Abstract
Hepatic steatosis continues to present a major challenge in liver transplantation. These organs have been shown to have an increased susceptibility to cold ischemia and reperfusion (CIR) injury compared to otherwise comparable lean livers; the mechanisms governing this increased susceptibility to CIR injury are not fully understood. Endoplasmic reticulum (ER) stress is an important link between hepatic steatosis, insulin resistance and the metabolic syndrome. In this study, we investigated ER stress signaling and blockade in the mediation of CIR injury in severely steatotic rodent allografts. Steatotic allografts from genetically leptin-resistant rodents had increased ER stress responses and increased markers of hepatocellular injury following liver transplantation into strain-matched lean recipients. ER stress response components were decreased by the chemical chaperone, TUDCA, resulting in improvement of the allograft injury. TUDCA treatment decreased NF-κB activation, and the pro-inflammatory cytokines IL-6 and IL-1β. However, the predominant response was decreased expression of the ER stress cell death mediator, CHOP. Further, activation of the inflammation-associated caspase 11 was decreased linking ER Stress/CHOP to pro-inflammatory cytokine production following steatotic liver transplantation. These data confirm ER stress in steatotic allografts, and implicate this as a mediating mechanism of inflammation and hepatocyte death in the steatotic liver allograft.
Keywords: Liver Transplantation, Endoplasmic Reticulum Stress, Hepatic Steatosis, Ischemia-Reperfusion Injury
Introduction
The epidemic of obesity in developed societies has led to an increase in its attendant complications, including the metabolic syndrome and nonalcoholic fatty liver disease (NAFLD)(1). NAFLD is currently defined as >5% steatosis in the liver either by light microscopic evaluation or magnetic resonance spectroscopy (2). NAFLD is recognized as a marker of the metabolic syndrome (1, 3) and is one of the most rapidly increasing and important causes of liver disease. Currently, hepatic steatosis is the most prevalent underlying condition affecting human liver donors(1), presenting a particular challenge in the setting of liver transplantation. Steatosis is currently estimated to be present in up to 50%(1) of deceased donor livers and is recognized as a the key donor variable predicting post-transplant allograft function(4). Given the current epidemic of obesity, the impact of hepatic steatosis on liver transplantation will likely continue to increase.
Steatosis is thought to impact liver allograft function and survival primarily by increasing the susceptibility of these organs to cold ischemia and reperfusion (CIR) injury (5–6). Both the early clinical functional recovery as well as the regenerative capacity of steatotic allografts are significantly impaired compared to lean allografts resulting in increased primary non-function and initial poor function rates in these organs (1, 6–7). There is no universally accepted measurement of liver steatosis and the majority of centers rely on estimates based on frozen section histologic evaluation. While many centers routinely use organs with mild macrosteatosis (usually defined as < 30% macrosteatosis via histologic estimation), the presence of moderate (31% to 59% macrosteatosis) to severe (> 60% macrosteatosis) steatosis continues to account for up to an estimated 40% of non-utilized and discarded liver allografts (8–9).
The mechanisms underlying CIR injury in steatotic livers differ significantly from similar injuries occurring in lean livers (5, 10–11). Steatotic livers have a predominance of rapid necrotic cell death which may be related to altered energy homeostasis in the steatotic liver (5, 10–12). However, much about the underlying cellular and molecular mechanisms affecting CIR injury in steatotic allografts remain poorly understood. NAFLD is strongly associated with the metabolic syndrome, a combination of abnormalities related to insulin resistance and adipose tissue-derived pro-inflammatory factors which increase the hepatic inflammatory response (1, 13–14). Endoplasmic reticulum (ER) stress is emerging as an important component of inflammatory responses in the liver associated with insulin resistance. ER stress in NAFLD is linked to alterations of hepatic lipid metabolism as well as hepatic insulin resistance (15–17).
A multitude of insults, including ischemia-reperfusion, can induce an ER stress response which is designed to dampen the injury and allow cellular adaptation. Following a cellular stressor, ER chaperones (glucose related/binding immunoglobulin protein 78, GRP-78) recognize mis-folded proteins and trigger downstream responses designed to globally reduce gene expression and alleviate the stress (18–20). Chaperone recognition of unfolded proteins activates three major ER stress mediators: pancreatic eukaryotic translation initiation factor kinase (PERK), inositol-requiring enzyme-1 (IRE1α), and activating transcription factor-6 (ATF6)(19–20). While the initial ER stress response is adaptive, increased or sustained ER stress can result in cell death via several pathways. ER stress mediators can activate NF-κB and a pro-inflammatory response usually via the IRE1α/X-Box Protein-1 (XBP-1) pathway (21). In addition, the ER stress cell-death responses are often associated with activating transcription factor-4 (ATF4) activation of C/EBP-homologous protein (CHOP) a cell death mediator (18, 22). In lean livers, ER stress has been implicated as a mediator of warm IR injury (23), and ER stress responses are known to occur following lean liver transplantation (24). However, little is known about the ER stress response and its affect on the steatotic liver following CIR.
We hypothesized that steatotic livers would have an increased ER stress response following CIR potentiating the injury response. In this report, we demonstrate an exaggerated acute ER stress response in severely steatotic allografts and postulate that this is involved in steatotic allograft injury.
Materials and Methods
Steatotic Liver Transplant Model
Our laboratory has an established steatotic liver transplant model in rats that is approved by the Animal Studies Committee at Washington University in St. Louis. Our method is a modification of the cuff technique described by Kamada and others (25–27). The donor operation is carried out under isoflourane anesthesia in obese (leptin mis-sense) and lean (control) Zucker rats following systemic heparinization. The hepatic artery is ligated, the portal vein is cannulated and the liver is flushed with cold (4°C) histidine-tryptophan-ketoglutarate (HTK) solution. The liver is subsequently extirpated with the vena cava and stored in cold HTK for 2 hours. Vascular cuffs are prepared for the portal vein and the infrahepatic vena cava. While the donor liver is being cold stored, the lean Zucker recipient is prepared under isoflourane anesthesia. Via a midline incision, the recipient hepatectomy is performed by ligating and dividing the hepatic artery, and clamping the infra- and supra-hepatic vena cava as well as the portal vein. The vena cava and portal veins are then divided and the liver removed. The donor liver is now brought to the operative field and the suprahepatic vena cava anastomosis is performed with 9-0 monofilament suture. The infra-hepatic and portal vein anastomoses are performed using the previously placed cuffs. The bile duct is anastomosed over a cannula. Following reperfusion of the liver, the recipient receives 1mL of saline IP. The abdomen is closed and the animal allowed to recover under close observation. At the appropriate time points, animals were euthanized and the allografts recovered for analysis.
Sham obese and lean animals underwent a laparotomy of similar anesthesia time as the transplant procedure. Animals were the euthanized at the appropriate time points for analysis.
Chemical Chaperone Treatment
Previous studies have suggested that the ER stress response can be modulated using the chemical chaperone taurine-conjugated ursodeoxycholic acid (TUDCA) (15). In the current study, we first investigated the effect of treating obese donor animals with TUDCA (0.5mg/kg in saline) daily for 7 days. Control obese donor animals received vehicle (saline). Serum was obtained on days 2, 4, 6, and 7 and the animals underwent liver procurement on day 7. These organs were flushed and cold preserved in HTK.
Next we investigated the effect of TUDCA treatment of steatotic donor organs during cold storage and reperfusion. This was carried out by adding TUDCA (10mM) to the HTK solution in which the donor organs were flushed and cold stored. Immediately following reperfusion, the lean recipient animals subsequently received TUDCA (0.5 mg/kg) or saline IP.
At the time of euthanasia of the recipient animal, serum was obtained and aliquots of the liver allograft were paraffin embedded for histological analysis or flash frozen for RNA, protein, and lipid extraction.
Quantitative Real-Time PCR
RNA was extracted from frozen liver tissue using RNA-Bee (Tel-Test, Inc, Friendswood, TX) and treated with DNAse. Reverse transcription was performed using the SuperScript II First-strand Synthesis System (Invitrogen, CA), with 1 ug of total RNA and random hexamers, to generate cDNA. QRTPCR assays were performed in triplicate on an ABI Prim 7000 Sequence Detection System using SYBR Green PCR Master Mix (Applied Biosystems, CA). The mRNA level of individual genes was quantified and normalized against a control reaction for rat 18S mRNA. Following normalization to 18S, relative mRNA abundance of individual genes in experimental groups was determined by comparison to sham operated livers and then expressed mRNA fold change by comparing different groups. The primer sequences used are summarized in Table 1.
TABLE 1.
Oligodeoxyribonucleotide primer sequences for quantitative real-time polymerase chain reaction studies.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| ATF-4 | 5’-tggccatctcccagaaagtg-3’ | 5’-gggaagaggctgcaagaatg-3’ |
| GRP78 | 5’-acgtccaacccggagaaca-3’ | 5’-ttccaagtgcgtccgatga-3’ |
| IRE1α | 5’-ctctcctgtggtggccttcta-3’ | 5’-acgttgatgtgcaccaccttt-3’ |
| CHOP | 5’-ttgaccctgcatccctagct-3’ | 5’-gggctttgggaggtgctt-3’ |
| 18S | 5’-ttgattaagtccctgccctttg-3’ | 5’-cgatccgagggcctcacta-3’ |
Determination of lipids and insulin resistance
Approximately 100 mg of frozen rat liver was homogenized to prepare lipid extracts for an enzymatic assay of triglyceride (28). Determination of serum and hepatic triglyceride and free fatty acids contents was carried according to methods described by the supplier (Wako Diagnostics, Richmond, VA). Insulin was quantified in the rat serum using rat insulin ELISA kit (Mercodia AB, Sweden). Quantification of serum glucose was performed using a commercially available assay (Wako Diagnostics, Richmond, VA). Insulin resistance was calculated using the homeostasis model assessment method (HOMA) model (29).
Western Blot Analysis
Western blot analysis was carried out as previously reported (30–32). Briefly, cytoplasmic extracts (20 μg to 60 μg protein) were subjected to polyacrylamide gel electrophoresis and transferred overnight to nitrocellulose membranes (Invitrogen, CA). Rat anti-XBP1 (1–2ug /ml, Pro Sci Incorporated, Poway, CA), anti-CHOP (1:1000 dilution, Cell Signaling Technology, Danvers, MA), anti-activated caspase-11, p20, (1:1000, Santa Cruz Biotechnololgy, CA), anti- caspase 11 (1:200, Santa Cruz Biotechnology, CA), anti-actin (1:1000, Santa Cruz Biotechnology, CA), and anti-HMGB1 (1:1000 dilution, Sigma-Aldrich, MO) were used as primary antibodies along with respective HRP-conjugated secondary antibodies. Semiquantitation via densitometry estimation was performed using ImageJ (ImageJ 1.41o, Wayne Rasband, National Institutes of Health). All experiments were performed in triplicate.
Determination of NF-κB Activation by Immunoprecipitation
The change in NF-κB activation upon treatment with TUDCA was determined using an immunoprecipitation method. Nuclear extracts were prepared from the liver as described by Burstein et al(33). Briefly the 5’ biotinylated canonical oligonucleotide sequence: 5’-TGCAAGGGACTTTCCGCTGGGGACTTTCCTGCA-3’ was coated on streptavidin beads and used to pull down the activated NF-κB. After overnight incubation the beads were washed and heat denatured at 70 °C. The pull down protein was loaded on 4–12% Bis-Tris gel and probed with p50 antibody (Santa Cruz Biotech, LA).
Histopathology
Paraffin embedded liver tissue sections were stained with hematoxylin and eosin and examined in a blinded fashion by an experienced hepatopathologist (EMB) for macrovesicular steatosis, inflammation, evidence of apoptosis, ischemic necrosis, and other lesions of hepatic injury.
Determination of pro-inflammatory cytokines
Rat cytokine (TNFα, IL-6, IL-1β) assays were done according to the ELISA protocol given by the supplier (Invitrogen Corporation, CA). 25ul or 50ul rat serum and 50ug of whole liver protein extracts were used for Elisa assay.
Statistical Analysis
Direct comparison of study cohorts was carried out using student’s t test. P-values less than 0.05 were considered significant.
Results
CIR injury is associated with an increased ER stress response in the steatotic liver
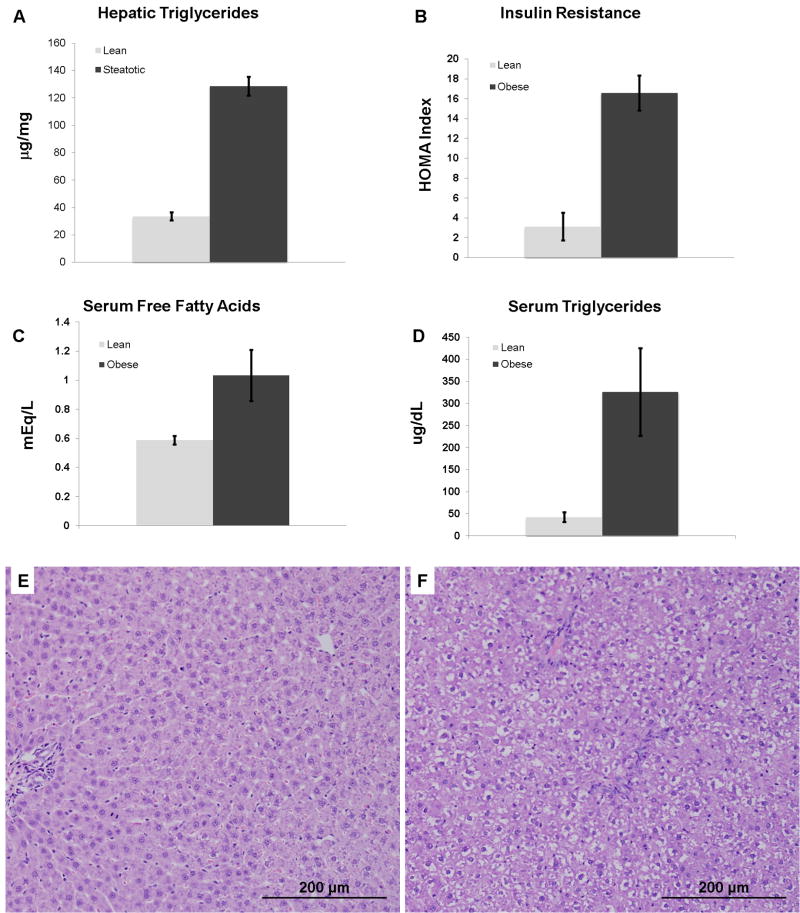
The obese Zucker rat is a model of hepatic steatosis in the context of the metabolic syndrome. These animals served as donors, and the allografts were transplanted into lean Zucker animals as a model of severely steatotic liver transplantation. The steatotic allografts demonstrated severe (> 60%) macrovesicular steatosis histologically and had a hepatic triglyceride content 4 fold greater than the lean allograft controls (p < 0.001, figure 1a). The obese donor animals also exhibited increased systemic insulin resistance (p < 0.005) and a significant increase in both serum free fatty acids (p < 0.02) and triglycerides (p < 0.001) consistent with the metabolic syndrome (figure 1b–d). Following the CIR injury from liver transplantation, the severely steatotic allografts had a significantly increased injury compared to the lean control (p < 0.001, figure 2).
Figure 1.
The obese Zucker rat livers (donors) have a 4-fold higher triglyceride content (A, p < 0.001), increased insulin resistance (B, p< 0.005), increased serum free fatty acids (C, p < 0.02), and increased serum triglycerides (D, p < 0.001) compared to lean. Representative photomicrographs (each 200x magnification) of (E) lean and (F) obese donor Zucker rat livers used for transplantation. The liver from the obese animal demonstrates severe macrovesicular steatosis. The macrovesicular steatosis is predominantly located in zone1 with relative sparing of zone 3.
Figure 2.
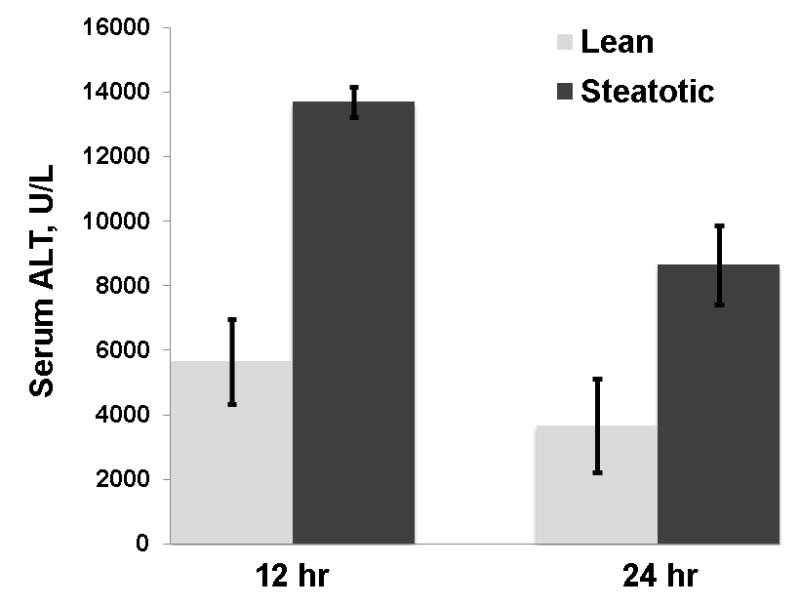
Allograft injury, measured by serum ALT levels, was significantly increased in the steatotic allograft at 12 and 24 hours compared to the lean (p < 0.001for each)
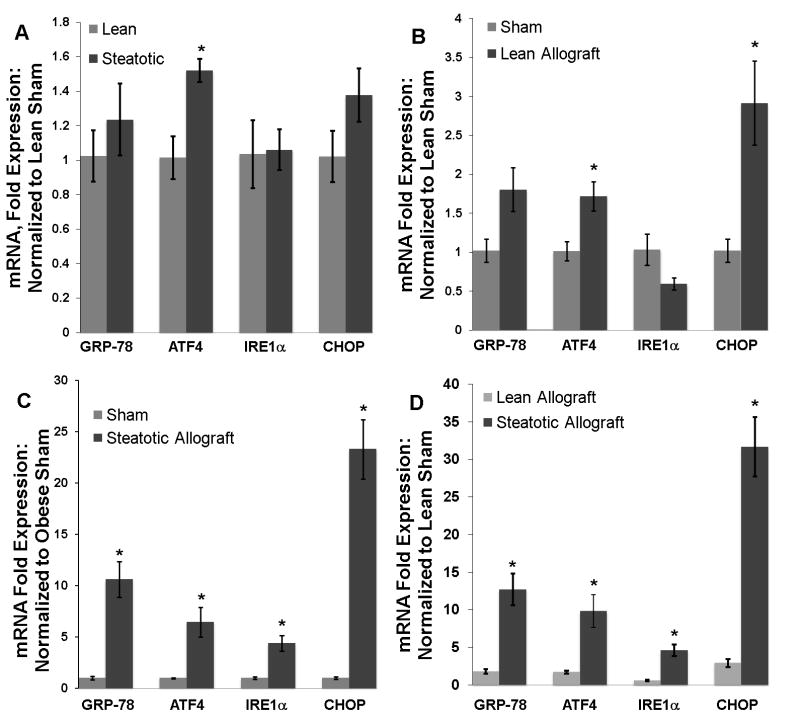
To investigate the ER stress response following CIR injury, we used quantitative real-time PCR (QRTPCR) to determine the hepatic mRNA expression of GRP-78, inositol-requiring enzyme-1 (IRE1α), activating transcription factor-4 (ATF4), and C/EBP-homologous protein (CHOP) in transplanted allografts. Sham operated animals were evaluated to determine the chronic expression of these ER stress genes in the steatotic and lean livers. ATF4 is significantly upregulated in the steatotic liver (1.5 fold, p < 0.05) with a trend toward increased expression of CHOP (1.37 fold, p> 0.05) (figure 3a). Transplantation triggered an ER stress response in both the lean and steatotic allografts. In the lean allograft, there was a significant increase in ATF4 and CHOP mRNA (p < 0.05 for both) (figure 3b) compared to the lean sham. The steatotic allograft, however, showed significant increases in all ER stress genes compared to the steatotic sham (p < 0.05 for all, figure 3c). The ER stress response following transplantation was more robust in the steatotic allograft than in the lean. Direct comparison of the cohorts by normalization to the lean sham values showed that the magnitude of the acute increase of ER stress gene expression following CIR was significantly greater in the steatotic allograft (p < 0.05 for all) (figure 3d) compared to the lean. The greatest example of this difference is in the acute 23 fold increase in CHOP mRNA at 12 hours in the steatotic allograft compared to a 2.9 fold increase in the lean group.
Figure 3.
(A) Hepatic mRNA expression of GRP-78, ATF4, IRE1α, and CHOP in sham operated lean and obese Zucker rats (n=3 in each group). ATF4 is chronically expressed at higher levels (*, p< 0.04) in the steatotic liver. In this panel, all samples are normalized to lean sham mRNA expression. (B) 12 hours following liver transplantation, hepatic mRNA levels of ATF4 and CHOP are significantly elevated in the lean allograft (*, p < 0.04 for both, n=3). The changes in GRP-78 and IRE1α are not significant (p > 0.07 for each). (C) In the steatotic allograft, transplantation triggers a significant increase of mRNA levels for all ER stress genes (*, p < 0.03 for all, n=5). (D) Normalizing both lean and steatotic allograft mRNA expression to the lean sham allows direct comparison between the cohorts. The magnitude of the ER stress response in the steatotic allograft following CIR is significantly greater for all markers (p < 0.03 for all).
Following reperfusion, the acute ER stress response genes in the steatotic allograft were increased significantly at 6-hrs, peaked at 12-hrs and remained elevated significantly at 24-hrs (p < 0.05 for all, figure 4a). ATF4, which is the most highly expressed marker at 6-hrs post-reperfusion, is a downstream mediator of the PERK pathway which can subsequently stimulate CHOP expression. As expected, CHOP expression peaked after ATF4 at 12-hrs post-reperfusion. IRE1α mRNA showed a more gradual increase in expression which peaked at 4.4 fold 12-hr following reperfusion. We next measured downstream activation of the IRE1α pathway by performing western analysis for X-box protein-1 (XBP-1). In sham operated animals, total XPB-1 protein expression was similar in lean and steatotic animals (normalized ratio of 1.0 and 0.8 respectively; p > 0.05). In addition, the ratio of spliced (active) to unspliced XBP-1 protein was similar in the sham lean and steatotic livers (normalized ratio of 1 and 1.11 respectively; p > 0.05). Both the lean and steatotic allografts showed a similar significant increase in the ratio of the spliced form of XBP-1 following CIR (figure 4b). However, transplantation caused an increase in total XBP-1 protein in the steatotic (normalized ratio of 0.8 vs. 1.87; p < 0.005), but not the lean allograft (normalized ratio of 0.79 vs. 1.0; p > 0.05).
Figure 4.
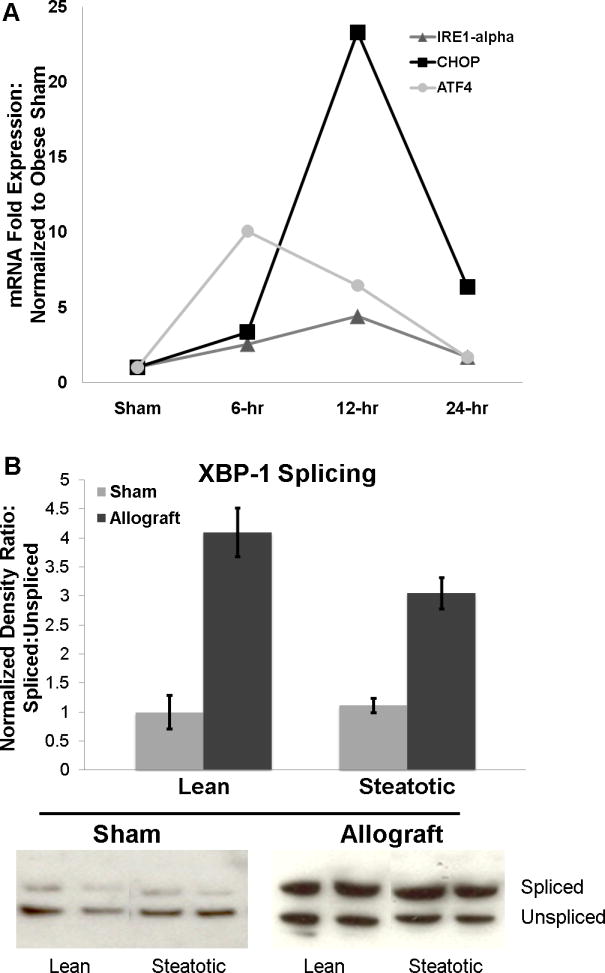
A) Time course of ER stress mRNA expression in the steatotic allograft. Shown are mRNA expression levels for IRE1α, CHOP, and ATF4 in sham, 6-, 12-, and 24-hr following transplantation. ATF4 expression peaks at 6-hr followed by the downstream upregulation of CHOP which shows peak expression at 12-hrs. All 3 genes remain upregulated at 24-hr following transplantation. (D) Western analysis for XBP-1 splicing with representative images and densitometry analysis. In the sham operated lean and steatotic livers, the ratio of spliced (upper band) to unspliced (lower band) is similar. CIR increased the ratio of XBP-1 splicing in both lean and steatotic allografts. When densitometry ratios are normalized to the lean sham liver, there are no significant differences in XBP-1 splicing between the lean and steatotic liver before or following transplantation.
Decreased chronic ER stress in the donor steatotic liver does not alter the acute post-CIR ER stress response
Chronic administration of TUDCA has been shown to decrease hepatic ER stress in animals with the metabolic syndrome(15). To determine if short-term TUDCA therapy in obese donor animals could alter the post-transplantation ER stress induction, we treated obese donor animals with TUDCA (0.5mg/kg daily) or vehicle (saline) for 7 days. Similar to prior reports, the TUDCA treated donors exhibited decreased expression of IRE1α and CHOP (figure 5a). Interestingly, GRP-78 was induced in the TUDCA group compared to vehicle. As expected, these changes resulted in improved insulin resistance profile in the donor animals. The insulin resistance in the TUDCA group was significantly lower than the saline group by day 7 (p < 0.03 ) (figure 5b). However, when the organs from these donor animals were used for transplant, there was no change in the acute ER stress response between TUDCA and vehicle (figure 5c). Further, the post-CIR serum ALT levels were unchanged between the 2 groups (9900 U/L vs. 8600 U/L, p > 0.5).
Figure 5.
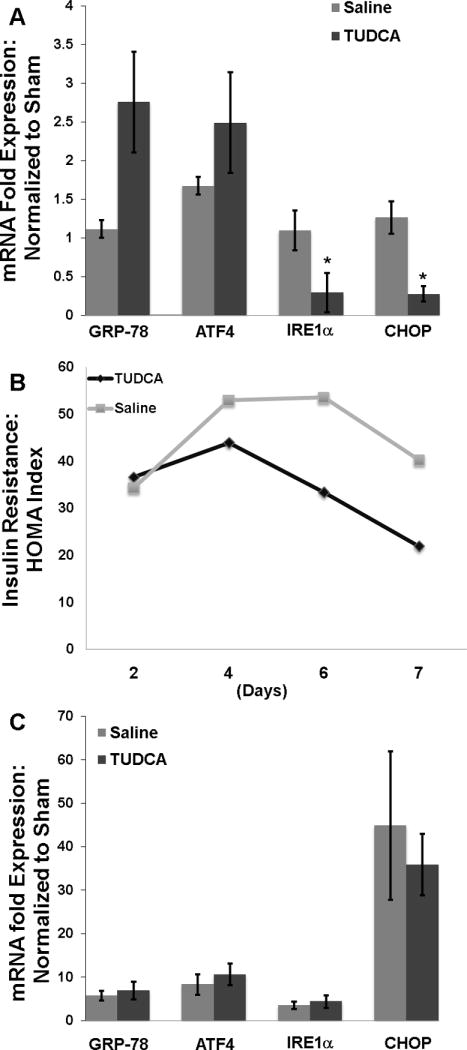
A) Obese donor rats showed decreased expression of IRE1α and CHOP (*, p < 0.05 for each) following 7 days of TUDCA therapy. B) The TUDCA therapy also decreased insulin resistance in the donor animals by day 7 (p < 0.03). C) Following 7 days of TUDCA therapy, the steatotic allografts were procured and used for transplant. Post-CIR, there was no difference in the ER stress response between the TUDCA therapy and the vehicle group.
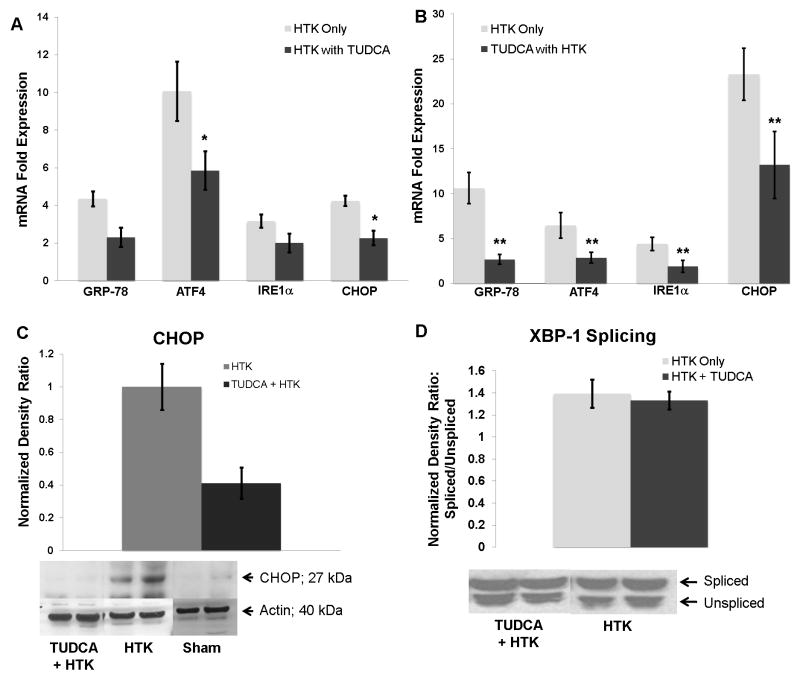
Chemical chaperone treatment decreases the acute ER stress response to CIR in the steatotic allograft
To determine if delivery of TUDCA during cold storage and reperfusion could alter the post-transplant acute ER stress response in the steatotic allografts, we added TUDCA (10mM) to the HTK storage solution in which the steatotic donor livers were cold preserved. In addition, we injected the recipients of these allografts with TUDCA (0.5mg/kg in saline, IP) at the time of reperfusion. This cohort was compared to steatotic livers stored in HTK alone whose recipients were injected with vehicle (saline) at reperfusion. After 6 hours of reperfusion, ATF4 and CHOP expression were both lower (p < 0.03) in the TUDCA treated cohort (figure 6a). At 12 hours following reperfusion, hepatic expression of all of the ER stress markers was significantly decreased (p< 0.04) in the TUDCA treated cohort (figure 6b). In the TUDCA treated cohort, hepatic expression of GRP-78, ATF4, and IRE1α peaked at 6 hours. CHOP expression continued to increase and peaked at 12 hours following reperfusion, but was reduced by approximately 50% (13 vs. 23 fold respectively, p < 0.02) by TUDCA treatment. The decrease in CHOP mRNA at 12 hrs correlated with a decrease in CHOP protein detected by western analysis (figure 6c). However, despite the significant down regulation of IRE1α, the total expression of XBP-1 protein was not altered by TUDCA (normalized ratio of 1.87 vs. 2.15; p > 0.05). Similarly, the splicing of XBP-1 was not inhibited by TUDCA (figure 6d).
Figure 6.
(A) Delivering TUDCA to the steatotic allograft during cold storage and at the time of reperfusion decreased ATF4 and CHOP mRNA levels 6 hours following reperfusion (*, p < 0.05 for each, n=4). (B) At 12 hours following reperfusion, the mRNA levels of all ER stress markers were decreased (**, p < 0.05 for all, n=5). (C) Western analysis of hepatic extracts for CHOP revealed decreased CHOP protein expression in the TUDCA cohort. One representative western blot is shown (n=5 for TUDCA + HTK and n=4 for HTK). Densitometry demonstrates a decrease in CHOP protein expression (p < 0.05). (D) Representative western blots of XBP-1 in steatotic allografts. There was no change in the ratio of the spliced form of XBP-1 (upper band) to the unspliced form (lower band) in the steatotic allografts treated with TUDCA.
TUDCA treatment decreased steatotic allograft injury and inflammation
TUDCA treatment decreased allograft injury as measured by serum ALT levels (figure 7a). In addition, recipients of the TUDCA treated allografts had no increase in the serum ALT from 6 to 12 hours after reperfusion compared to control. Consistent with decreased hepatic cell death and injury, there was less high-mobility group box protein 1 (HMGB-1) in the TUDCA treated steatotic allografts 12 hours following reperfusion (figure 7b). At 6 and 12 hours post-reperfusion, there was no quantifiable change in changes of ischemic necrosis between the cohorts (data not shown).
Figure 7.
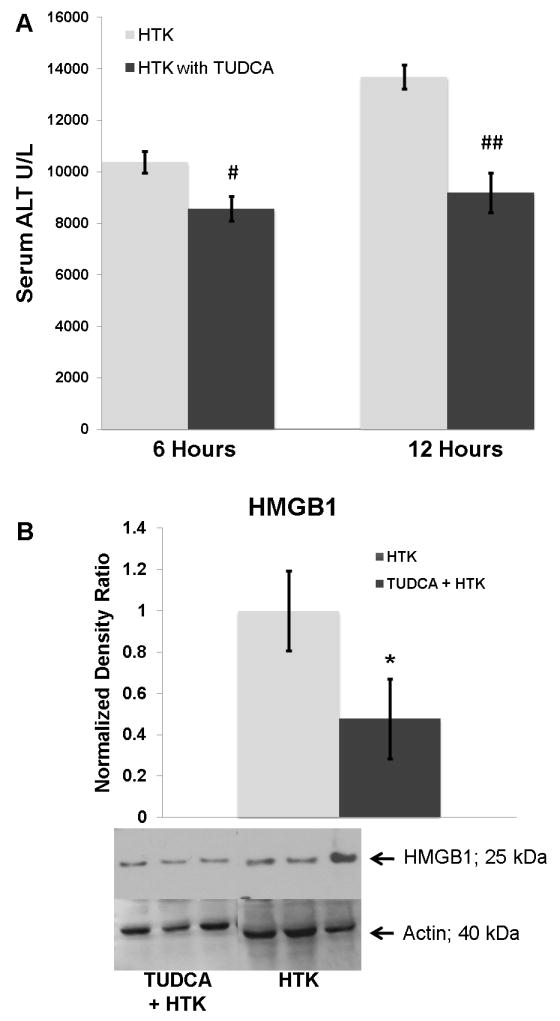
(A) Serum ALT levels were decreased significantly in the TUDCA cohort at 6 (#) and 12 (##) hours following reperfusion (p < 0.05 for each). (B) Consistent with decreased cell death and injury, HMGB1 was decreased in the TUDCA cohort at 12 hours following reperfusion (*, p< 0.03).
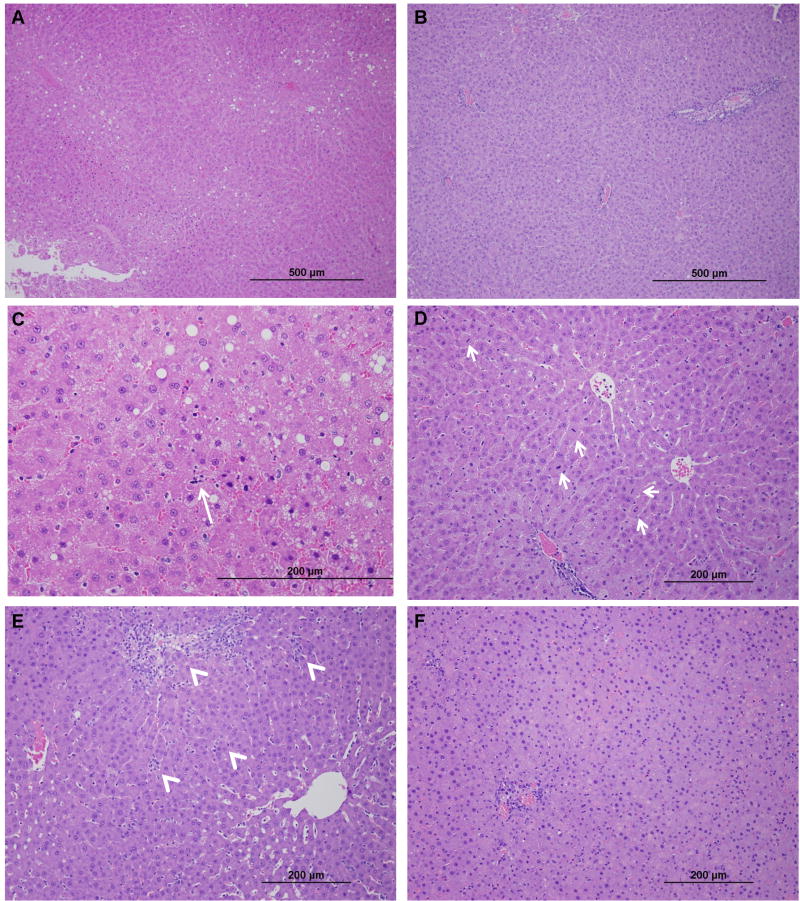
Histological examination of the non-necrotic liver revealed that the macrovesicular steatosis in the HTK cohort had a zone 3 predominance (figure 8a). In the TUDCA cohort, the zone 1 predominance observed in the sham animals remained (figure 8b). There were increased apoptotic hepatocytes and mitotic figures in the HTK group compared to the TUDCA cohort (figures 8c, 8d). By 12 hours post-reperfusion, a pattern of decreased inflammation could be seen between the TUDCA and HTK groups. There was a decrease in both portal and lobular inflammation, and decreased neutrophil infiltration was documented in the TUDCA group (figures 8e, 8f). There was not a significant difference in the hepatic content of triglyceride between the HTK and the TUDCA cohorts 12 hours following transplantation (112.2 ± 17.3 μg/mg vs. 86.5 ± 7.4 μg/mg; p = 0.2).
Figure 8.
Representative photomicrographs of non-necrotic allograft parenchyma: (A) Steatotic allograft 6-hr post-reperfusion (100x magnification) showing a predominance of zone 3 macrosteatosis with global evidence of early injury changes. (B) The TUDCA cohort has decreased histologic evidence of allograft injury and maintains a zone 1 distribution of macrosteatosis which is the distribution observed in the sham animal. (C) Control allograft (400x) at 6-hr demonstrating apoptotic changes (long arrow) which were absent in the TUDCA treated allografts. (D) Mitotic bodies (200x) in hepatocytes seen in the controls but not the TUDCA cohort after 6 hours of reperfusion. (E) Steatotic allograft 12-hr post-reperfusion (100x) with marked portal tract edema and inflammatory infiltrates (arrow heads). Lobular inflammation is also widely present (arrow heads). (F) In the TUDCA cohort, following 12-hr of reperfusion, the changes of allograft inflammation are decreased (100x).
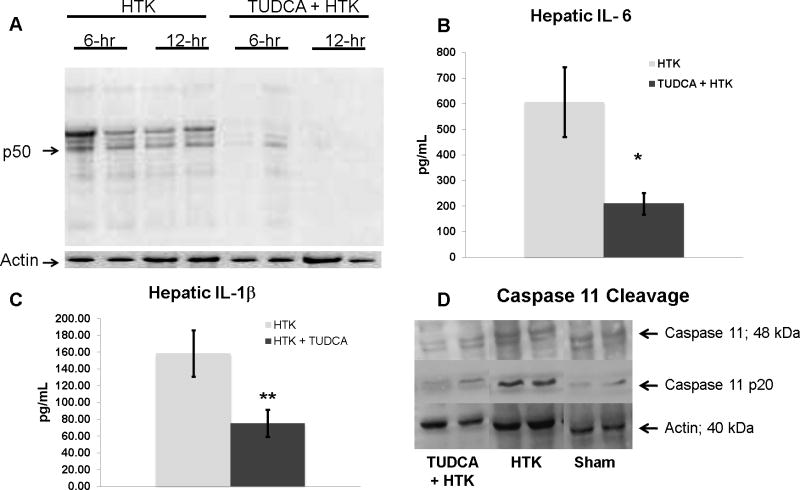
TUDCA treatment decreased NF-κB activation, IL-6, IL-1β, and caspase 11 activation
Because of the association of decreased histologic allograft inflammation with decreased CHOP expression, we examined the expression of inflammatory mediators following steatotic liver transplantation. TUDCA decreased NF-κB activation in the steatotic allograft at both 6 and 12 hours following reperfusion compared to HTK alone (figure 9a). There was no change in the hepatic levels of TNFα protein between groups (ELISA, data not shown). However, TUDCA treatment significantly decreased hepatic expression of IL-1β and IL-6 (figure 9b). Because caspase 11 activation is known to link CHOP to both the pro-inflammatory mediator, IL-1β and apoptosis (34–37), we examined caspase 11 activation in the steatotic allograft. At 12 hours following reperfusion, the p20 form of caspase 11 was present in the HTK cohort of severely steatotic allografts. The presence of this cleavage product was diminished in the TUDCA cohort.
Figure 9.
(A) Representative immunoprecipitation assay demonstrating decreased NF-κB p50 activation in the TUDCA treated steatotic allograft at both 6 and 12 hours following reperfusion compared to HTK alone. (B) TUDCA treatment significantly decreased the hepatic levels of IL-6 (p< 0.05, n= 5). (C) TUDCA treatment significantly decreased the hepatic levels of IL-1β (p< 0.05, n=5). (D) Representative western blot demonstrating the presence of activated caspase 11 (p20) at 12 hours following reperfusion. This was diminished in the TUDCA cohort.
Discussion
NAFLD in donor organs is an increasingly important problem in liver transplantation. Hepatic steatosis increases the susceptibility of the allograft to CIR injury leading to increased rates of allograft failure and/or slow functional recovery. Many centers currently utilize mildly steatotic livers for transplantation with good results; however, even with mild steatosis there is an increase in primary delayed function and increased resource utilization post-transplant compared to lean donor allografts (4, 7, 38–39). It has been demonstrated that the pathophysiology of CIR injury in the steatotic liver differs from that in the lean organ, and few successful strategies have been developed to attenuate this injury (5, 10–11, 40–41). Considering that hepatic steatosis is closely linked to the metabolic syndrome in the donor (1), the exploration of factors linking the steatosis to systemic insulin resistance and inflammation could yield important information on the behavior of the steatotic allograft following a CIR injury.
Hepatic ER stress is recognized as an important mechanistic link between obesity, insulin resistance and hepatic steatosis (15–17). Although, ER stress is, in general, an adaptive response, prolonged or sudden increases in ER stress can initiate pathways that result in cell death (22, 42–43). There are multiple pathways though which ER stress is known to mediate cell death (21–22, 43–46). Although their mechanisms are not entirely clear, drugs such as TUDCA are classified as chemical chaperones due to their ability to enhance ER protein folding and thus decrease the ER stress response (43). Studies using chemical chaperones to target the PERK and IRE1α pathways of hepatic ER stress have demonstrated improvements in the insulin resistance of obese rodents (15, 17). In this study, we investigated the ER stress response in the steatotic liver allograft as a component of the post-reperfusion injury observed in severely steatotic livers. Our results demonstrate that acute ER stress plays an important role in the CIR injury of the steatotic allograft.
An ER stress response occurs following liver transplantation in both lean and steatotic allografts. While lean allografts showed an ER stress response, the magnitude of the response seen in the steatotic liver was far greater and more prolonged. The ER stress response in the steatotic allograft peaked at 12 hours but remained significantly elevated at 24 hours following reperfusion. The magnitude of ER stress gene induction and the prominence of CHOP expression suggested the hypothesis that ER stress associated cell death pathways may play a mediating role in the CIR injury incurred by the steatotic allograft. Because chemical chaperones are well accepted ER stress inhibitors, we initially treated the obese donor animals to determine if decreasing chronic hepatic ER stress in the steatotic liver would affect the post-CIR injury. When TUDCA was given to donor animals for 7 days, we observed an improvement in the animal’s insulin resistance accompanied by a decrease in the hepatic expression of IRE1α and CHOP. These findings are similar to previous reports of TUDCA administration in ob/ob mice(15). Despite the successful decrease in the chronic hepatic IRE1α and CHOP levels, the steatotic allografts procured from the TUDCA treated donors had similar post-transplant ER stress responses and allograft injuries as donors treated with vehicle. These results suggest that the acute ER stress response in the steatotic liver is not directly related to chronic ER stress levels.
In contrast, the addition of TUDCA to the cold storage solution in addition to delivery at the time of reperfusion was a successful strategy to decrease the acute post-transplant ER stress response in the steatotic allograft. While this effect could be measured at 6 hours following transplant, the greatest improvement was seen at 12 hours. The effect on allograft injury followed the pattern of ER stress change, with a small improvement in serum ALT noted at 6 hours and a greater change at 12 hours. While TUDCA was able to decrease HMGB1 production in the steatotic allograft, qualitatively there was not a clear change in histologic necrosis. The histologic changes following TUDCA treatment were improvement of decreased portal and lobular inflammation. This suggested that TUDCA decreased a secondary component of the CIR injury. The improvements in histologic allograft inflammation are consistent with the observed decreased NF-κB activation, and decreased IL-6, and IL-1β levels in the TUDCA treated steatotic allografts. This data implicates ER stress as a mediator of the inflammatory component of CIR injury in the steatotic allograft.
Because the component of injury which seemed to be improved by TUDCA was inflammatory, we suspected that the IRE1α pathway with its well described ability to stimulate NF-κB and pro-inflammatory cytokines was a mediating pathway. However, we observed no difference in downstream IRE1α pathway activation (XBP-1 splicing) between the lean and steatotic allografts. Further, TUDCA had no effect on XBP-1 splicing. Together, these observations suggest that the IRE1α pathway may not be an important mediator of allograft injury in our model.
CHOP, a specific cell death mediator, was the most prominently upregulated ER stress marker in the steatotic allograft following CIR injury, and the improvements observed in allograft injury were accompanied by an almost 50% reduction in CHOP expression at 12 hours of reperfusion. The correlation of CHOP’s over-expression with CIR injury and the decrease of this expression with TUDCA suggested that the CHOP was an important effector of ER stress mediated cell death in the steatotic allograft. Our observation that caspase 11 activity was inhibited by TUDCA supports this hypothesis as caspase 11 links CHOP to IL-1β production (34–35). This is also one of many pathways linking CHOP to cell death (36–37). This observation, combined with the lack of support to implicate the IRE1α pathway, suggest that the ER Stress-CHOP pathway plays a mediating role in steatotic liver allograft injury via induction of inflammation associated caspases.
The relationship of ER stress mediated cell death and CIR injury in the steatotic liver will require further definition. However, this study serves to implicate ER stress on a broad scale as an important factor in this injury. The rodent transplant model is a good clinical approximation of the clinical scenario of steatotic liver transplantation, but it has limitations in the definition of specific cellular mechanisms involved in this multi-faceted problem. The non-arterialized model is useful for making observations about acute CIR injury such as in this study, but is less useful for more long term survival studies. In addition, this is a severely steatotic model; the extent of the reperfusion injury can often obscure the potential benefit of an intervention. This potentially explains the lack of correlation between histologic necrosis and decreased HMGB1 release. In this study we utilized a leptin deficient model of obesity, metabolic syndrome and hepatic steatosis. Steatotic livers from leptin deficient rodents and high fat diet treated rodents have been shown to have similar ER stress responses and it is unlikely that the leptin mutation in our model had any significant effect on hepatic ER stress response following CIR injury (16).
In summary, the steatotic liver allograft has an exaggerated ER stress response following CIR injury. These data implicate ER stress as a mediating factor in cell death and inflammation of the steatotic liver following transplantation. The definition of the exact mechanisms involved in ER stress mediated steatotic allograft injury will require further investigation, but the results of this study suggest that the ER stress pathways, specifically the CHOP-caspase 11-IL-1β pathway, are potential targets to improve steatotic liver allograft function following liver transplantation.
Acknowledgments
Funding Sources: This work was supported in part by the American Society of Transplant Surgeons/Astellas Faculty Development Award (CDA), and National Institute of Health Grants: P30 DK056341-09 (CDA), HL-38180 (NOD), DK-56260 (NOD), and DK-52574 (NOD)
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- CIR
cold ischemia and reperfusion
- ER
endoplasmic reticulum
- GRP-78
glucose related/binding immunoglobulin protein
- IRE1α
inositol-requiring enzyme-1
- ATF4
activating transcription factor-4
- CHOP
C/EBP-homologous protein
- XBP-1
X-Box Protein 1
- PERK
PRK-like ER kinase
- QRTPCR
quantitative real-time PCR
- TUDCA
taurine-conjugated ursodeoxycholic acid
- ALT
alanine aminotransferase
References
- 1.Angulo P. Nonalcoholic fatty liver disease and liver transplantation. Liver Transpl. 2006;12(4):523–34. doi: 10.1002/lt.20738. [DOI] [PubMed] [Google Scholar]
- 2.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288(2):E462–8. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 3.Brunt EM. Pathology of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 7(4):195–203. doi: 10.1038/nrgastro.2010.21. [DOI] [PubMed] [Google Scholar]
- 4.Nocito A, El-Badry AM, Clavien PA. When is steatosis too much for transplantation? J Hepatol. 2006;45(4):494–9. doi: 10.1016/j.jhep.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Selzner M, Rudiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32(6):1280–8. doi: 10.1053/jhep.2000.20528. [DOI] [PubMed] [Google Scholar]
- 6.Durand F, Renz JF, Alkofer B, Burra P, Clavien PA, Porte RJ, et al. Report of the Paris consensus meeting on expanded criteria donors in liver transplantation. Liver Transpl. 2008;14(12):1694–707. doi: 10.1002/lt.21668. [DOI] [PubMed] [Google Scholar]
- 7.McCormack L, Petrowsky H, Jochum W, Mullhaupt B, Weber M, Clavien PA. Use of severely steatotic grafts in liver transplantation: a matched case-control study. Ann Surg. 2007;246(6):940–6. doi: 10.1097/SLA.0b013e31815c2a3f. discussion 6–8. [DOI] [PubMed] [Google Scholar]
- 8.Escartin A, Castro E, Dopazo C, Bueno J, Bilbao I, Margarit C. Analysis of discarded livers for transplantation. Transplant Proc. 2005;37(9):3859–60. doi: 10.1016/j.transproceed.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 9.Sayuk GS, Leet TL, Schnitzler MA, Hayashi PH. Nontransplantation of livers from deceased donors who are able to donate another solid organ: how often and why it happens. Am J Transplant. 2007;7(1):151–60. doi: 10.1111/j.1600-6143.2006.01600.x. [DOI] [PubMed] [Google Scholar]
- 10.Chavin KD, Fiorini RN, Shafizadeh S, Cheng G, Wan C, Evans Z, et al. Fatty acid synthase blockade protects steatotic livers from warm ischemia reperfusion injury and transplantation. Am J Transplant. 2004;4(9):1440–7. doi: 10.1111/j.1600-6143.2004.00546.x. [DOI] [PubMed] [Google Scholar]
- 11.Evans ZP, Ellett JD, Schmidt MG, Schnellmann RG, Chavin KD. Mitochondrial uncoupling protein-2 mediates steatotic liver injury following ischemia/reperfusion. J Biol Chem. 2008;283(13):8573–9. doi: 10.1074/jbc.M706784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selzner N, Selzner M, Jochum W, Clavien PA. Ischemic preconditioning protects the steatotic mouse liver against reperfusion injury: an ATP dependent mechanism. J Hepatol. 2003;39(1):55–61. doi: 10.1016/s0168-8278(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 13.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293(5535):1673–7. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 14.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420(6913):333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 15.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 17.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9(1):35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Kaplowitz N, Than TA, Shinohara M, Ji C. Endoplasmic reticulum stress and liver injury. Semin Liver Dis. 2007;27(4):367–77. doi: 10.1055/s-2007-991513. [DOI] [PubMed] [Google Scholar]
- 19.Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15(6):829–40. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569(1–2):29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 21.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26(8):3071–84. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci. 2007;32(10):469–76. doi: 10.1016/j.tibs.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Vilatoba M, Eckstein C, Bilbao G, Smyth CA, Jenkins S, Thompson JA, et al. Sodium 4-phenylbutyrate protects against liver ischemia reperfusion injury by inhibition of endoplasmic reticulum-stress mediated apoptosis. Surgery. 2005;138(2):342–51. doi: 10.1016/j.surg.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Emadali A, Nguyen DT, Rochon C, Tzimas GN, Metrakos PP, Chevet E. Distinct endoplasmic reticulum stress responses are triggered during human liver transplantation. J Pathol. 2005;207(1):111–8. doi: 10.1002/path.1798. [DOI] [PubMed] [Google Scholar]
- 25.Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28(1):47–50. [PubMed] [Google Scholar]
- 26.Sun CK, Zhang XY, Zimmermann A, Wheatley AM. The metabolic and microcirculatory impact of orthotopic liver transplantation on the obese Zucker rat. Transplantation. 2003;75(6):761–9. doi: 10.1097/01.TP.0000054680.70476.70. [DOI] [PubMed] [Google Scholar]
- 27.Peng Y, Gong JP, Yan LN, Li SB, Li XH. Improved two-cuff technique for orthotopic liver transplantation in rat. Hepatobiliary Pancreat Dis Int. 2004;3(1):33–7. [PubMed] [Google Scholar]
- 28.Newberry EP, Xie Y, Kennedy S, Han X, Buhman KK, Luo J, et al. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J Biol Chem. 2003;278(51):51664–72. doi: 10.1074/jbc.M309377200. [DOI] [PubMed] [Google Scholar]
- 29.Vieira VJ, Valentine RJ, Wilund KR, Woods JA. Effects of diet and exercise on metabolic disturbances in high-fat diet-fed mice. Cytokine. 2009;46(3):339–45. doi: 10.1016/j.cyto.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson CD, Belous A, Pierce J, Nicoud IB, Knox C, Wakata A, et al. Mitochondrial calcium uptake regulates cold preservation-induced Bax translocation and early reperfusion apoptosis. Am J Transplant. 2004;4(3):352–62. doi: 10.1111/j.1600-6143.2004.00357.x. [DOI] [PubMed] [Google Scholar]
- 31.Anderson CD, Pierce J, Nicoud I, Belous A, Knox CD, Chari RS. Modulation of mitochondrial calcium management attenuates hepatic warm ischemia-reperfusion injury. Liver Transpl. 2005;11(6):663–8. doi: 10.1002/lt.20407. [DOI] [PubMed] [Google Scholar]
- 32.Anderson CD, Pierce J, Nicoud IB, Belous AE, Jones CM, Chari RS. Purinergic receptor antagonism prevents cold preservation-induced cell death independent of cellular ATP levels. J Surg Res. 2007;141(2):234–40. doi: 10.1016/j.jss.2006.12.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burstein E, Hoberg JE, Wilkinson AS, Rumble JM, Csomos RA, Komarck CM, et al. COMMD proteins, a novel family of structural and functional homologs of MURR1. J Biol Chem. 2005;280(23):22222–32. doi: 10.1074/jbc.M501928200. [DOI] [PubMed] [Google Scholar]
- 34.Endo M, Mori M, Akira S, Gotoh T. C/EBP homologous protein (CHOP) is crucial for the induction of caspase-11 and the pathogenesis of lipopolysaccharide-induced inflammation. J Immunol. 2006;176(10):6245–53. doi: 10.4049/jimmunol.176.10.6245. [DOI] [PubMed] [Google Scholar]
- 35.Endo M, Oyadomari S, Suga M, Mori M, Gotoh T. The ER stress pathway involving CHOP is activated in the lungs of LPS-treated mice. J Biochem. 2005;138(4):501–7. doi: 10.1093/jb/mvi143. [DOI] [PubMed] [Google Scholar]
- 36.Kang SJ, Wang S, Hara H, Peterson EP, Namura S, Amin-Hanjani S, et al. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J Cell Biol. 2000;149(3):613–22. doi: 10.1083/jcb.149.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang SJ, Wang S, Kuida K, Yuan J. Distinct downstream pathways of caspase-11 in regulating apoptosis and cytokine maturation during septic shock response. Cell Death Differ. 2002;9(10):1115–25. doi: 10.1038/sj.cdd.4401087. [DOI] [PubMed] [Google Scholar]
- 38.Feng S. Steatotic livers for liver transplantation--life-saving but at a cost. Nat Clin Pract Gastroenterol Hepatol. 2008;5(7):360–1. doi: 10.1038/ncpgasthep1159. [DOI] [PubMed] [Google Scholar]
- 39.Verran D, Kusyk T, Painter D, Fisher J, Koorey D, Strasser S, et al. Clinical experience gained from the use of 120 steatotic donor livers for orthotopic liver transplantation. Liver Transpl. 2003;9(5):500–5. doi: 10.1053/jlts.2003.50099. [DOI] [PubMed] [Google Scholar]
- 40.Birsner JH, Wan C, Cheng G, Evans ZP, Polito CC, Fiorini RN, et al. Steatotic liver transplantation in the mouse: a model of primary nonfunction. J Surg Res. 2004;120(1):97–101. doi: 10.1016/j.jss.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 41.Selzner N, Selzner M, Jochum W, Amann-Vesti B, Graf R, Clavien PA. Mouse livers with macrosteatosis are more susceptible to normothermic ischemic injury than those with microsteatosis. J Hepatol. 2006;44(4):694–701. doi: 10.1016/j.jhep.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 42.Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006;66(2 Suppl 1):S102–9. doi: 10.1212/01.wnl.0000192306.98198.ec. [DOI] [PubMed] [Google Scholar]
- 43.Gregor MF, Hotamisligil GS. Thematic review series: Adipocyte Biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res. 2007;48(9):1905–14. doi: 10.1194/jlr.R700007-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129(7):1337–49. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 45.Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, et al. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology. 2002;36(3):592–601. doi: 10.1053/jhep.2002.35441. [DOI] [PubMed] [Google Scholar]
- 46.Szegezdi E, Macdonald DC, Ni Chonghaile T, Gupta S, Samali A. Bcl-2 family on guard at the ER. Am J Physiol Cell Physiol. 2009;296(5):C941–53. doi: 10.1152/ajpcell.00612.2008. [DOI] [PubMed] [Google Scholar]