite margins. Dent Mater 24:129-133 [DOI] [PubMed] [Google Scholar]
- Weigand A, Buchalla W, Attin T. (2007). Review on fluoride-releasing restorative materials—fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater 23:343-362 [DOI] [PubMed] [Google Scholar]
- Wilson AD, McLean JW. (1988). Glass-ionomer cement. Chicago, IL, USA: Quintessence Publishing Co [Google Scholar]
- Wilson KS, Zhang K, Antonucci JM. (2005). Systematic variation of interfacial phase reactivity in dental nanocomposites. Biomaterials 26:5095-5103 [DOI] [PubMed] [Google Scholar]
- Xia Y, Zhang F, Xie H, Gu N. (2008). Nanoparticle-reinforced resin-based dental composites. J Dent 36:450-455 [DOI] [PubMed] [Google Scholar]
- Xu HH. (2003). Long-term water aging of whisker-reinforced polymer-matrix composites. J Dent Res 82:48-52; erratum in J Dent Res 82:326, 2003 [DOI] [PubMed] [Google Scholar]
- Xu HH, Eichmiller FC, Giuseppetti AA, Ives LK, Parry EE, Schumacher GE. (1999). Three-body wear of a hand-consolidated silver alternative to amalgam. J Dent Res 78:1560-1567 [DOI] [PubMed] [Google Scholar]
- Xu HH, Eichmiller FC, Antonucci JM, Flaim GM. (2000). Single-crystalline ceramic whisker-reinforced carboxylic acid-resin composites with fluoride release. Oper Dent 25:90-97 [PubMed] [Google Scholar]
- Xu HH, Eichmiller FC, Smith DT, Schumacher GE, Giuseppetti AA, Antonucci JM. (2002). Effect of thermal cycling on whisker-reinforced dental resin composites. J Mater Sci: Mater in Med 13:875-883 [DOI] [PubMed] [Google Scholar]
- Xu HH, Quinn JB, Giuseppetti AA. (2004a). Wear and mechanical properties of nano-silica-fused whisker composites. J Dent Res 83:930-935 [DOI] [PubMed] [Google Scholar]
- Xu HH, Smith DT, Simon CG. (2004b). Strong and bioactive composites containing nano-silica-fused whiskers for bone repair. Biomaterials 25:4615-4626 [DOI] [PubMed] [Google Scholar]
- Xu HH, Sun L, Weir MD, Antonucci JM, Takagi S, Chow LC, et al. (2006). Nano DCPA-whisker composites with high strength and Ca and PO4 release. J Dent Res 85:722-727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HH, Weir MD, Sun L, Takagi S, Chow LC. (2007a). Effects of calcium phosphate nanoparticles on Ca-PO4 composite. J Dent Res 86:378-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HH, Weir MD, Sun L. (2007b). Nanocomposites with Ca-PO4 release: effects of reinforcement, dicalcium phosphate particle size and silanization. Dent Mater 23:1482-1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HH, Moreau JL, Sun L, Chow LC. (2008a). Strength and fluoride release characteristics of a calcium fluoride-based dental nanocomposite. Biomaterials 29:4261-4267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HH, Weir MD, Sun L, Moreau JL, Takagi S, Chow LC. (2008b). Development of stress-bearing, caries-inhibiting nanocomposites (abstract). J Dent Res 87(Spec Iss A):abstract 577 http://iadr.confex.com/iadr/2008Dallas/techprogram/session_18562.htm [Google Scholar]
- Xu X, Burgess JO. (2003). Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials 24:2451-2461 [DOI] [PubMed] [Google Scholar]