In chronic heart failure (HF) from systolic cardiac dysfunction, the degree of exercise intolerance is not directly related to the degree of cardiac weakness (1–5). Somewhat surprisingly symptoms that typify HF, including shortness of breath and fatigue, are often directly related to the abnormalities of the skeletal musculature in HF. Our understanding of the features of skeletal myopathy is evolving, as therapy for HF is changing and improving. Understanding the skeletal myopathy of HF is important for 2 reasons: 1) therapies, including heart transplantation, only directed at improving cardiac function, will not immediately, and may never, improve exercise capacity in HF, and 2) with greater understanding of the mechanisms underlying the skeletal myopathy of HF, specific therapies targeting these abnormalities can be developed.
I will review the features of the skeletal myopathy of HF, as currently understood, based on state-of-the-art research in humans and animal models. I will then examine the mechanisms underlying the skeletal myopathy, which appears not to be unique to HF, but are shared by other chronic diseases such as chronic obstructive pulmonary disease (COPD) and chronic renal disease (3). Specifically, the role of “coordinated adaptation,” an energy conserving strategy to maximize efficiency in a damaged system, will be examined in the development of the skeletal myopathy in all of these chronic diseases (6). Further, I will present evidence supporting the concept that sympathetic nerve activation (SNA) initiates and maintains this systemic, coordinated adaptation. Finally, the impact of current and future pharmacological and non-pharmacological therapies will be related to the above pathophysiology of the skeletal myopathy of HF.
What are the salient features of the “skeletal myopathy” of HF, and do these features correlate with diminished exercise capacity?
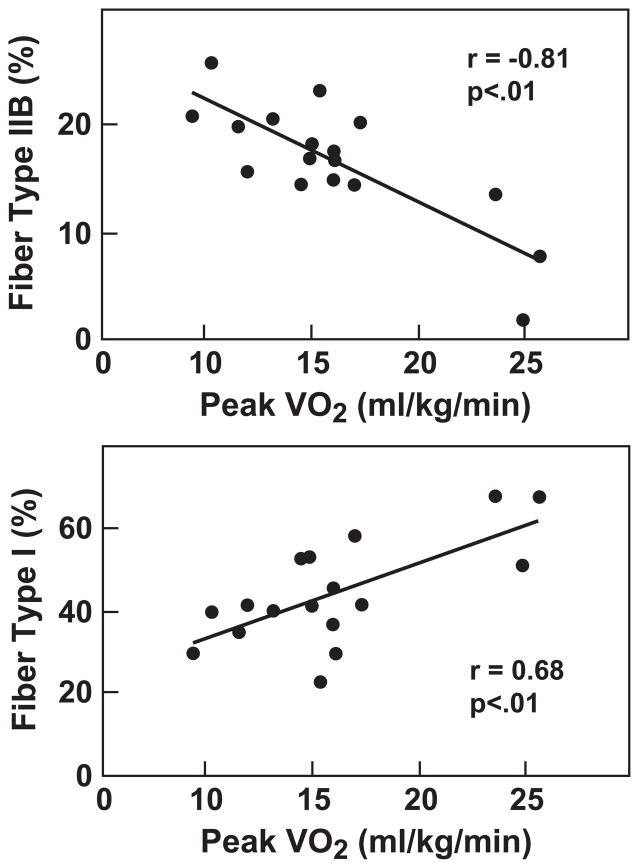
There are several features of the skeletal myopathy that have been described and confirmed by many different investigative groups. Patients with chronic HF on stable medical therapy have decreased muscle bulk compared to healthy humans (7,8). The cross sectional area of lower extremity musculature in HF is significantly smaller than that in healthy humans, and importantly, is correlated with decreased peak oxygen consumption. Morphometric analyses of muscle biopsies from patients with chronic HF have revealed a shift in fiber type, from slow twitch, oxidative type I fibers to fast twitch, glycolytic type IIb fibers (9–12). Once again, the shift in fiber type has been correlated with diminished exercise capacity as estimated by peak oxygen consumption (9) (Figure 1A). Capillary density is inconsistently reported in HF. Mancini and colleagues (9) have reported that the number of capillaries per area of muscle is actually increased. However, they attributed this to muscle fiber atrophy. When capillaries are quantified per fiber, the number of capillaries is not different, or is less, compared to that of controls (10–12). Duscha and colleagues (13) used cell-specific antibodies to measure capillary density, and found that capillary density was significantly decreased in HF patients compared with controls. Importantly, when quantified in this manner, capillary density was directly correlated with maximal oxygen consumption.
Figure 1.
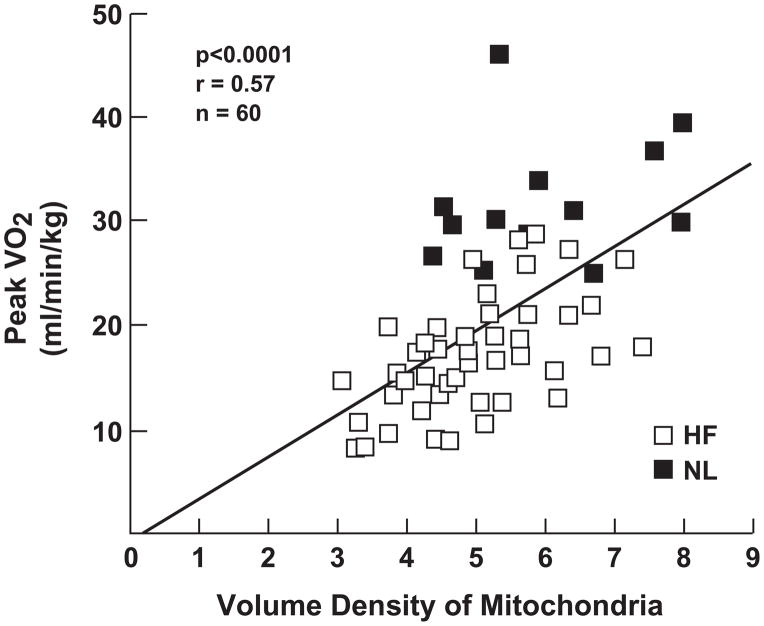
Panel A. Fiber type shift correlates with decreased exercise capacity. Morphometric analyses of muscle biopsies from HF patients have revealed a shift in fiber type, from slow twitch, oxidative type I fibers to fast twitch, glycolytic type IIb fibers. The shift in fiber type correlates with diminished exercise capacity as estimated by peak oxygen consumption. Panel B. Decreased mitochondrial density correlates with decreased exercise capacity. Quantified by electron microscopy, volume density of the mitochondrial in HF is significantly diminished compared to controls. See text for discussion. Filled squares=HF, open squares=controls.
Reprinted with permission from reference 9.
Muscle metabolism is abnormal during exercise in patients with HF. Using 31P nuclear magnetic resonance spectroscopy, Several groups have (9–14) reported greater phosphohocreatinine depletion, and early and excessive acidification in the skeletal muscle of HF patients compared to controls, consistent with reliance on glycolytic rather than oxidative metabolism. Not surprisingly, the patients with the lowest phosphocreatinine and pH levels are the patients with the most impaired exercise ability (14).
Abnormalities at the mitochondrial level have been correlated with a decrease exercise capacity in patients with HF (Figure 1B). Drexler and colleagues (10) used electron microscopy to quantify mitochondrial density in HF patients compared with controls and found it to be significantly diminished. Several other investigators have reported significantly diminished activity of mitochondrial enzymes involved in oxidative metabolism. Significantly, the abnormalities in mitochondrial density and activity are strongly related to decreased peak oxygen consumption in HF patients (15).
In summary, the salient features of the skeletal myopathy of HF are muscle fiber atrophy and fiber type shift from oxidative to glycolytic fiber types, decreased capillary number per muscle fiber, rapid depletion of high energy phosphates and rapid decrease in muscle pH during exercise, and decreased mitochondrial density and oxidative enzyme content. Consistent with the hypothesis that the skeletal myopathy in HF limits exercise capacity, each of these features has been correlated with decreased exercise capacity in patients with chronic HF.
Controversy in the role of mitochondrial oxidative metabolism
Although a compelling and appealing explanation for the exercise dysfunction in HF, the story of the skeletal myopathy of HF as outlined thus far may be incomplete.
Intrinsic mitochondrial function
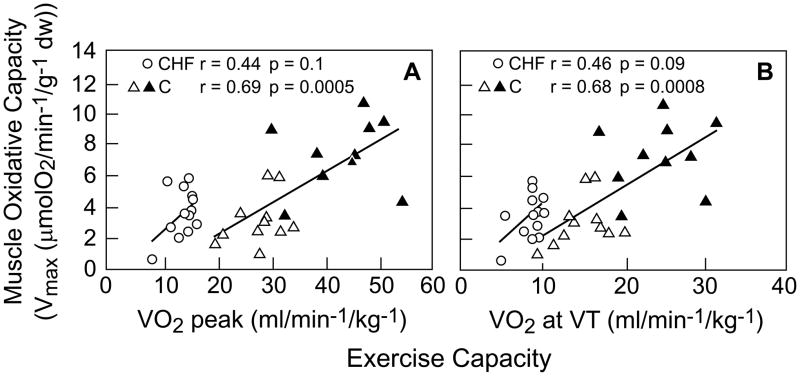
Mettauer and colleagues (16) examined the intrinsic mitochondrial function in situ, rather than selective enzyme activity in homogenized specimens, in intact muscle biopsies from patients with chronic HF on optimal therapy, and compared them to true sedentary, as well as active controls. HF patients compared to sedentary and active controls had diminished exercise capacity as measured by peak oxygen consumption and ventilation threshold; selective mitochondrial enzyme activity was diminished as well. Surprisingly, however, the muscle (mitochondrial) oxidative consumption was not different in HF patients compared to the sedentary controls (Figure 2). Muscle mitochondrial oxidation was diminished in both of these groups compared to the active control group. The investigators point out that the mitochondrial oxygen consumption (the tricarboxylic acid cycle) is not limited by a rate limiting enzyme, and that although individual enzyme activity levels are diminished in HF, they are apparently still in sufficient quantity to maintain normal mitochondrial respiration. These findings have been confirmed by other investigators (17).
Figure 2.
Correlation between exercise capacity and muscle oxidative capacity. HF patients have lower exercise capacity but similar muscle oxidative capacity compared to sedentary controls; both exercise capacity and muscle oxidative capacity are lower in these groups compared with active controls. The decreased exercise capacity in HF, therefore, cannot be entirely attributed to decreased muscle oxidative capacity. Open circles=HF, open triangles=sedentary controls, closed triangles=active controls. Modified from reference 16.
These investigators have reopened the question of what additional factors in the skeletal musculature, other than mitochondrial oxidative metabolism, may lead to decreased exercise capacity in chronic HF patients on optimal therapy?
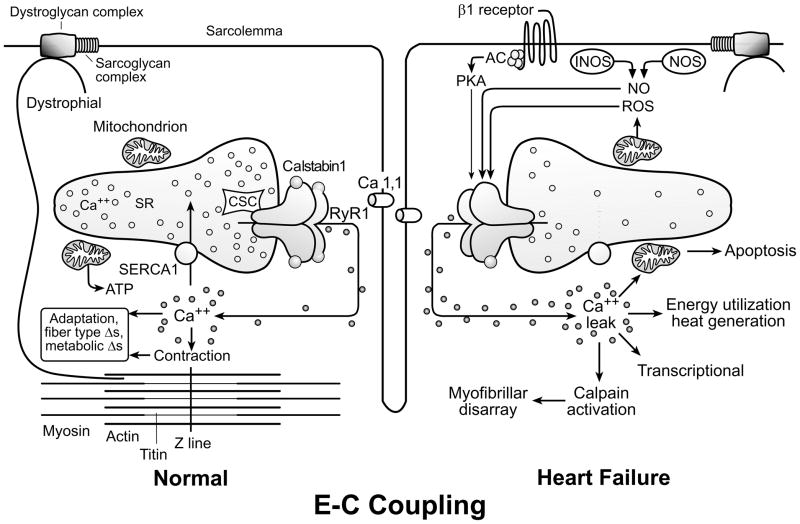
Skeletal muscle excitation-contraction coupling. (Figure 3)
Figure 3.
Abnormal excitation-contraction coupling in HF. See text for discussion. Modified from reference 19.
Following depolarization of the sarcolemma, calcium is released from the intra-cellular calcium storage site, the sarcoplasmic reticulum (SR), through type 1 ryanodine receptors (RyR1). Intracellular calcium concentration rapidly increases, binding troponin C, allowing myosin and actin cross bridges to form, leading to muscle contraction in a process called excitation-contraction (E-C) coupling. Calcium is then rapidly sequestered in an energy-requiring reaction utilizing the sarco (endo) plasmic reticulum Ca++-ATPase (SERCA). Abnormalities of E-C coupling have been identified in HF, although much of the work has focused on cardiac, not skeletal muscle E-C coupling.
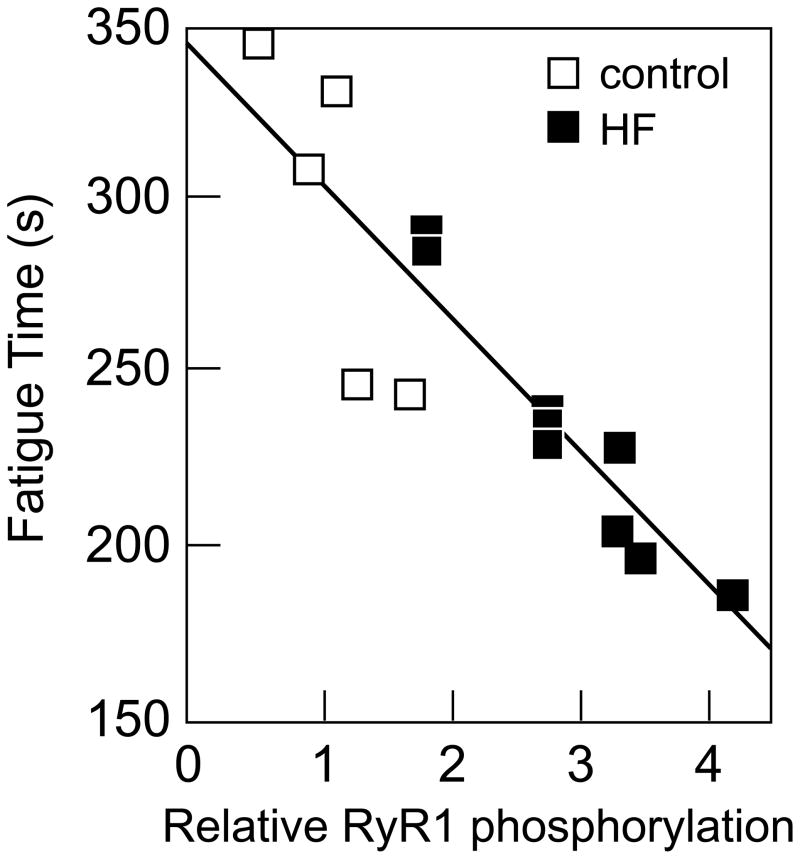
In animal models of HF, some, but not all, investigators have found the skeletal muscle RyR1 to be “leaky,” that is, prone to opening at rest, allowing calcium to leak into the cytoplasm from the SR (18–21). Leaky RyR1 channels result in reduced calcium release from the SR during contraction, and slow calcium reuptake following contraction, thereby negatively impacting muscle contraction. Further, increased cytoplasmic calcium concentration may have toxic effects, including excessive energy utilization, apoptosis, myofibrillar disarray, and transcriptional changes. Importantly, in a rat model of HF, the level of RyR1 dysfunction is correlated with early fatigue in isolated muscle experiments (20) (Figure 4). Although controversial, these data have contributed to the concept that the skeletal myopathy of HF may essentially be an example of abnormal E-C coupling.
Figure 4.
RyR1 phosphorylation and muscle fatigue. Time to fatigue was directly correlated to RyR1 PKA phosphorylation in rat skeletal muscle from sham operated (open squares) and HF (closed squares) animals. Reprinted with permission from reference 20.
Following calcium release, calcium is rapidly sequestered via SERCA isoforms back into the SR (22,23). In animal models of HF and in studies of the explanted heart in HF patients undergoing cardiac transplantation, cardiac SERCA2a levels are often diminished compared to normal controls, and calcium sequestration is slowed. Whether skeletal muscle SERCA levels and activity are also diminished has not been studied. Interestingly, in the skeletal muscle of patients with COPD, another chronic disease characterized by skeletal myopathy, SERCA activity was indeed found to be reduced, and calcium uptake was found to be slower compared to controls (24). These investigators postulated that the presence of reactive oxygen species (ROS) in skeletal muscle in COPD may lead to SERCA damage and dysfunction. ROS are, of course, also present in the skeletal muscles of patients with HF.
In summary, abnormalities of calcium cycling have been identified in the heart and skeletal musculature in patients with HF, and other chronic diseases. These studies support the concept that the skeletal myopathy of chronic HF is an example of abnormal E-C coupling.
Stress and the concept of “coordinated adaptation”
It has been hypothesized that the skeletal myopathy of HF and other chronic diseases such as COPD and chronic renal failure, represent a strategy to conserve energy, and maintain the operation of the damaged biologic system in the most efficient manner. Thus, the skeletal myopathy of chronic HF may be the predictable and logical sequelae following a primary cardiac disturbance (6). According to the concept of “coordinated adaptation,” the development of an abnormality in one step of a multi-step pathway initiates a readjustment of all other steps in the pathway to achieve a new equilibrium. Coordinated adaptation has replaced the concept of a rate-limiting step in governing many biological processes, including oxygen transport, the tricarboxylic acid cycle, and photosynthesis (25). In a system governed by coordinated adaptation, all steps contribute similarly and in a coordinated fashion to regulation of substrate flux. In a system governed by coordinated adaptation, no excessive reserves are maintained, since maintaining excessive reserves is metabolically expensive and inefficient.
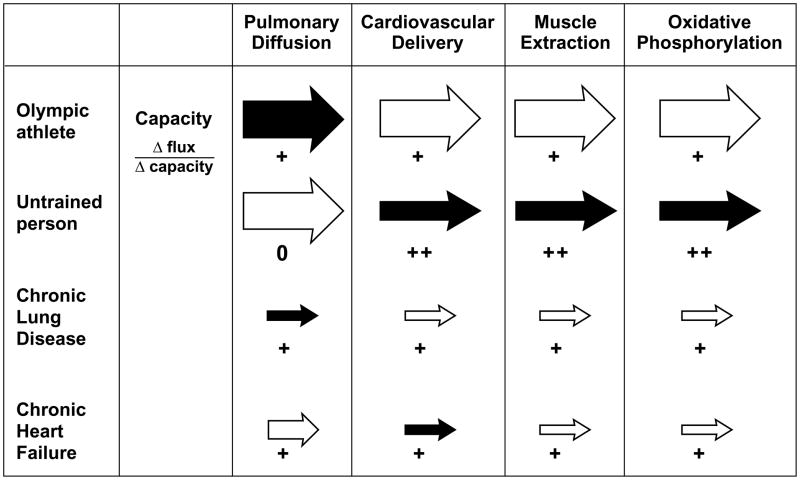
Exercise necessitates adequate oxygen transport and delivery to meet the increased oxygen requirements in exercising muscle. Oxygen transport is a multi-step pathway in which the cardiovascular system works alongside the pulmonary, hematological, and metabolic systems to ensure adequate oxygen transport. According to the theory of coordinated adaptation, when an acute, primary cardiac disorder becomes chronic, such as when an acute myocardial infarction progresses to chronic systolic dysfunction, the reserves of oxygen transport capacity in the periphery are unused and superfluous. In this setting, maintaining an excessive oxidative muscle mass in a patient with a severely limited cardiac output might impose large energetic requirements to maintain a reserve capacity unlikely ever to be utilized. Thus a system governed by coordinated adaptation ensures that the overall efficiency of the system is optimized by matching all steps at a lower functional level (Figure 5).
Figure 5.
Coordinated adaptation in oxygen transport. Arrow size indicates capacity; shaded arrows indicate sites of greatest limitation. The number of plus signs indicates the effect of changing capacity on changing maximal oxygen flux. The athlete has matched capacities at all steps. The untrained person is limited by cardiovascular delivery and muscle extraction. The chronic lung disease patient is limited by pulmonary diffusion. In chronic HF, the limited maximal cardiac output leads to down regulation of muscle oxygen extraction and mitochondrial utilization. Modified from reference 6.
The chronic condition of COPD may be an additional example of coordinated adaptation (6), in which a skeletal myopathy, sharing many of the features of the skeletal myopathy of HF, is present (3,26). Chronic renal failure, although more heterogeneous, may be yet another example (27). In these chronic conditions, reduced muscle mass, fiber atrophy and switch from oxidative to glycolytic fiber types, decreased oxidative enzyme content and mitochondrial density have all been described.
How is the down-regulation of all systems involved in oxygen transport signaled and coordinated throughout the body?
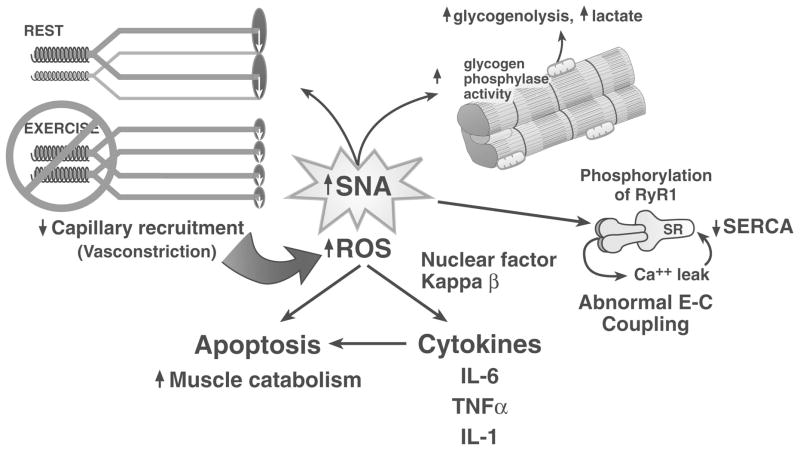
All of these chronic conditions, HF, COPD, and chronic renal disease, share the common feature of a stressed system. In each case, SNA is chronically elevated, approaching a chronic flight or fight condition. Although acutely life saving, chronic activation of the sympathetic nervous system has many deleterious consequences. In chronic HF, I hypothesize that sympathetic excitation provides an important signal to the skeletal musculature that there has been a primary cardiac injury (Figure 6). SNA may directly and indirectly, through cytokine release, then lead to the coordinated, secondary down-regulation of the other steps in oxygen transport, especially down-regulation of the skeletal muscle capacity.
Figure 6.
Mechanisms by which sympathetic nerve activation may contribute to the skeletal myopathy of HF. See text for discussion.
In summary, increased SNA during acute cardiac injury becomes chronic, and may be the signal to other organs and tissues to decrease oxygen transport capacity.
How could sympathetic nerve activation signal this coordinated down-regulation of the oxygen capacity in skeletal muscle, resulting in this skeletal myopathy?
SNA leads to inadequate blood flow
Increased SNA is a major contributor to the peripheral vasoconstriction in HF. Resting and reflex increases in SNA are the major mechanisms limiting increased limb blood flow during exercise in HF (28). In addition to restricting the quantity of limb blood flow during exercise, increased SNA may also alter the blood flow distribution in patients with HF. During exercise in healthy humans, vasoconstrictor SNA is offset by the generation of metabolic by products that produce vasodilatation. However, when SNA is exaggerated, as is the case in HF, the balance between vasoconstriction and vasodilatation shifts towards vasoconstriction. Inadequate perfusion may lead to areas of underperfusion and ischemia, followed by release of ROS, triggering muscle inflammation, and contributing to the skeletal myopathy of HF (1–3,29).
SNA leads to inflammation
Ample evidence supports the concept that HF from systolic dysfunction leads to a systemic inflammatory response (30). This inflammation involves the skeletal musculature, and contributes importantly to the skeletal myopathy of heart failure (1–3,30–39). HF patients have increased circulating tumor necrosis factor-alpha, associated with protein loss and cachexia. TNF-alpha and interleukin-6 have been linked to increased skeletal muscle catabolism, and apoptosis (32–34). In experimental models, increased levels of TNF-alpha are correlated with the degree of apoptosis in skeletal muscle in HF (33). Interleukin-6 levels are inversely correlated to muscle fiber thickness in heart failure patients (35). Adams and colleagues found that interleukin-1beta and iNOS levels were significantly increased in muscle biopsies from HF patients compared with controls (40). Nuclear factor-kappa B (NF-kB) is an important transcription factor for proinflammatory gene expression. IL-1beta mediates iNOS induction, which could be inhibited in cultures of skeletal muscle by inhibition of NF-kB. Excessive iNOS levels may interfere with mitochondrial oxidative enzyme activity, thereby reducing oxygen uptake and exercise capacity.
Although the release of TNF-alpha from the heart, and bacterial translocation across the bowel wall both may trigger inflammation, sympathetic hyperactivity is the major activator of inflammation in HF (39). Sympathetic hyperactivity leads to local areas of underperfusion in skeletal muscle leading to generation of ROS, a well known stimulus for TNF-alpha (29). Chronic HF patients with cachexia have been found to have markedly increased catecholamine and TNF-alpha levels, compared to non-cachectic patients, in whom levels were near normal. Sympathetic activation typifies other chronic diseases, such as COPD and chronic renal failure, which are also characterized by systemic inflammation and skeletal myopathy.
Increased SNA leads to abnormal metabolism
In addition to producing tissue hypoxia, oxidative stress, and inflammation, sympathetic nerve activation has direct effects on metabolic processes (41,42). Infusion of catecholamines into working muscles in healthy animals and humans produces an increased lactic acid output, even in the absence of hypoxia. Thus one explanation for the premature and excessive production of lactic acid in humans with HF during exercise may be a direct metabolic effect of catecholamines in myocyte metabolism.
Increased SNA contributes to E-C coupling abnormalities
Chronic sympathetic nerve activation in chronic HF leads to protein kinase A phosphorylation of the tetrameric RyR1 complexes, leading to dissociation of the calstabin molecule from the channel complex (18,19). Calstabin stabilizes the RyR1 channel in the closed state, and in its absence the RyR1 becomes leaky.
Increase SNA leads to areas of skeletal muscle hypoperfusion and generation of ROS. ROS–mediated damage to SERCA has been hypothesized as another potential mechanism of diminished calcium sequestration following calcium release from the SR. Other hyperadrenergic states, including COPD, also manifest this altered calcium cycling, leading to similar impairment of skeletal muscle function (24).
In summary, following an acute cardiac injury, such as a myocardial infarction, SNA acutely increases in order to maintain blood pressure and cardiac output. However, chronically elevated SNA also has detrimental effects, including vasoconstriction, tissue hypoxia, ROS generation, abnormal E-C coupling, and initiation of inflammation.
But what about deconditioning?
Deconditioning contributes to the skeletal myopathy of HF. Deconditioning can cause several, but not all, of the features present in patients with HF (43–45). Increased physical activity reverses many of the features of skeletal myopathy, and even more importantly, reverses the purported instigators of the myopathy, elevated SNA and inflammation.
Deconditioning is not the sole mechanism of skeletal muscle dysfunction in HF, however. In the rat infarct model of HF, HF rats and sham-operated rats had a similar activity level in the 8 weeks following operation (left coronary ligation or sham), yet the HF rats developed skeletal myopathy (45). HF rats exhibited a fiber shift from oxidative to glycolytic fiber type, and a decrease in mitochondrial enzyme activity. Furthermore, mRNA for cytochrome oxidase and beta-myosin heavy chains were decreased, and message for glycolytic myosin heavy chain IIx and IIb were increased. The skeletal myopathy in this rat model of HF, which resembled that in human HF, could not be explained by disuse.
There are important differences in the skeletal myopathy of disuse and that of chronic HF. In deconditioning, atrophy in both fiber muscle types I and II are present, but preferentially type II. In HF, type I is predominantly atrophied. Deconditioning preferentially affects the postural muscles, sparing small muscles of the arms and the muscles of respiration. The skeletal myopathy of HF is systemic. Finally, HF is characterized by early and severe muscle fatigue that exceeds the degree of mitochondrial enzyme deficiency. In summary, although disuse contributes to the skeletal myopathy of chronic HF, there are other important, contributing factors.
Why is resting and exercise-related SNA elevated in chronic HF?
Sympathetic activation after an acute cardiac injury is intuitive and easily understood. Initially sympathetic activation increases contractility, force of contraction, regulates blood flow, maintains blood pressure, and modulates glucose metabolism. However, prolonged, chronic sympathetic activation is maladaptive, leading to decreased capillary recruitment, and generation of ROS, initiation of inflammation, and the myopathic features of HF, as well as hyperphosphorylation of ryanodine receptors, leading to a E-C coupling myopathy (1–5). Persistent sympathetic activation in HF has been attributed to 1) decreased inhibitory reflexes, including blunted arterial or cardiopulmonary reflexes; 2) augmented excitatory reflexes, including heightened arterial chemoreceptor sensitivity and muscle somatic afferents; and/or 3) enhanced central nervous system influences. Below is a brief summary of the muscle somatic afferent contribution; the reader is directed to several excellent reviews (4,46–48).
In the 1990s, Coats and colleagues developed the “Muscle Hypothesis,” in which they postulated that over-activity of the somatic afferents located in skeletal muscle, maintains increased SNA both at rest and during exercise (49). Muscle metaboreceptors are somatic afferents located in the skeletal muscle, which are sensitive to ischemic metabolites generated during exercise. This receptor type plays a major role in increasing blood pressure and SNA (“the exercise pressor reflex”) during exercise in healthy humans. Muscle mechanoreceptors are another type of somatic afferent nerve fiber, which are sensitive to stretch, and also play a role, albeit minor, in mediating the exercise pressor reflex in healthy humans. Coats and colleagues attributed the over-activity of muscle afferents to the abnormal skeletal muscle milieu of HF.
My group and others (46,47) have reported that muscle metaboreceptor sensitivity is blunted and muscle mechanoreceptor sensitivity is increased in patients with HF, thereby potentially identifying this nerve fiber type as the culprit in mediating increased SNA at rest and during exercise in chronic HF. However, other investigators (46,47) have reported exaggerated muscle metaboreceptor sensitivity during exercise in HF. Thus, although investigations in patients with HF have reported exaggerated sympathetic and hemodynamic responses during exercise, the relative contributions of the muscle metaboreceptors and muscle mechanoreceptors is controversial (50), perhaps due to heterogeneity of patient populations, and failure of current experimental techniques to isolate entirely specific nerve fiber types.
Animal models provide a more homogeneous study group, and the potential to isolate selectively and precisely the specific nerve endings of interest for study (47). In the decerebrate rat infarct model of HF, capsaicin was used to selectively activate muscle metaboreceptors of the hindlimb skeletal muscle (47). Capsaicin elicited dose-related increases in blood pressure and heart rate, but these were blunted compared to non-HF control rats. In this same model, activation of the muscle mechanoreceptors by passive stretch of the hindlimb, greater increases in blood pressure and heart rate were elicited in HF compared to control rats. In summary, findings in animal models of HF support the findings in humans with HF that muscle mechanoreceptors underlie the exaggerated neurovascular responses to exercise in chronic HF.
How do our current therapies impact the skeletal myopathy of HF, and more interestingly, how does our present understanding of the skeletal myopathy of HF impact future therapies?
Pharmacological therapies
Interruption of the effects of angiotensin II in HF, either with angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blockade, results in improvements in the skeletal myopathy of HF. As noted above, in HF patients on chronic therapy with ACEI, mitochondrial oxidative function is not significantly different from that of healthy, but sedentary controls (16). In the infarct model of HF in rats, treatment with ACEI, initiated 7 days after MI, protected mitochondrial oxidative function and mRNA transcription of mitochondrial enzymes, which were abnormal in the untreated HF rats (51). Vescovo and colleagues (52) examined muscle biopsies in humans with HF before and after 6 months of treatment with ACEI (n=8) or losartan (n=8). Slow myosin heavy chain MHC1 increased, and fast oxidative MHC2A and fast glycolytic MHC2b significantly decreased on each therapy, and were associated with increased exercise capacity as estimated by increased peak oxygen capacity in each group (52). Mechanisms underlying the improvement in the skeletal myopathy with ACEI are unknown. One intriguing possibility is the inhibition of the deleterious effects of angiotensin on insulin-like growth factor-1 (IGF-1). In rats, angiotensin II infusion down-regulates IGF-1, leading to muscle proteolysis, specifically, actin cleavage and apoptosis (53). Thus, therapy with ACEI may potentially block this IGF-1 down-regulation. Therapy specifically developed to increase local IGF-1 in HF may be a novel strategy to reverse the skeletal myopathy of HF (53).
Beta-adrenergic blockade has been associated with improvement in exercise capacity in humans with HF. In animal models of HF, less protein oxidation is present in animals treated with beta-blockade compared to placebo, and the findings are greatest in animals treated wth carvedilol, which has anti-oxidative properties as well (54).
We have preliminary data (unpublished observations) in muscle biopsies obtained in 7 chronic HF patients, who were optimized on medical therapy including ACEI and beta-adrenergic blockers for at least 3 months. Many of the classic findings of this skeletal myopathy of HF, including changes in capillary density, mitochondrial enzyme activity, and myofibrillar size, were all, with rare exceptions, within normal limits. Nonetheless, exercise capacity remained depressed in this small group of heart transplant candidates, peak VO2 15.9 mg/kg. Analysis of E-C coupling in these HF patients is planned.
Therapies directed at the mediators of inflammation in HF have not been successful (55,56). Trials to interrupt TNF-alpha activity with recombinant chimeric soluble TNF receptor type 2 were halted due to lack of efficacy. Anti-TNF-alpha chimeric monoclonal antibody, infliximab was halted early due to increased deaths and hospitalizations in the drug group compared to placebo. These disappointing results do not disprove the important role of TNF-alpha, and inflammation, in the progression of HF, yet do challenge us to find alternate targets and directed therapies in advanced HF patients.
To restore E-C coupling in skeletal muscle in chronic HF patients, a pharmacological approach to prevent the dissociation of calstabin I from the RyR1 has been proposed (18). In mice with HF, rycals, small molecules that essentially fix the leak in RyR1, have been shown to improve muscle fatigue. Trials of the pharmacological agents to enhance SERCA activity, and even gene transfer of SERCA2a, have been shown to improve cardiac function; the impact of these strategies on skeletal muscle function have not been reported (57,58). Further work is necessary to determine if these approaches will improve exercise capacity in HF.
Chronic HF is associated with endocrine deficiencies that may have catabolic effects (59,60). Many HF patients are growth hormone (GH) deficient, and many men with HF may be testosterone deficient. Therapy with GH in those with low GH levels, or with testosterone in male HF patients, has each been shown to improve exercise performance in placebo-controlled trials (59,60). Unfortunately, muscle biopsies were not done, so the effect of these therapies on the skeletal myopathy remains unknown.
Finally, molecular therapies for skeletal myopathy are on the horizon. Gene therapy with SERCA2 was mentioned above (57,58). Myoblast transplantation is also being studied. Myoblasts are easily procured and grown, but so far, clinical trials have been hampered by poor myoblast survival and migratory ability (61).
In summary, present therapies with beta-blockade and ACEI, although beneficial to the heart, may actually have their greatest impact on reversing the skeletal myopathy of HF. Nonetheless, exercise capacity remains diminished, mandating the development of future therapies directed at abnormalities of E-C coupling, proteolysis and apoptosis.
Non-Pharmacological Therapies
Cardiac Resynchronization Therapy
Cardiac resynchronization therapy (CRT) has been shown to improve exercise capacity in chronic HF patients (62,63). This benefit may be attributable to improvements in ejection fraction or hemodynamics. Additionally, reduced SNA in patients treated chronically with CRT may lead to improved skeletal muscle function. Grassi and colleagues (64) recorded SNA directly using the technique of microneurography, and found that SNA was decreased by approximately 30% 2 months following biventricular pacemaker implantation. Unfortunately muscle biopsies were not obtained to assess whether the decrease in SNA was accompanied by improvement in muscle inflammation and the skeletal myopathy of HF. These studies are ongoing in our own laboratory.
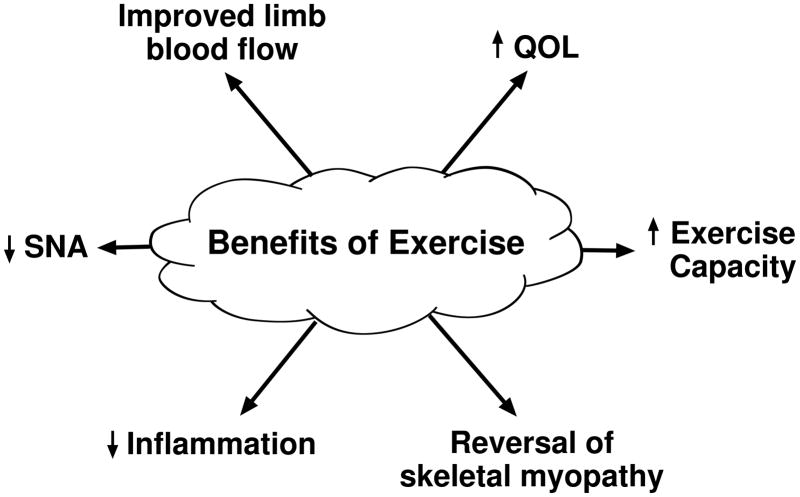
Exercise training. (Figure 7)
Figure 7.
Benefits of exercise. Exercise training decreases resting SNA, decreases inflammation, reverses morphometric and histochemical features of skeletal myopathy, and leads to improved blood flow, and increases quality of life and overall exercise capacity.
Exercise training in chronic HF improves quality of life, exercise capacity, and functional class, and has been the subject of several excellent reviews (46,65–67). These improvements are associated with reversal of many of the histochemical and morphometric features of skeletal myopathy, and may be seen within 2 months of initiation of an exercise program. Skeletal muscle atrophy and fiber shift can be largely reversed with exercise training. Volume density of mitochondria is improved, and oxidative capacity of the mitochondria, estimated by individual enzyme activity, is increased. Exercise training also reduces the early phosphocreatine depletion and early and excessive acidification that occurs during exercise. The marked improvement of the skeletal myopathy with exercise in HF lends support to the concept that deconditioning is one mechanism underlying this skeletal myopathy. Additional mechanisms underlying the reversal of the skeletal myopathy with exercise training include improvements in limb blood flow, decreased SNA, and reduced muscle inflammation (34,46,65–67,68).
Exercise training improves endothelially-mediated (nitric oxide-dependent) peripheral blood flow without improving cardiac function (67,69,70). Exercise training may both increase availability of nitric oxide by increasing endothelial nitric oxide expression, and/or increasing anti-oxidant enzymes that decrease oxygen free radicals that interfere with nitric oxide (69,70).
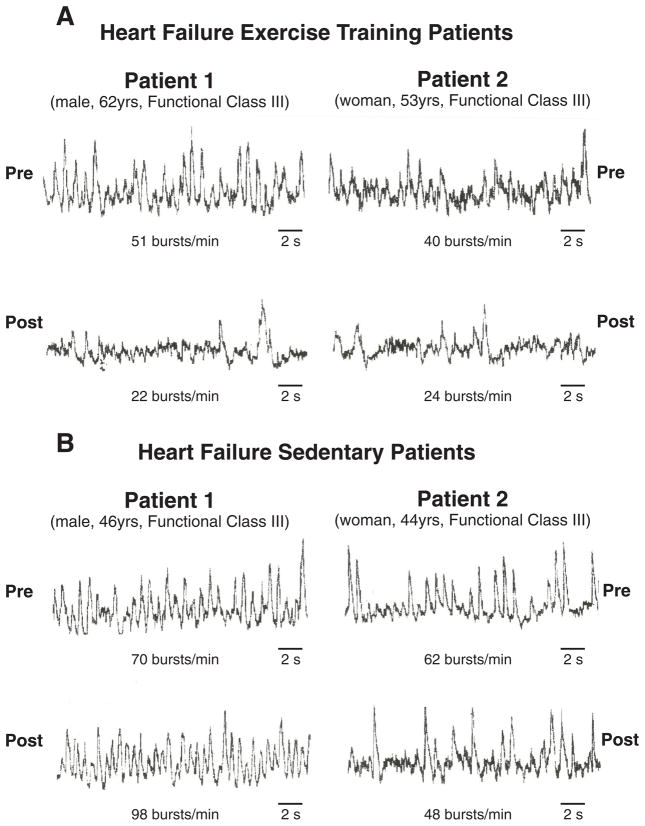
Exercise training in chronic HF patients reduces resting SNA (68,71). Coats and colleagues (71) reported that 8 weeks of exercise training in chronic HF patients significantly reduced whole body norepinephrine spillover, and improved heart rate variability. Negrao and colleagues (68) reported that 4 months of exercise training significantly reduced SNA, directly measured with miconeurography, in advanced HF patients randomized to exercise training, but not in the advanced HF patients randomized to no training, or the healthy, trained controls. In fact, SNA in the HF group after training was no longer greater than the SNA in the trained controls (Figure 8). The mechanisms underlying this normalization of SNA with training in HF were not explored, but a modulation of peripheral reflexive influences on central sympathetic outflow, or the reduction of excitatory influences located centrally may be involved (72). Studies are ongoing to investigate peripheral reflexive influences.
Figure 8.
Sympathetic nerve recordings before and after exercise training period (panel A) or sedentary period (panel B). In pre-training patients and pre-sedentary control patients, SNA is markedly increased. Following the training period, but not the sedentary period, SNA is significantly reduced. Reproduced with permission from reference 68.
Exercise training has chronic anti-inflammatory effects on the skeletal musculature in HF (34,69). Gielen and colleagues (34) studied 20 chronic HF patients, 10 randomized to 6 months of exercise training, and 10 sedentary controls. Peak oxygen consumption was significantly improved in the trained group. Skeletal muscle TNF-alpha, IL-6, and IL-1-beta were significantly decreased in the trained HF group, but were unchanged in the sedentary group. Further, skeletal muscle iNOS was significantly reduced in trained HF patients.
In summary, exercise leads to improved peripheral blood flow, decreased sympathetic activation, and decreased skeletal muscle inflammation. Whether all of these mechanisms are linked and mechanistically intertwined, remains unknown, but likely.
Persistent questions
Although it is generally agreed that exercise limitation in chronic systolic HF is attributable to abnormalities of the skeletal musculature, the exact nature of those abnormalities is uncertain. Previously described abnormalities of fiber shift from oxidative to glycolytic types, and insufficient limb blood flow are markedly improved by pharmacological therapies, yet patients still have exercise limitations. Abnormalities in mitochondrial activity seem also to have been largely corrected by pharmacological therapy. Reduced mitochondrial oxidative capacity may have been overstated; mitochondrial oxidative capacity appears to be similar to that of sedentary controls when approached with newer experimental techniques. Might not the abnormalities of the E-C coupling, increased proteolysis mediated by suppression of IGF-1, or other abnormalities, such as hormone deficiencies, play a role in the pathophysiology, and thus serve as therapeutic targets? Finally, since the skeletal musculature is the limiting factor in exercise ability in patients with HF, it follows that increased activity improves this limitation. How much exercise, and what type(s) of exercise of training are necessary to see a sympatholytic effect remain to be determined.
Acknowledgments
Finding Sources
Holly Middlekauff, MD is supported by NIH-RO1 HL084525, and the University of California, Los Angeles General Clinical Research Center NIH-MO1-RR00865.
Footnotes
Disclosures
None.
References
- 1.Vantura-Clapier R, De Sousa E, Veksler V. Metabolic myopathy in HF. New Physiol Sci. 2002;17:191–196. doi: 10.1152/nips.01392.2002. [DOI] [PubMed] [Google Scholar]
- 2.Duscha B, Schulze PC, Robbins JL, Forman DE. Implications of chronic HF on peripheral vasculature and skeletal muscle before and after exercise training. Heart Fail Rev. 2008;13:21–37. doi: 10.1007/s10741-007-9056-8. [DOI] [PubMed] [Google Scholar]
- 3.Troosters T, Gosselink R, Decramer M. COPD and chronic HF: Two muscle diseases? J Cardiopul Rehab. 2004;24:137–145. doi: 10.1097/00008483-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Sinoway LI, Li J. A perspective on the muscle reflex:implications for congestive HF. J Appl Physiol. 2005;99:5–22. doi: 10.1152/japplphysiol.01405.2004. [DOI] [PubMed] [Google Scholar]
- 5.Witte KK, Clark AL. Why does chronic HF cause breathlessness and fatigue? Prog Cardiovasc Dis. 2007;49:366–384. doi: 10.1016/j.pcad.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Hsia CCW. Coordinated adaptation of oxygen transport in cardiopulmonary disease. Circulation. 2001;104:963–969. doi: 10.1161/hc3401.094928. [DOI] [PubMed] [Google Scholar]
- 7.Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, Mullen JL, Wilson JR. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in HF. Circulation. 1992;85:1364–73. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- 8.Harrington D, Anker SD, Chua TP, Webb-Peploe KM, Ponikowski PP, Poole-Wilson PA, Coats AJ. Skeletal muscle function and its relation to exercise tolerance in chronic HF. J Am Coll Cardiol. 1997;30:1758–64. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 9.Mancini DM, Coyle E, Coggan A, Beltz J, Ferraro N, Montain S, Wilson JR. Contribution of intrinsic skeletal muscle changes to 31P NMR skeletal muscle metabolic abnormalities in patients with chronic HF. Circulation. 1989;80:1338–1346. doi: 10.1161/01.cir.80.5.1338. [DOI] [PubMed] [Google Scholar]
- 10.Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic HF. Circulation. 1992;85:1751–59. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term HF. Circulation. 1990;81:518–527. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- 12.Schaufelberger M, Eriksson BO, Grimby G, Held P, Swedberg K. Skeletal muscle alterations in patients with chronic HF. Eur Heart J. 1997;18:971–980. doi: 10.1093/oxfordjournals.eurheartj.a015386. [DOI] [PubMed] [Google Scholar]
- 13.Duscha BD, Kraus WE, Keteyian SJ, Sullivan MJ, Green HJ, Schachat FH, Pippen AM, Brawner CA, Blank JM, Annex BH. Capillary Density of skeletal muscle: a contributing mechanism for exercise intolerance in class II–III chronic HF independent of other peripheral alterations. J Am Coll Cardiol. 1999;33:1956–63. doi: 10.1016/s0735-1097(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 14.Massie B, Conway M, Yonge R, Frostick S, Ledingham J, Sleight P, Radda G, Rajagopalan B. Skeletal muscle metabolism in patients with congestive HF: relation to clinical severity and blood flow. Circulation. 1987;76:1009–1019. doi: 10.1161/01.cir.76.5.1009. [DOI] [PubMed] [Google Scholar]
- 15.Massie BM, Simonini A, Sahgal P, Wells L, Dudley GA. Relation of systemic and local muscle exercise capacity to skeletal muscle characteristics in men with congestive HF. J Am Coll Cardiol. 1996;27:140–5. doi: 10.1016/0735-1097(95)00416-5. [DOI] [PubMed] [Google Scholar]
- 16.Mettauer B, Zoll J, Sanchez H, Lampert E, Ribera F, Veksler V, Bigard X, Mateo P, Epailly E, Lonsdorfer J, Ventura-Clapier R. Oxidative capacity of skeletal muscle in HF patients versus sedentary or active control subjects. J Am Coll Cardiol. 2001;38:947–54. doi: 10.1016/s0735-1097(01)01460-7. [DOI] [PubMed] [Google Scholar]
- 17.Williams AD, Selig S, Hare DL, Hayes A, Krum H, Patterson J, Geerling RH, Toia D, Carey MF. Reduced exercise tolerance in CHF may be related to factors other than impaired skeletal muscle oxidative capacity. J Card Failure. 2004;10:141–48. doi: 10.1016/j.cardfail.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Lehnart SE. Novel targets for treating heart and muscle disease – stabilizing ryanodine receptors and preventing intracellular calcium leak. Current opinion in pharmacology. 2007;7:225–232. doi: 10.1016/j.coph.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Bellinger AM, Mongillo M, Marks AR. Stressed out: the skeletal muscle ryanodine receptor as a target of stress. J Clin Invest. 2008;118:445–453. doi: 10.1172/JCI34006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiken S, Lacampagne A, Zhou H, Kherani A, Lehnart SE, Ward C, Huang F, Gaburjakova M, Gaburjakova J, Rosemblit N, Warren MS, He K, Yi G, Wang J, Burkhoff D, Vassort G, Marks AR. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in HF. J Cell Biol. 2003;160:919–28. doi: 10.1083/jcb.200211012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danila CI, Hamilton SL. Phosphorylation of ryanodine receptors. Biol Res. 2004;37:521–5. doi: 10.4067/s0716-97602004000400005. [DOI] [PubMed] [Google Scholar]
- 22.Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–42. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- 23.Hovnanian A. SERCA pumps and human diseases. Subcell Biochem. 2007;45:337–63. doi: 10.1007/978-1-4020-6191-2_12. [DOI] [PubMed] [Google Scholar]
- 24.Green HJ, Burnett M, Duhamel TA, D’Arsigny C, O’Donnell DE, Webb KA, Ouyang J. Abnormal sarcoplasmic reticulum Ca2+-sequestering properties in skeletal muscle in COPD. Am J Physiol Cell Physiol. 2008;295:C350–C357. doi: 10.1152/ajpcell.00224.2008. [DOI] [PubMed] [Google Scholar]
- 25.Weibel ER, Taylor CR, Hoppeler H. The concept of symmorphosis: A testable hypothesis of structure-function relationship. PNAS. 1991;88:10357–10361. doi: 10.1073/pnas.88.22.10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ATS, ERS. Skeletal muscle dysfunction in COPD: a joint statement of the American Thoracic Society and European Respiratory Society. Am J Respir Crit Care med. 1999;159:S1–S40. doi: 10.1164/ajrccm.159.supplement_1.99titlepage. [DOI] [PubMed] [Google Scholar]
- 27.Adams GR, Vaziri ND. Skeletal muscle dysfunction in chronic renal failure: effects of exercise. Am J Physiol Renal Physiol. 2006;290:F753–F761. doi: 10.1152/ajprenal.00296.2005. [DOI] [PubMed] [Google Scholar]
- 28.Alves MJ, Rondon MU, Santos AC, Dias RG, Barretto AC, Krieger EM, Middlekauff HR, Negrao CE. SNA restrains reflex vasodilatation in HF. Clin Auton Res. 2007;17:364–9. doi: 10.1007/s10286-007-0448-6. [DOI] [PubMed] [Google Scholar]
- 29.Tsutue H, Ide T, Hayashidani S, Suematsu N, Shiomi T, Wen J, Nakamura K, Ichikawa K, Utsumi H, Takeshita A. Enhanced generation of reactive oxygen species in the limb skeletal muscles from a murine infarct model of HF. Circulation. 2001;104:134–136. doi: 10.1161/01.cir.104.2.134. [DOI] [PubMed] [Google Scholar]
- 30.Muller-Wedan U, Werdan K. Immune modulation by catecholamines-a potential mechanism of cytokine release in HF? Herz. 2000;25:271–273. doi: 10.1007/s000590050019. [DOI] [PubMed] [Google Scholar]
- 31.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic HF. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 32.Reid MB, Li YP. Cytokines and oxidative signaling in skeletal muscle. Acta Physiol Scand. 2001;171:225–232. doi: 10.1046/j.1365-201x.2001.00824.x. [DOI] [PubMed] [Google Scholar]
- 33.Libera LD, Sabbadini R, Renken C, Ravara B, Sandri M, betto R, Angelini A, Vescovo G. Apoptosis in the skeletal muscle of rats with HF is associated with increased serum levels of TNF-alpha and sphingosine. J Mol Cell Cardiol. 2001;33:1871–1878. doi: 10.1006/jmcc.2001.1453. [DOI] [PubMed] [Google Scholar]
- 34.Gielen S, Adams V, Mobius-Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic HF. J Am Coll Cardiol. 2003;42:861–868. doi: 10.1016/s0735-1097(03)00848-9. [DOI] [PubMed] [Google Scholar]
- 35.Larsen AI, Lindal S, Aukrust P, Toft I, Aarsland T, Dickstein K. Effect of exercise training on skeletal muscle fibre characteristics in men with chronic HF. Correlation between skeletal muscle alterations, cytokines and exercise capacity. Int J Cardiol. 2002;83:25–32. doi: 10.1016/s0167-5273(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 36.MacGowan GA, Mann DL, Kormos RL, Feldman AM, Murali S. Circulating interleukin-6 in severe HF. Am J Cardiol. 1997;79:1128–1131. doi: 10.1016/s0002-9149(96)00063-x. [DOI] [PubMed] [Google Scholar]
- 37.Torre-Amione T, Kapadia S, Benedict Cl. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the SOLVD. J Am Coll Cardiol. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 38.Ostrowski K, Rohde T, Zacho M. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol. 1998;508:949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baeuerle PA, Baltimore D. NF-kappa-Beta: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 40.Adams V, Jiang H, Yu J, Mobius-Winkler S, Fiehn E, Linke A, Weigl C, Schuler G, Hambrecht R. Apoptosis in skeletal myocytes of patients with chronic HF is associated with exercise intolerance. J Am Coll Cardiol. 1999;33:959–65. doi: 10.1016/s0735-1097(98)00626-3. [DOI] [PubMed] [Google Scholar]
- 41.Hartling OJ, Trap-Jansen J. Stimulation of beta-adrenoceptors in the exercising human forearm. Clin Physiol. 1982;2:363–371. doi: 10.1111/j.1475-097x.1982.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 42.Stainsby WN, Sumners C. Effects of catecholamines on lactic acid output during progressive working contractions. J Appl Physiol. 1985;59:1809–1814. doi: 10.1152/jappl.1985.59.6.1809. [DOI] [PubMed] [Google Scholar]
- 43.Vescovo G, Serfini F, Facchin Ll. Specific changes in skeletal muscle myosin heavy chain composition in cardiac failure: differences compared with disuse atrophy as assessed on microbiopsies by high resolution electrophoresis. Heart. 1996;76:337–343. doi: 10.1136/hrt.76.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franssen FME, Wouters AFM, Schols AMWJ. The contribution of starvation, deconditioning and ageing to the observed alterations in peripheral skeletal muscle in chronic organ diseases. 2002;21:1–14. doi: 10.1054/clnu.2001.0485. [DOI] [PubMed] [Google Scholar]
- 45.Simonini A, Long CS, Dudley GA, Yue P, McElhinny J, Massie BM. HF in rats causes changes in skeletal muscle morphology and gene expression that are not explained by reduced activity. Circ Res. 1996;79:128–136. doi: 10.1161/01.res.79.1.128. [DOI] [PubMed] [Google Scholar]
- 46.Khan MH, Sinoway LI. Muscle reflex control of SNA in HF: the role of exercise conditioning. Heart Fail Rev. 2000;5:87–100. doi: 10.1023/A:1009802308872. [DOI] [PubMed] [Google Scholar]
- 47.Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol. 2005;91:89–102. doi: 10.1113/expphysiol.2005.032367. [DOI] [PubMed] [Google Scholar]
- 48.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in HF: physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–1762. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 49.Clark AL, Poole-Wilson PA, Coats AJS. Exercise limitation in chronic HF: central role of the periphery. J Am Coll Cardiol. 1996;28:1092–1002. doi: 10.1016/S0735-1097(96)00323-3. [DOI] [PubMed] [Google Scholar]
- 50.Middlekauff HR, Sinoway LI. Point: Counterpoint: Increased mechanoreceptor/metaboreceptor stimulation explains the exaggerated exercise pressor reflex seen in HF. J Appl Physiol. 2006;102:492–494. doi: 10.1152/japplphysiol.00994.2006. [DOI] [PubMed] [Google Scholar]
- 51.Zoll J, Monassier L, Garnier A, N’Guessan B, Mettauer B, Veksler V, Piquard F, Ventura-Calpier R, Geny B. ACE inhibition prevents myocardial infarction-induced skeletal muscle mitochondrial dysfunction. J Appl Physiol. 2006;101:385–91. doi: 10.1152/japplphysiol.01486.2005. [DOI] [PubMed] [Google Scholar]
- 52.Vescovo G, Libera LD, Serafini F, Leprotti C, Facchin L, Volterrani M, Cecpmo C, Ambrosio GB. Improved exercise tolerance after losartan and enalapril in HF: Correlation with changes in skeletal muscle myosin heavy chain composition. Circulation. 1998;98:1742–1749. doi: 10.1161/01.cir.98.17.1742. [DOI] [PubMed] [Google Scholar]
- 53.Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest. 2005;115:451–458. doi: 10.1172/JCI22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Libera LD, Ravara B, Gobbo V, Betto DD, Germinario E, Angelini A, Vescovo Skeletal muscle myofibrillar protein oxidation in heart failure and the protective effect of carvedilol. J Mol Cell Cardiol. 2005;38:803–7. doi: 10.1016/j.yjmcc.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 55.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT Anti-TNF therapy against congestive heart failure investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe HF: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–40. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 56.Mann DL, McMurray JJ, Packer M, Swedburg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westhein A, Zannad F, Fleming T. Targeted anti-cytokine therapy in patients with chronic HF: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 57.Gupta D, Palma J, Molina E, Gaughan JP, Long W, Houser S, Macha M. Improved exercise capacity and reduced systemic inflammation after adenoviral-mediated SERCA-2a gene transfer. J Surg Res. 2008;145:257–65. doi: 10.1016/j.jss.2007.03.081. [DOI] [PubMed] [Google Scholar]
- 58.Khan H, Metra M, Blair JEA, Vogel M, Harinstein ME, Filippatos GS, Sabbah HN, Porchet H, Valentini G, Gheorghiade M. Istaroxime, a first in class new chemical entity exhibiting SERCA-2 activation and Na-K-ATPase inhibition: a new promising treatment for acute HF syndromes. Heart Fail Rev. 2009 doi: 10.1007/s10741-009-9136-z. [DOI] [PubMed] [Google Scholar]
- 59.Cittadini A, Saldamarco L, Marr AM, Arco M, Carlomagn G, Imbriaco M, DelForno D, Vigorito C, Merola B, Oliviero U, Fazio S, Sacca L. Growth hormone deficiency in patients with chronic HF and beneficial effects of its correction. J Clin Endocrinol Metab. 2009;94:3329–3336. doi: 10.1210/jc.2009-0533. [DOI] [PubMed] [Google Scholar]
- 60.Caminiti G, Volterrani M, Iellamo F, Marazzi G, Massaro R, Miceli M, Mammi C, Piepoli M, Fini M, Rosano GMC. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic HF. J Am Coll Cardiol. 2009;54:919–927. doi: 10.1016/j.jacc.2009.04.078. [DOI] [PubMed] [Google Scholar]
- 61.Vilquin JT. Myoblast transplantation: clinical trials and perspectives. Acta Myol. 2005;24:119–127. [PubMed] [Google Scholar]
- 62.Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, Garrigue S, Kappenberger L, Haywood GA, Santini M, Bailleul C, Daubert JC for the Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. Effects of multisite biventricular pacing in patients with HF and intra-ventricular conduction delay. N Engl J Med. 2001;344:873–80. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 63.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon A, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Messenger J for the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Investigators and Coordinators. Double-blind, randomized controlled trial of cardiac resynchronization in chronic HF. N Eng J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 64.Grassi G, Vincenti A, Brambilla R, Trevano FQ, Dell’Oro R, Trocino G, Vincenzi A, Mancia G. Sustained sympathoinhibitory effects of cardiac resynchronization therapy in severe HF. Hypertension. 2004;44:727–31. doi: 10.1161/01.HYP.0000144271.59333.a7. [DOI] [PubMed] [Google Scholar]
- 65.Corra U, Mezzani A, Giannuzzi P, Tavazzi L. Chronic heart failure-related myopathy and exercise training: a developing therapy for HF symptoms. Prog Cardiovsc Dis. 2002;44:157–72. doi: 10.1053/pcad.2002.127490. [DOI] [PubMed] [Google Scholar]
- 66.McKelvie RS, Teo KK, McCartney N, Humen D, Montague T, Yusuf S. Effects of exercise training in patients with congestive HF: A critical review. J Am Coll Cardiol. 1995:789–96. doi: 10.1016/0735-1097(94)00428-S. [DOI] [PubMed] [Google Scholar]
- 67.Ventura-Clapier R, Mettauer B, Bigard X. Beneficial effects of endurance training on cardiac and skeletal muscle energy metabolism in HF. Cardiovascular Res. 2007;73:10–18. doi: 10.1016/j.cardiores.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Roveda F, Middlekauff HR, Reis S, De Souza M, Barretto AC, Rondon MUB, Nastari L, Krieger EM, Negrao CE. Effects of exercise training on neurovascular control during exercise in HF patients. J Am Coll Cardiol. 2003;42:854–60. doi: 10.1016/s0735-1097(03)00831-3. [DOI] [PubMed] [Google Scholar]
- 69.Varin R, Mulder P, Richard V, Tamion F, Devaux C, Henry JP, Lallemand F, Lerebours G, Thuillez c. Exercise improves flow-mediated vasodilatation of skeletal muscle arteries in rats with chronic HF. Role of nitric oxide, prostanoids, and oxidant stress. Circulation. 1999;99:2951–7. doi: 10.1161/01.cir.99.22.2951. [DOI] [PubMed] [Google Scholar]
- 70.Ennezat PV, Malendowicz SL, Testa M, Colombo PC, Cohen-Solal A, Evans T, LeJemtel TH. Physical training in patients with chronic HF enhances the expression of genes encoding anti-oxidative enzymes. J Am Coll Cardiol. 2001;38:194–8. doi: 10.1016/s0735-1097(01)01321-3. [DOI] [PubMed] [Google Scholar]
- 71.Coats AJ, Adamopoulos S, Radaelli A, McCance A, Meyer TE, Bernardi L, Solda PL, Davey P, Ormerod O, Forfar C. Controlled trial of physical training in chronic HF. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation. 1992;85:2119–31. doi: 10.1161/01.cir.85.6.2119. [DOI] [PubMed] [Google Scholar]
- 72.Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic HF. Circulation. 2007;115:3042–4. doi: 10.1161/CIRCULATIONAHA.106.677989. [DOI] [PubMed] [Google Scholar]