Abstract
Adoptive immunotherapy with in vitro expanded cytotoxic T lymphocytes specific for Epstein-Barr virus (EBV) can successfully treat post-transplant lymphoproliferative disease (PTLD). However, extension of a similar strategy to Hodgkin’s disease (HD) and nasopharyngeal carcinoma (NPC) is limited by the poor immunogenicity of the limited set of EBV latency antigens expressed in these malignancies, making T-cell expansion difficult. Retroviral transduction of LMP-specific T-cell receptors (TCR) into activated T lymphocytes may provide a universal, MHC-restricted, means to generate effector cells without the need for tissue culture based methods of CTL expansion. We report the transfer of two LMP2-specific TCRs from human T-cell clones (HLA-A2 and HLA-A23,24 restricted) that confer the ability to lyse EBV-immortalized B-lymphoblastoid cell lines (B-LCL). B-LCL are the best model for native expression of LMP2. We also demonstrate the rapid transfer of the TCR by nucleofection of primary T cells using a simple plasmid-based vector. The ability to detect nucleofected TCRVβ chain by antibody, fully assembled TCR by tetramer, and peptide-MHC-specific lytic activity indicates that nucleofection can serve as a tool for rapid screening of TCR specificity.
Keywords: Immunotherapy, adoptive, Epstein-Barr virus, T-cell receptor, retroviral transduction, gene therapy
INTRODUCTION
The human cancers associated with Epstein-Barr virus (EBV) can be categorized according to the EBV-latency gene transcripts expressed in the transformed cells of the tumor (1). Post-transplant lymphoma (PTLD), a type III EBV malignancy, expresses the full complement of EBV latency antigens. This includes theEBNA-3 family of antigens, the primary targets of the polyclonal T-cell lines used to prevent or treat post-transplant lymphoma by adoptive immunotherapy (2). Like the strong immune response seen against the pp65 and gp100 antigens of CMV, the EBNA-3 antigens of EBV induce a robust cellular immune response in the host and may in fact be part of natural biology of establishing a latent and permanent infection in the host (3). The EBV antigens expressed in EBV-positive Hodgkin’s disease (HD) and nasopharyngeal carcinoma (NPC, type II EBV malignancies) are restricted to latency membrane protein (LMP) 1, LMP2, and EBNA-1. CD8+ CTL to EBNA-1 have yet to be detected in primary culture and the cellular immune response to LMP1 and LMP2 is weak at best (4). Thus, NPC and EBV-associated Hodgkin lymphoma (HD) express only weak or subdominant EBV-encoded tumor-associated antigens. These proteins are therefore more akin to endogenous tumor antigens than highly expressed viral antigens. In studies seeking to ascertain the prevalence of even low levels of CTL activity specific for LMP1 and LMP2 it appears that half of seropositive healthy volunteers have at least a minimal response (5). The ability to detect LMP1- and LMP2-specific CTL responses is likely to be even more difficult in patients with malignancy, especially in those that have received cytotoxic chemotherapy. The generation of an effective immune response to HD may be even more difficult in vivo given the Th2 cytokine-rich character of the lesion, which has been shown to contain IL-10, IL-6, and TGF-β (6–8). Rather than relying on a tumor vaccine to induce a response to a protein that is marginally recognized in healthy volunteers, we propose that adoptive transfer with lymphocytes transduced with EBV-specific TCR could be used to treat EBV malignancy in patients.
The clinical adoptive transfer of antigen-specific cytotoxic T lymphocytes to patients has been a logical outgrowth of in vitro studies demonstrating the ability of patient-derived antigen-specific T lymphocytes to recognize and lyse tumor cells in an MHC-restricted manner. Using techniques developed for T-cell-based gene therapy, populations of tumor-specific CTL can now be produced by retroviral transduction with vectors encoding the alpha (α) and beta (β) chains of the T-cell receptor (TCR). Transfer of lytic function by retroviral vectors encoding the α and β chains of the TCR was first successfully demonstrated by Clay et al. in 1999, and was expanded into the EBV system the following year (9, 10). The ability to transfer TCR genes means that within the confines of MHC restriction, if an antigen-specific T-cell clone exists, sequences encoding the native TCR from this clone can be introduced into activated peripheral blood lymphocytes from any patient sharing that MHC allele. Thus, independent of the ability to clone and expand CTL from a single patient, an antigen-specific effector population can be generated for all patients.
In previous work, we demonstrated that retroviral transduction of activated PBMC with TCRs cloned from cytotoxic T clones raised against an HLA-A2-binding LMP2 peptide required target cells to be peptide pulsed as well. Transduced PBMC were unable to lyse unpulsed B-LCL, which model the expression level of LMP2 in tumors (10). Here we demonstrate the transfer of B-LCL lytic activity by transduction of activated lymphocytes with a vector encoding the TCRα and -β chains from two CTL clones (NB-20 and CSIC7) specific for LMP2 (11).Native TCR were cloned into A7-based retroviral vectors and the resulting A7-LMP2.A2 and A7-LMP2.A23 vectors were used to transduce activated PBMC. We report for the first time the production of a transduced T-cell population that has lytic activity for endogenously expressed LMP2, as demonstrated by the lysis B lymphoblastoid cell lines (B-LCL) as well as peptide-pulsed T2 targets. We also evaluated a new technique for transfection, nucleofection, as a means of rapid TCR transfer into activated PBMC. The newly generated plasmid vector pBudA7-LMP2.A2, upon nucleofection into activated PBMCs, generated a CTL population capable of specifically lysing peptide-pulsed T2 cells in less than 24 h. The ability to use direct DNA transfection technology to transfer functional TCRs is a novel and rapid means for screening multiple recombinant TCR specificities for potential suitability in adoptive immunotherapy protocols.
MATERIALS AND METHODS
TCR Cloning and Vector Construction
RNA from the CSIC7 and NB20 human CTL clones was isolated using Trizol per manufacturer’s recommendations (Invitrogen, Carlsbad, CA). Briefly, 1 × 107 were lysed in Trizol, extracted with chloroform and precipitated in isopropanol. RNA pellets were washed in ethanol and resuspended in DNase, RNase-free dH2O. cDNA was produced from CTL clone total RNA using an oligo-dT primer in the presence of RNasin (Promega, Madison, WI), DTT, dNTP, 5X first strand buffer, and SuperScript II reverse transcriptase (Invitrogen). The oligo dT/RNasin mixture was incubated at 75°C for 10 min, additional reagents added and incubated at 42°C for 50 min and inactivated at 70°C for 15 min. The cDNA was spectratyped with the human primer set developed by Yassai et al. (12). The α and β full-length subunits were amplified from the cDNA using Pfu turbo Polymerase, tailed with Taq Polymerase (Invitrogen), and cloned into the pCR2.1 TOPO-TA vector (Invitrogen). The primers for amplification were Vα15, 5UTVA15: 5′CTCTCTTGGCTGGAGATTG′, Vα2 5UTLOVA2: 5′TCCTCAGTGAACCAGGGCA, and the common 3′ primer ALPH-C3′: 5′AGCACAGGCTGTCTTACAATCTTGC; Vβ13, 5UT13S1: 5′GCATCTGCCATGAGCATC; β21, 5UTVβ21S1: 5′CATCCTGCCCTGACCCTG, and the downstream primers 3utcrB1anti: 5′GCAGAGAGGTGAGAGCAGC for TCRCβ1 or 3utcrB2anti: 5′GGACACAGATTGGGAGCAGG for TCRCβ2. The α15 and α2 subunits were modified to add Sal I sites to their 5′ and 3′ regions by PCR and cloned into the A7 retroviral vector (9). Sequence integrity and orientation were verified by sequencing. The β subunits were modified in a similar way to include flanking Xho I sites and cloned into the A7 vector. TCR DNA sequences from LMP2-specific CTL clones have been submitted to Genebank under the following accession numbers, NB20 Vβ21: AY4755218, Vα2: AY475219; for CSIC7, Vβ13: AY475217, Vα15: AY457220.
Infectious Retroviral Vector Production and T-Cell Transduction
Infectious retroviral vector supernatant was produced by transient transfection of amphotropic Phoenix cells (kindly provided by Dr. G. Nolan, Stanford University) with retroviral vectors using Lipofectamine 2000 (Invitrogen) per manufacturer’s instructions. Transfections were performed using 20 µg of endotoxin-free plasmid (Endo-Free, Qiagen, Valencia, CA) per 10 cm dish. Forty-eight hours post-transfection, supernatant was collected, spun at 900 rpm to pellet cellular debris and passed through a 0.45-µm filter. Supernatant was stored at −80°C if not used immediately for PBMC transduction. Infectious retroviral vector supernatants were titered on 2 × 105 NIH 3T3 cells in the presence of 8 µg/mL polybrene by spinfection in 6-well plates. Twenty-four hours later, cells were split 1:4 and cultured in cDMEM containing 0.75 µg/mL G418 (Geneticin, Invitrogen). Surviving colonies were counted at 1 week. Titers obtained ranged from 1 × 105 to 1 × 106 cfu/mL. Retroviral transduction of activated T cells was as described previously in cLyEM (40% RPMI-1640, 40% EHAA, supplemented with 10 mM HEPES, 4 mM l-glutamine and penicillin/streptomycin, all media components from Life technologies, Gaithersburg, MD, 10% FBS from Gemini Bio-Products, Woodland, CA) (10). Briefly, PBMC purified on density gradients (Ficoll-Paque, Amersam Biosciences, Uppsala, Sweden) were activated in 24-well plates with 10 ng/mL OKT3 (Ortho Biotech, Raritan, NJ) and 600U IL-2/mL (Proleukin, Chiron), and transduced via spinfection by centrifuging the PBMCs in 24-well plates for 90min at 32°C at 1000 × g in media containing a 1:3 dilution of retroviral supernatant, 8 µg/mL polybrene and IL-2 for three consecutive days. PBMCs were maintained in 0.75 µg/mL G418 and 600U IL-2/mL for 5–6 days, rested 1 day, and then screened for lytic activity in 51Cr release assays. At 0.75 µg/mL G418, these culture conditions result in a four-fold decrease in the number of non-transfected cells, allowing for the selective expansion of transduced cells. Cells were transduced on day 3 after activation. When indicated, CD8+ cells from transduced PBMCs populations were enriched using CD8 paramagnetic microbeads (Miltenyi Biotec, Auburn, CA) and sorted on an AutoMACs cell separator.
Nucleofection Vector Construction
The α and β subunits from NB20 CTL were cloned into the plasmid pBudCE4.1 (Invitrogen) to create pBudA7-LMP2.2A by standard molecular methods. The α chain was cloned into a multi-cloning site of pBudCE4.1 whose expression is driven by a CMV-IE promoter. The β subunit was cloned into a second multi-cloning site provided in the pBud plasmid with its expression driven by the human elongation factor 1α promoter (EF1α). Plasmid vectors expressing single TCRα (pCI-NB20α, pCI-5.04α) and TCRβ chains (pCI-NB20β, pCI-PL5.04β) from a CMV-IE promoter were also produced using the pCINeo (Promega) mammalian expression vector.
PBMC Nucleofection
CTL from activated PBMCs (as above) were purified by depletion of CD56- and in some experiments also CD4-expressing cells on an AutoMACs cell separator. The CD8-containing cell fraction was nucleofected per manufacturer’s instructions using the “Human T-cell Nucleofetor Kit,” (Amaxa, Inc., Gaithersburg, MD). Briefly, 270 ng of pBud A7-LMP2.2Awas combined with 1 × 106 CD8+ PBMCs in 100 µL room temperature Human T Cell Nucleofector Solution. This mixture was transferred to the supplied cuvette and nucleofected with program T20 in the nucleofector device. Nucleofected cells were then placed in pre-warmed media and allowed to recover for 24 h prior to screening for lytic activity in 51Cr release assays.
T-Cell Functional Assays
T2 cells, K562, or EBV-transformed B-LCLs were used as targets in 4.5 h 51Cr release assays. One million targets were 51Cr labeled for 1.5 h at 37°C in 5% CO2, peptide (40 µg/mL) was added at 45 min where indicated. Peptides were synthesized by the Protein and Nucleic Acid Core Facility, Medical College of Wisconsin. Five thousand 51Cr-labeled APCs were incubated with the transduced PBMCs effector cells at the designated ratios for 4.5 h at 37°C in a humidified CO2 incubator. Assays were terminated by transferring 30 µL of co-culture media on to LumaPlates (Packard Instrument Company Inc.,Meriden, CT) and gamma emission counted on a Top-Count microplate scintillation counter (Packard). The percent lysis was determined using the formula: specific lysis = (target cell release − spontaneous release)/(total cell release − spontaneous release).
Cell Surface Protein Analysis
FITC-conjugated anti-human Vβ13.1 and Vβ21.3 were purchased from Immunotech (Marseille, France), PE-conjugated anti-human CD8 antibodies were from BD Biosciences-Pharmingen (San Diego, CA). The LMP2 peptide containing teteramers HLA-A-0201/CLGGLLTMV-PE and HLA-A-2301/PYLFWLAAI-PE were obtained from the NIAID MHC Tetramer Core Facility, Yerkes Regional Primate Research Center, Atlanta, GA. For tetramer staining, 1 × 106 cells were blocked in human A/B serum (10% A/B serum, Labquip Ltd., Niagara Falls, NY, 0.1% azide in PBS) on ice for a 0.5 h, washed twice in flow buffer (0.2% azide in PBS) and resuspended in antibody dilution solution (5% FBS, 0.1% azide in PBS). To 40 µL of resuspended cells, 1µL of stock tetramer and additional antibodies to cell surface markers were added and incubated at 37°C for 1 h in the dark. Cells were washed twice with flow buffer were resuspended in 500 µL flow media (0.5% FBS, 0.2 azide in PBS) and 1 µL 7AAD added. Cells were analyzed immediately. A FACScan flow cytometer (Becton-Dickinson, San Jose, CA) was used for all experiments and data analyzed using Win-MDI v2.8, ©Joseph Trotter. For analysis, cells were gated on live lymphocyte populations.
RESULTS
The human T-cell clones CSIC7 and NB20 are both specific for the EBV LMP2 (11). CSIC7 is HLA-23,24 restricted and is specific for LMP2 peptide PYLFWLAAI, residues 131–139. NB20 is HLA-2 restricted and specific for the LMP2 peptide CLGGLLTMV, residues 425–433 in the BNLF2a open reading frame of EBV. These cell clones are unique in that they have the ability to lyse not only peptide-pulsed or target cells infected with vaccinia virus-LMP2 expression vectors, but also B-LCL expressing what is considered native levels of LMP2 tumor protein. Therefore, cDNA was prepared by reverse transcription from total RNA and the TCRVα and -β subunits identity established by PCR analysis using V-region family-specific primers (12). The CDR3 regions for these clones are shown in Fig. 1. Full-length cDNA was amplified, using primers described in “Methods” section, and the single TCRVα and -β sequences subcloned into the A7 retroviral vector, creating A7-LMP2.A23 and A7-LMP2.A2, respectively. Infectious vector-containing supernatant was generated by transient transfection of the Phoenix amphotropic packaging cell line as previously described and titered on NIH3T3 cells (10).
Fig. 1. Complementarity Determining Region-3 (CDR3) of cloned TCR.
The sequences of the α and β chains CDR3 regions from the TCR donor clones NB20 and CSIC7 are illustrated using standard single amino acid letter codes. VA2, TCRVα sequences immediately preceding the J chain; JA41, Jα41; CA, the first four residues of the constant region of the TCR. VB21, TCRVβ21, TCRVβ sequence preceding the Diversity (D) and Jβ2.7 (JB2.7) regions. CB2, the first four resides of the beta chain constant region 2. Identical terminology is used for the CDR3 region of CSIC7.
To test the ability of A7-LMP2.A23 and A7-LMP2.A2 to confer B-LCL-specific lytic function, peripheral blood mononuclear cells (PBMCs) were activated for 3 days with OKT3 and IL-2 and then transduced by “spinfection” for three consecutive days (10). Transduced PBMCs were maintained in cLyEM media containing 600 U/mL IL-2 and 0.75 mg/mL G418 for 5–6 days of selection, then used in 51Cr release assays with B-LCL or peptide-pulsed targets.
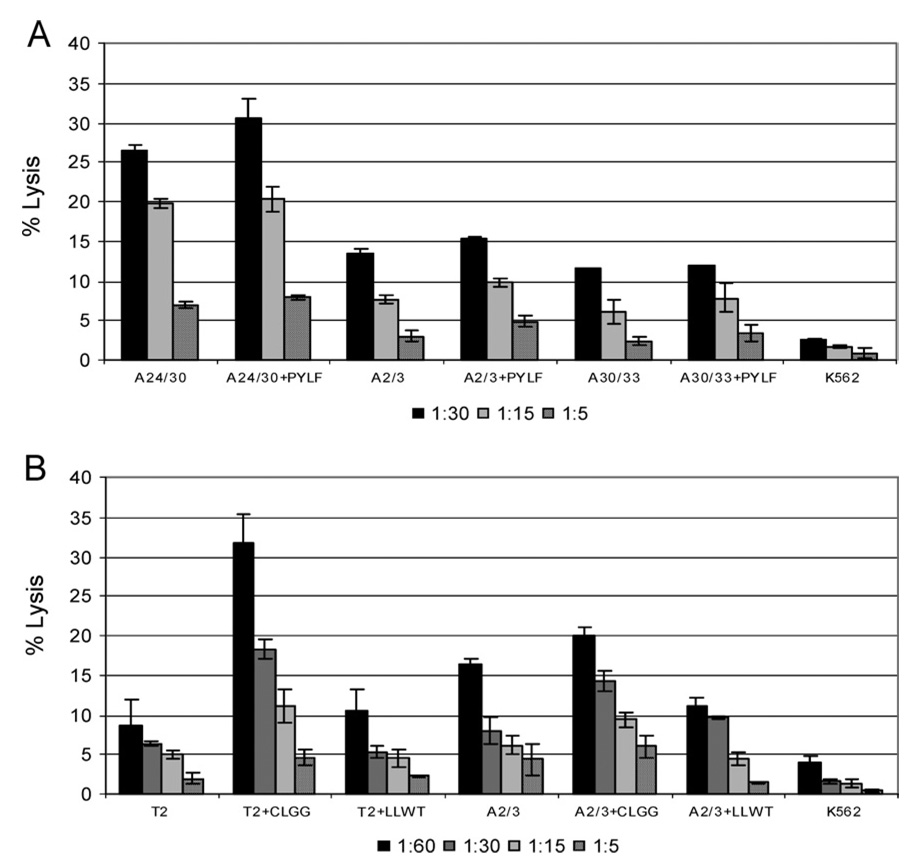
In previous reports we demonstrated the ability to lyse LMP2 peptide-pulsed targets with retroviral-vector expressed TCR (10). However, direct lysis of a B-LCL, the best model for native expression levels of LMP2, has not previously been demonstrated. The A7-LMP2.A23 retroviral vector, expressing CSIC7 TCRα and -β chains was able to confer the parental clone’s lytic specificity. B-LCL expressing HLA-A24 were efficiently lysed by transduced PBMCs (Fig. 2A). The A7-LMP2.A23 CD8+ effector cells, when magnetically separated from the bulk population by CD8-immunomagnetic beads, lysed both B-LCLs and peptide-loaded B-LCLs effectively with 27 and 31% lysis of target cells at target to effector cell ratio of 1:30, respectively (Fig. 2A). When HLA-mismatched LCLs were used as targets in the 51Cr release assays, the lysis was reduced to background regardless of the presence of specific peptide, indicating that lytic function was antigen and HLA specific in the context of B-LCL as well.
Fig. 2. Transduced PBMC specifically lyse HLA-matched B-LCL.
(A) Lytic activity of CD8+ cells selected from PBMC transduced with retroviral vector A7-LMP.A23 at the indicated effector to target cell ratios (E:T) was demonstrated in a 51Cr-release assay. Targets were matched LCL (A24/30), matched LCL pulsed with specific peptide (A24/30+PYLF), mismatched LCL (A2/3 or A30/33), or mismatched LCL pulsed with specific peptide (A2/3+PYLF and A30/33+PYLF). (B) Lytic activity of PBMC (non-CD8 selected) transduced with retroviral vector A7-LMP.A2. Targets were unpulsed T2 cells (T2), T2 cells loaded with specific peptide (T2+CLGG), T2 cells loaded with non-specific peptide (T2+LLWT), MHC-matched LCL (A2/3), matched LCL pulsed with specific peptide (A2/3+CLGG), matched LCL pulsed with non-specific peptide (A2/3+LLWT), or K562 cells. All figures are representative of three or more experiments using the same PBMC donor.
When B-LCL were used as CTL targets, PBMC transduced with the A7-LMP2.A2 retroviral construct displayed target specificity identical to the NB20 CTL clone (Fig. 2B). Transduced PBMCs had specific lysis of greater than 30% against T2 cells pulsed with the peptide CLGGLLTMV, for which the NB20 CTL TCR is specific, at an effector to target cell ratio of 60:1. To determine if B-LCL lysis could be maximized by peptide loading, B-LCLs were incubated with peptide during 51Cr labeling. A small increase in lysis was observed, suggesting that the TCR function on the A7-LMP2.A2 transduced cells was not limited by availability of TCR, but specific peptide class I MHC complexes. The lysis observed with the A7-LMP2.A2 transduced PBMCs was clearly peptide-restricted as T2 cells that were not peptide loaded or were loaded with a different HLA-A2 binding peptide also encoded by LMP2 (LLWTLVLL), exhibited only background lysis. K562 lysis was less than 5% at all target to effector cell ratios, indicating that lysis was not attributable to the presence of NK cells in the transduced PBMC population. These results demonstrate the ability to transfer lytic activity from a CTL antigen-specific clone to an activated PBMC population by the transfer of the TCR by retroviral transduction.
The production of retroviral vectors requires a significant investment of time and effort. In an effort to accelerate the process of TCR analysis, we explored non-viral means of gene transfer. To this end, nucleofection (developed by Amaxa Inc.), which transfects plasmid DNA directly into the cell nucleus by electroporation in a proprietary solution, was evaluated. Theα2 and β21 subunits from the NB20 CTL donor were cloned into the plasmid pBudCE4.1 (Invitrogen). This vector is designed for simultaneous expression of two genes from the CMV-IE and EF1α promoters. The resulting plasmid, pBudA7-LMP2.A2, was nucleofected into PBMCs on day 3, 5 or 7 following OKT3 and IL-2 activation. Twenty-four hours post-nucleofection, CD56+ and CD4+ cells were removed from the nucleofected cell population by AUTOMACs magnetic cell separation using anti-CD56 and anti-CD4 microbeads and the isolated CD8+ cells were used in chromium release assays. Activated PBMCs nucleofected on day 3 with pBudA7-LMP2.A2 demonstrated specific lysis of greater than 40% when tested against antigen-specific (CLGGLLWT) peptide-loaded T2 cells at both 1:30 and 1:15 target to effector cell ratios (Fig. 3). For PBMCs nucleofected without DNA present, lysis was similar to background and NK activity (K562 lysis) was negligible (Fig. 3). PBMCs nucleofected on day 3 with pBudA7-LMP2.A2 or with no DNA were analyzed for cell surface expression of the NB20 Vβ21 subunit. Cells nucleofected in the absence of DNA had 1.74% surface expression of the Vβ21 subunit, while the cells nucleofected with pBudA7-LMP2.A2 had an increase in cell surface expression to 3.58% (Fig. 4A and B). To demonstrate that the increased cell surface expression of the Vβ21 subunit was a result of pBudA7-LMP2.A2 transfection, the cell surface expression of an irrelevant Vβ subunit, Vβ13, was also monitored by flow cytometry (Fig. 4C and D). In PBMCs nucleofected in the absence of vector DNA, Vβ13 expression was 0.74%. In the pBudA7-LMP2.A2 nucleofected cells Vβ13 expression was 0.49%, indicating that nucleofection does not increase TCRVβ cell surface expression in general.
Fig. 3. Nucleofected PBMC lyse peptide-pulsed T2 cells.
Activated PBMC were depleted of CD4+ and CD56+ cells by AutoMACS cell sorting (“Methods” section) and nucleofected with pBudA7-LMP.A2. The following day cells were co-incubated with T2 targets (T2), T2 cells pulsed with cognate peptide (T2+CLGG), or K562 cells at E:T ratios of 1:30 (black) or 1:15 (white). Activated PBMC nucleofected without DNA vector at E:T ratios of 1:30 (diagonal stripes) or 1:15 (stippled) served as a negative control.
Fig. 4. Demonstration of cell surface TCR expression in nucleofected PBMC.
Activated PBMC depleted of CD56+ cells by AutoMACS were nucleofected with pBudA7-LMP.A2 and stained with CD8-PE and Vβ21-FITC or Vβ13-FITC antibodies and analyzed by flow cytometry, see “Methods” section. The percent positive cell surface expression for each quadrant is indicated below each two-dimensional plot. Dot blots are representative of multiple experiments. (A) Activated day 3 PBMC nucleofected without DNA vector and stained for Vβ21 (x-axis) and CD8 (y-axis). (B) As in (A), but nucleofected with pBudA7-LMP.A2. (C) Activated day 3 PBMC nucleofected without DNA vector and stained for Vβ13 (x-axis) and CD8 (y-axis). (D) As in (C), but nucleofected with pBudA7-LMP.A2.
The increase of Vβ expression could be due either to successful assembly of the transfected TCRα and α chains into a functional CD3 complex or the displacement of the native TCRβ chain from the native α–β pairing and the subsequent assembly of a hybrid receptor. HLA-A0201 tetramers loaded with the cognate CLGGLLTMV peptide were therefore used to probe for the presence of the transduced α–β pair on the surface. When activated PBMC were nucleofected with the pBudA7-LMP2.A2 plasmid, peptide-loaded HLA-A2 tetramers demonstrated specific binding to nucleofected cells, Fig. 5. An increase in tetramer binding was seen in the CD8 population in nucleofected cells, 0.8% positive, as compared to negative controls. The increase in tetramer binding observed in the CD8 negative population as well (1.2% positive) is likely attributable to non-specific binding of tetramer to CD8-FITC antibody. Binding of a non-specific tetramer (HLA-A-2301/PYLFWLAAI-PE) was minimal. Although the change in the percentage of positively-gated cells in the pBudA7-LMP2.A2 nucleofected PBMC population was small, results were consistent and correlated with both Vβ expression and lytic activity.
Fig. 5. Tetramer staining of TCR-nucleofected primary T cells.
Activated PBMC, depleted of CD56+ cells, were nucleofected in the absence (A), or presence (B) of the TCR expression plasmid pBudA7-LMP.A2, cultured for 24 h, and then analyzed for expression of recombinant TCR by co-incubation with the HLA-A-0201/CLGGLLTMV-PE tetramer and anti-CD8-FITC, as described in “Methods” section. Numbers in the upper left and right quadrants report the percentage of cells present within those gates. Dot plots are representative of multiple experiments.
The timing of TCR nucleofection following PBMC activation is a crucial parameter. PBMC nucleofected on day 0 or on 7 cells gave poor lysis against peptide pulsed T2 target cells. It was found that intracellular granzyme B and perforin levels peaked on day 3 and subsequently decreased on each consecutive day (data not shown). Therefore, the expression of lytic activity in nucleofected T cells correlates with the expression of perforin and granzyme B in activated cells over the time course of T-cell activation.
DISCUSSION
Adoptive immunotherapy with cytotoxic T lymphocytes (CTL) holds great promise for the treatment of human malignancy. For the treatment of EBV-associated post-transplant lymphoma (PTLD) it is a clinical reality (2, 13). The first report of immunotherapy for PTLD featured unmodified PBMC from EBV-seropositive bone marrow donors (14). The use of unmodified PBMC was further refined by amplifying EBV-specific CTL in vitro by co-culture with B-LCL. This yielded a polyclonal population of CD4 and CD8 effector cells that could treat or prevent PTLD arising in autologous or allogeneic bone marrow transplant, or in solid-organ transplantation (15, 16). The ability to cross HLA boundaries (with respect to the amplified CTL population and the recipient) demonstrated that in vitro culture in the presence of a strong antigenic stimulus expanded EBV-reactive cells without expanding allo-reactive cells. However, clinical results using B-LCL expanded CTL in the treatment of EBV-associated HD and NPC have not been as successful. EBV-specific CTL infusion for the treatment of NPC has thus far demonstrated no improved tumor control (17) and in the treatment of HD, despite detection of genetically marked CTL in peripheral blood 9 months post-infusion and a reduced viral burden, only a single patient survived to 28 months post-infusion (18, 19). The inability of the immune system to mount a strong response to the LMP expressed in these type II EBV-associated malignancies likely hinders the success of these efforts. CTL responses to LMP1 and LMP2 occur naturally in some individuals, but at lower frequency and strength than responses against the immunodominant EBNA-3 antigens (20, 21). Escape of these EBV-associated malignancies from immunosurveillance may be due to both low levels of overall antigen expression and decreased entry in antigen-processing pathways (22). Therefore, the efficient generation of CTL with enhanced recognition of LMP1 and LMP2 is an important challenge.
In previous work, retroviral transfer of an LMP2 peptide-specific TCR into activated PBMC demonstrated lytic activity against peptide-loaded T2 cells, but not B-LCL. The inability of peptide-generated CTL to lyse B-LCL is a common finding in the EBV field (10, 23). In the present study, two CTL clones, generated in vitro by stimulation of PBMC with B-LCL as opposed stimulation with peptide were used as donors of TCRα and -β chains. These clones were unique in their ability to be maintained in culture long-term and to lyse B-LCL. Transduction of activated PBMC with retroviral vectors expressing the TCR chains from these clones, A7-LMP2.A23 and A7-LMP2.A2, produced effector T-cell populations capable of B-LCL lysis in an HLA-restricted manner (Fig. 2). In comparing the lytic activity of each vector, CD8+ cells transduced with the A7-LMP2.A23 vector lysed target cells more effectively than A7-LMP2.A2 transduced PBMCs. CD8 cells could not be selected from the A7-LMP2.A2 transduced cells (HLA-A2 donor for both vectors) due to the level of cell loss during G418 selection. The lower level of lysis seen in Fig. 2B may be due to measurement of a more dilute effector cell population (PBMC vs. CD8 selected), lower TCR affinity for peptide-MHC, or a lesser ability of the TCR chains present in the A7-LMP2.A2 vector to out-compete endogenous TCR for assembly into functional TCR complexes (as compared to A7-LMP2.A23). Although surface plasmon resonance has provided a kinetic model for TCR–MHC peptide interaction, the crystallographic evidence that significant conformational changes take place upon TCR interaction with its ligand reveals that a much more complex interaction, that must also take into account multimeric interactions at a close distance, will be required for a full explanation of TCR kinetics that encompasses both the specificity and cross-reactivity of the TCR (24, 25).
The physiology of the transduced cell population, which will be influenced by methods of isolation, culture, and activation, also influences the lytic activity of the newly generated CTL. Although we have documented that levels of transduced TCR mRNA expression is one of the main drivers of lytic activity, and that it can vary considerably, why either high or low levels of transduced TCR are expressed on the cell surface remains to be determined (26).
We also demonstrate here for the first time the successful nucleofection of plasmid-based TCR expression vectors, that nucleofected PBMC are functional in lytic assays, that transfected Vβ chains can be detected by cell-surface staining with antibody, and that binding of HLA-A2 tetramer loaded with antigenic peptide can bind these TCR (Figs. 4 and 5). Although not presented, a major drawback to nucleofection at this point in time is the large degree of cell death (at least 50%) associated with the process. Secondly, after extensive experimentation, we were not able to render activated lymphocytes capable of lysing LCL using nucleofection technology. Current research is focused on elucidating a mechanism behind the failure of nucleofection to recapitulate the lytic activity seen with retrovirally transduced lymphocytes. When nucleofected lymphocytes were re-stimulated with PHA, lytic activity mediated by the transfected TCR was not maintained (not shown). This is most likely due to the loss of transfected plasmid from the nucleofected plasmid, as rapidly dividing tumor cell lines do not maintain plasmid DNA in the nucleus beyond 2 days (manuscript in preparation).
Other methods of generating effector T-cell populations for adoptive immunotherapy of type II EBV malignancies are also being developed. Dendritic cells (DC) transduced with adenovirus expressing LMP2B generated a significant increase in CTL activity to antigen peptide and demonstrated specific lysis of EBV LCL (27). DC infected with adeno-LMP2A also stimulated the generated CTL able to lyse autologous fibroblasts infected with vaccinia virus-expressing LMP2A and autologous LCL (28). In this second study, EBV-specific CTL generated by stimulation with autologous LCL showed minimal lysis of LMP2 vaccinia virus-infected fibroblasts. This discrepancy is of interest, as it suggests that EBV-specific CTL expanded using DC infected with viral gene expression vectors for LMP2, as opposed native B-LCL expression level, generates an effector cell population with greatly increased activity. This may be due to the increased amount of antigen being expressed by an exogenous viral promoter. New LMP2-specific TCRs could be generated either from DC-generated effectors or potentially from HLA-A2.1 transgenic mice that have been shown to produce efficient anti-tumor responses when partially humanized TCRs are used in retroviral TCR-transfer vectors (29). Both of these approaches are attractive means to generate high-affinity/avidity TCR that could be cloned and transferred into activated PBMC. The use of TCR transduction as means to generate therapeutic effector cells is further supported by data demonstrating that the transferred TCR maintains the avidity of the parental CTL clone (30).
TCR transfer using genetic vectors encoding native TCRα and -β chains provides a direct immunotherapeutic approach for treating type II EBV-associated malignancies. We propose that the vectors reported here could be used in patients with severe or relapsed disease that express an HLA-A2 or HLA-A23,24 allele. Our approach, direct TCR transfer to patient PBMC, is a novel way to rapidly generate a large effector cell population. With a single qualified vector product, we would no longer be required to culture and expand CTL from individual patients but could directly generate CTL effectors from the peripheral blood lymphocytes of a wide range of patients. While the production of a lytic TCR population is readily accomplished by retroviral transduction, a number of questions must be answered prior to clinical application including the long-term ability of transduced T cells to maintain TCR expression, demonstration that specificity is maintained over multiple stimulations, and the requirement for IL-2 in vivo. The neo gene is likely to be immunogenic, and thus the ability to produce effective lytic populations without neo or other transgene expression remains to be explored. Another possible drawback is the evolution of a viral escape mutant. One possible solution may be transduction of separate lymphocyte populations with vectors specific for other viral epitopes. Currently, OKT3 and IL-2 are used to produce cells used for retroviral transduction. In vivo experiments demonstrate that these cells are cytotoxic, and the use of class-I-MHC-restricted TCRs orients the newly transduced cells toward recognizing targets expressing class I MHC, normally considered targets of Th1-oriented immunity. The production of a population that is both cytotoxic and Th1-like could be optimized further by manipulating culture conditions, perhaps through the inclusion of IL-15, stimulation of T cells via CD137, or other agents thought to promote Th1-like immunity.
ACKNOWLEDGMENTS
This work was supported by NIH RO1-CA82781 (to R.O.) and the Midwest Athletes Against Childhood Cancer (MACC Fund Inc.).
REFERENCES
- 1.Ambinder RF, Orentas R, Robertson KD. Epstein-Barr virus and Hodgkin’ disease. In: Armitage J, Newland A, Keating A, Burnett A, editors. Cambridge Medical Reviews: Heamtological Oncology. Vol. 4. Cambridge: Cambridge University Press; 1995. pp. 1–20. [Google Scholar]
- 2.Rooney CM, Smith CA, Ng CYC, Loftin S, Li C, Krance RA, Brenner MK, Heslop HE. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 3.Riddell SR, Greenberg PD. T-cell therapy of cytomegalovirus and human immunodeficiency virus infection. J Antimicrob Chemoth. 2000;45:35–43. doi: 10.1093/jac/45.suppl_4.35. [DOI] [PubMed] [Google Scholar]
- 4.Levitskaya J, Coram M, Levitsky V. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature. 1995;375:685–688. doi: 10.1038/375685a0. [DOI] [PubMed] [Google Scholar]
- 5.Lee SP, Thomas WA, Blake NW, Rickinson AB. Transporter (TAP)-independent processing of a multiple membrane-spanning protein, the Epstein-Barr virus latent membrane protein 2. Eur J Immunol. 1996;26:1875–1883. doi: 10.1002/eji.1830260831. [DOI] [PubMed] [Google Scholar]
- 6.Hsu SM, Lin J, Xie SS, Hsu PL, Rich S. Abundant expression of transforming growth factor-beta 1 and -beta 2 by Hodgkin’s Reed–Sternberg cells and by reactive T lymphocytes in Hodgkin’s disease. Hum Pathol. 1993;24:249–255. doi: 10.1016/0046-8177(93)90034-e. [DOI] [PubMed] [Google Scholar]
- 7.Herbst H, Foss HD, Samol J, Araujol I, Klotzbach H, Krause H, Agathanggelou A, Niedobitek G, Stein H. Frequent expression of interleukin-10 by Epstein-Barr virus-harboring tumor cells of Hodgkin’s disease. Blood. 1996;87:2918–2929. [PubMed] [Google Scholar]
- 8.Herbst H, Samol J, Foss HD, Raff T, Niedobitek G. Modulation of interleukin-6 expression in Hodgkin and Reed–Sternberg cells by Epstein-Barr virus. J Pathol. 1997;182:299–306. doi: 10.1002/(SICI)1096-9896(199707)182:3<299::AID-PATH856>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Clay TM, Custer MC, Sachs H, Hwu P, Rosenberg SA, Nishimura MI. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163:507–513. [PubMed] [Google Scholar]
- 10.Orentas RJ, Roskopf SJ, Nolan GP, Nishimura MI. Retroviral transduction of a T cell receptor specific for an Epstein-Barr virus-encoded peptide. Clin Immunol. 2001;98:220–228. doi: 10.1006/clim.2000.4977. [DOI] [PubMed] [Google Scholar]
- 11.Khanna R, Burrows SR, Moss DJ, Silins SL. Peptide transporter (TAP-1 and TAP-2)-independent endogenous processing of Epstein-Barr virus (EBV) latent membrane protein 2A: Implications for cytotoxic T-lymphocyte control of EBV-associated malignancies. J Virol. 1996;70:5357–5362. doi: 10.1128/jvi.70.8.5357-5362.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yassai M, Naumova E, Gorski J. Generation of TCR spectratypes by multiplex PCR for T cell repertoire analysis. In: Oksenberg JR, editor. The Antigen T Cell Receptor: Selected Protocols and Applications. Austin, TX: Landes Bioscience; 1997. pp. 326–372. [Google Scholar]
- 13.Heslop HE, Ng CYC, Li C, Smith CA, Loftin SK, Krance RA, Brenner MA, Rooney CM. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 14.Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH, Castro-Malaspina H, Childs BH, Gillio AP, Small TN, Childs BH, Gillio AP, Small TN, Young JW, Kernan NA, O’Reilly RJ. Infusions of donor leukocytes to treat Epstein-Barr Virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994;330:1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 15.Khanna R, Bell S, Sherritt M, Gailbraith A, Burrows SR, Rafter L, Clarke B, Slaughter R, Falk MC, Douglas J, Williams T, Elliot SL, Moss DJ. Activation and adoptive transfer of Epstein-Barr virus-specific cytotoxic T cells in solid organ transplant patients with posttransplant lymphoproliferative disease. Proc Natl Acad Sci USA. 1999;96:10391–10396. doi: 10.1073/pnas.96.18.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comoli P, Labirio M, Basso S, Baldanti F, Grossi P, Furione M, Vigano M, Fiocchi R, Rossi G, Ginevri F, Gridelli B, Moretta A, Montagna D, Locatelli F, Gerna G, Maccario R. Infusions of autologous Epstein-Barr virus (EBV)-specific cytotoxic T cells for prevention of EBV-related lymphoproliferative disorder in solid organ transplant recipients with evidence of active virus replication. Blood. 2002;99:2592–2598. doi: 10.1182/blood.v99.7.2592. [DOI] [PubMed] [Google Scholar]
- 17.Chua D, Huang J, Zheng B, Lau SY, Luk W, Kwong DL, Sham JS, Moss D, Yuen KY, Im SW, Ng MH. Adoptive transfer of autologous Epstein-Barr virus-specific cytotoxic T Cells for nasopharygeal carcinoma. Int J Cancer. 2001;94:73–80. doi: 10.1002/ijc.1430. [DOI] [PubMed] [Google Scholar]
- 18.Roskrow MA, Suzuki N, Gan YJ, Sixbey JW, Ng CY, Kimbrough S, Hudson M, Brenner MK, Heslop HE, Rooney CM. Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for the treatment of patients with EBV-positive relapsed Hodgkin’s disease. Blood. 1998;91:2925–2934. [PubMed] [Google Scholar]
- 19.Straathof K, Bollard CM, Rooney C, Heslop HE. Immunotherapy for Epstein-Barr virus-associated cancers in children. Oncologist. 2003;8:83–98. doi: 10.1634/theoncologist.8-1-83. [DOI] [PubMed] [Google Scholar]
- 20.Rickinson AB, Murray RJ, Brooks JM, Moss DJ, Masucci M. T cell recognition of Epstein-Barr virus associated lymphomas. Cancer Surv. 1992;13:53–79. [PubMed] [Google Scholar]
- 21.Lee SP, Chan ATC, Cheung ST, Thomas WA, Croom-Carter D, Dawson CW, Tsai CH, Leung SF, Johnson PJ, Huang DP. CTL Control of EBV in nasopharyngeal carcinoma (NPC): EBV-specific CTL responses in the blood and tumors of NPC patients and the antigen-processing function of the tumor cells. J Immunol. 2000;165:573–582. doi: 10.4049/jimmunol.165.1.573. [DOI] [PubMed] [Google Scholar]
- 22.Brooks L, Yao QY, Rickinson AB, Young LS. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J Virol. 1992;66:2689–2697. doi: 10.1128/jvi.66.5.2689-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redchenko IV, Rickinson AB. Accessing Epstein-Barr virus-specific T-cell memory with peptide-loaded dendritic cells. J Virol. 1999;73:334–342. doi: 10.1128/jvi.73.1.334-342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gakamsky DM, Luescher IF, Pecht I. T cell receptor–ligand interactions: A conformational preequilibrium or an induced fit. Proc Natl Acad Sci USA. 2004;101:9063–9066. doi: 10.1073/pnas.0402840101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubio-Godoy V, Dutoit V, Rimoldi D, Lienard D, Lejeune F, Speiser D, Guillame P, Serottini JC, Romero P, Valmori D. Discrepancy between ELISPOT IFN-γ secretion and binding of A2/peptide multimers to TCR reveals interclonal dissociation of CTL effector function from TCR-peptide/MHC complexes half-life. Proc Natl Acad Sci USA. 2001;98:10302–10307. doi: 10.1073/pnas.181348898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orentas RJ, Bircher LA, Roskopf S. Retroviral transfer of T-cell receptor genes produces cells with a broad range of lytic activity. Scand J Immunol. 2003;58:33–42. doi: 10.1046/j.1365-3083.2003.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranieri E, Herr W, Gambotta A, Olson W, Rowe D, Robbins PD, Kierstead LS, Watkins SC, Gesualdo L, Storkus WJ. Dendritic cells transduced with an adenovirus vector encoding Epstein-Barr virus latent membrane protein 2B: A new modality for vaccination. J Virol. 1999;73:10416–10425. doi: 10.1128/jvi.73.12.10416-10425.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gahn B, Siller-Lopez F, Pirooz AD, Yvon E, Gottschalk S, Longnecker R, Brenner MK, Heslop HE, Aguilar-Cordova E, Rooney CM. Adenoviral gene transfer into dendritic cells efficiently amplifies the immune response to LMP2A antigen: A potential treatment strategy for Epstein-Barr virus-positive Hodgkin’s lymphoma. Int J Cancer. 2001;93:706–713. doi: 10.1002/ijc.1396. [DOI] [PubMed] [Google Scholar]
- 29.Stanislawski T, Voss RH, Lotz C, Sadovnikova E, Willemsen RA, Kuball J, Ruppert T, Bolhuis RL, Melief CJ, Huber S, Stauss HJ, Theobold M. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat Immunol. 2001;2:962–970. doi: 10.1038/ni1001-962. [DOI] [PubMed] [Google Scholar]
- 30.Rubinstein MP, Kadina AM, Salem ML, Nguyen CL, Gillanders WE, Nishimura ML, Cole DJ. Transfer of TCR genes into mature T cells is accompanied by the maintenance of parental T cell avidity. J Immunol. 2003;170:1209–1217. doi: 10.4049/jimmunol.170.3.1209. [DOI] [PubMed] [Google Scholar]