Abstract
Background: Reducing the excessive accumulation of amyloid β-protein (Aβ) in Alzheimer's disease (AD) is a key objective of most AD therapies. Several studies suggest that pharmacological inhibition of angiotensin-converting enzyme (ACE) or its by-product angiotensin II may delay onset or progression of dementia and it has been suggested that this occurs via regulation of Aβ. Intraneuronal oligomeric accumulation of Aβ is postulated to be one of the earliest pathological events. Thus this study investigated the effect of an ACE-inhibitor, captopril, and two angiotensin II receptor blockers (ARBs), eprosartan and valsartan, on intraneuronal Aβ pathology and oligomeric Aβ levels in a triple transgenic (3xTGAD) mouse model of AD. Methods: Male, adult (3-4 month old) 3xTgAD mice (n=39) were randomly assigned to 4 treatment groups: valsartan (0.17g/l), eprosartan (0.8g/l), captopril (5g/l) or normal drinking water and the drugs given ad libitum for 2 months. Mean arterial blood pressure (MABP) was measured at baseline, at 2 weeks and at 2 months when the mice were sacrificed and the brains hemisected for analysis. One hemisphere was processed for Aβ and amyloid precursor protein (APP) immunohistochemistry and the other for biochemical measurement of oligomeric Aβ and APP. ACE activity was measured in the brain and kidney. Results: MABP was significantly reduced at 2 weeks and 2 months in the ACE-I group (p=0.0006) but was unaltered in the ARB groups compared to vehicle. Neither ACE-I nor ARB treatment altered Aβ and APP immunolabelling or the level of Aβ or APP in brain tissue homogenates. Similarly neither ACE-I nor ARB treatment altered ACE activity in either brain or kidney compared to control tissue. Conclusions: ACE-I or ARB administration over 2 months did not affect APP levels or either intraneuronal Aβ or oligomeric Aβ levels in 3xTGAD mice. While ARBs did not alter MABP, captopril did mediate reductions in MABP in the 3xTGAD mice which appeared to be independent of ACE activity. Further studies are needed to examine the effects of these drugs over a longer term and in older mice (i.e. when AD-like changes are more pronounced).
Keywords: Angiotensin converting enzyme inhibitor, angiotensin-II receptor blocker, triple transgenic mouse model, Alzheimer's disease, amyloid-beta (Aβ)
Introduction
One of the key pathological hallmarks of Alzheimer's disease (AD) is the accumulation of amyloid β peptide (Aβ) which can be detected in the extracellular space, vasculature and within neurons. Intraneuronal accumulation of Aβ is suggested to be one of the earliest pathological events and has been shown to be associated with cognitive deficits and neurodegeneration (see [1] for review). Vascular risk factors are implicated in the aetiology of AD and hypertension in mid-life, in particular, they are associated with AD and cognitive decline (reviews [2-5]) In addition to epidemiological studies, neuropathological studies have also indicated that there is a link between hypertension and risk of AD, via the demonstration that hypertension increases accumulation
However, whilst several studies have indicated that hypertension may play an important role in development of cognitive decline or AD there is conflicting evidence from the study of anti-hypertensives. In some [6-12], but not all [13-14]), observational studies to date, antihyper-tensive drugs slowed the incidence or rate of cognitive decline in patients with mild cognitive impairment (MCI) or AD. The disparities in these studies may reflect methodological limitations such as small sample size and low statistical power [15-16]. Of the most promising findings, have been those associated with the use of the anti-hypertensives, angiotensin converting enzyme inhibitors (ACE-Is) or angiotensin receptor blockers (ARBs). ARBs, are associated with a lower incidence and rate of progression of AD and dementia than that seen in patients given ACE-Is or other anti-hypertensive medication [8]. Moreover, ARBs have also been reported to enhance cognitive function in normal people [17]. ARBs are newer, more expensive and less widely used than ACE-Is and as a result, pre-clinical and epidemiological data on their use and relevance in experimental models of dementia and in human subjects is currently limited. Nonetheless a recent trial involving 25,475 patients demonstrated a significant improvement in the mini-mental state examination score over a 6-month period of treatment with eprosartan [18]. Losartan also improved performance of immediate and delayed memory tasks in very elderly hypertensive patients [19]. These findings have prompted calls for further study of the utility of these drugs in preserving cognition or at least slowing the progression of dementia [8, 12, 20-22].
The mechanism by which anti-hypertensives may be beneficial is unclear and may be related to direct effects on lowering blood pressure and by improving blood flow, and there is evidence that they might have direct actions on Aβ accumulation. However these latter links are confounded by several in vitro, in vivo and ex vivo laboratory studies that demonstrate ACE promotes the degradation of Aβ [23-28] whereas ACE-I can result in an increase or accumulation of Aβ [24, 27]. These findings raise concern that long term use of ACE-Is may exacerbate accumulation of Aβ and perhaps accelerate cognitive decline [21, 27-29]. To date, there has been a lack of investigation of the long-term effects of ACE-I although one in vivo study has indicated that the ACE-I , captopril, has no effect on Aβ deposition in a mutant mouse model of AD [26].
The major vasotonic product of ACE activity, angiotensin II (AngII), is prevented from binding to its receptors by ARBs without altering ACE activity. ARB treatment has been shown to improve cognitive performance and reduce Aβ pathology in in vivo models of AD [27, 30-31] and while in animal studies valsartan reduced Aβ accumulation and improved cognitive performance in Tg2576 mice [27], telmisartan improved cognitive function in mice given in-tracerebral injections of Aβ1-40 [30], and olme-sartan improved cognitive and cerebrovascular function in mice that were given intracerebral injections of Aβ1-40 (actions independent of the antihypertensive activity of the drug) and reduced cerebrovascular dysfunction in young APP23 mice [32]. Thus, ARBs may be more beneficial as a treatment strategy, acting to reduce Aβ load as compared to ACE-I that may increase accumulation of Aβ.
In view of the links between anti-hypertensives and Aβ accumulation and potential disparities between their modes of action, the present study sought to compare the effects of an ACE-I (captopril) and ARBs (valsartan, eprosartan) on Aβ accumulation. Since recent evidence emphasises the importance of intraneuronal and oli-gomeric Aβ as a precursor of degeneration and cognitive decline, we examined the effects of these compounds in a 3xTGAD mouse model of AD at an age in which intraneuronal Aβ is present but extracellular amyloid deposition absent. The accumulation of intraneuronal Aβ is associated with cognitive deficits in this model [34] and thus alterations in intraneuronal Aβ as a result of ACE-I or ARBs may be indicative of cognitive alterations.
Methods
Animals
Adult (3-4 months old), male triple transgenic (3xTGAD; PS1m146vKI; Thy1.2-APPswe; Thy1.2-tauP301L) mice [33] were used in this study. The animals were group-housed under a 12-hour light/dark cycle with access to food and water ad libitum until the day of the experiment. Room temperature in the animal house was maintained at approximately 21 ± 2°C. Mice (n=39 total; all groups n=10, except Valsartan n=9) were randomly assigned to a treatment group prior to study commencement. Researchers were blinded to drug treatment at all times. All animal procedures were performed according to the Animal Scientific Procedures Act of 1986 under license from the United Kingdom Home Office.
Drug treatment
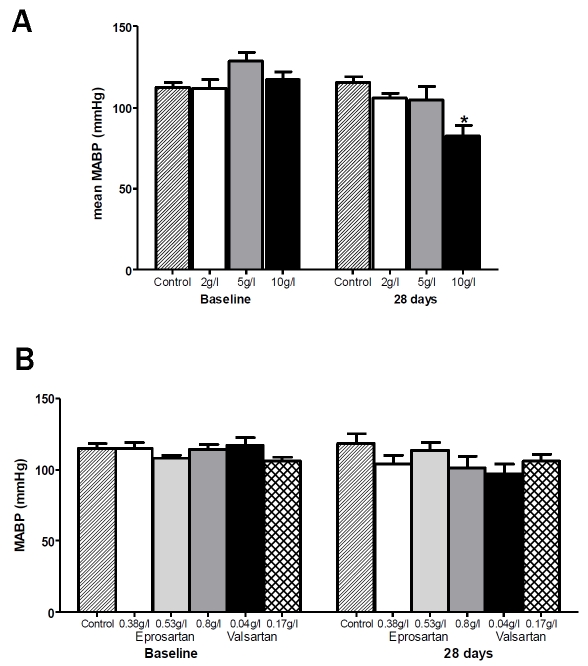
The drugs were administered in drinking water to avoid the stress of repeated injections over a 2-month period and to mimic the human situation. The ARBs used were eprosartan (0.8g/l) and valsartan (0.17g/l) and the ACE-I was cap-topril (5g/l) and a control group received drinking water only. The doses selected were informed by pilot studies on C57Bl/6J mice in which each of the drugs was administered in several different concentrations (captopril 2, 5 and 10g/l; eprosartan 0.3, 0.6 and 0.9g/l, valsartan 0.05 and 0.2g/l) and mean arterial blood pressure (MABP) monitored over 28 days. None of the final doses selected had any significant effect upon MABP (Figure 1) and so hypotension and vascular insufficiency were removed as potential confounding factors. The dose of eprosartan (eprosartan monosodium salt, Solvay pharmaceuticals) equates to ∼13mg/kg in humans, which is the maximum that may be prescribed in human patients (www.bnf.org). That of valsartan (valsartan monosodium salt, LGM Pharma) equates to approximately twice the average dose administered to human patients, and half the maximum prescribable dose and is comparable to previous studies in which doses of 10-40mg/kg inhibited Aβ accumulation in vitro and in vivo [27]. The dose of captopril (LGM Pharma) was approximately 30 times greater than the maximum dose normally prescribed for human patients.
Figure 1.
Effect of ACE-inhibitor and ARB upon MABP in C57Bl6/J mice. The data illustrate the range and maximal drug doses of, a) Captopril and b) Eprosartan and Valsartan, which were well tolerated but did not alter mean arterial blood pressure (MABP) over a period of 28 days.
The drugs used in this study are all weak acids, and are thus well absorbed in their non-ionised form from the stomach. To prepare the ARBs for administration in drinking water, they were first dissolved in 3-4ml sterile distilled water (dH2O) plus a small amount of 5M sodium hydroxide (NaOH) titrated to the amount of drug being used. This solution was vigorously vortexed until the drug was completely dissolved, then added to the appropriate volume of sterile dH2O with continuous stirring. The final solution was further neutralized to pH 7.0 by addition of 5M NaOH. Captopril was prepared by adding the drug to the appropriate volume of sterile dH2O and neutralizingto pH 7.0 by the addition of 5M NaOH with continuous stirring. All drinking solutions were freshly prepared twice each week, the containers wrapped in aluminium foil to avoid potential photochemical changes and kept at room temperature to avoid potential precipitation from solution. Fluid consumption was monitored twice weekly throughout the study.
Blood pressure measurement
MABP was monitored non-invasively with a tail sphygmomanometer (Panlab, LE5002). MABP was measured at baseline, prior to the start of drug delivery, 2wk after drug delivery had begun and immediately before termination of the experiment (at 2mo). Ten measurements were taken each day and the average for each mouse determined from the last 5 measurements.
Mice were habituated to the procedure for 5d before each measurement day to minimise stress and anxiety. Visual inspection of the pulse trace was carried out to ensure that movement artefacts had not occurred during the measurement; if these were detected the measurement was discarded. Any measurement that coincided with a heart rate of over 700 beats per minute (bpm) was discarded as this is indicative of excessive stress in the animal [34].
Tissue preparation
After 2mo the animals were anaesthetised with isoflurane and sacrificed by transcardial perfu-sion with cold saline. Brains were harvested and hemisected midsagittally. One hemisphere was snap frozen in liquid nitrogen for biochemical analyses, the other fixed in 4% paraformalde-hyde for 48h for histology and immunohisto-chemistry. From each mouse, a kidney was isolated and snap-frozen for subsequent analysis of ACE activity.
ACE activity assay
ACE activity was determined using the fluoro-genic substrate (Abz-FRK(Dnp)-P; BIOMOL). Brain tissue or kidney was homogenised in lysis buffer with Biospec 2.3mm Zirconia/Silica beads using a PrecellyS automatic homogeniser for 2 × 15s at 6000 rpm. The homogenates were then spun for 15min at 13000 rpm in a centrifuge refrigerated to 4°C. The protein concentration of each sample was measured using Total Protein kit (Sigma). This value was used to calculate the volume of each sample to be used subsequently in the ACE activity assay, in which the protein concentration in each sample was standardised to 50ug/ml total protein. Samples were diluted in HEPES buffer (595mg in 50ml dH2O; pH 6.5) and 50μl of each sample was incubated for 18h at 26°C with Abz-FRK(Dnp)-P. Triplicates of each sample were incubated with 10ug (100mM) captopril (Biomol International, Exeter, UK) which inhibits the reaction by over 90% [35]. The fluorescence of Abz-FRK(Dnp)-P was measured with excitation at 320nm and emission at 405nm in a 96-well plate using a FLUOstar OPTIMA plate reader, and the difference between captopril-inhibited and non-inhibited samples used to calculate ACE activity. ACE activity was expressed in arbitrary units. Treatment groups were compared by 1-way ANOVA, with significance set at P<0.05.
Immunohistochemistry
The fixed brain tissue was sliced coronally at 3mm intervals and the slices embedded in paraffin wax. Sections 6μm in thickness were collected onto Superfrost slides and dried on a hotplate. The sections were dewaxed and hy-drated through a series of graded alcohols and washed in phosphate-buffered saline (PBS). Endogenous peroxidase activity was blocked by incubation in 3% hydrogen peroxide in methanol for 30min. For labelling of Aβ, antigen retrieval was carried out by covering the sections in 80% formic acid for 10min. Antigen retrieval for APP was carried out by boiling the sections in citric acid buffer twice for 5min. Sections were blocked in normal horse serum for 1h at room temperature then incubated overnight with the anti-Aβ antibody 4G8 (Millipore) or anti-APP (Chemicon Clone 22C11), both diluted 1:1000, at 4°C. Sections were washed in PBS, incubated with anti-mouse biotinylated secondary antibody (1:100) for 1h at room temperature, washed and bound antibody visualised by the avidin/ biotin method (ABC Elite kit, Vector laboratories) with diaminobenzidine (DAB) as the chromogen.
In 3xTGAD mice, aged 5-6mo, Aβ is detectable intraneuronally but at this age there is no extracellular deposition of Aβ. To quantify intra-neuronal Aβ and APP, three non-overlapping images of the CA1 field of the hippocampus and three from somatosensory cortex were captured from each immunostained section [×40 magnification] and the percentage area of section labelled for Aβ or APP was determined using ImageJ software. Four separate sets of measurements were made for each animal in each region of interest and the mean for each region calculated. Treatment groups were compared by 1-way ANOVA with significance set at P<0.05.
Tissue homogenisation
Brains were homogenized in 10 volumes (wt:vol) of tissue homogenization buffer (THB; 250mM sucrose, 20mM Tris base, 1mM EDTA, 1mM EGTA). The homogenate was mixed 1:1 with 0.4% diethanolamine buffer (DEA; 200μl DEA, 1ml 5M NaCl, ddH2O to 50ml) and centri-fuged at 135,000xg at 4°C for 1h. The supernatant was saved as the soluble fraction. The insoluble pellet was re-suspended in cold formic acid, briefly sonicated and spun at 135,000xg at 4°C for 1h. The resulting supernatant was diluted (210μl:4ml of formic acid neutralization buffer, comprising 50.57g Tris base, 35.5g Na2HPO4, 2.5ml 10% NaN3, H2O to 500ml) and saved as the insoluble fraction.
Quantification of full length APP
Soluble proteins were extracted from brain tissue as described. 10μgwas loaded onto a Tris-bis 4-12% gel and the proteins separated by electrophoresis at 150V for 1.5h. Each of 3 gels contained samples from the control group and samples from an individual drug treatment group, to avoid intergel comparisons. Proteins were transferred to a PVDF membrane and probed with MAB348 (Millipore) to detect full length APP and GAPDH antibody (Abcam) to assess protein loading. Bands were visualised with the appropriate Odyssey infrared secondary antibodies (IRDye; LiCor) and band intensity measured using Odyssey Infrared Imaging System (LiCor). APP measurements were adjusted for (the slight) variations in GAPDH band intensity. Each treatment group was compared to control using Student's t test with significance set at P>0.05 and the mean for control and treatment group was calculated for each membrane and visualised in a histogram.
Levels of oligomeric Aβ
The levels of oligomeric Aβ were measured in the soluble fraction by dot blot analysis with the A11 antibody. 2ug of soluble protein was loaded in duplicate into the 96-well manifold of the bio-dot microfiltration apparatus (Bio-rad) below which a pre-wetted nitrocellulose membrane had been placed. Samples were rapidly filtered through the membrane, which was removed and placed into blocking buffer (Odyssey) for 1h at room temperature with constant agitation. Membranes were incubated overnight with A11 antibody at 4°C with constant agitation. After they had been washed, membranes were incubated with infrared secondary antibodies (IRDye; LiCor) for 45min at room temperature and scanned using Odyssey infrared Imaging System (LiCor). Relative optical density was measured and the mean for each sample calculated. Treatment groups were compared by 1-way ANOVA with Dunn's post hoc multiple comparison test and significance set at P<0.05.
Statistical analysis
Statistical tests were performed with GraphPad Prism, version 5 (GraphPad Software, La Jolla, CA). Changes in MABP were analysed by repeated measures ANOVA. ACE activity and Aβ and APP immunolabellingwere analysed by oneway ANOVA with Dunn's test for post hoc comparisons between experimental and control groups. Statistical significance was set at P<0.05 for all analyses.
Results
Mean arterial blood pressure
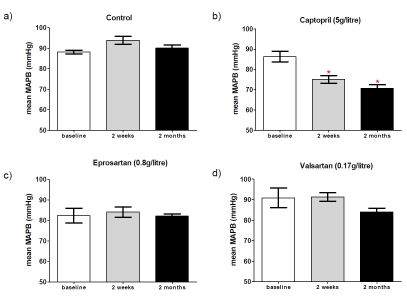
Mean arterial blood pressure (MABP) measurements at 2wk and 2mo after starting treatment were compared with that at baseline (Figure 2). There was no significant change in MABP in controls, mice given eprosartan (0.8g/l of drinking water) or valsartan (0.17g/l). However captopril (5g/l) induced a significant reduction in MABP in 3xTGAD mice at 2wk and 2mo after the commencement of treatment (p=0.0006, Figure 2).
Figure 2.
Mean arterial blood pressure at baseline and following 2 weeks and 2 months of treatment with either (a) drinking water only (control), (b) captopril (5g/litre), (c) eprosar-tan (0.8g/litre) or (d) valsartan (0.17g/litre). * indicates significantly different from baseline; data were analysed using repeated measures 1-way ANOVA with Dunn's post-hoc for multiple comparisons. Significance was set at p<0.05.
ACE activity
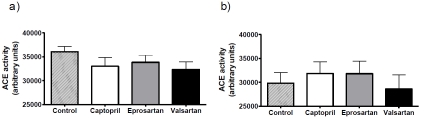
ACE activity was assessed in homogenates of both brain and kidney in all groups to determine the effects of treatment on central and peripheral ACE activity (Figure 3). ACE activity tended to be higher in the brain than the kidney. No significant effect of drug treatment on ACE activity was detected in either brain or kidney, this despite the clear physiological effect of captopril on blood pressure.
Figure 3.
ACE inhibitor and ARB treatment does not affect ACE activity in the 3xTGAD mouse. 3xTGAD mice were treated with captopril (5g/litre), eprosartan (0.8g/litre), valsartan (0.17g/litre) and drinking water for 2 months. ACE activity was determined in brain (a) and kidney (b) tissue using a fluorogenic substrate for Angiotensin converting enzyme; ACE activity is expressed in arbitrary units.
Immunohistochemistry
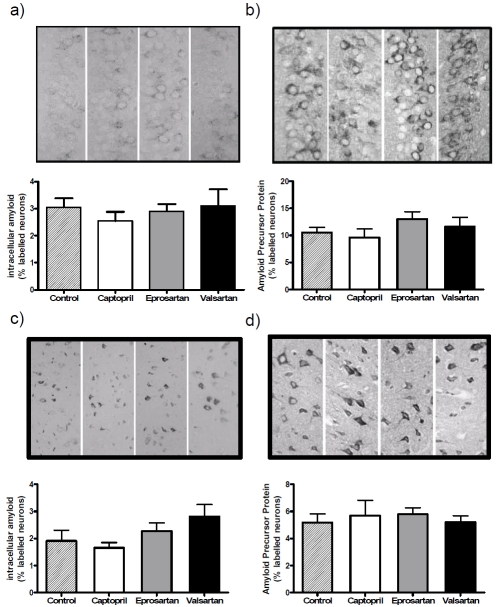
Intraneuronal Aβ and APP were detected in the hippocampus and cortex in the control treated group and drug-treated groups (Figure 4). No extracellular Aβ deposition was seen in any of the groups. Quantification of intraneuronal Aβ immunolabelling revealed only slight variation between the groups in the area of the CA1 field of the hippocampus which was immunopositive for Aβ, and the differences were not significant.
Figure 4.
ACE inhibitor and ARB treatment does not affect intraneuronal levels of amyloid or amyloid precursor protein (APP) in the 3xTGAD mouse. 3xTGAD mice were treated with captopril (5g/litre), eprosartan (0.8g/litre), valsartan (0.17g/litre) and drinking water for 2 months. Brain sections were stained for amyloid (a,c) and APP (b,d) and visualised in hippocampus CA1 (a, b) and somatosenory cortex (c, d). The percentage area stained was determined using computer-assisted image analysis (ImageJ).
There was greater variation in the cortex, where the labelling between groups varied by as much as 50%, being lowest in captopril-treated mice and highest in those given valsartan, but once again these differences did not reach statistical significance. APP labelling varied only slightly (and not significantly) between the groups in both the hippocampus and the cortex.
Levels of APP and oligomeric Aβ
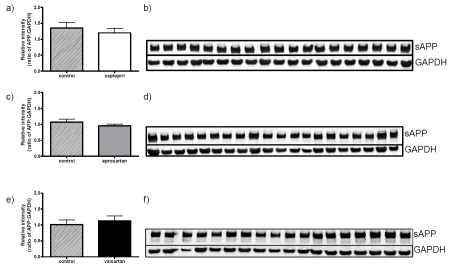
The total protein levels of full-length APP were determined in Western blots prepared from brain tissue homogenates of all control and drug-treated mice. The protein levels of APP did not differ signficantly between any of the drug exposure groups and controls (Figure 5).
Figure 5.
ACE inhibitor and ARB treatment does not affect the protein levels of full-length amyloid precursor protein (APP) in the 3xTGAD mouse. 3xTGAD mice were treated with captopril (5g/litre), eprosartan (0.8g/litre), valsartan (0.17g/litre) and drinking water for 2 months. Soluble proteins were isolated from brain tissue samples as described and expression of APP was examined in 10μg of protein by western blot analysis. Each drug treatment group was randomly loaded onto a single gel alongside samples from the control group [(a) captopril, (c) eprosartan and (e) valsartan]; representative images are shown from each treatment group [(b) captopril, (d) eprosartan and (f) valsartan]. The membranes were probed with anti-APP antibody and GAPDH, and the relative intensity normalised to GAPDH for each gel. Data were analysed using Student's t test with significance set at P>0.05.
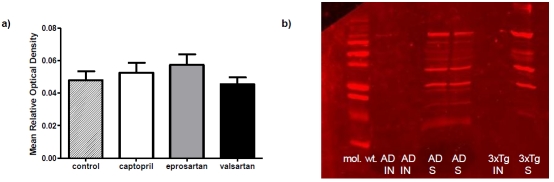
Oligomeric Aβ could be detected by the antibody A11 as shown in Western blots of the soluble and not insoluble fraction in both 3xTGAD mice and AD patients (Figure 6). Subsequently the levels of total oligomeric Aβ species were compared between the control and drug-treated groups by dot blot analysis. There were no significant differences in the levels of oligomeric Aβ between the controls and any of the treatment groups.
Figure 6.
ACE-inhibitor and ARB treatment does not affect oligomeric Aβ species (A11) in young 3xTGAD mice treated with captopril (5g/litre), eprosartan (0.8g/litre), valsartan (0.17g/litre) and drinking water for 2 months. Levels of oligomeric Aβ were assessed by dot blot but there was no statistical difference in the levels of oligomeric Aβ determined between the drug-treated and control groups (a) . Western blot analysis of Aβ as detected by A11 antibody showing presence of multiple oligomeric species in soluble (S) fractions, but not insoluble (IN) fractions, prepared from brain homogenates of Alzheimer disease (AD) cases and 3xTGAD mice (b).
Discussion
Reduction in blood pressure and improvements in cerebral blood flow have been the most commonly suggested mechanisms to explain observations that anti-hypertensive treatments may be beneficial in AD, although there has been some suggestion of a more direct action on Aβ accumulation. However, conflicting indications from pre-clinical studies suggest that ACE-Is, by inhibiting ACE, may have an adverse effect on Aβ [23, 25-26, 36-37]. This study investigated the potential impact of ACE-Is and ARBs on in-traneuronal Aβ and oligomeric levels of Aβ in the 3xTGAD mouse model of AD.
Present findings in relation to previous research
Intraneuronal Aβ and APP were detected in all groups of mice, consistent with other reports on animals of the same age (5-6mo) in this trans-genic model [33] and at this age there was no evidence of extracellular Aβ deposition in any of the groups at 6mo. Thus we were able to address whether anti-hypertensives had an effect on early intraneuronal and oligomeric Aβ. Our data robustly demonstrate that treatment over 2 months with an ACE-I, captopril, or two ARBs, valsartan and eprosartan; have no significant effect on the levels of soluble APP (sAPP), intraneuronal oligomeric Aβ or oligomeric Aβ levels. These observations partly agree with those of Hemming and colleagues [38] who also found no differences in levels of soluble and insoluble Aβ (total), Aβ40 and Aβ42 in captopril or losar-tan treated 3xTGAD mice as well as in an older captopril treated J20 transgenic mice. In contrast, perindopril, another reported brain-penetrating ACE-I, improved cognitive deficits in Aβ25-35-injected mice [39] and Aβ1-40-injected Sprague Dawley rats [24]. In both of these studies reductions in brain ACE activity were also reported after short treatment periods but the reductions did not approach the abolition of ACE activity reported by Hemming and colleagues [38].
In other studies using ARBs, Wang and colleagues found diminished Aβ pathology in Tg2576 mice treated with valsartan for 5mo which coincided with better cognitive performance [27]. Takeda and colleagues also showed that a short course of olmesartan (4-5 weeks) ameliorated cerebrovascular dysfunction and oxidative stress in young APP23 mice but did not coincide with any changes to soluble or insoluble levels of Aβ1-40 and Aβ1-42. Similarly effects were also found by the same group in an Aβ1-40-injected mouse model [32].
Levels of brain and kidney ACE activity were comparable in this study in contrast to the findings of Hemming and colleagues [38] who used slightly younger (3mo) 3xTGAD mice which were treated for 28d with 2g/l of captopril and a different ARB (losartan at 0.6g/l). While the brain ACE activity data were similar between the studies, in that no differences were found across groups of animals, our kidney measurements differed in not showing a detectable reduction in ACE activity in captopril treated mice, despite a clear hypotensive effect. [38]. The ARBs used in both studies had no effects on ACE activity, although the losartan used by Hemming et al showed a modest reduction in ACE activity which we did not find for either valsartan or ep-rosartan.
In contrast to other studies we attempted to disentangle possible anti-hypertensive and other unknown drug specific effects on the disease phenotype by selecting from pilot studies the maximal drug doses that were well tolerated but did not alter mean arterial blood pressure (MABP) in otherwise normotensive wild-type mice. However the same doses applied to 3xTGAD mice evoked a significant progressive and sustained reduction (19%) in MABP (p=0.0006) in the captopril treated group although not in the valsartan or eprosartan groups. No blood pressure measurements were reported by Hemming and colleagues for the 3xTGAD mice [38]. The absence of any MABP-lowering effects from valsartan in our 3xTGADs is consistent with the observations of Wang et al on Tg2576 mice, in which valsartan (10 or 40mg/kg/d) ameliorated Aβ pathology and improved spatial memory deficits [27].
Possible explanations for the differences
Noteworthy in the current study was the lack of extracellular Aβ deposition in contrast to some reports in 3xTGAD mice in the age-range studied [33], and the lack of captopril-mediated reductions in ACE activity in the kidney [38]. Studies of other ACE-Is in other murine [39] and rodent [24] models documented reductions in brain ACE activity which neither this nor another study [38] of 3xTGAD found. The studies on ACE in 3xTGAD mice differed in the methods used. Hemming and colleagues [38] administered 2g/l of captopril for 28d in contrast to our 5g/l for 2mo. ACE activity was also measured differently in the studies. While both studies used detergent-free homogenates Hemming and colleagues (personal communication) used manual homogenisation whereas we used rapid, high-throughput machine-based homogenisation with ceramic beads, which may have resulted in differing yields in the final proportions of membrane-bound and soluble forms of ACE that were represented in the resulting homogenates. This could be particularly relevant to the kidney which is more fibrous and resilient. Moreover, the ACE substrates used also differed: Hemming and colleagues used a hippuryl-L-histidyl-L-leucine (His-His-Leu) ACE substrate in contrast to our use of the ACE-specific fluoro-genic peptide substrate Abz-FRK(Dnp)-P as in previous studies [35, 40]. However the results produced by the two substrates were reported to be closely correlated (r2=0.90) in parallel measurements of plasma ACE activity [41] although the HHL assay has been shown possibly to underestimate ACE activity and this may well be a tissue-specific effect [42]. These methodological differences and the differences in the ages of the animals at the time of assay may have been relevant. In addition, the Hemming et al 3xTGAD mouse had extracellular Aβ pathology which ours did not.
The preparation of the captopril drug could also have been a contributing factor to the lack of apparent kidney ACE activity disparity. However that significant and sustained reduction in MABP was observed for the captopril-treated mice, consistent with other studies using lower doses of captopril (2g/l) [38] suggests similar bioavailability and makes this an unlikely factor. The absence of reported blood pressure measurement data by Hemming and colleagues limits comparisons on this point. Animal strain differences, as well as drug-specific differences, are likely to be important in these observations, particularly as the same dose of captopril had no effect on MABP in our pilot experiments on C57Bl6/J mice and neither eprosartan nor valsartan altered MABP in the 3xTGAD mice.
Limitations of the present study
In this study a high dose of captopril was used: 30 times greater than the maximum dose normally prescribed for human patients, although only 2.5 fold higher than that used by Hemming and colleagues in 3xTGAD [38]. Although in our pilot studies this dose did not affect blood pressure in C57Bl6/J mice, it is clear that optimisation in 3xTGAD would have been advisable. Another limitation of the present study was that we did not assess cognitive function; Takeda et al [32] found that the ARB olmesartan improved spatial learning in APP23 mice despite a lack of change in Aβ level. In addition, the lack of change in brain ACE activity after systemic captopril administration does raise questions regarding the alleged BBB penetrability of captopril [10, 12]. The brain penetrating ACE-I perin-dopril was shown to reduce brain ACE activity in another study [39]. Finally it is worth considering that we focussed our study on the measurement of intraneuronal oligomeric Aβ and levels but arguably anti-hypertensives may exert beneficial effects via other mechanisms such as by improving cerebral blood flow and haemody-namics which would impact positively on vascular integrity.
Implications of the present findings
We found no evidence that ACE-Is had a detrimental effect on neuronal or total oligomeric Aβ or precipitated extracellular Aβ pathology in the 3xTGAD mice studied. This would suggest that if any of these compounds did exert an action on Aβ that at this age the Aβ was able to be metabolised or cleared efficiently. Data from other similar studies provide reassurance that ACE-Is may not increase AD pathology [38] and may improve cognitive and cerebrovascular function in people[27, 30-32].
Conclusions, including plans for future studies
The present study was limited to a short-term treatment strategy with different anti-hypertensives in a 3xTGAD model but longer-term treatments should be assessed to define in a number of models both familial and sporadic. Future studies should assess blood pressure, cerebrovascular function, cognitive performance and ACE activity in different cellular and tissue compartments, as well as a range of markers of AD pathology.
Acknowledgments
This work was funded in part through an Alzheimer's Research Trust (ART) Network Cooperation grant. Linda Ferrington is supported by a Royal Society of Edinburgh/Lloyds TSB Foundation for Scotland Personal Research Fellowship, Scott Miners by an ART Research Fellowship and Patrick Kehoe a senior research fellow funded from Sigmund Gestetner Foundation Endowment grant to the University of Bristol. The work was also supported by PhD scholarship funding to Laura Palmer from Bristol Research into Alzheimer's and Care of the Elderly (BRACE) and equipment grants from BRACE and ART.
References
- 1.Bayer TA, Wirths O. Intracellular accumulation of amyloid-Beta - a predictor for synaptic dysfunction and neuron loss in Alzheimer's disease. Front Aging Neurosci. 2010;2:8. doi: 10.3389/fnagi.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kehoe PG. The renin-angiotensin-aldosterone system and Alzheimer s disease? J Renin Angiotensin Aldosterone Syst. 2003;4:80–93. doi: 10.3317/jraas.2003.017. [DOI] [PubMed] [Google Scholar]
- 3.Poon IO. Effects of antihypertensive drug treatment on the risk of dementia and cognitive impairment. Pharmacotherapy. 2008;28:366–375. doi: 10.1592/phco.28.3.366. [DOI] [PubMed] [Google Scholar]
- 4.Skoog I, Gustafson D. Update on hypertension and Alzheimer's disease. Neurol Res. 2006;28:605–611. doi: 10.1179/016164106X130506. [DOI] [PubMed] [Google Scholar]
- 5.Wolozin B, Bednar MM. Interventions for heart disease and their effects on Alzheimer's disease. Neurol Res. 2006;28:630–636. doi: 10.1179/016164106X130515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aalten P, Verhey FR, Boziki M, Brugnolo A, Bullock R, Byrne EJ, Camus V, Caputo M, Collins D, De Deyn PP, Elina K, Frisoni G, Holmes C, Hurt C, Marriott A, Mecocci P, Nobili F, Ousset PJ, Reynish E, Salmon E, Tsolaki M, Vellas B, Robert PH. Consistency of neuropsychiatric syndromes across dementias: results from the European Alzheimer Disease Consortium. Part II. Dement GeriatrCogn Disord. 2008;25:1–8. doi: 10.1159/000111082. [DOI] [PubMed] [Google Scholar]
- 7.Hajjar IM, Keown M, Lewis P, Almor A. Angiotensin converting enzyme inhibitors and cognitive and functional decline in patients with Alzheimer's disease: an observational study. Am J Alzheimers Dis Other Demen. 2008;23:77–83. doi: 10.1177/1533317507309803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li NC, Lee A, Whitmer RA, Kivipelto M, Lawler E, Kazis LE, Wolozin B. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ. 2010;340:b5465. doi: 10.1136/bmj.b5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohrui T, Matsui T, Yamaya M, Arai H, Ebihara S, Maruyama M, Sasaki H. Angiotensin-converting enzyme inhibitors and incidence of Alzheimer's disease in Japan. J Am Geriatr Soc. 2004;52:649–650. doi: 10.1111/j.1532-5415.2004.52178_7.x. [DOI] [PubMed] [Google Scholar]
- 10.Ohrui T, Tomita N, Sato-Nakagawa T, Matsui T, Maruyama M, Niwa K, Arai H, Sasaki H. Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology. 2004;63:1324–1325. doi: 10.1212/01.wnl.0000140705.23869.e9. [DOI] [PubMed] [Google Scholar]
- 11.Rozzini L, Chilovi BV, Bertoletti E, Conti M, Del Rio I, Trabucchi M, Padovani A. Angiotensin converting enzyme (ACE) inhibitors modulate the rate of progression of amnestic mild cognitive impairment. Int J Geriatr Psychiatry. 2006;21:550–555. doi: 10.1002/gps.1523. [DOI] [PubMed] [Google Scholar]
- 12.Sink KM, Leng X, Williamson J, Kritchevsky SB, Yaffe K, Kuller L, Yasar S, Atkinson H, Robbins M, Psaty B, Goff DC Jr. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the cardiovascular health study. Arch Intern Med. 2009;169:1195–1202. doi: 10.1001/archinternmed.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khachaturian AS, Zandi PP, Lyketsos CG, Hayden KM, Skoog I, Norton MC, Tschanz JT, Mayer LS, Welsh-Bohmer KA, Breitner JC. Anti-hypertensive Medication Use and Incident Alzheimer Disease: The Cache County Study. Arch Neurol. 2006 doi: 10.1001/archneur.63.5.noc60013. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg PB, Mielke MM, Tschanz J, Cook L, Corcoran C, Hayden KM, Norton M, Rabins PV, Green RC, Welsh-Bohmer KA, Breitner JC, Munger R, Lyketsos CG. Effects of cardiovascular medications on rate of functional decline in Alzheimer disease. Am J Geriatr Psychiatry. 2008;16:883–892. doi: 10.1097/JGP.0b013e318181276a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feigin VL. Stroke epidemiology in the developing world. Lancet. 2005;365:2160–2161. doi: 10.1016/S0140-6736(05)66755-4. [DOI] [PubMed] [Google Scholar]
- 16.Pathak A, Hanon O, Negre-Pages L, Sevenier F. Rationale, design and methods of the OSCAR study: observational study on cognitive function and systolic blood pressure reduction in hypertensive patients. Fundam Clin Pharmacol. 2007;21:199–205. doi: 10.1111/j.1472-8206.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 17.Gard PR, Rusted JM. Angiotensin and Alzheimer's disease: therapeutic prospects. Expert Rev Neurother. 2004;4:87–96. doi: 10.1586/14737175.4.1.87. [DOI] [PubMed] [Google Scholar]
- 18.Hanon O, Berrou JP, Negre-Pages L, Goch JH, Nadhazi Z, Petrella R, Sedefdjian A, Sevenier F, Shlyakhto EV, Pathak A. Effects of hypertension therapy based on eprosartan on systolic arterial blood pressure and cognitive function: primary results of the Observational Study on Cognitive function And Systolic Blood Pressure Reduction open-label study. J Hypertens. 2008;26:1642–1650. doi: 10.1097/HJH.0b013e328301a280. [DOI] [PubMed] [Google Scholar]
- 19.Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, Waldman A, Walton I, Poulter R, Ma S, Comsa M, Burch L, Fletcher A, Bulpitt C. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008;7:683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 20.Kehoe PG. Angiotensins and Alzheimer's disease: a bench to bedside overview. Alzheimers Res Ther. 2009;1:3. doi: 10.1186/alzrt3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kehoe PG, Miners S, Love S. Angiotensins in Alzheimer's disease - friend or foe? Trends Neurosci. 2009;32:619–628. doi: 10.1016/j.tins.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Kehoe PG, Wilcock GK. Is inhibition of the renin-angiotensin system a new treatment option for Alzheimer's disease? Lancet Neurol. 2007;6:373–378. doi: 10.1016/S1474-4422(07)70077-7. [DOI] [PubMed] [Google Scholar]
- 23.Hemming ML, Selkoe DJ. Amyloid beta -protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J Biol Chem. 2005;280:37644–37650. doi: 10.1074/jbc.M508460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou DR, Wang Y, Zhou L, Chen K, Tian Y, Song Z, Bao J, Yang QD. Altered angiotensin-converting enzyme and its effects on the brain in a rat model of Alzheimer disease. Chin Med J (Engl) 2008;121:2320–2323. [PubMed] [Google Scholar]
- 25.Hu J, Igarashi A, Kamata M, Nakagawa H. Angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide (A beta); retards A beta aggregation, deposition, fibril formation; and inhibits cytotoxicity. J Biol Chem. 2001;276:47863–47868. doi: 10.1074/jbc.M104068200. [DOI] [PubMed] [Google Scholar]
- 26.Oba R, Igarashi A, Kamata M, Nagata K, Takano S, Nakagawa H. The N-terminal active centre of human angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide. EurJ Neurosci. 2005;21:733–740. doi: 10.1111/j.1460-9568.2005.03912.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Ho L, Chen L, Zhao Z, Zhao W, Qian X, Humala N, Seror I, Bartholomew S, Rosendorff C, Pasinetti GM. Valsartan lowers brain beta -amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J Clin Invest. 2007;117:3393–3402. doi: 10.1172/JCI31547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou K, Yamaguchi H, Akatsu H, Sakamoto T, Ko M, Mizoguchi K, Gong JS, Yu W, Yamamoto T, Kosaka K, Yanagisawa K, Michikawa M. Angiotensin-converting enzyme converts amyloid beta-protein 1-42 (Abeta(1-42)) to Abeta(1-40), and its inhibition enhances brain Abeta deposition. J Neurosci. 2007;27:8628–8635. doi: 10.1523/JNEUROSCI.1549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kehoe PG, Wilcock GK. The Renin Angiotensin System in Alzheimer's Disease - Updates Highlight a Clinical and Biological Dichotomy? Current Alzheimer Research. 2006;3:171–173. [Google Scholar]
- 30.Mogi M, Li JM, Tsukuda K, Iwanami J, Min LJ, Sakata A, Fujita T, Iwai M, Horiuchi M. Telmisartan prevented cognitive decline partly due to PPAR-gamma activation. Biochem Biophys Res Commun. 2008;375:446–449. doi: 10.1016/j.bbrc.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 31.Mogi M, Tsukuda K, Li JM, Iwanami J, Min LJ, Sakata A, Fujita T, Iwai M, Horiuchi M. Inhibition of cognitive decline in mice fed a high-salt and cholesterol diet by the angiotensin receptor blocker, olmesartan. Neuropharmacology. 2007;53:899–905. doi: 10.1016/j.neuropharm.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 32.Takeda S, Sato N, Takeuchi D, Kurinami H, Shinohara M, Niisato K, Kano M, Ogihara T, Rakugi H, Morishita R. Angiotensin receptor blocker prevented beta-amyloid-induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension. 2009;54:1345–1352. doi: 10.1161/HYPERTENSIONAHA.109.138586. [DOI] [PubMed] [Google Scholar]
- 33.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 34.Lorenz JN. A practical guide to evaluating cardiovascular, renal, and pulmonary function in mice. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1565–R1582. doi: 10.1152/ajpregu.00759.2001. [DOI] [PubMed] [Google Scholar]
- 35.Miners JS, Ashby E, Van Helmond Z, Chalmers KA, Palmer LE, Love S, Kehoe PG. Angio-tensin-converting enzyme (ACE) levels and activity in Alzheimer's disease, and relationship of perivascular ACE-1 to cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2008;34:181–193. doi: 10.1111/j.1365-2990.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- 36.Sun X, Becker M, Pankow K, Krause E, Ringling M, Beyermann M, Maul B, Walther T, Siems W-E. Catabolic attacks of membrane-bound angiotensin-converting enzyme on the N-terminal part of species-specific amyloid-[beta] peptides. European Journal of Pharmacology. 2008;588:18–25. doi: 10.1016/j.ejphar.2008.03.058. [DOI] [PubMed] [Google Scholar]
- 37.Toropygin IY, Kugaevskaya EV, Mirgorodskaya OA, Elisseeva YE, Kozmin YP, Popov IA, Nikolaev EN, Makarov AA, Kozin SA. The N-domain of angiotensin-converting enzyme specifically hydrolyzes the Arg-5-His-6 bond of Alzheimer's Abeta-(1-16) peptide and its isoAsp-7 analogue with different efficiency as evidenced by quantitative matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:231–239. doi: 10.1002/rcm.3357. [DOI] [PubMed] [Google Scholar]
- 38.Hemming ML, Selkoe DJ, Farris W. Effects of prolonged angiotensin-converting enzyme inhibitor treatment on amyloid beta-protein metabolism in mouse models of Alzheimer disease. Neurobiol Dis. 2007;26:273–281. doi: 10.1016/j.nbd.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada K, Uchida S, Takahashi S, Takayama M, Nagata Y, Suzuki N, Shirakura S, Kanda T. Effect of a centrally active angiotensin-converting enzyme inhibitor, perindopril, on cognitive performance in a mouse model of Alzheimer's disease. Brain Res. 2010;1352:176–186. doi: 10.1016/j.brainres.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Miners S, Ashby E, Baig S, Harrison R, Tayler H, Speedy E, Prince JA, Love S, Kehoe PG. Angiotensin-converting enzyme levels and activity in Alzheimer's disease: differences in brain and CSF ACE and association with ACE1 genotypes. Am J Transl Res. 2009;1:163–177. [PMC free article] [PubMed] [Google Scholar]
- 41.Alves MF, Araujo MC, Juliano MA, Oliveira EM, Krieger JE, Casarini DE, Juliano L, Carmona AK. A continuous fluorescent assay for the determination of plasma and tissue angiotensin I-converting enzyme activity. Brazilian Journal of Medical and Biological Research. 2005;38:8. doi: 10.1590/s0100-879x2005000600007. [DOI] [PubMed] [Google Scholar]
- 42.Oliveira EM, Santos RAS, Krieger JE. Standardization of a fluorimetric assay for the determination of tissue angiotensin-converting enzyme activity in rats. Brazilian Journal of Medical and Biological Research. 2000;33:755–764. doi: 10.1590/s0100-879x2000000700005. [DOI] [PubMed] [Google Scholar]