Abstract
Two former biologists play at dice. In the center of the table there are several banknotes from a prize they had won a few years before they dropped out of science. The rule of the game is that each player gets a banknote whenever he correctly predicts how many throws it will take after throwing a 6 to throw the next 6. One of the two players, a former theoretical biologist, remembers that the frequency of throwing a 6 is one in six, so he always foretells that the waiting period will be 6. The other player's cause for failing in science was opposite: he believed in superstitions. As his lucky number is three, he guesses after each 6 that the next 6 will occur three throws later. Which of the two fellows will recover more from the prize money? And is there a waiting period that could be predicted that would make more money?
Waiting periods in molecular biology
I faced the equivalent problem during my diploma thesis in Professor Charles Weissmann's laboratory 15 years ago. I had not been thrown out of science and there was no money involved. My problem was to clone the hamster Scrapie Prp gene, but despite many controls and for reasons that will probably remain unknown forever, I could not identify a genomic Prp clone in the various phage libraries I made. As one of the positive controls, I monitored each time the conditions of hybridization by adding a Southern blot representing a few lanes of digested genomic hamster DNA. Hence I was reminded repeatedly that a large portion of the Prp gene was located either on a 3.0 kb EcoRI or a 7.0 kb HindIII fragment of the hamster genome. Desperate to succeed, I decided to clone at least one of these restriction fragments by making a new library from size-selected genomic fragments. Which fragment should I choose? For which fragment would a size selection (of say a 1 kb interval) be most effective and grant the highest enrichment? This led to the following more general problem. If cellular DNA is digested to completion with a restriction endonuclease generating a population of DNA fragments of different lengths, which size class is most abundant? There are, as always, three approaches to handling such a problem. I was pursuing each of them. One way to go forward is to ignore the question and just carry out the experiment. I cloned both fragments successfully and completed my diploma thesis (Basler et al., 1986). Alternatively, the question can be taken up from the theoretical side, or it can be addressed experimentally.
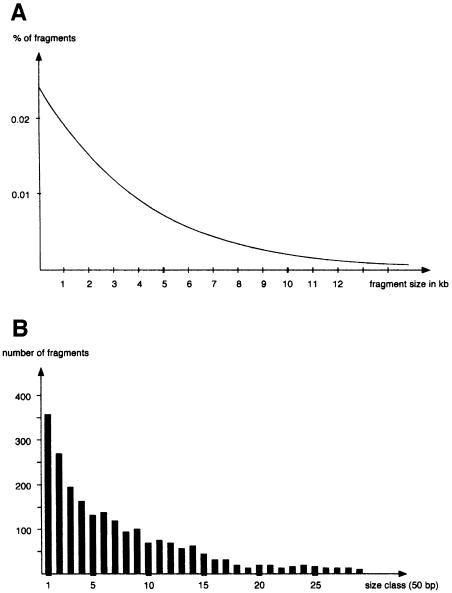
If a very long DNA strand of random sequence is scanned for the occurrence of a particular restriction site, what is the most frequent distance between two sites, or in other words, what are the waiting periods from leaving one site to arriving at the next? The probability p that at a certain position the given recognition sequence of r bases will be encountered is p = (1/4)r (e.g. 1/4096 for a hexa-nucleotide recognition sequence). How many fragments of a specific length n can be expected after complete digestion? The probability F(n) that the recognition pattern is occurring exactly n nucleotides 3′ of a certain position (moving 5′ to 3′) is F(n) = (1–p)n–1 ⋅ p. The first part of the formula expresses the condition of the site not occurring for the first n–1 bases. The value for the probability F(n) of generating a distinct size fragment of length n is, of course, also the value for the fraction of fragments with size n within the total population of fragments. The percentage of fragments of any length generated by an endonuclease with a 6–base recognition sequence is shown in Figure 1A, and reveals a counter-intuitive result: the most probable and frequent restriction fragments are predicted to be the smallest. The most abundant EcoRI fragment therefore corresponds to the one with length 6 (to be precise: fragments of lengths 6–11 are equally abundant due to the finite length of the EcoRI site). The larger the fragment, the fewer fragments will be in its size class and hence the more efficiently it should be enriched by size fractionation. This conclusion can be understood easily by considering the above game of dice. What is the most probable waiting period between two 6s? The answer is 1. It is more probable that another 6 will be thrown in the next attempt than that a 6 will be thrown precisely in the sixth attempt. The probability that a 6 occurs in the next throw is 1/6. The probability that a 6 occurs in six attempts (neither earlier nor later) is: (5/6)5 ⋅ 1/6, which is a number definitively smaller than 1/6.
Fig. 1. Distribution of restriction fragment lengths. (A) Fraction (as a percentage) of fragments with size n within the total population of fragments generated by an endonuclease with a 6–base recognition sequence. (B) Number of fragments, grouped in size classes of 50 bp, present in the first 939 848 nucleotides of GenBank (1985) after virtual digestion with MaeI (an endonuclease with a 4–base recognition sequence).
To test this prediction empirically, I wanted to join all DNA sequences that were available in GenBank in 1985 and scan this virtual genome for a particular restriction site. Luckily, Professor Martin Billeter had just obtained the latest IBM computer system a few weeks earlier, with software installed by a US company called DNA STAR and the entire GenBank database on its hard disc. This equipment was the institute's pride and joy. With my limited knowledge of computer programming, I wrote a short BASIC program that would join all DOS files to one large string of DNA sequence. This string was then scanned for MaeI sites (with a 4–bp recognition sequence) and each fragment would score a hit in its particular size class (50 bp intervals). On a Sunday afternoon I sneaked to the DNA STAR computer system, installed the program and made it run. It worked! The size classes filled in (Figure 1B), and showed the same result as expected from the theoretical considerations! I was extremely pleased. Unfortunately, my feelings abruptly changed when I found out that my program had accidentally deleted each and every GenBank entry on the computer system. Now I was in big trouble. I had not asked for permission to use the equipment (and there was a special introductory training required before being allowed to do so). And as a student I may not have been allowed to use it even with provable knowledge. It should be mentioned, for people who have never worked in the Institute of Molecular Biology at the University of Zurich, that the heads of the institute played a very authoritarian and intimidating role at the time. I was really going to have big difficulties. I suddenly wished that I had studied something else, anything else, just not molecular biology.
I am not going to reveal here the secret of how I got out of trouble. I would like to point out, however, that this incident was not the reason for me switching to developmental biology and doing my PhD with Ernst Hafen in the Zoology Institute. On the contrary, some may have noticed that I am now a member of the Molecular Biology Institute, so it must have had a happy ending.
I was asked to write an autobiographical review on my scientific activities. To evade the difficult task of going back chronologically and giving an account of how everything took its course over the past 15 years, I decided to deal with the biographical aspect by describing two very early events that must have had a motivating influence on my career. The first one is my launch into the waters of molecular biology (diploma thesis with Charles Weissmann, see above). The second event, equally stimulating, is my work on Sevenless with Ernst Hafen (represented here by my recollections of an EMBO-sponsored student course in Spetsai, see below). To comply with the scientific part I will then discuss from a completely different perspective some optimistic and some realistic aspects of our current research on organizers and positional information.
An early instructive signal from Spetsai
Spetsai, Greek islands, late summer in 1987, 10 months after I began my thesis on the role of Sevenless in Drosophila eye development. It was my first conference abroad. I was very proud of my first poster that I would present. I had spent a lot of time composing it. My itinerary via Athens brought me to Spetsai a day before the meeting started. I was simultaneously intimidated and excited about the caliber of the speakers that I would meet for the first time (such as S.Artavanis-Tsakonas, A.Bradley, T.Caskey, W.Gehring, P.Gruss, A.McLaren, G.Morata, R.Palmiter, W.Schaffner and many more). The accommodation for students consisted of a deteriorating former boarding school beautifully situated outside the main village. Most of my Swiss colleagues had not yet arrived. I was able to get hold of the keys to the dormitories and wrote small, but official looking notes, which I placed in their rooms. These memos advised each of them to prepare a short lecture on a given subject and to contact the corresponding speaker to coordinate the time for their presentation (for each case, I had chosen the most intimidating speaker in the respective field). It took them a few days into the conference and a few restless nights until my colleagues learned that their preparations were unnecessary.
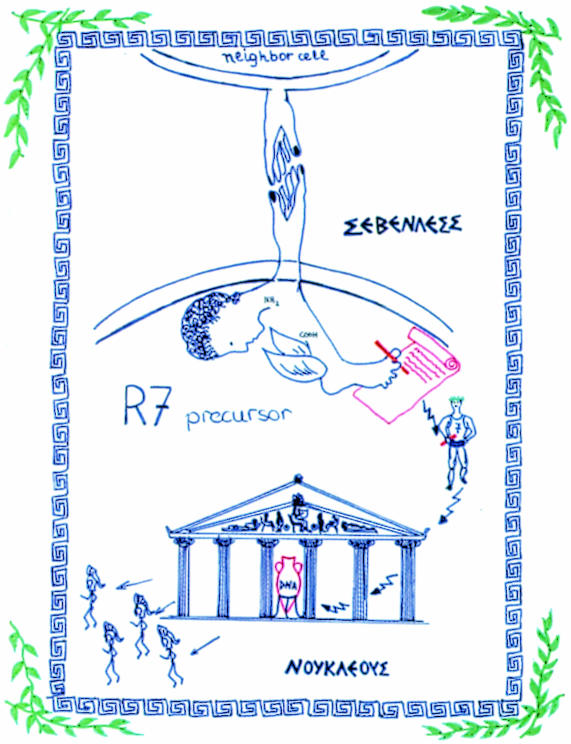
The conference was phenomenal in every respect. We had fascinating workshops on beaches that could only be reached by boats, wonderful dinners in open ‘Klimaterias’, and non-scientific late night programs causing a perpetual sleep shortage. Ironically, a handful of participants, including myself, were eventually selected to give an oral presentation of their work—ad hoc, with a single day of preparation, no slides, only a few empty transparencies for an overhead projector. I was terribly nervous. Fortunately, there was Lianna, the daughter of the local organizer, who had already been instrumental in giving me the keys to the dormitories. She was a talented artist. I explained to her the role of Sevenless and talked her into drawing a model that I could present in my talk (Figure 2). When I look at this scheme today, I realize how differently we were conceiving the role of Sevenless in determining the fate of the R7 photoreceptor cell during eye development at the time. The ligand Boss—which had at best only been postulated at the time—handing over the signal from the neighboring R8 cell to the presumptive R7 cell would activate the Sevenless receptor tyrosine kinase with a handshake. This message was transmitted by marathon runners from the cell surface to the ‘temple of chromosomes’ (the signal transduction pathway of receptor tyrosine kinases was not yet known then). Numerous warriors were emitted in response to the message to enforce the R7 specific developmental program. The diagram illustrates nicely our conviction that the Boss/Sevenless signal would be highly instructive (Basler and Hafen, 1988). It would take a few more years, and the discovery of the Ras-MAPK signal transduction cassette, before the specificity of the Boss/Sevenless signal was challenged. A decade later, the prevailing view is that such signals exhibit a shockingly low information content and that the specific response to such a signal (e.g. R7 development) is essentially determined by the properties of the receiving cell, which in turn are a function of the developmental history the cell has undergone (Freeman, 1997).
Fig. 2. Model of the role of Sevenless in 1987.
Positional information
The present model: compartment boundaries stabilize organizers
Since the beginning of the century, positional information has been proposed to play a central role in pattern formation during development. The simplest view implies that positional information is sent out in the form of extracellular signals by special cell groups. Such cell groups have been called ‘organizers’, because they have the ability to influence the fate of surrounding cells. A major break-through in developmental biology has been the ability to manipulate positional information. Originally restricted to experimental systems that permit implantation and transplantation experiments, the advent of more and more sophisticated transgenes has also allowed the exposure of cells in small model organisms to altered positional information. In Drosophila, I had already encountered the deductive power of altering positional information in experiments with Sevenless. Although we had no access to the positional information itself (the ligand Boss), we could alter the ability of cells to recognize positional information (Basler and Hafen, 1989), or bypass the latter by the use of a constitutively active receptor (Basler et al., 1991). But with the beginning of the 1990s the techniques for studying positional information became even more powerful with the advent of the FLP recombination method and the Gal4/UAS system. As described above, it also became increasingly clear that in many instances positional information consisted merely of a temporal trigger with relatively low information content (possibly only 1 bit). The search was on for signals that would specify more than only two states, and that would act over the distance of several cell diameters to do so. It was not so much the search for such molecules, since many secreted signaling molecules were known from genetic analysis. It was more a question of developing the tools and tests that would allow the range of action of, and the number of responses to, candidate signals to be assessed.
Rather than going through the strategies and experiments that have been applied to proving the existence of morphogen gradients and establishing the role of compartment boundaries, I would like to discuss the logic of the organizer that specifies the antero-posterior (A/P) axis of the Drosophila wing. The beauty of the A/P wing system is that the way the organizer becomes installed and how it functions is very simple. In fact, it was my colleague Markus Noll, a trained physicist, who suggested to me that the mechanism by which the A/P organizer is generated might reflect its evolution and hence is likely to have been selected for its simplest possible design. Let me first discuss this design from Noll's perspective and subsequently consider a few examples where the real system deviates from this theoretically simplest design.
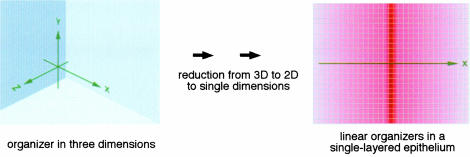
The simplest configuration of an organizer: a morphogen acting along a single dimension. The most drastic simplification that the Drosophila limb system appears to exploit is the reduction of dimensionality. Although Drosophila limbs (legs and wings) are three-dimensional organs, the patterning processes underlying the development of these organs take place in two-dimensional, single-layered cell populations (imaginal discs). It can be shown quantatively by theoretical considerations that dimensional reduction provides a dramatic advantage for biological systems to handle patterning problems that are based on diffusion processes (Adam and Delbrück, 1968).
The simplest way to pattern a two-dimensional tissue would be to break it down further into two one-dimensional systems. The most simplistic way to achieve this would be the use of independent organizer systems for the x and y axes. Instead of using point sources, linear organizer ‘stripes’ can be used such that each organizer has to operate only in a single dimension, as if it were part of a Cartesian coordinate system (Figure 3; Meinhardt, 1983).
Fig. 3. Reduction of dimensionality as the most effective means to simplify the task of an organizer.
The least complicated way by which such a simplified organizer could control pattern formation along one axis is the use of a morphogen gradient. Cells discover where they are along the axis and differentiate accordingly, by referring to the concentration of the morphogen substance. If a cell is close to the organizer it will experience a high concentration of morphogen and activate (or repress) one set of genes, but if further away it will experience a lower concentration and activate (or repress) a different set (Wolpert, 1969).
Thus, with a few key principles, the task of an organizer to pattern a complex tissue is significantly reduced: the reduction of dimensionality, the use of linear organizers and the premise that each of them acts via a single signaling molecule (morphogen).
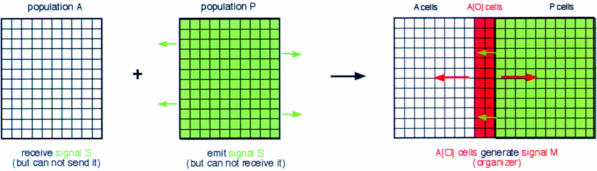
The simplest way of generating a linear organizer: two interacting cell populations. The prerequisite for setting up a linear organizer appears to consist of two adjacent cell populations that differ in their abilities to send and receive an inducing signal. One population (arbitrarily named ‘P cells’ here) is able to emit this signal, the other population (‘A cells’) is able to receive it (Figure 4).
Fig. 4. The simplest way to generate an organizer: the apposition of two different cell populations that differ in their ability to send or receive the short-range signal S results in the generation of long-range signal M.
Two properties suffice to delimit the response to this signal to only a stripe of cells at the interface of the two cell populations. First, the signal sent by P cells should not be able to diffuse over a large distance, such that only those A cells that are closest to the P cells can receive it (this subset of cells is designated ‘A[O]’ cells here, ‘O’ for organizer). Secondly, P cells that produce the signal may not be able to receive it, while A cells that receive it may not be able to generate it.
The A[O] cells are the organizer cells. They generate a long-range signal, M, which diffuses along the surface of the two-dimensional cell population and is received by both A and P cells (Figure 4). A and P cells respond to M differently (as they are already different from each other to begin with), and A and P cells respond differently at different distances from the A[O] cells.
This simple set-up of two cell populations, one emitting a signal, the other receiving it, is enough to explain the generation of a simple, linear organizer (cf. Lawrence and Struhl, 1996).
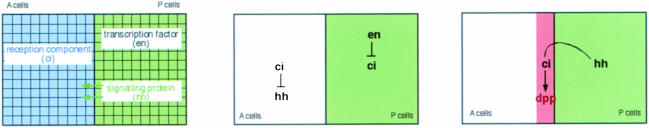
The simplest way to implement molecularly such a system. The very simplest way of making the two cell populations different from each other would be if one population expresses a transcription factor that the other does not express, let's say P cells express the factor ‘En’ and A cells do not. Based on this asymmetry, P cells somehow turn on another gene (hh), which encodes the short-range signal. Since only A, and not P, cells should be able to respond to the signal, a third gene (ci) is required that is ‘on’ in A cells but ‘off’ in P cells. The simplest way of achieving this is by having En repress ci, which is constitutively expressed in the absence of En. The presence of the ci gene product makes A cells competent to respond to the Hh signal, and its absence prevents P cells from responding to Hh (Figure 5).
Fig. 5. The minimal set-up to implement molecularly the generation of a linear organizer (see text for details).
The simplest way to achieve only P, and not A, cells being able to send the Hh signal is if Ci represses the generation of the Hh signal (being constitutively active in the absence of Ci) or if En activates hh directly.
Generating a morphogenetic signal in A[O] cells is simplest if these cells secrete another signal (Dpp) whose synthesis depends on the gene that is ‘on’ in A cells but ‘off’ in P cells (i.e. ci) and on the reception of the Hh signal.
Once the organizer is set up and carrying out its function [i.e. providing surrounding cells with positional information (Dpp)], cells have to recognize this information and translate it into different cell fates. One simple way this could be achieved is if different concentrations of the morphogen lead to a different degree of activation of its signal transduction pathway, ultimately establishing different activity states, or different nuclear levels, of a transcription factor. These quantitative differences are then converted into qualitatively different gene expression patterns by target genes that are turned on or off when a particular transcriptional threshold activity is met (Gurdon, 1998).
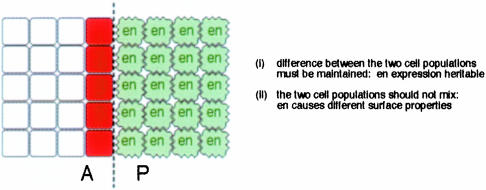
The simplest way of maintaining the organizer. So far, the facts that the organizer operates in a growing tissue and the final size of the tissue must be coordinated with the patterning activity of the morphogen have been ignored. The least complicated way to achieve this would be if one signal (Dpp) controlled both growth and patterning. Growth could continue, for example, until the slope of the morphogen gradient falls below a certain value. The notion that the morphogen controls growth as well as pattern necessitates, however, that the organizer functions over a long time period (the growth of the Drosophila imaginal discs, for example, occurs over a period of several days). Therefore, it is necessary that the organizer is stably maintained in a straight stripe. Two properties are required for this stability (Figure 6), as follows.
Fig. 6. The prolonged function of the organizer requires a maintenance system which ensures that the position and shape of the morphogen source is stably maintained during development. This is achieved by the establishment of a compartment boundary.
(i) The difference between the two cell populations must be maintained. The simplest way this could be achieved would be if the expression of the transcription factor En is heritable.
(ii) The two cell populations A and P should not mix, but should minimize their contact, preventing the generation of a wiggly, zig-zag-like border between them. The simplest way to achieve this would be if En programs P cells to exhibit different surface properties to A cells, which in turn would prevent A and P cells from mixing with each other (Figure 6).
The reality
Experiments from a number of laboratories indicate that the organizer controlling antero-posterior pattern formation in the Drosophila wing is nearly as simple as this simplest possible mechanism described above. There are, however, a few instances where the reality differs from the model by being slightly more complicated. These violations may reflect the evolutionary constraints of this organizer system or, in some cases, may be part of a backup system for maximal stability.
en becomes Hh inducible. The most severe violation is the late expression pattern of Engrailed (En). Although en expression is generally heritable and confined to P cells, en becomes expressed in a narrow zone of A cells along the A/P boundary at late stages of wing disc development (Blair, 1992). This A-specific expression of en is induced by high levels of Hedgehog (Hh) signaling (Strigini and Cohen, 1997) and manifests itself in the suppression of a few innervated sensory bristles at the distal tip of the wing (Hidalgo, 1994). It is an unresolved question why a potential minor advantage for flight performance would justify such a severe transgression of the compartmental organizer principle. The late En expression does not, however, appear to upset the circuitry or function of the A/P organizer, presumably because hh expression at this stage became heritable on its own and does not follow En, and because the affinity boundary that prevents A and P cells from intermixing is maintained by Hh signaling despite the anterior expression of En (Dahmann and Basler, 2000).
Cubitus interruptus (Ci) is not the only repressor of hh expression. As discussed above, hh expression is indeed confined to P cells through the repressor activity of Ci (Dominguez et al., 1996). However, in A cells that lack ci function, hh is only expressed at low levels (Méthot and Basler, 1999). Hence the absence of Ci in P cells cannot fully explain the high levels of hh expression in P cells. One possibility (which violates, however, the simple model) could be the existence of another repressor of hh whose expression is also negatively regulated by En.
dpp expression is controlled by Hh and En. Although Dpp nearly complies with the theoretical predictions, it does not do so completely. dpp is not only regulated by Ci and Hh signaling, but is also actively repressed in P cells by En (Méthot and Basler, 1999), an apparent redundancy since P cells do not express Ci and hence should not turn on dpp. However, the recent genetic analysis of ci indicated that while Ci functions as an activator in Hh-receiving cells to turn on dpp, Ci also functions as a repressor of low, basal dpp expression levels in cells that do not receive the Hh signal (cells further anterior; Méthot and Basler, 1999). Since the dpp gene is endowed with a constitutive, basal enhancer and since there is no Ci repressor in P cells, En fulfills the repressor function there to prevent ectopic organizer activity.
Hh also contributes to patterning. Anterior cells in the vicinity of the compartment boundary—the cells in the center of the wing—become patterned by the Hh signal, not by Dpp (Strigini and Cohen, 1997). This is not predicted by the simple model above. However, given the relatively broad stripe of dpp-expressing cells observed in vivo, it makes sense that diversity among them must be generated by the upstream signal Hh, as the positional information within the morphogen source is presumably uniform.
Hh and En contribute to the difference in cell affinities between A and P cells. The simplest way to keep the boundary between A and P cells straight, and hence an even shape of the linear Dpp morphogen source, would be if En programmed cells to sort out from non-En-expressing cells (Morata and Lawrence, 1975). While this is precisely what is observed, a significant portion of the segregation behavior of A versus P cells is mediated via the Hh signal. Hh-receiving cells sort out from non-Hh-receiving cells (Blair and Ralston, 1997; Rodriguez and Basler, 1997). It is not obvious why En utilizes Hh-dependent as well as Hh-independent branches to maintain the compartment boundary (Dahmann and Basler, 2000).
The Dpp gradient is first translated into a counter-gradient of Brinker. Although Dpp target genes such as optomoter-blind (omb) and spalt appeared to support the simple model of being turned on directly by different threshold activities of Dpp signaling (Nellen et al., 1996), it turns out that the first and probably most direct read-out of the Dpp morphogen gradient is the establishment of a counter-gradient of Brinker expression (Campbell and Tomlinson, 1999; Jawiska et al., 1999). Brinker functions as a transcriptional repressor of omb and spalt expression, antagonizing the graded, positive transcriptional activity of Mad, and thus critically contributing to the nested expression pattern of these genes. The employment of two counter-gradients of opposing transcriptional activities might help to define sharp expression domains of target genes.
Cytonemes may mediate the long-range action of the Dpp morphogen. Recently it was discovered that wing-disc cells project long, thin cytoplasmic extensions towards the A/P boundary (Ramírez-Weber and Kornberg, 1999). Although the simplest model predicts that the Dpp morphogen gradient is established over a long range by the extracellular movement of the ligand, it is possible that the long-range activity of Dpp is mediated by these cytonemes. Dpp ligand, or an intracellular mediator, may travel inside the cytonemes to the body of lateral cells. Thus, like axons, cytonemes may be involved in conveying signals over large distances. It should be pointed out that the role of cytonemes in mediating organizer functions has not been established. But it is clear that if cytonemes are involved in conveying positional information, then reality is significantly more complicated than anticipated.
The future
The A/P organizer represents a very simple prototype for an organizer. It consists of a stripe of cells expressing the Dpp morphogen. This stripe is induced by the apposition of two cell populations: a Hh-secreting and a Hh-responsive one. This asymmetry maintains a flow of Hh signal from P to A cells that results in Dpp expression and the continuous segregation of A and P cells. Despite its simplicity, there are a few questions for which there are no definitive answers yet. I would like to mention four.
(i) The first of these concerns the role of cytonemes. Are these structures conduits for positional information? Are cytonemes the solution for the long-standing debate on whether diffusion of extracellular molecules can be the basis for morphogen gradients? If not, the question shifts: what then is their purpose?
(ii) Another fundamental cellular mechanism is still unsolved: the molecular basis for compartmental cell segregation. Despite our advances in uncovering the genes required for the A/P and dorso-ventral (D/V) systems, one class of genes has remained elusive: the cell surface molecules that are thought to be responsible for the segregation of cells of opposite compartments. Are there different molecules involved at each boundary? How are the activities of different compartment systems (such as A/P and D/V) kept independent of each other, such that a cell can simultaneously belong to different subpopulations? The discovery of the first molecules controlling these processes will represent a fundamental advance in cell and developmental biology.
(iii) Despite the discovery of several morphogen systems, the number of distinct positional values that can be specified by a single morphogen remains a controversial issue. Although a distinction was made above between a temporal or positional ‘trigger’ with an information content of two states (1 bit for Boss) and a positional information system based on a morphogen gradient, there are only a handful of different responses known to a morphogen (2–3 bits for Dpp). If the number of threshold responses remains as low as it appears today, it will be a challenge to explain how a sequence of innumerable pattern elements in a vertebrate or insect limb, for example, can be generated along one axis.
(iv) Finally, while the above-described coupling of compartment boundaries and organizers appears to be a recurring theme during pattern formation of insects, it will be a challenging task to test whether compartmentalization serves a similar function in vertebrates. Examples exist for boundaries that appear to be stabilized by cell segregation mechanisms, yet in none of the cases have they been unambiguously linked to the stabilization of an organizer that conveys positional information.
Given the number of problems that need to be solved and the rate at which new questions arise, there should be enough to do to keep me and my colleagues firmly in science. Yet, given the low predictive value of models and intuitions, it may be good advice to spend the prize money right away.
Acknowledgments
Acknowledgements
I am deeply indebted to my mentors and collaborators Charles Weissmann, Ernst Hafen, Tom Jessell, Gary Struhl and Markus Noll.
References
- Adam G. and Delbrück,M. (1968) Reduction of dimensionality in biological diffusion processes. In Rich,A. and Davidson,N. (eds), Structural Chemistry and Molecular Biology. W.H. Freeman & Co. [Google Scholar]
- Basler K. and Hafen, E. (1988) Control of photoreceptor cell fate by the sevenless protein requires a functional tyrosine kinase domain. Cell, 54, 299–311. [DOI] [PubMed] [Google Scholar]
- Basler K. and Hafen, E. (1989) Ubiquitous expression of sevenless: position-dependent specification of cell fate. Science, 243, 931–934. [DOI] [PubMed] [Google Scholar]
- Basler K., Oesch, B., Scott, M., Westaway, D., Wälchli, M., Groth, D.F., McKinley, M.P., Prusiner, S.B. and Weissmann, C. (1986) Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell, 46, 417–428. [DOI] [PubMed] [Google Scholar]
- Basler K., Christen, B. and Hafen, E. (1991) Ligand-independent activation of the sevenless receptor tyrosine kinase changes the fate of cells in the developing Drosophila eye. Cell, 64, 1069–1081. [DOI] [PubMed] [Google Scholar]
- Blair S.S. (1992) Engrailed expression in the anterior lineage compartment of the developing wing blade of Drosophila.Development, 115, 21–33. [DOI] [PubMed] [Google Scholar]
- Blair S.S. and Ralston, A. (1997) Smoothened-mediated Hedgehog signalling is required for the maintenance of the anterior–posterior lineage restriction in the developing wing of Drosophila.Development, 124, 4053–4063. [DOI] [PubMed] [Google Scholar]
- Campbell G. and Tomlinson, A. (1999) Transducing the Dpp morphogen gradient in the wing of Drosophila: regulation of Dpp targets by brinker. Cell, 96, 553–562. [DOI] [PubMed] [Google Scholar]
- Dahmann C. and Basler, K. (2000) Opposing transcriptional outputs of Hedgehog signaling and Engrailed control compartmental cell sorting at the Drosophila A/P boundary. Cell, 100, 411–422. [DOI] [PubMed] [Google Scholar]
- Dominguez M., Brunner, M., Hafen, E. and Basler, K. (1996) Sending and receiving the Hedgehog signal: control by the Drosophila Gli protein Cubitus interruptus. Science, 272, 1621–1625. [DOI] [PubMed] [Google Scholar]
- Freeman M. (1997) Cell determination strategies in the Drosophila eye. Development, 124, 261–270. [DOI] [PubMed] [Google Scholar]
- Gurdon J.B., Cyson, S. and St Johnston, D. (1998) Cells' perception of position in a concentration gradient. Cell, 95, 159–162. [DOI] [PubMed] [Google Scholar]
- Hidalgo A. (1994) Three distinct roles for the engrailed gene in Drosophila wing development. Curr. Biol., 4, 1087–1098. [DOI] [PubMed] [Google Scholar]
- Jawiska A., Kirov, N., Wieschaus, E., Roth, S. and Rushlow, C. (1999) The Drosophila gene brinker reveals a novel mechanism of Dpp target gene regulation. Cell, 96, 563–573. [DOI] [PubMed] [Google Scholar]
- Lawrence P.A. and Struhl, G. (1996) Morphogens, compartments and pattern: lessons from Drosophila? Cell, 85, 951–961. [DOI] [PubMed] [Google Scholar]
- Meinhardt H. (1983) Cell determination boundaries as organizing regions for secondary embryonic fields. Dev. Biol., 96, 375–385. [DOI] [PubMed] [Google Scholar]
- Méthot N. and Basler, K. (1999) Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. Cell, 96, 819–831. [DOI] [PubMed] [Google Scholar]
- Morata G. and Lawrence, P.A. (1975) Control of compartment development by the engrailed gene in Drosophila.Nature, 255, 614–617. [DOI] [PubMed] [Google Scholar]
- Nellen D., Burke, R., Struhl, G. and Basler, K. (1996) Direct and long-range action of a DPP morphogen gradient. Cell, 85, 357–368. [DOI] [PubMed] [Google Scholar]
- Ramírez-Weber F.-A. and Kornberg, T.B. (1999) Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell, 97, 599–607. [DOI] [PubMed] [Google Scholar]
- Rodriguez I. and Basler, K. (1997) Control of compartmental affinity boundaries by Hedgehog. Nature, 389, 614–618. [DOI] [PubMed] [Google Scholar]
- Strigini M. and Cohen, S.M. (1997) A Hedgehog activity gradient contributes to AP axial patterning of the Drosophila wing. Development, 124, 4697–4705. [DOI] [PubMed] [Google Scholar]
- Wolpert L. (1969) Postional information and the spatial pattern of cellular differentiation. J. Theor. Biol., 25, 1–47. [DOI] [PubMed] [Google Scholar]