Abstract
Primary chronic venous disease (CVD) is an inflammatory pathology involving an erratic structural remodeling in the venous well leading to vascular incompetence and the development of varicose vein, characterized by altered collagen and elastin content. In the early steps of varicose vein formation is crucial the role of MMP/TIMP balance, implicated in both ECM and vascular degradation during inflammation processes in early and late stages of venous diseases. Although several pharmacological and surgical strategies are being utilized in the management of varicose vein and CVD with variable success and recurrence rate, inhibition of MMP through glycosaminoglycans may represent a novel therapeutic intervention to limit the progression of varicose vein to CVD and leg ulceration, suggesting possible opportunity to prevent future morbidity and enhancing clinical benefits and quality of life.
Keywords: Matrix metalloproteinase, glycosaminoglycan, chronic venous disease, inflammation, venous ulcer, leukocyte, fibroblast, dermatan sulphate, varicose vein, tissue inhibitor of MMP
Introduction
Primary chronic venous disease (CVD), with well-known diversity of symptoms, clinical signs and prevalence, is a common worldwide pathology affecting mainly the adult population[1]. In general, “degeneration” of the peripheral veins leads to dilatation of the lumen and insufficient closure of the valves resulting in a backflow of blood from deep to the superficial venous system [2], leading to ambulatory hypertension in the superficial venous system and recirculation of large amounts of draining blood remaining in the affected leg of venous drainage blood in the affected leg [3]. Without treatment this results in the long term in variable degrees of decompensation of the recirculation pathways and an increasing secondary insufficiency of the deep venous system [4]: the result corresponds to the clinical symptoms of chronic venous insufficiency (CVI), an advanced form of CVD characterized by a sustained ambulatory venous hypertension and venous-specific skin changes (e.g., leg edema, dermal hyperpigmentation, eczema, lipodermatosclerosis and finally ulceration) [5].
In patients with CVI, the lifetime of risk of chronic venous ulceration (CVU) is around 1% with approximately 10% of ulcers being open at any one time, and the incidence of skin changes disease is about 10 times greater (10%) [4,6,7]. However, many of the studies upon which these estimates are based are old and/or methodologically flawed, and there is reason to believe that the incidence, prevalence and characteristics of CVI/CVU may have changed considerably over the last 10-20 years and that future change in determining the actuarial numbers is likely [8].
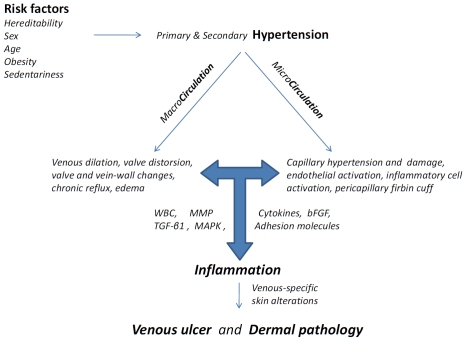
As schematized in Figure 1, CVI culminating in CVU is primarily the result of sustained ambulatory venous hypertension, which in turn arises from superficial and/or deep venous reflux with or without deep vein obstruction. However, there are many other elements to this complex condition, for example, microvascular dysfunction; congenital and acquired thrombophilia; obesity and diet; muscle pump efficiency; dermal inflammation; recruitment of white blood cells; production and secretion of cytokines; stimulation of adhesion molecules and metallo-proteinases; disordered fibroblast function and matrix rearrangement; bacterial colonization; failure of epithelialization; acute and chronic wound; ulcer and necrosis [1, 7].
Figure 1.
Schematic diagram of the pathophysiology of chronic venous disease and its progression leadingto skin alterations. The cascade emphasizes the presence of predisposing factors leadingto venous hypertension, causing changes in both the macrocirculation and microcirculation, and the central role of inflammatory cell activation, resulting in cytokine, growth factor, and MMP production and the unabated inflammatory and proteolytic environment that eventually leads to dermal skin changes and venous ulcer formation. WBC, white blood cells; MMP, matrix metallo-proteinase; TIMP, tissue inhibitor of metalloproteinase; TGFβ1, transforming growth factor β1; bFGF, basic fibroblast growth factor; MAPK, mitogen activated protein kinase.
Among the many proposed mechanisms linking venous hypertension to CVD, CVI and finally to CVU the complex interplay between inflammatory responses by leukocytes, cytokine cascade, stimulation of adhesion molecules and matrix metalloproteinase (MMP) activity may lead to cell dysfunction resulting in dermal changes observed clinically in patients [5].
Although we are beginning to understand the pathophysiologic mechanisms in CVD, several areas of our knowledge need to be addressed in order to perform translational studies reducing the risk of CVD progression. In particular, in this review we will focus attention on: 1) how glyco-saminoglycan components of the extracellular matrix (ECM) may be involved in the unrestrained MMP activity; 2) how the MMPs may contribute to the venous dilation, and during inflammation to the breakdown of ECM components promoting then the ulcer formation and impairing healing; 3) how pharmacologic agents based on glycosaminoglycans could attenuate various elements of both the inflammatory cascade and inhibit the crucial proteolytic step, offering through translational researche a greater opportunity to prevent future morbidity that is associated with CVD.
Valve and Vein-wall structural changes in CVD
CVD has been estimated to account for 1-3 % of the total health care budgets in countries with developed health care systems [8]. CVD is associated with a reduced quality of life, particularly in relation to symptom ascribed to aching, pain, heaviness, depression, cramps, itching, tingling and restless legs and ulcers [3]. In the absence of trophic skin changes, the two major mechanisms are 1) Hypoxia of the tunica media of the venous wall due to alteration of the vasa vasorum, and 2) Venous wall tension resulting from both dilatation of the vein due to hypertension and valvular insufficiency, and causing superficial and/or deep venous reflux [4]. Despite the diversity of signs and symptoms associated with CVD, it seems likely that all are related to Venous Hypertension, linked to altered hydrostatic and hydrodynamic components (e.g., elevated leg venous pressure for prolonged periods) and significantly influenced by the action of venous valves (due to structural changes in valve vein-wall changes) [1].
In this respect, ultrastructural morphology and biochemical studies of varicose veins have found hypertrophy of the vein wall with altered collagen and elastin content [9-12], together with disruption of the glycosaminoglycan rearrangements [13-20]. In particular, disturbed collagen synthesis with elevated ratios of type I to type III collagen, may significantly contribute to the weakness and reduced elasticity of varicose veins [21-23]. A complicating factor is the heterogeneity of the varicose vein wall; in fact, in CVD patients hypertrophic segments can alternate with thinner atrophic segments with reduced ECM and increased number of inflammatory cells (e.g., mast cells, macrophages, and neutrophils) [4], suggesting that both inflammation and degradation of ECM proteins would be crucial in the early etiopathogenesis of CVD [24].
Interestingly, the role of different glycosaminoglycan (GAG) species from the vessel walls has been extensively studied, suggesting that 1) the saphenous vein (the more frequent locus of CVD) contains high percentage of collagen and dermatan sulfate (DS) [17,18,20]; 2) the content of sulfated GAG was significantly increased in varicose vein, in particular in the presence of thrombophlebitis [19]; 3) the DS may have anticoagulant and antithrombotic activity [13]; 4) the disorders of vascular DS metabolism may contribute to vascular pathology and remodeling [14].
ECM proteolytic degradation and CVD initiation/ progression
The ECM is an important structural and functional scaffolding made up of proteins (such as collagen, elastin, fibronectin, growth factors, proteoglycans, and glycosaminoglycans) that are necessary for a variety of cell functions, including cell differentiation and signaling, cellular migration, angiogenesis, blood vessel support, epithelialization, and wound repair [25]. Degradation of ECM is mainly caused by an array of proteolytic enzymes, including matrix metallo-proteinases (MMPs) and serine proteases, which are produced by both vascular (endothelial cells, fibroblasts) and white blood cells [26,27], in particular during inflammation [28,29]. MMPs are released as inactive proen-zymes that are finely regulated [30,31,27] in order to achieve the appropriate function during physio-pathologic conditions, such as activation by other proteinases, formation of complexes with regulatory proteins, inactivation by endogenous tissue inhibitors TIMP, and reciprocal interactions/controls with ECM peptidoglycans [32,33].
The involvement of MMP and TIMP in vascular diseases is a matter of a strong and continuous scientific interest, especially in the study of effective MMP modulators that would be important in the management of patients with arterial and venous diseases [34,35]. In particular, for what concerns CVD it has been suggested that the balance between MMP and TIMP play a crucial role in early steps of varicose vein formation in the lower extremities as well as in their progression to thrombophlebitis and venous leg ulcers [5,36].
It has been widely documented that the effects of MMP and TIMP on ECM degradation may result in a significant venous tissue remodeling [37,38], degenerative and structural changes in the vein wall [9,39], leading to venous dilation and valve dysfunction [2,40]. Taken together, increased MMP activity and altered MMP/TIMP balance [41,42] may also induce early modifications in the endothelium and venous smooth muscle function in the absence of significant ECM degradation or structural changes in the vein wall. In addition, evidence suggests that increased activity of MMP is also present in the advanced stages of CVD encompassing skin changes and CVU [43-46], as well as in the wound fluid microenvironment [47,48].
Studies have highlighted that chronic wound fluid contained up to tenfold increased levels of gelatinase MMP [49-52], suggesting a high tissue turnover in ulcers and an imbalance of MMP/TIMP ratio; moreover, the inhibition of MMP activity by TIMP in ECM (by fibroblast and probably also glycosaminoglycans) may cause impaired ability to reorganize the ECM in chronic wounds leading to delayed healing [5,29,53-58]. These studies should indicate that although there is significantly increased MMP proteolytic activity in the venous ulcer and wound fluid, both cellular and extracellular components may be compensating by altering their expression of MMP and TIMP [5,54,59].
The altered MMP/TIMP balance may be crucial in CVD initiation and progression to CVI and finally CVU, due to the increased secretion of proteolytic enzymes from vascular cells and inflammatory cells (like macrophages, neutro-phils and mast cells) [4,39,60,61]. The abnormalities in structure and healing processes seen in inflammatory and lipodermatosclerotic skin have also been attributed to MMP-mediated pathophysiology [62]; in fact, in lipodermatosclerotic and chronically inflamed skin, which are precursors to venous ulcer formation there is an unrestrained MMP activity [45,63], an imbalance of MMP/TIMP ratio [46,48] and an excessive ECM turnover [18-20], with an altered glycosaminoglycan accumulation/ degradation in vein wall [13,14,64,65].
For all these reasons, MMPs have been directly implicated in the pathophysiology of many arterial and venous disorders (in particular in CVD) and remain an important potential therapeutic target [66], even though more studies are needed to further demonstrate the clinical benefits of MMP modulating agents [67].
Future perspective in therapeutic interventions
Several pharmacological therapies and surgical strategies are being utilized in the management of varicose veins and CVU, with variable success and recurrence rates, and have been recently reviewed [1,3-5,7,8,68].
Although the causal and temporal sequences of events occurring in the development and progression of CVD (as well as suggested also by the diversity of signs and symptoms during the variable rates of disease evolution) have not been fully ascertained, the emerging themes of disturbed venous-flow patterns, chronic inflammation and in situ proteolysis may underlie all the clinical manifestations of the CVD (Figure 1), leading nevertheless to a blunted perception of cause and effect among clinicians [3].
In this respect, early treatments aimed at preventing venous hypertension and reflux, as well as treatments focused to inhibit the proinflam-matory process, could offer an even larger opportunity to prevent future morbidity, alleviating symptoms of CVD and reducing the risk of CVI and ulcers, both of which reduce the quality of life and are expensive to treat. Rapidly advancing understanding of both cellular and bio-molecular mechanisms involved in CVD initiation and progression has allowed the identification of new targets for possible future pharma-cologic intervention, which are mainly related to the cellular and biomolecular aspects of disease, like collagen type I and III, transforming growth factor β1, basic fibroblast growth factor, vascular endothelial growth factor, cytokines, mast and neutrophils cells, fibroblast, integrin CD11b, iron, ICAM1, L-selectin, TIMP, and MMPs [3]. Even though currently and future available drugs/agents deserve more detailed studies, it therefore seems reasonable to speculate that the treatment of such targets could reduce the risk of ulcers if administered early in the course of CVD, especially inhibiting inflammation and preventing disease-related complications [4].
A proactive approach to the treatment of the early and late stages of CVD may be focused on the inflammation-related and MMP-dependent proteolysis [59]. In fact, actually the inhibition of MMPs may represent a realistic, novel and possible therapeutic intervention to limit the progression of varicose vein to CVI and leg ulcera-tion [5]. The therapeutic hypothesis is based on the well known role of glycosaminoglycans (especially dermatan sulfate) in health and disease, in wound healing and vein remodeling [13,69,70]. In particular, it has been found that the amounts of collagen and dermatan sulfate were higher in the saphenous vein than in the mammary artery [17], suggesting also the involvement of vessel glycosaminoglycans in the process of atherosclerosis [18]. Moreover, it has been found that the most abundant glyco-saminoglycan in human veins is dermatan sulfate whereas chondroitin 4/6-sulfate is preponderant in arteries [20], and that normal and varicose saphenous veins differed in their glyco-saminoglycan contents [19].
Given the importance of GAG in vessel biology and disease, it is noteworthy to highlight the role of GAG (in particular dermatan sulfate) in the proteinases activity regulation [65,71], especially for MMP in fibroblasts dermal explants [72] and TIMP-3 [73]. We have recently studied the in vitro effects on MMP circulating in blood treated with the glycosaminoglycan sulodexide [74], to evaluate the possible modulation of MMP activity/secretion by the highly purified glycosaminoglycan sulodexide, a drug useful in venous ulcer treatment [75-77]. Sulodexide is a highly purified mixture of glycosaminoglycans composed of 80% low molecular weight heparin and 20% dermatan sulfate, which exhibits anti-thrombotic and pro-fibrinolytic properties. Sulodexide main pharmacologic effects are characterized by a prolonged half-life and involving effects on global coagulation (e.g., increasing the bleeding parameters INR, PTT, and bleeding time) [78].
Sulodexide decreases blood viscosity and enhances fibrinolytic and lipolytic activities, which is useful in vascular-related pathologies of an aging population (e.g., diabetic nephropathy, peripheral vascular diseases, and post-thrombotic syndrome) [79,80].
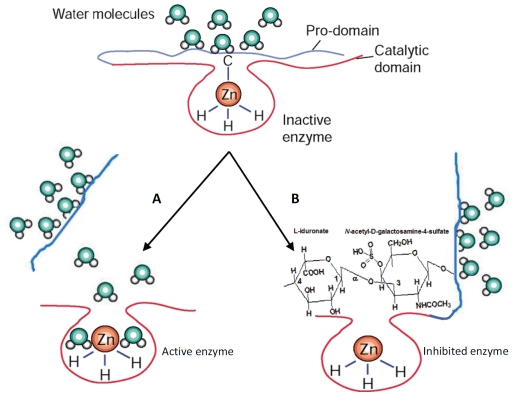
Studying the proteolytic activity, enzyme levels, and active secretion of both MMP-2 and MMP-9 in peripheral blood collected from healthy subjects, as well as in cultured leukemia cells, we found that increasing amounts of sulodexide may modulate both proenzyme and complexed forms of MMP-9, with significant decrease of MMP-9 secretion from white blood cells in a dose-dependent fashion[74], without any displacement of the MMP prodomains, indicating that sulodexide possible mechanism is by directly inhibiting the active zinc binding site of the proMMP-9 molecule (Figure 2). Our suggested model of sulodexide-induced MMP-9 inhibition is in agreement with the well-known mechanism of inhibition of MMP latent enzyme, which can also be brought about by several agents, such as synthetic collagen peptidomimetic compounds, and tetracycline derivative [81,82], which inhibit the enzyme activity by stabilizing the conformation of the gelatinase B protein where the cysteine is spatially displaced from the active site zinc atom without loss of pro-domain and change of molecular weight. Understanding the mechanism of reduced release of MMP-9 forms from leukocytes and inhibition of proteolytic activity due to sulodexide treatment may provide novel therapeutic applications in chronic inflammatory diseases (e.g., chronic venous diseases associated with MMP activation in blood and limbs), according to the well-established clinical effects, but not the bio-molecular mechanisms of sulodexide in the treatment of venous diseases and leg venous ulcers [75,77,80], and the established involvement of MMP-9 in venous tissue remodeling and pathology [59,5].
Figure 2.
Scheme of MMP-9 modulation mechanisms. A) The classical chemical or proteolytic multistep removal of pro-domain, resulting in gelatinase B activated form with lower molecular weight. In the zymogen enzyme, the sulfhydryl group of the cysteine (-C) present in the pro-domain is coordinated to the zinc (Zn) atom of the catalytic site in a manner that covers the active site and renders the enzyme latent. Note that the zinc atom is bound by three histidine molecules (-H), which is characteristic of the conserved zinc-binding motif in the catalytic site of MMPs. The cysteine residue of the pro-domain is fully dissociated on removal of the zinc atom by EDTA (a divalent ion chelator) and ortho-phenanthroline (an inhibitor of MMPs). It is also released by proteolytic loss of pro-domain after autolytic activation, or activation by trypsin or other proteinases. Finally, the enzyme lacking the pro-domain results in fully activated gelatinase B form with lower molecular weight. B) In our hypothetical model of MMP-9 inhibition induced by the dermatan sulphate sulodexide, the pro-domain is only opened and not detached (e.g., via hinge-like opening), allowing the interaction between hydrated form of the dermatan sulfate and the zinc atom at the catalytic site. By this interaction, sulodexide stabilizes gelatinase B conformation and inhibits the enzyme activity without loss of the pro-domain.
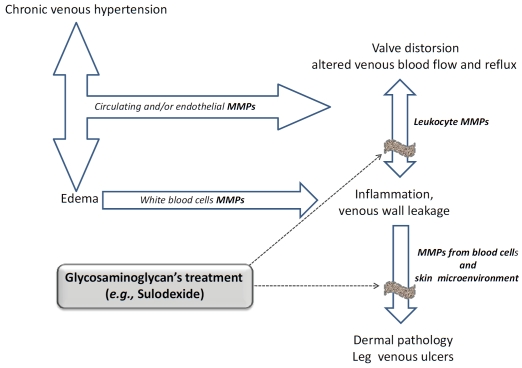
Although the possible mechanism(s) of the reduction/inhibition of MMP-9 release from blood cells through sulodexide treatment is not actually known, pharmacological agents that could attenuate MMP release from leukocytes and into the venous diseases microenvironment may offer a further opportunity to limit the progression of vein pathology, preventing future morbidity (Figure 3). In fact, previous evidence showed that sulodexide, administered topically as a local treatment in addition to compression bandaging [77], was associated with more frequent and faster venous ulcer healing without clarifying the mechanism of sulodexide-dependent ulcer healing.
Figure 3.
Risk factors for Chronic Venous Disease: involvement of Matrix Metalloproteinases and possible therapeutic application of glycosaminoglycans (e.g., Sulodexide).
Based on our in vitro preclinical results [74], and clinical studies that are actually in progress (Raffetto and Mannello, personal communications), we are investigating which component of sulodexide is responsible for the best MMP-modulation, in order to set the basis for future randomized controlled studies evaluating the safety and efficacy of new formulations in patients with venous diseases. These studies will improve the understanding of venous patho-physiology by allowing the following: 1) translating the research into clinical medicine; 2) elucidate the sulodexide-dependent mechanism of MMP-9 release/inhibition; 3) provide therapeutic benefit in reducing the symptoms of chronic venous disease, a worldwide pathology with high economic implication that accounts for 3-5 % of the national health expenditures in many western countries [59,5,3].
Finally, although these studies deserve more detailed investigations, in the long term the improved understanding of the cellular and bio-molecular mechanisms involved in CVD and CVU and the clinical evaluation of possible agents as sulodexide that attenuate crucial inflammatory-linked MMP proteolysis, could enhance the translational research to slow or prevent disease progression from the earliest stages, improving the quality of life and reducing expenditure of intractable ulcer treatment.
References
- 1.Meissner MH, Gloviczki P, Bergan J, Kistner RL, Morrison N, Pannier F, Pappas PJ, Rabe E, Raju S, Villavicencio JL. Primary chronic venous disorders. J Vasc Surg. 2007;46(Suppl S):54S–67S. doi: 10.1016/j.jvs.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 2.Raffetto JD, Khalil RA. Mechanisms of varicose vein formation: valve dysfunction and wall dilation. Phlebology. 2008;23(2):85–98. doi: 10.1258/phleb.2007.007027. [DOI] [PubMed] [Google Scholar]
- 3.Bergan JJ, Schmid-Schonbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355(5):488–498. doi: 10.1056/NEJMra055289. [DOI] [PubMed] [Google Scholar]
- 4.Nicolaides AN. Chronic venous disease and the leukocyte-endothelium interaction: from symptoms to ulceration. Angiology. 2005;56(Suppl 1):S11–S19. doi: 10.1177/00033197050560i103. [DOI] [PubMed] [Google Scholar]
- 5.Raffetto JD. Dermal pathology, cellular biology, and inflammation in chronic venous disease. Thromb Res. 2009;123(Suppl 4):S66–S71. doi: 10.1016/S0049-3848(09)70147-1. [DOI] [PubMed] [Google Scholar]
- 6.Moore K, Huddleston E, Stacey MC, Harding KG. Venous leg ulcers - the search for a prognostic indicator. Int Wound J. 2007;4(2):163–172. doi: 10.1111/j.1742-481X.2007.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meissner MH, Eklof B, Smith PC, Dalsing MC, DePalma RG, Gloviczki P, Moneta G, Neglen P, O’ DT, Partsch H, Raju S. Secondary chronic venous disorders. J Vasc Surg. 2007;46(Suppl S):68S–83S. doi: 10.1016/j.jvs.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 8.Fiebig A, Krusche P, Wolf A, Krawczak M, Timm B, Nikolaus S, Frings N, Schreiber S. Heritability of chronic venous disease. Hum Genet. 2010;127(6):669–674. doi: 10.1007/s00439-010-0812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wali MA, Eid RA. Intimal changes in varicose veins: an ultrastructural study. J Smooth Muscle Res. 2002;38(3):63–74. doi: 10.1540/jsmr.38.63. [DOI] [PubMed] [Google Scholar]
- 10.Wali MA, Eid RA. Changes of elastic and collagen fibers in varicose veins. Int Angiol. 2002;21(4):337–343. [PubMed] [Google Scholar]
- 11.Wali MA, Dewan M, Eid RA. Histopa-thological changes in the wall of varicose veins. Int Angiol. 2003;22(2):188–193. [PubMed] [Google Scholar]
- 12.Porto LC, Ferreira MA, Costa AM, da Silveira PR. Immunolabeling of type IV collagen, laminin, and alpha-smooth muscle actin cells in the intima of normal and varicose saphenous veins. Angiology. 1998;49(5):391–398. doi: 10.1177/000331979804900508. [DOI] [PubMed] [Google Scholar]
- 13.Tovar AM, de Mattos DA, Stelling MP, Sarcinelli-Luz BS, Nazareth RA, Mourao PA. Dermatan sulfate is the predominant anti-thrombotic glycosaminoglycan in vessel walls: implications for a possible physiological function of heparin cofactor II. Biochim Biophys Acta. 2005;1740(1):45–53. doi: 10.1016/j.bbadis.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 14.He L, Giri TK, Vicente CP, Tollefsen DM. Vascular dermatan sulfate regulates the anti-thrombotic activity of heparin cofactor II. Blood. 2008;111(8):4118–4125. doi: 10.1182/blood-2007-12-127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yla-Herttuala S, Sumuvuori H, Karkola K, Mottonen M, Nikkari T. Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest. 1986;54(4):402–407. [PubMed] [Google Scholar]
- 16.Merrilees MJ, Beaumont B, Scott LJ. Comparison of deposits of versican, biglycan and decorin in saphenous vein and internal thoracic, radial and coronary arteries: correlation to patency. Coron Artery Dis. 2001;12(1):7–16. doi: 10.1097/00019501-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Sisto T, Yla-Herttuala S, Luoma J, Riekkinen H, Nikkari T. Biochemical composition of human internal mammary artery and saphenous vein. J Vasc Surg. 1990;11(3):418–422. doi: 10.1067/mva.1990.17248. [DOI] [PubMed] [Google Scholar]
- 18.Marquezini MV, Strunz CM, Dallan LA, Toledo OM. Glycosaminoglycan distribution in atherosclerotic saphenous vein grafts. Cardiology. 1995;86(2):143–146. doi: 10.1159/000176860. [DOI] [PubMed] [Google Scholar]
- 19.Wolanska M, Sobolewski K, Glowinski S, Kowalewski R, Plonski A. Glycosaminoglycans of normal veins and their alterations in varicose veins and varicose veins complicated by thrombophlebitis. Eur Surg Res. 2001;33(1):28–32. doi: 10.1159/000049689. [DOI] [PubMed] [Google Scholar]
- 20.Leta GC, Mourao PA, Tovar AM. Human venous and arterial glycosaminoglycans have similar affinity for plasma low-density lipoproteins. Biochim Biophys Acta. 2002;1586(3):243–253. doi: 10.1016/s0925-4439(01)00102-8. [DOI] [PubMed] [Google Scholar]
- 21.Sansilvestri-Morel P, Nonotte I, Fournet-Bourguignon MP, Rupin A, Fabiani JN, Verbeuren TJ, Vanhoutte PM. Abnormal deposition of extracellular matrix proteins by cultured smooth muscle cells from human varicose veins. J Vasc Res. 1998;35(2):115–123. doi: 10.1159/000025573. [DOI] [PubMed] [Google Scholar]
- 22.Sansilvestri-Morel P, Rupin A, Badier-Commander C, Kern P, Fabiani JN, Verbeuren TJ, Vanhoutte PM. Imbalance in the synthesis of collagen type I and collagen type III in smooth muscle cells derived from human varicose veins. J Vasc Res. 2001;38(6):560–568. doi: 10.1159/000051092. [DOI] [PubMed] [Google Scholar]
- 23.Sansilvestri-Morel P, Rupin A, Badier-Commander C, Fabiani JN, Verbeuren TJ. Chronic venous insufficiency: dysregulation of collagen synthesis. Angiology. 2003;54(Suppl 1):S13–S18. doi: 10.1177/0003319703054001S03. [DOI] [PubMed] [Google Scholar]
- 24.Takase S, Pascarella L, Lerond L, Bergan JJ, Schmid-Schonbein GW. Venous hypertension, inflammation and valve remodeling. Eur J Vasc Endovasc Surg. 2004;28(5):484–493. doi: 10.1016/j.ejvs.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Kirsch D, Schreiber J, Dienes HP, Bottger T, Junginger T. Alterations of the extracellular matrix of venous walls in varicous veins. Vasa. 1999;28(2):95–99. doi: 10.1024/0301-1526.28.2.95. [DOI] [PubMed] [Google Scholar]
- 26.Mannello F, Jung K, Tonti GA, Canestrari F. Heparin affects matrix metalloproteinases and tissue inhibitors of metalloproteinases circulating in peripheral blood. Clin Biochem. 2008;41(18):1466–1473. doi: 10.1016/j.clinbiochem.2008.09.104. [DOI] [PubMed] [Google Scholar]
- 27.Mannello F. Serum or plasma samples? The “Cinderella” role of blood collection procedures: preanalytical methodological issues influence the release and activity of circulating matrix metalloproteinases and their tissue inhibitors, hampering diagnostic trueness and leading to misinterpretation. Arterioscler Thromb Vasc Biol. 2008;28(4):611–614. doi: 10.1161/ATVBAHA.107.159608. [DOI] [PubMed] [Google Scholar]
- 28.Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6(6):480–498. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 29.Moali C, Hulmes DJ. Extracellular and cell surface proteases in wound healing: new players are still emerging. Eur J Dermatol. 2009;19(6):552–564. doi: 10.1684/ejd.2009.0770. [DOI] [PubMed] [Google Scholar]
- 30.Mannello F, Gazzanelli G. Tissue inhibitors of metalloproteinases and programmed cell death: conundrums, controversies and potential implications. Apoptosis. 2001;6(6):479–482. doi: 10.1023/a:1012493808790. [DOI] [PubMed] [Google Scholar]
- 31.Mannello F, Luchetti F, Falcieri E, Papa S. Multiple roles of matrix metalloproteinases during apoptosis. Apoptosis. 2005;10(1):19–24. doi: 10.1007/s10495-005-6058-7. [DOI] [PubMed] [Google Scholar]
- 32.Murphy G, Nagase H. Progress in matrix metalloproteinase research. Mol Aspects Med. 2008;29(5):290–308. doi: 10.1016/j.mam.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knauper V, Murphy G. Methods for studying activation of matrix metalloproteinases. Methods Mol Biol. 2010;622:233–243. doi: 10.1007/978-1-60327-299-5_14. [DOI] [PubMed] [Google Scholar]
- 34.Lim CS, Davies AH. Pathogenesis of primary varicose veins. Br J Surg. 2009;96(11):1231–1242. doi: 10.1002/bjs.6798. [DOI] [PubMed] [Google Scholar]
- 35.Papazafiropoulou A, Tentolouris N. Matrix metalloproteinases and cardiovascular diseases. Hippokratia. 2009;13(2):76–82. [PMC free article] [PubMed] [Google Scholar]
- 36.Simka M. Cellular and molecular mechanisms of venous leg ulcers development–the “puzzle” theory. Int Angiol. 2010;29(1):1–19. [PubMed] [Google Scholar]
- 37.Tarlton JF, Bailey AJ, Crawford E, Jones D, Moore K, Harding KD. Prognostic value of markers of collagen remodeling in venous ulcers. Wound Repair Regen. 1999;7(5):347–355. doi: 10.1046/j.1524-475x.1999.00347.x. [DOI] [PubMed] [Google Scholar]
- 38.Raffetto JD, Khalil RA. Matrix metalloproteinases in venous tissue remodeling and varicose vein formation. Curr Vasc Pharmacol. 2008;6(3):158–172. doi: 10.2174/157016108784911957. [DOI] [PubMed] [Google Scholar]
- 39.Jacob MP, Badier-Commander C, Fontaine V, Benazzoug Y, Feldman L, Michel JB. Extracellular matrix remodeling in the vascular wall. Pathol Biol (Paris) 2001;49(4):326–332. doi: 10.1016/s0369-8114(01)00151-1. [DOI] [PubMed] [Google Scholar]
- 40.Soini Y, Satta J, Maatta M, utio-Harmainen H. Expression of MMP2, MMP9, MT1-MMP, TIMP1, and TIMP2 mRNA in valvular lesions of the heart. J Pathol. 2001;194(2):225–231. doi: 10.1002/path.850. [DOI] [PubMed] [Google Scholar]
- 41.Raffetto JD, Barros YV, Wells AK, Khalil RA. MMP-2 induced vein relaxation via inhibition of [Ca2+]e-dependent mechanisms of venous smooth muscle contraction. Role of RGD peptides. J Surg Res. 2010;159(2):755–764. doi: 10.1016/j.jss.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Badier-Commander C, Verbeuren T, Lebard C, Michel JB, Jacob MP. Increased TIMP/ MMP ratio in varicose veins: a possible explanation for extracellular matrix accumulation. J Pathol. 2000;192(1):105–112. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH670>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 43.Peschen M. Cytokines in progressing stages of chronic venous insufficiency. Curr Probl Dermatol. 1999;27:13–19. doi: 10.1159/000060631. [DOI] [PubMed] [Google Scholar]
- 44.Saito S, Trovato MJ, You R, Lal BK, Fasehun F, Padberg FT, Jr, Hobson RW, Duran WN, Pappas PJ. Role of matrix metalloproteinases 1, 2, and 9 and tissue inhibitor of matrix metalloproteinase- 1 in chronic venous insufficiency. J Vasc Surg. 2001;34(5):930–938. doi: 10.1067/mva.2001.119503. [DOI] [PubMed] [Google Scholar]
- 45.Zamboni P, Scapoli G, Lanzara V, Izzo M, Fortini P, Legnaro R, Palazzo A, Tognazzo S, Gemmati D. Serum iron and matrix metalloproteinase- 9 variations in limbs affected by chronic venous disease and venous leg ulcers. Dermatol Surg. 2005;31(6):644–649. doi: 10.1111/j.1524-4725.2005.31611. [DOI] [PubMed] [Google Scholar]
- 46.Mwaura B, Mahendran B, Hynes N, Defreitas D, Avalos G, Adegbola T, Adham M, Connolly CE, Sultan S. The impact of differential expression of extracellular matrix metalloproteinase inducer, matrix metalloproteinase-2, tissue inhibitor of matrix metalloproteinase-2 and PDGF-AA on the chronicity of venous leg ulcers. Eur J Vasc Endovasc Surg. 2006;31(3):306–310. doi: 10.1016/j.ejvs.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Ulrich D, Lichtenegger F, Unglaub F, Smeets R, Pallua N. Effect of chronic wound exudates and MMP-2/-9 inhibitor on angiogenesis in vitro. Plast Reconstr Surg. 2005;116(2):539–545. doi: 10.1097/01.prs.0000173447.81513.7a. [DOI] [PubMed] [Google Scholar]
- 48.Subramaniam K, Pech CM, Stacey MC, Wallace HJ. Induction of MMP-1, MMP-3 and TIMP-1 in normal dermal fibroblasts by chronic venous leg ulcer wound fluid*. Int Wound J. 2008;5(1):79–86. doi: 10.1111/j.1742-481X.2007.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol. 1996;107(5):743–748. doi: 10.1111/1523-1747.ep12365637. [DOI] [PubMed] [Google Scholar]
- 50.Baker EA, Leaper DJ. Proteinases, their inhibitors, and cytokine profiles in acute wound fluid. Wound Repair Regen. 2000;8(5):392–398. doi: 10.1111/j.1524-475x.2000.00392.x. [DOI] [PubMed] [Google Scholar]
- 51.Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP-1 to TIMP-1 is a predictor of wound healing. Diabet Med. 2008;25(4):419–426. doi: 10.1111/j.1464-5491.2008.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rayment EA, Upton Z, Shooter GK. Increased matrix metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol. 2008;158(5):951–961. doi: 10.1111/j.1365-2133.2008.08462.x. [DOI] [PubMed] [Google Scholar]
- 53.Cook H, Davies KJ, Harding KG, Thomas DW. Defective extracellular matrix reorganization by chronic wound fibroblasts is associated with alterations in TIMP-1, TIMP-2, and MMP-2 activity. J Invest Dermatol. 2000;115(2):225–233. doi: 10.1046/j.1523-1747.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- 54.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17(2):153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, Min D, Bolton T, Nube V, Twigg SM, Yue DK, McLennan SV. Increased matrix metalloproteinase- 9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care. 2009;32(1):117–119. doi: 10.2337/dc08-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toriseva M, Kahari VM. Proteinases in cutaneous wound healing. Cell Mol Life Sci. 2009;66(2):203–224. doi: 10.1007/s00018-008-8388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mirastschijski U, Schnabel R, Claes J, Schneider W, Agren MS, Haaksma C, Tomasek JJ. Matrix metalloproteinase inhibition delays wound healing and blocks the latent transforming growth factor-beta1-promoted myofibroblast formation and function. Wound Repair Regen. 2010;18(2):223–234. doi: 10.1111/j.1524-475X.2010.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Utz ER, Elster EA, Tadaki DK, Gage F, Perdue PW, Forsberg JA, Stojadinovic A, Hawksworth JS, Brown TS. Metalloproteinase expression is associated with traumatic wound failure. J Surg Res. 2010;159(2):633–639. doi: 10.1016/j.jss.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 59.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75(2):346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacob SS, Shastry P, Sudhakaran PR. Monocyte-macrophage differentiation in vitro: modulation by extracellular matrix protein substratum. Mol Cell Biochem. 2002;233(1-2):9–17. doi: 10.1023/a:1015593232347. [DOI] [PubMed] [Google Scholar]
- 61.Johnson JL, Jackson CL, Angelini GD, George SJ. Activation of matrix-degrading metalloproteinases by mast cell proteases in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 1998;18(11):1707–1715. doi: 10.1161/01.atv.18.11.1707. [DOI] [PubMed] [Google Scholar]
- 62.Herouy Y, Nockowski P, Schopf E, Norgauer J. Lipodermatosclerosis and the significance of proteolytic remodeling in the pathogenesis of venous ulceration (Review) Int J Mol Med. 1999;3(5):511–515. doi: 10.3892/ijmm.3.5.511. [DOI] [PubMed] [Google Scholar]
- 63.Zamboni P. The big idea: iron-dependent inflammation in venous disease and proposed parallels in multiple sclerosis. J R Soc Med. 2006;99(11):589–593. doi: 10.1258/jrsm.99.11.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kowalewski R, Sobolewski K, Malkowski A, Wolanska M, Gacko M. Evaluation of enzymes involved in proteoglycan degradation in the wall of abdominal aortic aneurysms. J Vasc Res. 2006;43(1):95–100. doi: 10.1159/000089790. [DOI] [PubMed] [Google Scholar]
- 65.Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26(8):587–596. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sluijter JP, de Kleijn DP, Pasterkamp G. Vascular remodeling and protease inhibition– bench to bedside. Cardiovasc Res. 2006;69(3):595–603. doi: 10.1016/j.cardiores.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 67.Lim CS, Shalhoub J, Gohel MS, Shepherd AC, Davies AH. Matrix metalloproteinases in vascular disease–a potential therapeutic target? Curr Vasc Pharmacol. 2010;8(1):75–85. doi: 10.2174/157016110790226697. [DOI] [PubMed] [Google Scholar]
- 68.Hobeika MJ, Thompson RW, Muhs BE, Brooks PC, Gagne PJ. Matrix metalloproteinases in peripheral vascular disease. J Vasc Surg. 2007;45(4):849–857. doi: 10.1016/j.jvs.2006.09.066. [DOI] [PubMed] [Google Scholar]
- 69.Trowbridge JM, Gallo RL. Dermatan sulfate: new functions from an old glycosaminoglycan. Glycobiology. 2002;12(9):117R–125R. doi: 10.1093/glycob/cwf066. [DOI] [PubMed] [Google Scholar]
- 70.Malavaki C, Mizumoto S, Karamanos N, Sugahara K. Recent advances in the structural study of functional chondroitin sulfate and dermatan sulfate in health and disease. Connect Tissue Res. 2008;49(3):133–139. doi: 10.1080/03008200802148546. [DOI] [PubMed] [Google Scholar]
- 71.Tersariol IL, Pimenta DC, Chagas JR, Almeida PC. Proteinase activity regulation by glycosaminoglycans. Braz J Med Biol Res. 2002;35(2):135–144. doi: 10.1590/s0100-879x2002000200001. [DOI] [PubMed] [Google Scholar]
- 72.Isnard N, Robert L, Renard G. Effect of sulfated GAGs on the expression and activation of MMP-2 and MMP-9 in corneal and dermal explant cultures. Cell Biol Int. 2003;27(9):779–784. doi: 10.1016/s1065-6995(03)00167-7. [DOI] [PubMed] [Google Scholar]
- 73.Yu WH, Yu S, Meng Q, Brew K, Woessner JF., Jr TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J Biol Chem. 2000;275(40):31226–31232. doi: 10.1074/jbc.M000907200. [DOI] [PubMed] [Google Scholar]
- 74.Mannello F, Tonti GA, Medda V, Raffetto JD. In vitro inhibition of MMP-9 forms by glycosaminoglycan Sulodexide. A new direction for therapeutic applications involving chronic inflammatory conditions. Clin Biochem. 2010 in press. [Google Scholar]
- 75.Scondotto G, Aloisi D, Ferrari P, Martini L. Treatment of venous leg ulcers with sulodexide. Angiology. 1999;50(11):883–889. doi: 10.1177/000331979905001102. [DOI] [PubMed] [Google Scholar]
- 76.Coccheri S, Scondotto G, Agnelli G, Palazzini E, Zamboni V. Sulodexide in the treatment of intermittent claudication. Results of a randomized, double-blind, multicentre, placebocontrolled study. Eur Heart J. 2002;23(13):1057–1065. doi: 10.1053/euhj.2001.3033. [DOI] [PubMed] [Google Scholar]
- 77.Coccheri S, Scondotto G, Agnelli G, Aloisi D, Palazzini E, Zamboni V. Randomised, double blind, multicentre, placebo controlled study of sulodexide in the treatment of venous leg ulcers. Thromb Haemost. 2002;87(6):947–952. [PubMed] [Google Scholar]
- 78.Ofosu FA. Pharmacological actions of sulodexide. Semin Thromb Hemost. 1998;24(2):127–138. doi: 10.1055/s-2007-995831. [DOI] [PubMed] [Google Scholar]
- 79.Lauver DA, Booth EA, White AJ, Poradosu E, Lucchesi BR. Sulodexide attenuates myocardial ischemia/reperfusion injury and the deposition of C-reactive protein in areas of infarction without affecting hemostasis. J Pharmacol Exp Ther. 2005;312(2):794–800. doi: 10.1124/jpet.104.075283. [DOI] [PubMed] [Google Scholar]
- 80.Lauver DA, Lucchesi BR. Sulodexide: a renewed interest in this glycosaminoglycan. Cardiovasc Drug Rev. 2006;24(3-4):214–226. doi: 10.1111/j.1527-3466.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- 81.Mannello F, Tonti G, Papa S. Matrix metalloproteinase inhibitors as anticancer therapeutics. Curr Cancer Drug Targets. 2005;5(4):285–298. doi: 10.2174/1568009054064615. [DOI] [PubMed] [Google Scholar]
- 82.Mannello F. Natural bio-drugs as matrix metalloproteinase inhibitors: new perspectives on the horizon? Recent Pat Anticancer Drug Discov. 2006;1(1):91–103. doi: 10.2174/157489206775246421. [DOI] [PubMed] [Google Scholar]