Abstract
Predictive biomarkers may be beneficial for detecting, diagnosing, and assessing the risk of restenosis and vascular injury. We utilized proteomic profiling to identify protein markers in the blood following vascular injury, and corroborated the differential protein expression with immunological approaches. Rats underwent carotid artery injury, and plasma was collected after 2 or 5 weeks. Proteomic profiling was carried out by two-dimensional differential in-gel electrophoresis. The differentially expressed plasma proteins were identified by mass spectroscopy and confirmed by immunoblotting. Proteomic profiling by two-dimensional differential in-gel electrophoresis and mass spectroscopy revealed plasma proteins that were differentially expressed at 2 weeks after injury. Among the proteins identified included vitamin D binding protein (VDBP), aldolase A (aldo A), and apolipoproteinE (apoE). Immunoblotting results validated a significant reduction in these proteins in the plasma at 2 or 5 weeks after vascular injury, in comparison to control animals without vascular injury. These findings suggest that VDBP, aldo A, and apoE may be biomarkers for vascular injury, which will have important prognostic and diagnostic implications.
Keywords: Vascular injury, angioplasty, apolipoprotein E, atherosclerosis, plasma marker, vitamin D binding protein, proteomic profiling, aldolase
Introduction
Cardiovascular disease (CVD) is one of the leading causes of death, accounting for the mortality of over eighty million people in the United States [1]. Among the various kinds of CVDs, myocardial infarction, stroke, and peripheral vascular disease can result from the narrowing and occlusion of blood vessels over an atherosclerotic lesion [2]. Percutaneous coronary intervention (PCI) by balloon angioplasty and stenting has demonstrated promising clinical results for patients having atherosclerosis, but the vascular injury after surgical intervention often causes restenosis.
Vascular diseases are usually initiated by vascular injuries. Therefore, it will be beneficial to detect vascular injuries at an early stage the disease development. Since blood composition is related to a wide range of disease states [3-6], we postulated that vascular injury might cause a change of proteins in the blood, which could be used as biomarkers associated with vascular injury. Although pharmacological and genetic approaches have been employed to study the pathological remodeling process after vascular injury [7-10], recent technological advancements in differential proteomics enable high-throughput screening of the proteome to identify blood proteins associated with CVDs [3, 5, 11-13]. However, the application of proteomic profiling to identify predictive biomarkers for vascular injury remains limited.
The purpose of this study was to utilize proteomic profiling to identify predictive markers of vascular injury in blood. Using a rodent model of carotid artery vascular injury, we identified plasma proteins that were differentially expressed at 2 and 5 weeks after injury, and corroborated the differential protein expression with immunological approaches.
Materials and methods
Rodent vascular injury model
Male Sprague-Dawley rats (400g) were randomized into one of 4 groups, namely control (2 weeks), injury (2 weeks), control (5 weeks), or injury (5 weeks) (n=5 each group). We induced mechanical injury to the carotid artery based on published reports [14]. Vascular injury was induced by a custom-made wire probe that consisted of a 10-cm-long stainless steel wire soldered with a copper-beaded tip (1.0 mm in diameter). The animals were first anesthetized by intraperitoneal injections of ketamine (75-100 mg/kg) and xylazine (10 mg/kg) and then maintained on 2.5% isoflurane (1.5-2 L/min) for the duration of the procedure. Once reliably anesthetized, the rats were intubated and placed on a ventilator (Harvard Rodent Ventilator, Model 683, South Natick, MA) at a respiratory rate of 100-115 per minute, and then placed in a supine position. Using sterile technique, a midline incision and blunt dissection was carried out to expose the left external carotid artery. The left external carotid artery was then ligated near its distal end with 5-0 Prolene sutures. Hemostatic clamps were placed on the internal and common carotid artery. After removingthe clamp on the common carotid artery, transluminal mechanical injury was induced by the insertion of the wire probe through an arteriotomy of the external carotid artery. The wire was passed back and forth 5 times within a 2-cm segment of the common carotid artery. In all cases, obvious distention of the vessel was observed. After the procedure, the left external artery was completely ligated near its proximal end. All clamps were removed, and the neck incision region was finally sutured and closed. For comparison, control animals received the same surgical liga-tion of the left external carotid artery without mechanical injury. Animal studies were approved by the Committee for Animal Research of the University of California San Francisco (San Francisco, CA).
Rodent blood collection
At specified time points, the animals were overdosed with pentobarbital (200 mg/kg body weight), and blood was drawn from the inferior vena cava and placed into an EDTA blood collection tube (BD Biosciences, Franklin Lakes, NJ). The blood samples were subjected to centrifugation at 400xg for 10 minutes at4°C, and the plasma supernatants were collected and stored in microcentrifuge tubes at-80 °C.
Histology and immunohistochemistry
At 2 or 5 weeks after injury, the common carotid arteries were explanted, embedded in OCT compound (TissueTek, Elkhart, IN), and immediately immersed in methyl butane chilled in dry ice for histological analysis. Transverse sections of samples were cryosectioned at 10μm in thickness. Routine hematoxylin and eosin (H&E) staining was performed to assess the tissue morphology. To assess neointimal formation and reendothelization, the sections were stained by antibodies against endothelial cell marker CD31 (BD Pharmingen, San Diego, CA) and smooth muscle α-actin (α-SMA, Sigma, St. Louis, MO) for smooth muscle cells using the Animal Research Kit (Dako, Carpinteria, CA). Under a Zeiss microscope, at least 4 tissue sections spanning the length of the carotids were analyzed for each sample (n=5 per group).
Two-dimensional differential in-gel electrophoresis (2D-DIGE)
2D-DIGE was performed at Applied Biomics (Hayward, CA) following established methods [15, 16]. Briefly, equal volumes of plasma proteins were denatured by the addition of a lysis buffer containing 7M Urea, 2M thiourea, 4% 3-((3-cholamidopropyl)dimethylammonio)-1-pro-panesulfonate (CHAPS) and 30 mM Tris-HCl, pH 8.8, at a 5:1 ratio of lysis buffer:plasma. Next, the samples were labeled with CyDye DIGE fluors (Cy3 and Cy5, GE Healthcare) on ice for 30 minutes. The labeled samples were then subjected to isoelectric focusing (IEF) on a 13-cm precast non-linear IPG (immobilized pH gradient) strip (pH 3-10, Amersham) based on pI in the first dimension using an Amersham Pharmacia IPGPHOR unit with a power supply (EPS 3501XL) in gradient mode. Next, the samples were separated by sodium dodecyl sulfate poly-acrylamide gel electrophoresis (SDS-PAGE) in the second dimension in a 13.5% gel based on size. The gels were scanned using Typhoon Trio scanner (Amersham Biosciences), and images were analyzed using ImageQuant and DeCyder software (Amersham). The individual fluorescent dye signals were converted to black and white images that correspond to the individual samples. The volume ratio of injury/control samples (n=3) was calculated for each protein spot.
Mass spectroscopy (MS)
Based on 2D-DIGE assessment, proteins showing statistically significant differences in intensity were excised from the gel using an Ettan spot picker (Amersham Biosciences), digested with trypsin (Promega Corporation, Madison WI) at 37 °C, extracted with 2% trifluoroacetic acid and 40 μl of acetonitrile, and desalted with a C-18 ZipTip (Millipore). Each sample was mixed with matrix buffer and spotted onto a MALDI plate. MALDI-TOF MS was performed using the ABI4700/ABI4800 (Applied Biosystems, Foster City, CA) proteomic analyzer according to the manufacturer's instructions. The top ten most abundant peptides were further subjected to fragmentation (MS/MS). Both MS and MS/MS spectra were submitted for database search using GPS explorer equipped with MASCOT search engine, and the NCBI and SwissProt protein databases were searched (two variable modifications, carbamidomethyl and oxidation, one missed cleavage; precursor tolerance, 100 ppm; MS/MS tolerance, 0.3D). Successful matches were obtained when de novo sequences were derived from high-quality mass spectra and the peptide score was > 95%.
Immunoblotting
Proteins were separated using either SDS-PAGE or native-PAGE, followed by membrane transfer, and then immunoblotting. For SDS-PAGE, equal amounts of protein were mixed with loading buffer containing 2% SDS and 5% 2-mercaptoethanol, and then denatured at 85 °C for 3 min. Samples were loaded in a 10% SDS-polyacrylamide gel and separated by electropho-resis. A similar procedure was used for native-PAGE, but in the absence of SDS. The proteins were transferred to nitrocellulose or polyvi-nylidene difluoride membranes, blocked with 3% non-fat milk, and then incubated with one of the following primary antibodies: anti-Aldolase A (Abcam, Cambridge, MA), rat-specific anti-ApoE (Abcam), human-specific anti-ApoE4 (Medical and Biological Laboratories, Woburn, MA), anti-VDBP (Abcam), anti-fibronectin (Chemicon, Te-mecula, CA), and anti-human serum albumin (HSA, Abcam). This was followed by the incubation with horse radish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Protein bands were visualized using the ECL detection system (Amersham Biosciences) or SuperSignal West Pico chemiluminescence detection reagent (Thermo Fisher Scientific, Waltham, MA). Signal intensity was analyzed using Image J (National Institutes of Health, Bethesda, MD). Equal loading was verified by both Coomassie staining and abundance of fibronectin or albumin.
Statistical analysis
Data is shown as mean ± standard deviation. Statistical analysis of two groups was quantified by a Student's t-test. Statistical significance was accepted at P<0.05.
Results
Validation of vascular injury model
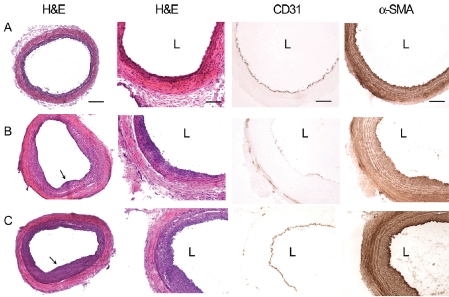
To validate the vascular injury model, we first characterized the morphology of the mechanically denuded samples after 2 and 5 weeks by histological analysis. In comparison to control samples (Figure 1A), the injured samples demonstrated progressive intimal thickening from 2 weeks (Figure 1B) to 5 weeks (Figure 1C). Im-munohistochemical staining demonstrated that α-SMA-expressing smooth muscle cells populated the thickness of the neointima, and endo-thelial cells initiated formation of an endothelial lining at 2 weeks that was fully complete after 5 weeks (Figures 1B-1C). Based on histological assessment, successful neointimal thickening was observed in all animals that underwent vascular injury.
Figure 1.
Histological and immunohistochemical assessment of neointimal formation after vascular injury. Shown in panel A are histological cross sections of control carotid arteries without vascular injury. Shown in Panel B are carotid arteries at 2 weeks after injury, and Panel C are carotid arteries at 5 weeks after injury. Neointimal formation can be visualized by H&E staining at low and high magnification. Vascular cells could be visualized by CD31 staining for endothelial cells and α-SMA staining for smooth muscle cells. Arrows point to locations of neointimal formation. L denotes the location of the arterial lumen. Scale bar: 200 μm in the left-most panel and 100 μm elsewhere.
2D-DIGE and protein identification
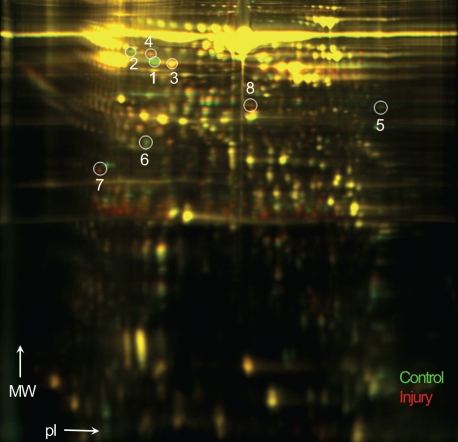
To identify early biomarkers of vascular injury, we profiled the proteome for differentially expressed proteins at 2 weeks after endothelial denudation using 2D-DIGE, which separated proteins based on pI and molecular weight (Figure 2). The differential expression between the injury and control samples were assessed by relative volume ratios (injury/control). Due to the large number of protein spots that showed statistically significant differential expression between the 2 groups (P<0.05), 8 protein spots with most dramatic changes were selected for further analysis.
Figure 2.
2D-DIGE showing differentially expressed proteins after 2 weeks of carotid artery injury in rats. Green spots represent proteins from the control plasma, whereas red spots indicate proteins from vascular injury samples. Proteins were separated according to molecular weight (MW) and isoelec-tric point (pI). Circles and numbers refer to spots in which proteins were identified by MS.
Based on MS analysis of the 8 proteins, we identified 5 proteins and their isoforms (Table 1). The spots labeled 1-4 were isoforms of the prepeptide form of vitamin D binding protein (VDBP), also known as group-specific component globulin, a carrier for vitamin D and its plasma metabolites (Figure 3). Among the 4 spots, spots 1 and 2 showed differentially lower expression in the injury samples, when compared to the control samples, with respective volume ratios (injury/control) of 0.09 ± 0.01 and 0.41 ± 0.16 (Table 1). On the other hand, spots 3 and 4 were identified as isoforms with increased expression in the injured samples (2.01 ± 0.08 and 1.84 ± 0.21, respectively).
Table 1.
Differentially expressed proteins at 2 weeks after vascular injury
| Spot No. | Volume Ratio (Injury/Control) | Protein Name | Accession No. | Molecular Weight (Da) | pI | P < |
|---|---|---|---|---|---|---|
| 1 | 0.09 ± 0.01 | VDBP prepeptide | gi|203927 | 55089.6 | 5.65 | 0.001 |
| 2 | 0.41 ± 0.16 | VDBP prepeptide | gi|203927 | 55089.6 | 5.65 | 0.003 |
| 3 | 2.01 ± 0.08 | VDBP prepeptide | gi|203927 | 55089.6 | 5.65 | 0.001 |
| 4 | 1.84 ± 0.21 | VDBP precursor | gi|203941 | 53509 | 5.65 | 0.002 |
| 5 | 0.42 ± 0.11 | Aldolase A | gi|202837 | 39691.4 | 8.31 | 0.001 |
| 6 | 0.50 ± 0.09 | ApoE | gi|55824759 | 35788.4 | 5.23 | 0.001 |
| 7 | 2.79 ± 0.79 | Ig γ2 chain C region | gi|125899 | 11310.6 | 5.76 | 0.017 |
| 8 | 1.94 ± 0.12 | GFAP | gi|115311597 | 49912.6 | 5.35 | 0.007 |
pI, isolectric point
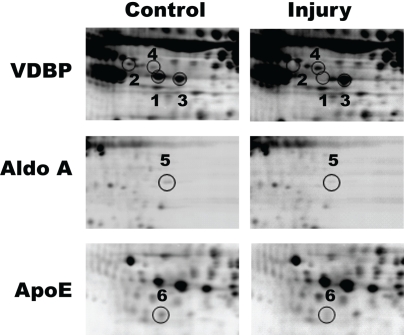
Figure 3.
Identification of differentially expressed rat plasma proteins after 2 weeks of vascular injury. As shown in magnified view, the identified proteins included multiple isoforms of VDBP, apoE, and aldo A (P<0.05).
Another metabolism-related protein was spot 5, which was identified to be aldolase A (aldo A), an enzyme that plays a critical role in muscle-specific metabolism (Figure 3). Aldo A was significantly reduced in vascular injury samples, in comparison to control samples (0.42 ± 0.11) as shown in Table 1. In addition, we also observed that the injury samples had a significant reduction in spot 6 that was identified to be plasma Apolipoprotein E (ApoE), an important ligand in receptor-mediated uptake of triglyceride-rich lipoproteins (Figure 3). The volume ratio was 0.50 ± 0.09, which represented a 2-fold lower abundance of protein expression than the control samples (Table 1). The remaining spots showed increased expression in the injury samples (Table 1). Spot 7 corresponded with immunoglobulin lambda-2 chain C region, whose volume ratio was 2.79 ± 0.79. Glial fibrillary acidic protein (GFAP), an intermediate filament expressed in astrocytes and glial cells, was identified as spot 8, and the volume ratio for GFAP was 1.94 ± 0.12.
Immunoblotting analysis of rat plasma
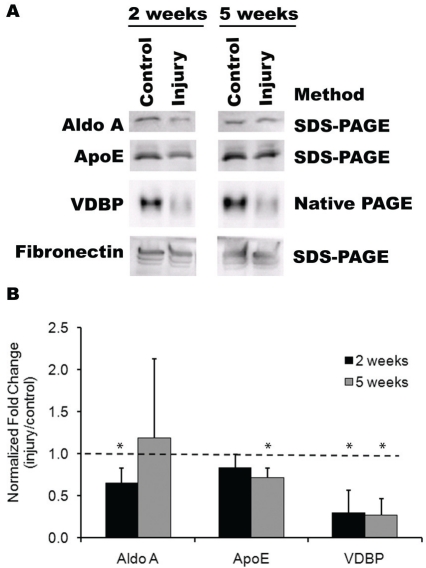
Following the identification of a number of differentially expressed proteins at 2 weeks post-injury by 2D-DIGE, we sought to verify these results for a subset of the proteins, namely VDBP, aldo A, and apoE. Since multiple isoforms of VDBP were identified, a native PAGE gel was run prior to immunoblotting to preserve the protein integrity of VDBP isoforms. Indeed, as shown in Figure 4, the differential expression of VDBP could be confirmed. The normalized relative ratio (injury/control) of VDBP expression after 2 weeks of injury was 0.30 ± 0.27 (P<0.05), representing about a 3-fold reduction in the injury samples, in comparison to control samples. Furthermore, the relative ratio of aldo A was 0.65 ± 0.18, which indicated a significant decrease compared to control samples (P<0.05). On the other hand, ApoE did not demonstrate a significant difference between injury and control samples.
Figure 4.
Validation of differentially expressed proteins by immunoblotting. (A) Representative im-munoblots for rodent plasma proteins. For the assessment of aldo A and apoE, plasma samples were resolved on an SDS-PAGE gel before immunoblotting. For VDBP, a native PAGE gel was run to preserve protein integrity of VDBP isoforms. Equal quantities of protein were loaded and confirmed by fibronectin. (B) Immunoblot data was quantified as a ratio of injury/control at each timepoint. * indicates statistically significant from control sample at the same time point (P<0.05). The horizontal line depicts the relative level of the control sample.
To determine whether the levels of differential expression could be sustained beyond 2 weeks, we further investigated the protein expression levels at 5 weeks post-injury by immunoblotting. Indeed, the relative ratio of VDBP expression remained significantly reduced at a volume ratio of 0.28 ± 0.19 (P<0.05), indicating that the decrease in VDBP expression was sustained for up to 5 weeks after injury. In addition to differential expression of VDBP, the relative ratio of apoE was also significantly lower in injured samples (0.71 ± 0.11, P<0.05), suggesting that a reduction in plasma apoE may be associated with later time points of injured vessels. Aldo A levels were not significantly different between injured and control samples after 5 weeks.
Discussion
With conventional molecular biological approaches, studies on proteins can only be conducted on a limited number of proteins. Advances in proteomic analysis now enable direct monitoring of global changes in protein expression and post-translational modifications, which will help identify new biomarkers for CVDs and potentially provide more insight into the treatment of CVDs [17-22]. In particular, 2D-DIGE utilizes mass-and charge-matched spectrally resolvable fluorescent dyes (Cy3 and Cy5) to label two different protein samples in vitro prior to two-dimensional electrophoresis (2DE). To date, 2DE/DIGE is still one of the central technologies in proteomics for the separation and differential comparison of thousands of proteins in a complex mixture [18, 23, 24].
Since blood is easily accessible and harbors many proteins that remain to be fully characterized, it may be a valuable source for identifying diagnostic or prognostic markers of CVDs by proteomic profiling. Based on the 2D-DIGE results from the rat injury model, we identified a proteomic signature of blood proteins that may be useful as biomarkers of vascular injury. These proteins consisted of VDBP, aldo A, apoE, immunoglobulin lambda-2 chain C region and GFAP, and they were differentially expressed in high confidence intervals. Among them, VDBP, aldo A and apoE were potential biomarkers for vascular injury.
One of the main findings of this study was that VDBP protein levels change in the presence of vascular injury. VDBP is a member of the albumin superfamily of binding proteins and exists in 3 dominant full-length isoforms, in addition to over 120 genetic variants [25]. Besides serving as a transporter of vitamin D, VDBP also plays an important role in response to tissue injury, in which VDBP can be converted to a macrophage-activating factor (VDBP-MAF) to stimulate macrophages [26]. Another role of VDBP in response to injury is to scavenge for vascular and extracellular actin as a result of cellular necrosis, and VDBP has been shown to be in lower circulating concentrations in the presence of inflammatory or necrotic diseases [25, 27, 28]. Furthermore, VDBP is also implicated in a numerous diseases, including chronic obstructive pulmonary disease, cancer, and trauma [29-31]. Using proteomic profiling, we identified 4 isoforms of VDBP, of which included isoforms with significant induction or reduction at 2 weeks after vascular injury in rat plasma. The differential regulation of VDBP isoforms suggests that there may be a unique VDBP expression pattern that characterizes the progression of vascular injury and restenosis. Immunoblot-ting data showed an overall reduction of VDBP expression in the plasma of injured rats, and this trend of reduced VDBP expression remained consistent at both 2 weeks and 5 weeks after vascular injury. However, further studies are necessary to elucidate the mechanism by which VDBP is regulated in the development of vascular injury.
ApoE is an important ligand for receptor-mediated uptake of triglyceride-rich lipoproteins and mediator for extracellular lipoprotein ho-meostasis [32]. Its function in the low density lipoprotein receptor-related protein (LRP)-mediated pathway includes binding to the lipoprotein for delivery to the receptor, binding to heparin sulfate proteoglycans to mediate ligand -receptor interactions, and interacting with the LRP receptor to regulate uptake of the lipoprotein [33]. ApoE deficiency or defect is known to be associated with hyperlipidemia [34], atherosclerosis [35], and Alzheimer's disease [36]. In accordance with previous reports [37], significant apoE reduction could be detected in rat plasma after 5 weeks of vascular injury, concomitant with neointimal thickening. These results suggest that apoE reduction may be associated with vascular injury, but further research would be required to explore this possibility.
Another metabolism-related marker identified by proteomic profiling was aldo A. Aldo A is 1 of 3 isoforms of aldolase, whose functional form is a homo or hetero-tetramer [38]. Aldo A plays an important role in muscle-specific glycolysis by catalyzing the cleavage of fructose-1, 6-bisphosphate into glyceraldehyde-3-phosphate and dihydroacetate phosphate [39]. Although the role of aldo A in diseases is largely unknown, the data from the rodent vascular injury study suggests that aldo A reduction is evident in the plasma at 2 weeks after injury and implicates aldo A as a biomarker of vascular injury.
Previous studies have shown evidence of plasma proteins that are associated with vascular injury, including interleukin-18, thrombo-modulin, and soluble CD39 [40-42]. These proteins, when combined with the ones identified by our proteomic analysis, may serve as a robust biomarker panel that is predictive of vascular injury.
Although the rodent model of vascular injury is limited by the absence of cardiovascular risk factors, it distinguishes the proteomic changes associated with injury from those resulting from other disease factors. This model provides initial clues to unravel the complex events that underlie proteomic changes in response to pathophysiological events. Further studies using established disease models are warranted and will further elucidate the roles of VDBP, aldo A, and apoE in vascular disease in the presence of cardiovascular risk factors. In addition, future clinical studies may provide further validation of these biomarkers.
In summary, we utilized high-throughput proteomic profiling and traditional immunological approaches and identified VDBP, aldo A, and apoE levels to be differentially expressed in an experimental model of rat carotid injury. These finding have important prognostic and diagnostic implications in the treatment of vascular injury.
Acknowledgments
We thank Yuqiao Shen and Jian Liao at Applied Biomics Inc. for their technical assistance in DIGE analysis. This work was supported in part by grants (HL079419 and HL083900) from the National Institutes of Health to S.L. N.H. was funded by a fellowship from the National Science Foundation.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics-2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 3.Mateos-Caceres PJ, Garcia-Mendez A, Lopez Farre A, Macaya C, Nunez A, Gomez J, Alonso-Orgaz S, Carrasco C, Burgos ME, de Andres R, Granizo JJ, Farre J, Rico LA. Proteomic analysis of plasma from patients during an acute coronary syndrome. J Am Coll Cardiol. 2004;44:1578–1583. doi: 10.1016/j.jacc.2004.06.073. [DOI] [PubMed] [Google Scholar]
- 4.Hori T, Naishiro Y, Sohma H, Suzuki N, Hatakeyama N, Yamamoto M, Sonoda T, Mizue Y, Imai K, Tsutsumi H, Kokai Y. CCL8 is a potential molecular candidate for the diagnosis of graft-versus-host disease. Blood. 2008;111:4403–4412. doi: 10.1182/blood-2007-06-097287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson AM, Kimura E, Harada RK, Nair N, Narasimhan B, Meng XY, Zhang F, Beck KR, Olin JW, Fung ET, Cooke JP. Beta2-microglobulin as a biomarker in peripheral arterial disease: proteomic profiling and clinical studies. Circulation. 2007;116:1396–1403. doi: 10.1161/CIRCULATIONAHA.106.683722. [DOI] [PubMed] [Google Scholar]
- 6.Kim SS, Kim MH, Shin BK, Na HJ, Choi JY, Kee MK, Chong SA, Nam MJ. Different isoforms of apolipoprotein AI present heterologous post-translational expression in HIV infected patients. J Proteome Res. 2007;6:180–184. doi: 10.1021/pr060323f. [DOI] [PubMed] [Google Scholar]
- 7.Shagdarsuren E, Bidzhekov K, Djalali-Talab Y, Liehn EA, Hristov M, Matthijsen RA, Buurman WA, Zernecke A, Weber C. C1-esterase inhibitor protects against neointima formation after arterial injury in atherosclerosis-prone mice. Circulation. 2008;117:70–78. doi: 10.1161/CIRCULATIONAHA.107.715649. [DOI] [PubMed] [Google Scholar]
- 8.Bailey SR, Mitra S, Flavahan S, Bergdall VK, Flavahan NA. In vivo endothelial denudation disrupts smooth muscle caveolae and differentially impairs agonist-induced constriction in small arteries. J Cardiovasc Pharmacol. 2007;49:183–190. doi: 10.1097/FJC.0b013e318031d5dd. [DOI] [PubMed] [Google Scholar]
- 9.Liu P, Patil S, Rojas M, Fong AM, Smyth SS, Patel DD. CX3CR1 deficiency confers protection from intimal hyperplasia after arterial injury. Arterioscler Thromb Vasc Biol. 2006;26:2056–2062. doi: 10.1161/01.ATV.0000234947.47788.8c. [DOI] [PubMed] [Google Scholar]
- 10.Chadjichristos CE, Matter CM, Roth I, Sutter E, Pelli G, Luscher TF, Chanson M, Kwak BR. Reduced connexin43 expression limits neointima formation after balloon distension injury in hypercholesterolemic mice. Circulation. 2006;113:2835–2843. doi: 10.1161/CIRCULATIONAHA.106.627703. [DOI] [PubMed] [Google Scholar]
- 11.Marshall J, Kupchak P, Zhu W, Yantha J, Vrees T, Furesz S, Jacks K, Smith C, Kireeva I, Zhang R, Takahashi M, Stanton E, Jackowski G. Processing of serum proteins underlies the mass spectral fingerprinting of myocardial infarction. J Proteome Res. 2003;2:361–372. doi: 10.1021/pr030003l. [DOI] [PubMed] [Google Scholar]
- 12.Mayr M, Zhang J, Greene AS, Gutterman D, Perloff J, Ping P. Proteomics-based development of biomarkers in cardiovascular disease: mechanistic, clinical, and therapeutic insights. Mol Cell Proteomics. 2006;5:1853–1864. doi: 10.1074/mcp.R600007-MCP200. [DOI] [PubMed] [Google Scholar]
- 13.Mayr M, Yusuf S, Weir G, Chung YL, Mayr U, Yin X, Ladroue C, Madhu B, Roberts N, De Souza A, Fredericks S, Stubbs M, Griffiths JR, Jahangiri M, Xu Q, Camm AJ. Combined metabolomic and proteomic analysis of human atrial fibrillation. J Am Coll Cardiol. 2008;51:585–594. doi: 10.1016/j.jacc.2007.09.055. [DOI] [PubMed] [Google Scholar]
- 14.Leidenfrost JE, Khan MF, Boc KP, Villa BR, Collins ET, Parks WC, Abendschein DR, Choi ET. A model of primary atherosclerosis and post-angioplasty restenosis in mice. Am J Pathol. 2003;163:773–778. doi: 10.1016/S0002-9440(10)63704-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de la Fuente J, Blouin EF, Manzano-Roman R, Naranjo V, Almazan C, Perez de la Lastra JM, Zivkovic Z, Jongejan F, Kocan KM. Functional genomic studies of tick cells in response to infection with the cattle pathogen, Anaplasma marginale. Genomics. 2007;90:712–722. doi: 10.1016/j.ygeno.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Corena-McLeod Mdel P, Oliveros A, Charlesworth C, Madden B, Liang YQ, Boules M, Shaw A, Williams K, Richelson E. Paliperidone as a mood stabilizer: a pre-frontal cortex synaptoneuro-somal proteomics comparison with lithium and valproic acid after chronic treatment reveals similarities in protein expression. Brain Res. 2008;1233:8–19. doi: 10.1016/j.brainres.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Mo W, Karger BL. Analytical aspects of mass spectrometry and proteomics. Curr Opin Chem Biol. 2002;6:666–675. doi: 10.1016/s1367-5931(02)00379-4. [DOI] [PubMed] [Google Scholar]
- 18.Rabilloud T. Two-dimensional gel electrophoresis in proteomics: old, old fashioned, but it still climbs up the mountains. Proteomics. 2002;2:3–10. [PubMed] [Google Scholar]
- 19.Patterson SD, Aebersold RH. Proteomics: the first decade and beyond. Nat Genet. 2003;33(Suppl):311–323. doi: 10.1038/ng1106. [DOI] [PubMed] [Google Scholar]
- 20.MacCoss MJ, Yates JR 3rd. Proteomics: analytical tools and techniques. Curr Opin Clin Nutr Metab Care. 2001;4:369–375. doi: 10.1097/00075197-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Yarmush ML, Jayaraman A. Advances in proteomic technologies. Annu Rev Biomed Eng. 2002;4:349–373. doi: 10.1146/annurev.bioeng.4.020702.153443. [DOI] [PubMed] [Google Scholar]
- 22.Srinivas PR, Verma M, Zhao Y, Srivastava S. Proteomics for cancer biomarker discovery. Clin Chem. 2002;48:1160–1169. [PubMed] [Google Scholar]
- 23.Witzmann FA, Li J. Cuttingedge technology. II. Proteomics: core technologies and applications in physiology. Am J Physiol Gastrointest Liver Physiol. 2002;282:G735–741. doi: 10.1152/ajpgi.00510.2001. [DOI] [PubMed] [Google Scholar]
- 24.Lilley KS, Razzaq A, Dupree P. Two-dimensional gel electrophoresis: recent advances in sample preparation, detection and quantitation. Curr Opin Chem Biol. 2002;6:46–50. doi: 10.1016/s1367-5931(01)00275-7. [DOI] [PubMed] [Google Scholar]
- 25.Meier U, Gressner O, Lammert F, Gressner AM. Gc-globulroles in response to injury. Clin Chem. 2006;52:1247–1253. doi: 10.1373/clinchem.2005.065680. [DOI] [PubMed] [Google Scholar]
- 26.Gomme PT, Bertolini J. Therapeutic potential of vitamin D-binding protein. Trends Biotechnol. 2004;22:340–345. doi: 10.1016/j.tibtech.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 27.White P, Cooke N. The multifunctional properties and characteristics of vitamin D-binding protein. Trends Endocrinol Metab. 2000;11:320–327. doi: 10.1016/s1043-2760(00)00317-9. [DOI] [PubMed] [Google Scholar]
- 28.Lee WM, Galbraith RM. The extracellular actin-scavenger system and actin toxicity. N Engl J Med. 1992;326:1335–1341. doi: 10.1056/NEJM199205143262006. [DOI] [PubMed] [Google Scholar]
- 29.Schellenberg D, Pare PD, Weir TD, Spinelli JJ, Walker BA, Sandford AJ. Vitamin D binding protein variants and the risk of COPD. Am J RespirCritCare Med. 1998;157:957–961. doi: 10.1164/ajrccm.157.3.9706106. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto N, Naraparaju VR. Immunotherapy of BALB/c mice bearing Ehrlich ascites tumor with vitamin D-binding protein-derived macrophage activating factor. Cancer Res. 1997;57:2187–2192. [PubMed] [Google Scholar]
- 31.Dahl B, Schiodt FV, Rudolph S, Ott P, Kiaer T, Heslet L. Trauma stimulates the synthesis of Gc-globulin. Intensive Care Med. 2001;27:394–399. doi: 10.1007/s001340000837. [DOI] [PubMed] [Google Scholar]
- 32.Miserez AR, Scharnagl H, Muller PY, Mirsaidi R, Stahelin HB, Monsch A, Marz W, Hoffmann MM. Apolipoprotein E3Basel: new insights into a highly conserved protein region. Eur J Clin Invest. 2003;33:677–685. doi: 10.1046/j.1365-2362.2003.01180.x. [DOI] [PubMed] [Google Scholar]
- 33.Moghadasian MH, McManus BM, Nguyen LB, Shefer S, Nadji M, Godin DV, Green TJ, Hill J, Yang Y, Scudamore CH, Frohlich JJ. Patho-physiology of apolipoprotein E deficiency in mice: relevance to apo E-related disorders in humans. Faseb J. 2001;15:2623–2630. doi: 10.1096/fj.01-0463com. [DOI] [PubMed] [Google Scholar]
- 34.Ghiselli G, Schaefer EJ, Gascon P, Breser HB Jr. Type III hyperlipoproteinemia associated with apolipoprotein E deficiency. Science. 1981;214:1239–1241. doi: 10.1126/science.6795720. [DOI] [PubMed] [Google Scholar]
- 35.FazioS, Babaev VR, Murray AB, Hasty AH, Carter KJ, Gleaves LA, Atkinson JB, Linton MF. Increased atherosclerosis in mice reconstituted with apolipoprotein E null macrophages. Proc Natl Acad Sci U S A. 1997;94:4647–4652. doi: 10.1073/pnas.94.9.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poirier J, Delisle MC, Quirion R, Aubert I, Farlow M, Lahiri D, Hui S, Bertrand P, Nalbantoglu J, Gilfix BM, Gauthier S. Apolipoprotein E4 al-lele as a predictor of cholinergic deficits and treatment outcome in Alzheimer disease. Proc Natl Acad Sci U S A. 1995;92:12260–12264. doi: 10.1073/pnas.92.26.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wientgen H, Thorngate FE, Omerhodzic S, Rolnitzky L, Fallon JT, Williams DL, Fisher EA. Subphysiologic apolipoprotein E (ApoE) plasma levels inhibit neointimal formation after arterial injury in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:1460–1465. doi: 10.1161/01.ATV.0000134297.61979.3c. [DOI] [PubMed] [Google Scholar]
- 38.Hori K, Mukai T, Joh K, Arai Y, Sakakibara M, Yatsuki H. Structure and expression of human and rat aldolase isozyme genes: multiple mRNA species of aldolase A produced from a single gene. Isozymes Curr Top Biol Med Res. 1987;14:153–175. [PubMed] [Google Scholar]
- 39.Hers HG, Kusaka T. [The metabolism of fructose-1-phosphate in the liver.] Biochim Biophys Acta. 1953;11:427–437. doi: 10.1016/0006-3002(53)90062-6. [DOI] [PubMed] [Google Scholar]
- 40.Maffia P, Grassia G, Di Meglio P, Carnuccio R, Berrino L, Garside P, Ianaro A, Ialenti A. Neutralization of interleukin-18 inhibits neointimal formation in a rat model of vascular injury. Circulation. 2006;114:430–437. doi: 10.1161/CIRCULATIONAHA.105.602714. [DOI] [PubMed] [Google Scholar]
- 41.Strijbos MH, Rao C, Schmitz PI, Kraan J, Lamers CH, Sleijfer S, Terstappen LW, Gratama JW. Correlation between circulating endothelial cell counts and plasma thrombomodulin levels as markers for endothelial damage. Thromb Haemost. 2008;100:642–647. [PubMed] [Google Scholar]
- 42.Drosopoulos JH, Kraemer R, Shen H, Upmacis RK, Marcus AJ, Musi E. Human solCD39 inhibits injury-induced development of neointimal hyperplasia. Thromb Haemost. 103:426–434. doi: 10.1160/TH09-05-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]