Abstract
Elevated blood glucose is a major component in metabolic syndrome and pre-diabetes, sometimes leading to type 2 diabetes mellitus (DM II). Additionally, it may lead to adipose deposits when left elevated for long periods. The epidemiology of DM II clearly shows that uncontrolled blood glucose levels leads to many adverse conditions including heart disease, retinal damage, renal failure, erectile dysfunction, and other significant medical conditions. Here we conducted a single-center, prospective, randomized, double-blinded, placebo-controlled, parallel-group- clinical trial of a nutraceutical supplement vs. placebo to measure its glucose lowering effect in generally healthy adults before and after a simple sugars meal. Subjects reported to the test clinic on multiple days to receive placebo or treatment, a simple sugars meal, as well as pre-and postprandial blood glucose measurement (modified oral glucose tolerance test). Each subject served as his or her own control and thirty-one subjects completed the trial with at least one oral glucose tolerance test (OGTT) with the nutraceutical supplement and placebo. Statistical analysis revealed the nutraceutical supplement significantly lowered postprandial glucose levels by 36% and 59% at 45 and 60 minutes, respectively (***P<.001). The study was limited by its composition of primarily overweight females. Future studies will be required over longer periods in more heterogeneous and larger groups to determine the long-term effect of this supplement on blood glucose levels in terms of prophylaxis or treatment for DM II.
Keywords: Glucose tolerance test, nutraceutical supplement, diet, hyperglycemia, diabetes mellitus II, insulin resistance
Introduction
Treatment for unregulated blood sugar levels in diabetics is generally complex. A controlled combination of diet restriction, intensive monitoring, medication, lifestyle modification, and exercise is necessary for most diabetics to maintain healthy blood glucose levels. In non-diabetics, diet, lifestyle modification and exercise are recommended for general maintenance of healthy blood glucose levels [1]. Drawbacks of standard treatments for unregulated blood sugar include time commitment, pain, expense, complexity, noncompliance, and ineffectiveness. Thus alternative methods for modulating blood glucose at the pre-diabetes level are necessary [1].
Current therapies in prevention and treatment of Type 2 diabetes (DM II) include diet and drugs targeting obesity, glucose-lowering medications (metformin, glyburide, acarbose), and more recently, insulin sensitizers such as the peroxisome proliferator-activated receptor (PPAR)-α and PPAR-γ agonists, and thiazolidie-nodiones (TZDs). Unfortunately, therapies involving existing drugs have limited efficacy or tolerability and can have significant side effects [2].
During recent years, some natural products have been reported to reduce hyperglycemia and lipid disorders in individuals with DM II. Vuksan et al reported that a single oral administration of ginseng supplement (containing 200-500 mg ginsenosides) reduced postprandial glycemia in healthy subjects and in individuals with DM II [3]. It has also been shown that blood glucose and insulin levels can also be influenced by other natural compounds [1, 4]; including chromium picolinate at levels between 200 and 1000 mcg [1, 4, 5]. A combination of zinc and chromium has been suggested to offer potential beneficial effects to individuals with diabetes, in terms of increased antioxidant status [6]. Zinc itself may also aid in glucose assimilation, due to an insulin-like effect, and has been shown to be deficient in individuals with DM II [7-9]. Cinnamon and cinnamon extracts have been suggested to reduce glucose levels, to induce insulin excretion, and to offer insulin-like effects, in human, animal and in vitro studies [10-13]. Additionally, cinnamon has been shown to support lower blood glucose levels [14] by delaying the gastric emptying rate [12, 13]. Recent evidence suggests that oral administration of transglucosidase (TG) can be useful for decreasing postprandial glucose concentrations [15].
The active ingredients in the treatment formulation (capsules) tested here are transglucosidase, lipase, protease (Glucoreductase™) blend, chromium picolinate, zinc gluconate, and cinnamon extract. The purpose of this formulation is to prevent the absorption of dietary sugars and to promote the movement of blood glucose into muscle and brain cells. The actions of the enzymes are to generate long-chained polymers from individual sugar molecules that then pass out of the gut.
An oral glucose tolerance test (OGTT) is recommended as a tool in the diagnosis of impaired glucose tolerance and diabetes. By World Health Organization (WHO) definition, subjects with impaired glucose tolerance have a blood glucose level between 7.8 and 11.1 mmol/L, 120 minutes after a 75 goral glucose load [16]. A modified OGTT that uses jellybeans is commonly used as an alternative to the traditional glucose beverage because there are fewer reported side effects [17].
The purpose of the current human clinical trial is to examine the benefits of a proprietary formulation of nutraceutical supplements designed to support healthy glucose levels and as an alternative or adjuvant therapy to support a variety of conditions in which insulin resistance may be a disease modifier.
Research design and methods
This clinical trial was reviewed and approved by Liberty Institutional Review Board (Deland, FL). It was a single-center, prospective, randomized, double-blinded, placebo-controlled, parallel-group-design clinical trial of the treatment formulation versus placebo in adult subjects. Participants were recruited by TV advertising. All participants in the study were able and willingto consent to clinical trial participation and were interested in assessing their serum glucose following a simple sugars challenge to better understand their serum glucose patterns. Informed consent was obtained. All subjects were deemed qualified for the study after evaluation for predetermined inclusion and exclusion criteria, which are listed in Table 1. These criteria are based upon recommendations made by the study physician, confounders identified by the biostatistician, or information identified in product ingredients’ research.
Table 1.
Inclusion and exclusion criteria
| Inclusion Criteria: |
|---|
| Men and women wanting to test their response to starch and simple sugars, with a Body Mass Index between 22 and 45 (BMI > 22 and < 45 m/kg2); |
| Women and men who wished to promote healthy blood glucose management; |
| Women and men who were 18 to 60 years of age, inclusive, at the Initial Visit; |
| Exclusion Criteria: |
| Were unwilling or unable to comply with any aspect of the clinical trial protocol; |
| Were allergic to or express problems with ingredients in the Enzymedica GR Plus™ product or placebo; |
| Who had lost or gained 10 or more pounds of body weight in the last 3 months; |
| Had severe co-morbid disease including cardiac, pulmonary, renal, hepatic, or active cancer; |
| Consumed alcohol at an elevated level (Defined as consumption of more than 10 standard alcoholic drinks per week. A standard alcoholic drink is defined as one bottle/can of beer (12 ounces) equals one glass of wine (4 ounces) equals one ounce of hard liquor). |
| Had uncontrolled diabetics (as defined by having a self-reported A1c greater than 8.0, use daily varying amounts of insulin, or fail the fasting blood glucose test: greater than 126 mg/dl); |
| Had uncontrolled hypertension: Systolic blood pressure (SBP) > 180 mm Hg or diastolic blood pressure (DBP) > 100 mm Hg, upon two of three repeated measures, if not on medications for hypertension. Systolic blood pressure (SBP) > 150 mm Hg or diastolic blood pressure (DBP) > 90 mm Hg, upon two of three repeated measures, if on medications for hypertension; |
| Had a surgery or a hospitalization within the past 3 months; |
| Had a recent cardiovascular event; |
| Had an acute illness (e.g., heavy cold, flu, flu-like symptoms) |
| Had elevated liver enzymes (as defined by SGOT levels 1.5 times the upper limit or CPK levels 2 times the upper limit) |
| Had a Body Mass Index (BMI) of less than 22 or greater than 45 m/kg2; |
| Had participated in a clinical trial in the past 4 weeks; |
| Took methadone, insulin, anticoagulants (blood thinners), daily narcotics, or similar medications; |
| The anticipated need for major surgery during the entire study; |
| Women who were nursing, pregnant, or actively trying to become pregnant; |
| Had any disease or condition that in the investigator's opinion compromises the integrity of the clinical trial or the safety of the subject; |
All subjects were surveyed for a variety of demographic factors. These subject population statistics can be found in Table 2. Key parameters to take into consideration are that the subject population consisted mainly of middle-aged, overweight, Caucasian females. Additionally, almost 9% of the subject population reported that they were diabetic.
Table 2.
Subject demographics
| Medical Conditions | Prevalence (%) | |
|---|---|---|
| Diabetes | 8.8 | |
| Hypertension | 35.3 | |
| Thyroid Dysfunction | 14.7 | |
| Asthma | 23.5 | |
| Heart Disease | 2.9 | |
| Gallbladder or gallstones | 17.6 | |
| Gastrointestinal Issues | 20.6 | |
| Reflux (GERD) | 26.5 | |
| Cholesterol Issues | 20.6 | |
| Cancer | 5.9 | |
| Arthritis | 35.3 | |
| Neurological Diseases | 2.9 | |
| Blood disorders | 2.9 | |
| Migraines | 20.6 | |
| Skin conditions | 20.6 | |
| Diet Restrictions (lactose intolerance, allergies) | 17.6 | |
| Allergies | 50 | |
| Severe Injuries (past 10 years) | 20.6 | |
| Surgeries (past 15 years) | 64.7 | |
| Other medical conditions | 17.6 | |
| On prescription medication | 55.9 | |
| Prevalence (%) | ||
| Female gender | 85.3 | |
| Caucasian | 94.1 | |
| Black | 2.9 | |
| Other | 2.9 | |
| Mean | Std. Dev | |
| Age | 45.2 yrs | 14.7 |
| Height | 65.6 inches | 2.9 |
| Weight | 215.1 lbs | 43.8 |
Subjects were asked to keep their diet and exercise as stable as possible throughout the trial. There was a two-week run-in period for the purpose of assessing normal existing patterns of diet and exercise, to test compliance, and to determine that each subject's fasting blood glucose levels did not exceed the limits defined by the predetermined inclusion/exclusion criteria.
The clinical trial flow chart can be found in Figure 1. Subjects were randomized into one of two groups. Randomization determined which of the two formulations (treatment or placebo) were administered first. Randomization was equal: Using permuted blocks of six, three subjects were assigned into each group. All subjects reported to the clinic after a 10-hour fast (water only). Blood glucose level was obtained using an OneTouch® Ultra® Meter. Test product was administered (treatment or placebo). Five minutes later, the testing meal was administered (40 g of jellybeans). Following the completion of the test meal (approx. 2-5 minutes), blood glucose was measured at 15, 30, 45 and 60-minute intervals.
Figure 1.
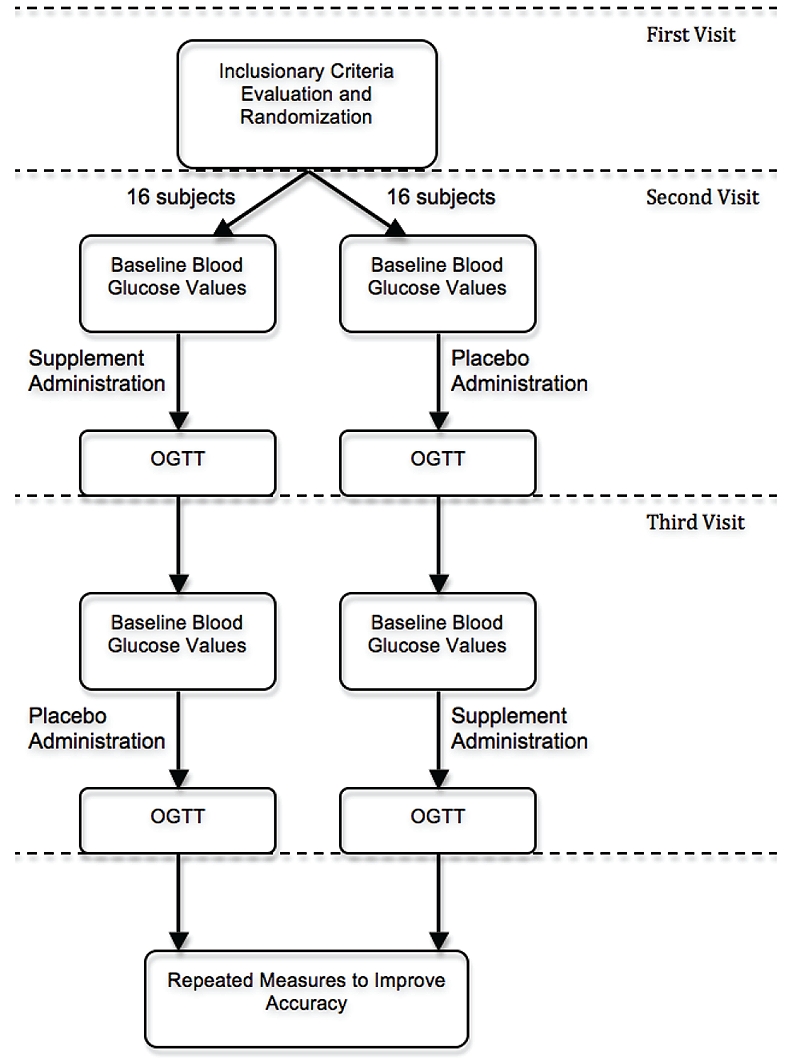
Clinical trial flow chart.
Statistical analysis
GraphPad Prism version 5.01 was used to create the graphs, calculate the area under the curve, and for statistical analysis (San Diego, CA). There were a total of 84 placebo runs and 56 treatment runs. In cases where multiple runs were taken for one subject, the mean blood glucose measurement at each time point was calculated and used for the final analysis. The mean change was calculated by subtracting the baseline measurement (before glucose challenge) from each additional blood glucose measurement. Each subject served as his or her own control. The multivariate analysis combined all the groups in an overall ANOVA (analysis of variants). The Greenhouse-Geisser statistic was used to test for sphericity, which allows the Tests of Within-Subjects Effects. Post hoc analysis consisted of student's two-tailed t-tests at each time point to assess statistical significance of the difference between the treatment and placebo groups. The criterion for rejection of the null hypothesis was P<0.05. Additionally, the area under the curve (AUC) for each subject was calculated and presented as mean ± SEM. A two-tailed t-test was used to assess the statistical significance.
Results
Dampened rise in blood glucose
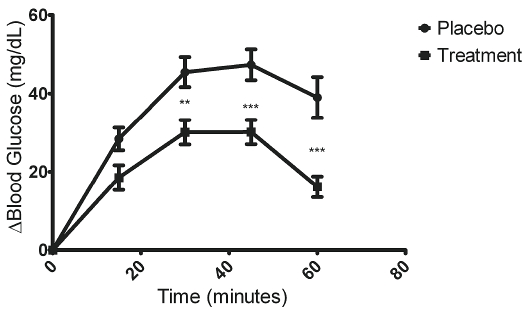
To assess the effectiveness of the supplement for reducing blood glucose following a simple sugars challenge, the change in blood glucose was calculated so that each subject served as his or her own control. Figure 2 illustrates the mean change in blood glucose measured at each time point ± SEM. The treatment not only dampened the rise in blood glucose, but it also promoted a faster return to the baseline levels. In fact, at t=60 minutes blood glucose levels for the treatment group had almost completely returned to the baseline levels (before the test meal). The placebo group at t=60 minutes had a mean blood glucose level that was higher than the maximum level reached by the treatment group. Thus, if the blood glucose had been monitored for a longer period of time, there would likely be an even larger difference between the treatment and placebo group. Interestingly, two of the subjects exhibited a mild transient drop in blood glucose from the baseline measurement during the treatment run. Within subject testing revealed that the data conform to a quadratic model (F=177.9). Post-hoc analysis of two-tailed t-tests at each time point revealed statistical significance (*P<.05, **P<.01, ***P<.001) at t=30, 45, and 60 minutes (Figure 2).
Figure 2.
Oral supplement dampens rise in blood glucose. To assess the effectiveness of the supplement for reducing blood glucose following a simple sugars challenge, the change in blood glucose was calculated so that each subject served as his or her own control. Within subject testing revealed that the data conform to a quadratic model (F=177.9). Data is presented as mean change ± SEM at each time point Post-hoc analysis of two-tailed t-tests at each time point revealed statistical significance (*P<.05, **P<.01, ***P<.001) att=30, 45, and 60 minutes.
Total blood glucose load
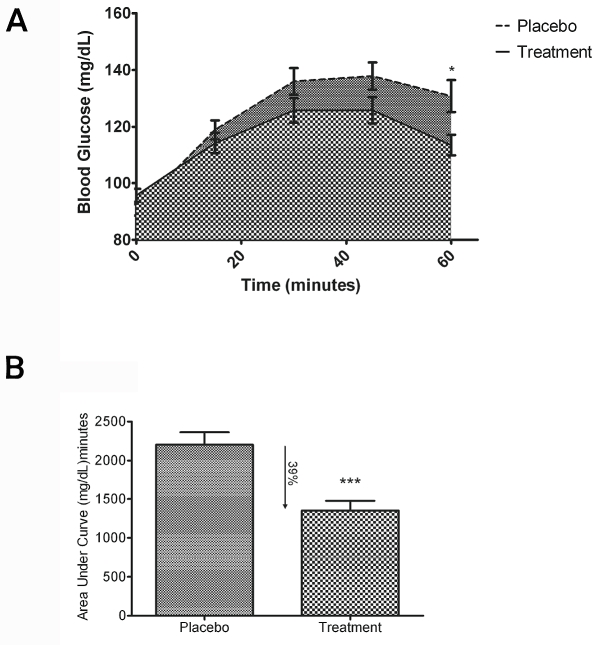
The blood glucose curves observed in this study were within the expected range as defined by the American Diabetes Association [18]. This was expected because the subject population consisted mostly of individuals without any type of hyperglycemic disorder (Table 2). Figure 3A lists the mean blood glucose levels (mg/dL) and standard error of the mean (SEM) for the two groups at all five time periods. Statistical significance was observed at t= 60 minutes (*P<.05).
Figure 3.
Oral supplement lowers total blood glucose load: (A) The mean blood glucose levels (mg/dL) and standard error of the mean (SEM) for placebo vs. supplement at time points from 0-60 min indicate a statistical significance at t= 60 minutes (*P<.05). (B) To better illustrate the observed decrease in blood glucose levels, the area under curve (AUC) values were calculated for each subject ± SEM. Administration of the supplement prior to the test meal resulted in a 39% reduction in the area under the curve (AUC) over the 60-minute test period (***P<.001).
Area under curve comparison
To better illustrate the observed decrease in blood glucose levels, the area under curve values were calculated for each subject. Figure 3B illustrates the mean area under the curve (AUC) for each group ± SEM. Administration of the supplement prior to the test meal resulted in a 39% reduction in the area under the curve (AUC) over the one-hour test period.
Discussion
The estimated cost of diabetes in the United States in 2007 was $174 billion [19]. The total number of people with diabetes is expected to more than double from 171 million in 2000, to 366 million in 2030 [20]. These projections illustrate the importance of diabetes prevention. Recent studies have suggested that nutraceuticals might play a very important role in diabetes care and prevention [2, 21]. Additionally, a preventative nutraceutical targeting patients unwilling or unable to make necessary lifestyle changes to prevent the onset of type 2 diabetes could be very important [2].
This preliminary clinical evaluation indicates that using a proprietary blend of several active ingredients can effectively lower blood glucose levels following a simple sugars challenge. A 39% reduction of the change in blood glucose over the first hour following the test meal is significant and supports the effectiveness of the supplement tested. This supplement might not only be useful for the promotion of glucose tolerance and insulin sensitivity, but also as a potential preventative measure for those at-risk for diabetes or metabolic syndrome. As seen in the Table 2, many of the participants in this clinical trial exhibited symptoms (hyperglycemia, B.M.I. >30, etc.) that might qualify them as “at risk” for hyperglycemic disorders. Therefore, this study was conducted on people who could benefit from the inclusion of the test supplement in their daily regimen.
Although there is evidence to support the use of each ingredient in this supplement, there is also conflicting evidence. Some researchers have reported that cinnamon is ineffective at reducing blood glucose levels [22], while others report that cinnamon is effective at reducing blood glucose levels [23]. It is important for future studies to be mechanism-based in order to better elucidate the bioactivity and possible synergies of the ingredients. Additionally, because two of the participants in this study exhibited a mild drop in blood glucose after consumption of the nutraceutical supplement, further studies should evaluate the risk of hypoglycemia. However, there were no instances in this study that the drop in blood glucose was large enough to put the subject at risk for hypoglycemia.
Potential limitations include the intake of different carbohydrates, the interaction of carbohydrates, fats, and proteins during the same meal, the timing and amounts of the supplement ingested, as well as the overall health of the individual. Additionally, the generalizability is limited as the study was focused mainly on overweight females. Another limitation to this clinical trial is that commercially available jellybeans were used. We assumed that these jellybeans were reasonably consistent in composition. Jellybeans were used instead of the traditional glucose drink because there have been fewer reported side effects with the jellybeans and a comparable blood glucose response [17]. Further testing will help define some of these potential limitations.
The clinical trial documented a statistically significant reduction of blood glucose levels in the subjects who had received the supplement prior to the test meal. This supports the use of this nutraceutical supplement for promoting healthy levels of blood glucose. The proprietary formulation represents a potential alternative or adjuvant therapy for people with a broad range of hyperglycemic disorders. The ingredients have a long history of human consumption and are well tolerated. Individuals with various conditions that include obesity, insulin-resistance, diabetes, and others could benefit from this product.
Acknowledgments
Enzymedica Inc. of Port Charlotte, FL funded this study.
Author disclosure statement
Dr. Blum was funded by Enzymedica to carry out this double blind clinical evaluation of their formulation.
References
- 1.Balch JF, Balch PA. Garden City Park, N.Y.: Avery Pub. Group; 1997. Prescription for nutritional healing. [Google Scholar]
- 2.McCarty MF. Nutraceutical resources for diabetes prevention–an update. Med Hypotheses. 2005;64:151–158. doi: 10.1016/j.mehy.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 3.Vuksan V, Sievenpiper JL, Koo VY, Francis T, Beljan-Zdravkovic U, Xu Z, Vidgen E. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med. 2000;160:1009–1013. doi: 10.1001/archinte.160.7.1009. [DOI] [PubMed] [Google Scholar]
- 4.Roberts AJ, O'Brien ME, Subak-Sharpe GJ. New York: Perigee Book; 2001. Nutraceuticals : the complete encyclopedia of supplements, herbs, vitamins, and healing foods. [Google Scholar]
- 5.Pattar GR, Tackett L, Liu P, Elmendorf JS. Chromium picolinate positively influences the glucose transporter system via affecting cholesterol homeostasis in adipocytes cultured under hyperglycemic diabetic conditions. Mutat Res. 2006;610:93–100. doi: 10.1016/j.mrgentox.2006.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson RA, Roussel AM, Zouari N, Mahjoub S, Matheau JM, Kerkeni A. Potential antioxi-dant effects of zinc and chromium supplementation in people with type 2 diabetes mellitus. J Am Coll Nutr. 2001;20:212–218. doi: 10.1080/07315724.2001.10719034. [DOI] [PubMed] [Google Scholar]
- 7.Golik A, Cohen N, Ramot Y, Maor J, Moses R, Weissgarten J, Leonov Y, Modai D. Type II diabetes mellitus, congestive heart failure, and zinc metabolism. Biol Trace Elem Res. 1993;39:171–175. doi: 10.1007/BF02783187. [DOI] [PubMed] [Google Scholar]
- 8.Niewoehner CB, Allen JI, Boosalis M, Levine AS, Morley JE. Role of zinc supplementation in type II diabetes mellitus. Am J Med. 1986;81:63–68. doi: 10.1016/0002-9343(86)90183-x. [DOI] [PubMed] [Google Scholar]
- 9.Kinlaw WB, Levine AS, Morley JE, Silvis SE, McClain CJ. Abnormal zinc metabolism in type II diabetes mellitus. Am J Med. 1983;75:273–277. doi: 10.1016/0002-9343(83)91205-6. [DOI] [PubMed] [Google Scholar]
- 10.Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26:3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 11.Broadhurst CL, Polansky MM, Anderson RA. Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. J Agric Food Chem. 2000;48:849–852. doi: 10.1021/jf9904517. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Hyun SH, ChoungSY Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J Ethnopharmacol. 2006;104:119–123. doi: 10.1016/j.jep.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 13.Hlebowicz J, Darwiche G, Bjorgell O, Almer LO. Effect of cinnamon on postprandial blood glucose, gastric emptying, and satiety in healthy subjects. Am J Clin Nutr. 2007;85:1552–1556. doi: 10.1093/ajcn/85.6.1552. [DOI] [PubMed] [Google Scholar]
- 14.Mang B, Wolters M, Schmitt B, Kelb K, Lichtinghagen R, Stichtenoth DO, Hahn A. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type 2. EurJ Clin Invest. 2006;36:340–344. doi: 10.1111/j.1365-2362.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki M, Joh T, Koikeda S, Kataoka H, Tanida S, Oshima T, Ogasawara N, Ohara H, Nakao H, Kamiya T. A novel strategy in production of oligosaccharides in digestive tract: prevention of postprandial hyperglycemia and hyperinsu-linemia. J Clin Biochem Nutr. 2007;41:191–196. doi: 10.3164/jcbn.2007027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 17.Lamar ME, Kuehl TJ, Cooney AT, Gayle LJ, Holleman S, Allen SR. Jelly beans as an alternative to a fifty-gram glucose beverage for gestational diabetes screening. Am J Obstet Gynecol. 1999;181:1154–1157. doi: 10.1016/s0002-9378(99)70099-2. [DOI] [PubMed] [Google Scholar]
- 18.Standards of medical care in diabetes–2008. Diabetes Care. 2008;31(Suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 19.Economic costs of diabetes in the U.S. In 2007. Diabetes Care. 2008;31:596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 20.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 21.Preuss HG, Echard B, Polansky MM, Anderson R. Whole cinnamon and aqueous extracts ameliorate sucrose-induced blood pressure elevations in spontaneously hypertensive rats. J Am Coll Nutr. 2006;25:144–150. doi: 10.1080/07315724.2006.10719525. [DOI] [PubMed] [Google Scholar]
- 22.Hlebowicz J, Hlebowicz A, Lindstedt S, Bjorgell O, Hoglund P, Holst JJ, Darwiche G, Almer LO. Effects of 1 and 3 g cinnamon on gastric emptying, satiety, and postprandial blood glucose, insulin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and ghrelin concentrations in healthy subjects. Am J Clin Nutr. 2009;89:815–821. doi: 10.3945/ajcn.2008.26807. [DOI] [PubMed] [Google Scholar]
- 23.Pham AQ, Kourlas H, Pham DQ. Cinnamon supplementation in patients with type 2 diabetes mellitus. Pharmacotherapy. 2007;27:595–599. doi: 10.1592/phco.27.4.595. [DOI] [PubMed] [Google Scholar]