Abstract
Background
Giant cell arteritis (GCA) is a granulomatous vasculitis of the aorta and its branches that causes blindness, stroke and aortic aneurysm. CD4 T-cells are key pathogenic regulators, instructed by vessel wall dendritic cells (DC) to differentiate into vasculitic T cells. The unique pathways driving this DC-T-cell interaction are incompletely understood but may provide novel therapeutic targets for a disease in which the only established therapy is chronic treatment with high doses of corticosteroids.
Methods and Results
Immunohistochemical and gene expression analysis of GCA-affected temporal arteries revealed abundant expression of the NOTCH receptor and its ligands Jagged1 and Delta1. Cleavage of the NOTCH intracellular domain (NICD) in wall-infiltrating T cells indicated ongoing NOTCH pathway activation in large vessel vasculitis. NOTCH activation did not occur in small vessel vasculitis affecting branches of the vasa vasorum tree. We devised two strategies to block NOTCH pathway activation; γ-secretase inhibitor treatment, preventing nuclear translocation of the NICD, and competing for receptor-ligand interactions through excess soluble ligand, Jagged1-Fc. In humanized mice carrying human arteries NOTCH pathway disruption had strong immunosuppressive effects, inhibiting T-cell activation in the early and established phase of vascular inflammation. NOTCH inhibition was particularly effective in downregulating Th17 responses, but also markedly suppressed Th1 responses.
Conclusions
Blocking NOTCH signaling depleted T cells from the vascular infiltrates, implicating NOTCH-NOTCH ligand interactions in regulating T-cell retention and survival in vessel wall inflammation. Modulating the NOTCH signaling cascade emerges as a promising new strategy for immunosuppressive therapy of large vessel vasculitis.
Keywords: Arteries, Inflammation, T-cell, NOTCH, Costimulation, IFN-γ, IL-17
Introduction
Giant cell arteritis (GCA) is characterized by intramural and perivascular granulomatous lesions that destroy the vascular wall structure and induce luminal occlusion through fast and concentric neointimal outgrowth.1 Clinical manifestations include blindness, stroke, and aortic aneurysm, and arterial inflammation is almost always combined with a syndrome of severe systemic inflammation.2
Vascular lesion formation is mediated by a maladaptive immune response, characterized by in situ activation of CD4 T-cells.3CD4 T-cells receive activating signals from tissue-resident vascular dendritic cells (vDC).4, 5 Apart from the strength of the antigen:T-cell receptor (TCR) signal, the microenvironment and accessory signals derived from the antigen-presenting cell (APC) are critical in T-cell activation. APC surface receptors costimulate or coinhibit TCR-mediated signals and ultimately shape the outcome of the T-cell activation cascade.
The current project has examined how NOTCH-NOTCH ligand interactions affect T-cell activation in vasculitis, with the goal of targeting such interactions therapeutically. NOTCH is critically involved in lymphocyte development 6; its over-expression in T-cell-acute lymphoblastic leukemia points to a potential central position for the pathway in regulating T-cell growth. Experimental data suggest cross-talk between the TCR signaling cascade and the canonical NOTCH signaling pathway.7 Signal transduction in the NOTCH pathway8 is initiated by ligand binding which leads to two proteolytic cleavage processes, catalyzed by ADAM metalloproteases and γ-secretase, respectively.9 The latter cleavage liberates the NOTCH intracellular domain (NICD) facilitating its nuclear translocation and induction of target genes, such as Hairy enhancer of split (Hes).10
As the instigator of T-cell activation in GCA is unknown current therapies are restricted to long-term high doses of corticosteroids. Interfering with in situ T-cell activation in the vascular microenvironment emerges as an attractive alternative. Here, we report that in humanized mice carrying human arteries and human T cells, NOTCH pathway blockade has profound implications, suppressing vessel wall inflammation, cytokine production and T-cell accumulation. Blockade of the NOTCH pathway using the γ-secretase inhibitor (GSI) N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT), and the soluble NOTCH ligand Jagged1-Fc, effectively inhibits vascular inflammation. NOTCH-targeted immunosuppression is effective during the early and established phase of the disease process, opening the possibility for novel therapeutic interventions in treating GCA.
Methods
Tissues and Cells
GCA-affected temporal arteries were derived from diagnostic biopsies. Normal human temporal and axillary arteries were collected from early postmortem tissues. Peripheral blood mononuclear cells (PBMC) were isolated from healthy donors and patients freshly diagnosed with GCA. CD4+ T-cells were positively selected with CD4 microbeads (Miltenyi Biotec). Monocyte-derived DCs were generated from CD14+ monocytes by 6-day culture in 1000 U/ml GM-CSF and 800 U/ml IL-4.
All protocols were approved by the Institutional Review Board and informed consent was obtained.
Antibodies and Reagents
Antibodies to human CD4-FITC and CD25-APC were purchased from BD PharMigen (San Diego, CA); anti-human NOTCH1-PE (clone 527425) was obtained from R&D systems. Anti-NOTCH1 (ab27526; Abcam) was used for extracellular detection of NOTCH1. Antibodies against NICD (ab8925; Abcam) were used for flow cytometry and immunohistochemistry. Goat-anti-rabbit-Alexa 488 was purchased from Stem Cell Technologies. The recombinant Jagged1-Fc fusion protein was manufactured by R&D systems. DAPT and LPS (Escherichia coli, 0127:B8) were obtained from Sigma-Aldrich (St. Louis, MO).
Proliferation Assay
CFSE-loaded T-cells preincubated with 10 μM DAPT or carrier DMSO were stimulated with plate bound α-CD3/CD28 or with monocyte derived DCs (1:10) activated with LPS. Proliferation was measured by flow cytometry on day 3. Alternatively, T-cells were cultured (2×105 cells/well) with plate bound α-CD3/CD28 (1 μg/ml) Abs and Jagged1-Fc (0.25-10 μg/ml) or isotype control IgG. After 48 h cells were pulsed with 1 μCi of 3H thymidine and 24 h later proliferation was determined by 3H thymidine incorporation.
Flow Cytometric Analysis
PBMC were stained with the relevant primary antibodies or appropriate isotype controls (30 min, 4°C). For detection of intracellular molecules, cells were fixed and permeabilized using Cytofix/Cytoperm (BD Biosciences). Permeabilized cells were incubated with anti-NOTCH1 antibody or isotype control followed by FITC-conjugated secondary Ab (30 min, 4°C). Data was acquired using the LSRII instrument (BD Biosciences), and analyzed with FlowJo software (Tree Star, Ashland, OR).
Immunohistochemistry
Immunohistochemical analysis of OCT or paraffin embedded arterial sections followed previously published procedures.4, 11 Isotype-matched primary antibodies were used as controls.
Human Artery–SCID Mouse Chimeras
Chimeras were generated as previously described.12 Three mice implanted with arteries from the same donor were assigned to two treatment arms and a control arm. On day 7 post-implantation mice received LPS (3 μg/mouse i.p.) or PBS. Twenty-four hours later, PBMC (4×107 cells/mouse) were pre-incubated with 10 μM DAPT for 30 min, and adoptively transferred into the chimeras. On the same day, the chimeras received 1 mg DAPT or vehicle (DMSO) i.p. PBMC derived from normal donors who had previously been tested for their ability to cause intrawall infiltrates in human artery-SCID chimeras. Alternatively, PBMC were isolated from patients with biopsy-proven GCA. Mice treated with soluble ligand received T-cells preincubated with 100 μg of Jagged1-Fc or control IgG i.p. on day 8, followed by 100 μg of Jagged1-Fc or control IgG on days 9 and 10. Grafts were harvested on day 15 and shock frozen for RNA isolation, or OCT embedded for immunostaining. For DAPT therapy of established disease, chimeras engrafted with human arteries were injected with LPS (day 7), received an adoptive cell transfer on day 8, and treated with 1 mg DAPT by i.p. injection on days 12-15. Tissues were harvested on day 20. All protocols were approved by the Animal Care and Use Committee.
Quantitative RT-PCR
Total RNA was isolated from human tissues, reverse transcribed and analyzed by real time PCR as described.4 Relative RNA was normalized to 2×105 copies of β-actin. Specific PCR primers are listed in Supplemental Table 1.
Statistical analysis
Statistical methods employed rely on an assumption of normality and homoskedasticity across groups. As data were skewed with large heteroskedasticity, data were first log-base 2-transformed prior to analyses. The data were then averaged over three replicated measurements, where appropriate. ANOVA techniques were used to assess differences between treatment conditions where independence across groups could be assumed (comparison of data obtained from control and GCA samples). Paired t-tests were used to evaluate differences between treatment conditions where responses were expected to be correlated, as in experiments in which chimeras were engrafted with arteries from the same donor. In experiments in which engrafted human arteries were either sham treated, treated with vehicle or treated with NOTCH pathway blockers, paired t-tests were used to first compare sham and vehicle treatment to assess for induction of vascular inflammation. Once this was established, a paired t-test was conducted to compare expression between vehicle and active treatment.
A mixed effects model that assessed a synergistic effect between dose level and treatment condition on expression and that allowed a non-linear relation between dose level and expression was fit to assess whether the effect of treatment conditions (control Fc versus Jagged 1-Fc) varied by dose. Such a model accounted for correlation of responses across dose levels.
All tests were two-sided and conducted with a 0.05 significance level. All analyses were performed in SAS V 9.2.
Results
NOTCH expression in the vascular lesions of giant cell arteritis
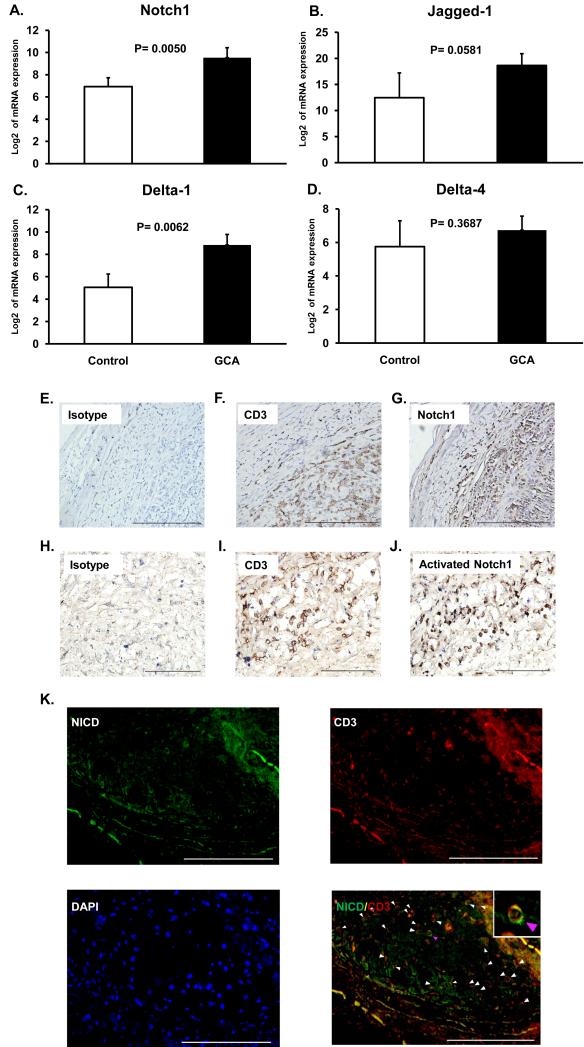
In GCA, granulomatous vessel wall infiltrates are composed of highly activated macrophages and T-cells.13 Intramural T-cells produce IL-2 and IFN-γ, but essentially no IL-4.3 The role of costimulatory receptor-ligand pairs for in situ T-cell activation is unexplored. To examine whether NOTCH1 and its major ligands are expressed in GCA lesions, temporal artery specimens from patients with typical histology were analyzed. NOTCH1 transcripts were 6-fold higher in tissue extracts from GCA arteries than in normal arteries (Figure 1A). Also, the NOTCH ligand Jagged1 was highly abundant, detected at more than 10-fold higher transcript levels in diseased vessels (Figure 1B). Similar to Jagged1, Delta1 transcripts were highly increased in vasculitic arteries (Figure 1C) whereas levels of Delta4-specific sequences were indistinguishable between normal and inflamed arteries (Figure 1D). Cellular analysis of tissue sections revealed dense infiltrates penetrating deep into the media, with almost all mononuclear cells positive for the T-cell marker CD3 (Figure 1F, I). The majority of intramural lymphocytes expressed NOTCH1 receptor (Figure 1G). Staining for the cleaved intracellular domain of NOTCH1 (NICD) (Figure 1J) demonstrated in-situ activation of the NOTCH pathway in the tissue. NOTCH activation in T-cell infiltrates was confirmed at the single cell level by co-staining for NICD and CD3 (Figure 1K). Widespread NICD detection provided confirmatory evidence that cells encountered NOTCH ligands in the tissue environment.
Figure 1. Activated NOTCH1 is abundant in GCA arteries.
RNA was isolated from temporal artery biopsies, which either had no evidence for inflammation (Control) or showed granulomatous infiltrates typical of GCA (GCA) (n=4). Expression levels of NOTCH1 (A), Jagged1 (B), Delta1 (C) and Delta 4 (D) transcripts were quantified by RT-PCR. Data shown are mean ± SD. Paraffin-embedded temporal artery specimens affected by GCA were stained with anti-human CD3 (F and I), anti-human NOTCH1 (G), or antibodies to the cleaved intracellular domain of the NOTCH1 receptor (J). Isotype antibodies served as control (E and H). Activated NOTCH1 and CD3 were visualized by immunofluorescence using Alexa 488-labeled (green) and Alexa 546-labeled (red) antibodies, respectively (K). Colocalization of NICD and CD3 resulted in a yellow cellular stain (merge). Original magnification, E: ×100, scale bar = 500 μm; F, G, H, K: ×200, scale bar = 200 μm; I, J: ×400, scale bar = 100 μm.
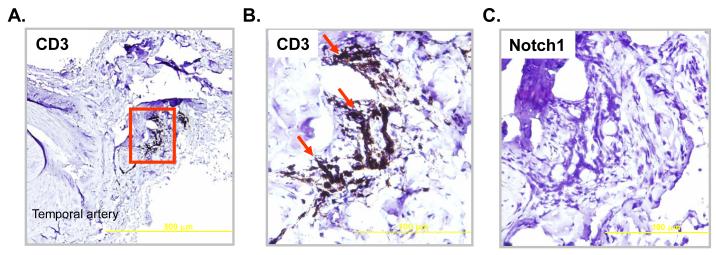
To explore whether NOTCH pathway activation is a feature of all T cells involved in vascular inflammation, a distinct, yet related type of vasculitis was examined. In a subset of GCA patients intramural infiltrates are localized in the small arteries of the vasa vasosurm and not in the main temporal artery.14 Although T cells were crowded in the walls of the vasa vasorum branches (Figure 2 A, B) there was no detectable expression of the NOTCH1 receptor (Figure 2C).
Figure 2. Tissue-infiltrating T cells in small vessel vasculitis are NOTCH1 negative.
Paraffin-embedded temporal artery specimens from patients with small vessel vasculitis in the vasa vasorum branches were stained with anti-human CD3 (A and B) or anti-human NOTCH1 (C). Original magnification, A: ×100, scale bar = 500 μm; B, C: ×400, scale bar = 100 μm.
NOTCH expression on circulating T cells in GCA
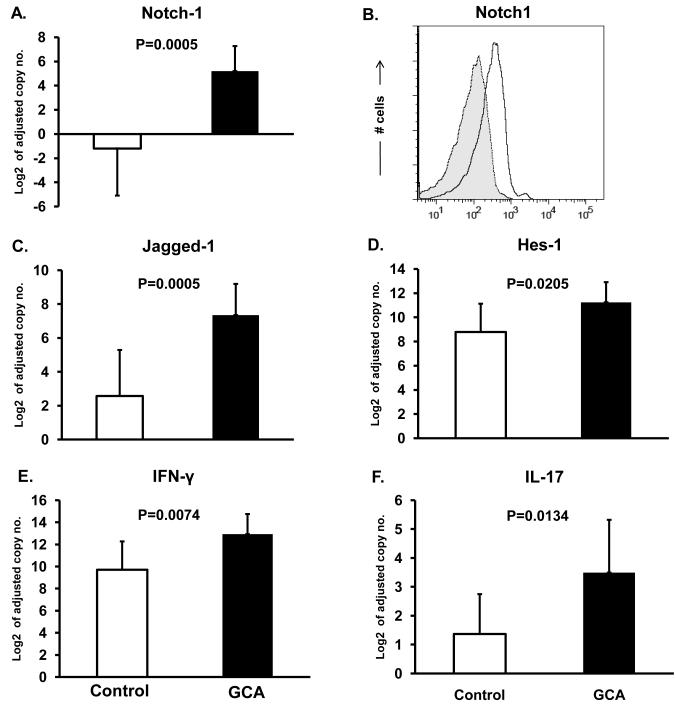
The intense systemic inflammation in GCA patients suggests that antigen encounter also occurs outside of the artery.15, 16 We therefore explored whether NOTCH expression is limited to the vascular lesions or also present in circulating immune cells. PBMC were collected from freshly diagnosed patients and examined for NOTCH1 and Jagged1 transcript expression. Induction of the NOTCH target gene Hes-1 was assessed (Figure 3) to provide evidence for ongoing stimulation of the NOTCH pathway. Compared to PBMC from age-matched controls, GCA PBMC contained 20-fold higher NOTCH1 transcript levels (Figure 3A). By flow cytometry essentially the entire population of peripheral CD4 T cells in GCA patients was strongly positive for NOTCH1 (Figure 3B).
Figure 3. Expression of NOTCH1 receptor and the proinflammatory cytokines IL-17 and IFN-γ by peripheral T cells in GCA.
Peripheral blood mononuclear cells were isolated from eight GCA patients and eight healthy controls (Control). NOTCH1 (A), Jagged1 (C) and Hes-1 (D), IL-17 (E) and IFN-γ (F) specific transcripts were quantified by RT-PCR. Data are shown as mean ± SD. Surface expression of NOTCH1 (black line) on CD4 T-cells was determined by flow cytometry (B). Shaded histogram =isotype control.
Similarly, Jagged1 sequences were abundantly expressed in GCA samples and barely detectable in controls (Figure 3C). Ongoing NOTCH signaling was confirmed by the upregulation of Hes-1 (Figure 3D). Thus, both NOTCH receptors and Jagged1 ligands are highly induced in GCA PBMC and NOTCH-derived signals are constantly reaching the nucleus.
To test whether NOTCH1 expression in circulating GCA T cells were associated with functional changes, expression patterns of two major T-cell cytokines recently implicated in GCA were assessed. PBMC from untreated GCA patients contained high levels of both IFN-γ and IL-17 sequences, demonstrating activation of the Th1 and the Th17 pathway (Figure 3E, F).17 The concentrations of IFN-γ-specific sequences were more than doubled in patients. The differences were even more pronounced for IL-17. A 4- to 5-fold increase in IL-17 transcript levels distinguished GCA PBMC from those of healthy controls.
NOTCH blockade inhibits CD4 T cell function
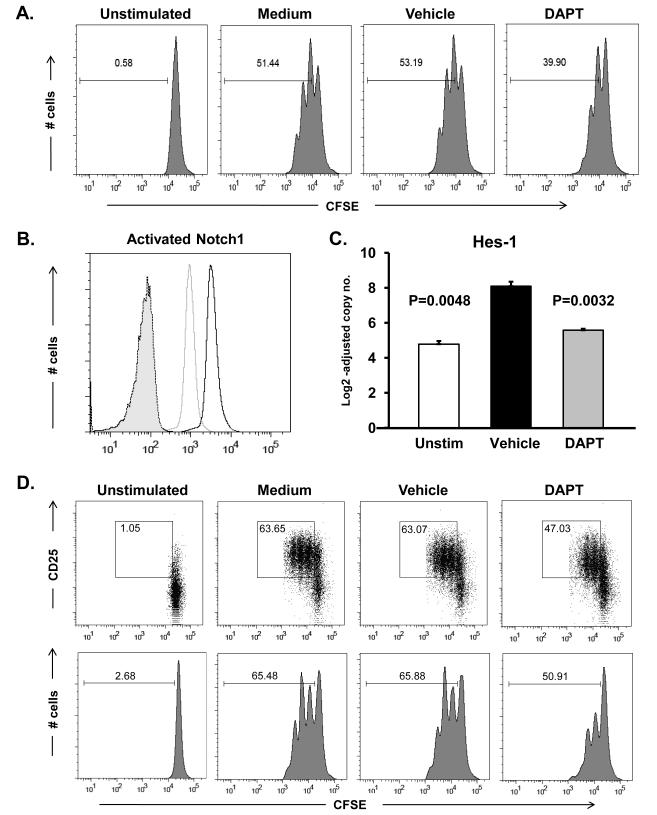
To explore functional implications of NOTCH activation we inhibited NOTCH activity using the γ-secretase inhibitor DAPT. Cross-linking of the CD3 and CD28 receptors resulted in vigorous T-cell proliferation; by day 3 >50% of cells had entered the cell cycle. Cell cycle kinetics were unaffected in DMSO vehicle controls (53.2%) (Figure 4A). Cells stimulated in the presence of DAPT showed a 25% decrease in proliferation with only 39% of cells progressing through the cell cycle (Figure 4A). To confirm that DAPT was efficient in disrupting NOTCH signaling we assessed the accumulation of NICD, a process directly dependent on enzymatic activity of the γ-secretase complex.9 DAPT treatment abrogated NICD accumulation, with intracellular NICD levels diminished after short-term and prolonged T cell activation (Figure 4B). In DAPT treated cells the level of the NOTCH target gene Hes-1 was reduced by 80% (Figure 4C), providing confirmation that γ-secretase inhibiton was actively targeting the NOTCH pathway.
Figure 4. NOTCH pathway blockade dampens T-cell responses.
Freshly isolated CD4 T-cells were stimulated with anti-CD3/CD28 mAbs (1 μg/ml) in the presence or absence of the γ-secretase inhibitor DAPT (10 μM) or vehicle control. CFSE dilution was evaluated by flow cytometry at 72 h (A). CD4 T-cells were stimulated for 24 h with anti-CD3/CD28 (5 μg/ml) in the presence of DAPT (light grey) or vehicle (black line) and analyzed for the cleaved intracellular domain of the NOTCH1 receptor (B). Shaded histogram = isotype control. Hes-1 transcripts were quantified by RT-PCR (C). CFSE-labeled T-cells were cultured with DC and anti-CD3 (1 μg/ml), in the presence or absence of DAPT or vehicle. On day 3 cells were stained for CD4 and CD25 (upper panels) and proliferation was assessed by flow cytometry using CD4 gates (upper and lower panels) (D). Percentage of cells that have divided are indicated. Data shown are representative of at least 6 independent experiments.
To more closely approximate in vivo T-cell-DC interactions we explored the impact of NOTCH inhibition in T cell-DC coculture assays. In control cultures 63% of the T cells upregulated the activation marker CD25 and a similar proportion of cells underwent activation in the presence of DMSO (Figure 4D). Inhibiting intracellular NOTCH cleavage reduced T-cell activation with only 47% of the T cells acquiring CD25 expression.
In essence, NOTCH-NOTCH ligand interactions provide co-stimulatory signals, amplifying the process of T-cell activation and regulating T-cell expansion.
Kinetic studies of NOTCH1 expression on T cells pointed towards interesting kinetics in the upregulation of this costimulatory receptor (Supplemental Figure 1). Anti-CD3/CD28 stimulation increased both the level of NOTCH1 transcripts and cell surface expression. By 24 h post-stimulation, levels were 4-fold higher, by 48 h even 8-fold higher (Supplemental Figure 1). Thus, changes in NOTCH1 receptor expression are an integral component of the T-cell activation program and NOTCH-NOTCH ligand interactions may not be relevant early after antigen recognition but days after TCR ligation.
NOTCH signaling inhibition restricts early CD4 T-cell infiltration into the arterial wall
To investigate the contribution of the NOTCH pathway in vascular inflammation, a global NOTCH inhibitor was applied in a humanized mouse model of vasculitis. In this disease model human arteries are implanted into SCID mice and human allogeneic T cells from selected healthy donors or from GCA patients are adoptively transferred into the chimeras. Tissue tolerance is broken through injection of Toll-like receptor ligands, e.g. LPS and cellular infiltrates start to accumulate at 72 h, progressing over the next 3 to 5 days.4 Kinetics of inflammatory responses in the blood vessels are delineated in Supplemental Figure 2. IL-6 derives from wall-residing dendritic cells; IFN-γ and IL-17 are produced by wall-infiltrating T cells.
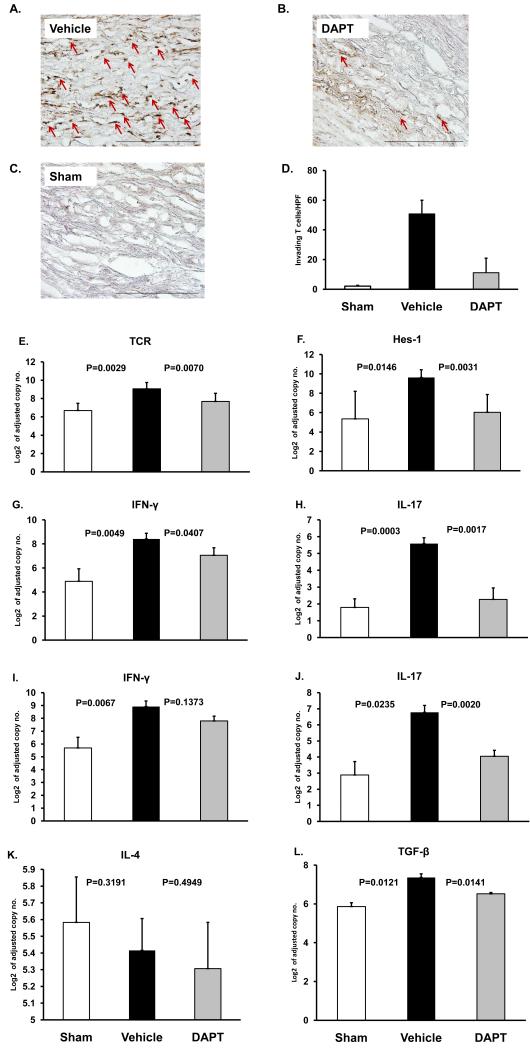
To study the role of NOTCH during the early phase of vascular inflammation, human T cells were co-administered with DAPT or vehicle (Figure 5). Explanted arteries were analyzed for TCR and Hes-1 transcript levels to monitor composition and activity of the infiltrates. NOTCH inhibition had a profound effect on T-cell invasion into the wall layers. The density of infiltrating T cells was measured by immunohistochemical quantification of CD3+ T cells in tissue sections (Figure 5A-C). Chimeras without vessel wall inflammation (did not receive LPS injection) served as controls. Arteries with fully developed inflammation harbored about 50 cells per high-powered field (Figure 5D). Co-administration of DAPT inhibited T-cell recruitment/retention, and only 10 cells/hpf were detected in the human grafts. T-cell depletion correlated with an almost 2-fold reduction of TCR transcript levels (Figure 5E).
Figure 5. Treatment with a γ-secretase inhibitor suppresses vessel wall inflammation.
For each experimental series segments from a human axillary artery were implanted into 3 SCID mice. The sham group received PBS (day 7) followed by intravenous adoptive transfer of PBMC (day 8). The vehicle group was injected with LPS (3 μg/mouse; day 7) followed by vehicle-pretreated PBMC (day 8) and vehicle injection i.p. the next day. DAPT treatment involved LPS injection (day 7) and adoptive transfer of DAPT-pretreated PBMC (day 8) followed by an injection of DAPT (1mg; day 9). PBMC derived from either healthy donors (A-H) or from patients with biopsy-proven GCA (I-K). Arteries were explanted 1 week later. Human T-cell infiltrates (brown color) in sham (A), vehicle- (B) or DAPT-treated (C) arteries were stained with rabbit anti-human CD3 Ab (A-C). Magnification: ×200. Vessel-infiltrating T-cells were enumerated in at least 10 randomly chosen high-powered fields (D). TCR (E), Hes-1 (F), IFN-γ (G and I), IL-17 (H and J), IL-4 (K) and TGFβ (L) transcripts in the tissues were quantified by RT-PCR. Results from five independent experiments are shown as mean ± S.D.
To test effectiveness of NOTCH pathway blockade we determined the expression levels of the NOTCH target gene Hes-1. Mice infused with DAPT-treated human T cells produced 4-fold less Hes-1 in the arterial grafts than mice with fully developed vascular infiltrates (Figure 5F). NOTCH pathway blockade resulted in a marked suppression of tissue IFN-γ (Figure 5G) and IL-17 (Figure 5H).
Similar results were obtained if the adoptively transferred PBMC derived from patients with biopsy-proven GCA. Infiltration of GCA PBMC into the human vessels elicited strong Th1 and Th17 responses (Figure 5 I, J). Both arms of T-cell immunity were susceptible to DAPT-dependent suppression. However, blocking NOTCH activation was more effective in downregulating IL-17 than IFN-γ (Figure 5 I, J).
Monitoring of TCR transcript levels as well as histochemical analysis of the explants from treated and untreated chimeras strongly suggested that the NOTCH pathway is involved in the recruitment/retention of proinflammatory T cells. Alternative mechanisms-of-action include the recruitment/activation of immunosuppressive Treg cells or swaying of immune responses from the Th1 to the Th2 lineage. To address these possibilities IL-4 as a marker of Th2 cells and TGFβ as a marker for Treg cells were evaluated in the explanted blood vessels (Figure 5 K, L). IL-4 transcripts were low in vehicle and DAPT-treated grafts; consistent with the absence of IL-4 in temporal artery biopsies from GCA patients (Supplemental Figure 3). TGFβ transcripts were detectable in vehicle-exposed arteries but declined with blocking NOTCH activation (Figure 5L). In essence, interfering with NOTCH cleavage and translocation had profound implications for the inflammatory infiltrates; essentially disrupting T cell recruitment/retention.
Jagged1-Fc treatment is protective against vessel wall inflammation
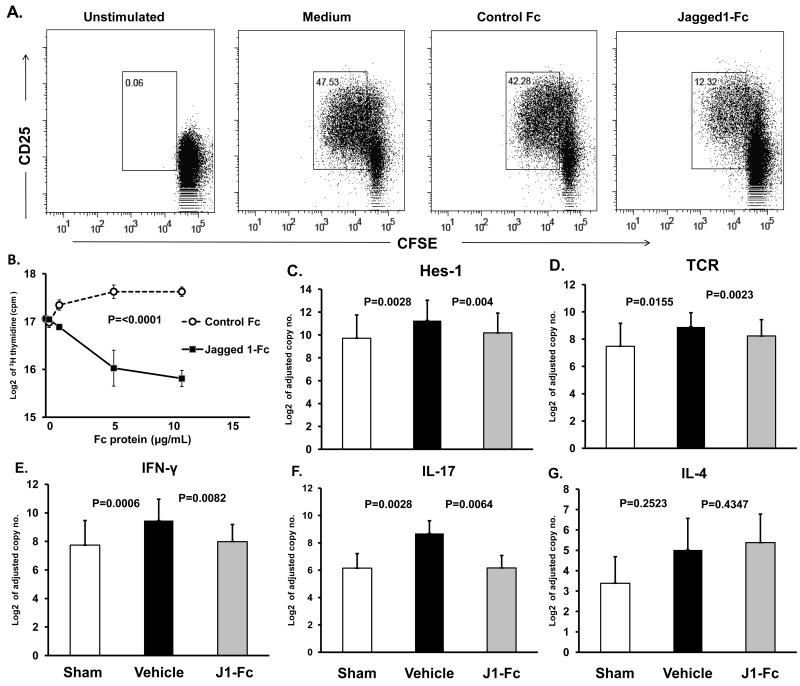
In a complementary approach, we utilized another strategy to prevent NOTCH pathway activation by occupying NOTCH1 with the exogenous decoy ligand Jagged1-Fc. Immunosuppressive effects of Jagged1-Fc were first explored in vitro. Triggering of CD4 T cells by TCR cross-linking induced the activation marker CD25 on essentially all T cells within 24 h. In the presence of exogenous soluble Jagged1-Fc protein, T-cell activation was curbed with a minor population acquiring CD25. Stimulation in the presence of 5 μg/ml Jagged1-Fc significantly diminished the entry into the cell cycle by 75% (Figure 6A). In alternative experiments, proliferation of CD4 T cells was measured 3 days post-stimulation by thymidine incorporation. Control Fc protein improved the proliferative expansion, likely by interacting with inhibitory Fc receptors. Jagged1-Fc protein impaired T-cell proliferation in a dose-dependent manner (Figure 6B). At doses of 5-10 μg/ml Jagged1-Fc T-cell proliferation reached only 50% of that in untreated T cells.
Figure 6. Soluble Jagged1 ligand inhibits T cell activation and abrogates vessel wall inflammation.
CFSE-labeled CD4 T cells were incubated with αCD3/CD28 antibodies in the presence or absence of Jagged1-Fc or control Fc for 72 h. Cells were stained with anti-CD25 and proliferation was analyzed by flow cytometry (A). The effect of increasing concentrations of Jagged1-Fc or control-Fc on T-cell proliferation was measured at 72 h by 3H-thymidine incorporation in triplicate cultures (B). For in vivo experiments, CD4 T cells pre-incubated with Jagged1-Fc or control Fc were adoptively transferred into SCID-chimeras 8 days after implantation of human arteries as described in Figure 5. Additional doses of Jagged1-Fc or control Fc were given 24 and 48 h later via i.p. injection. Arterial grafts explanted one week later were analyzed by RT-PCR for Hes-1 (C), TCR (D), IFN-γ (E), IL-17 (F), and IL-4 (G) gene expression. Data are shown as mean ± S.D.
In vivo therapeutic effects of Jagged1-Fc were probed in the humanized mouse model. To explore immunosuppressive effects of Jagged1-Fc, 100 μg of the soluble ligand was administered concurrent with the adoptively transferred human T cells. The chimeras received two additional doses 24 and 48 h later. Control animals were injected with human IgG. Treatment with Jagged1-Fc over a 3 day course effectively inhibited NOTCH-dependent signaling as indicated by the marked reduction of Hes-1 sequences (Figure 6C). The loss of NOTCH target gene induction was accompanied by a loss of TCR sequences, indicating reduced T-cell accumulation (Figure 6D). Administration of Jagged1-Fc suppressed in situ production of both, IFN-γ (approximately 4-fold reduction) (Figure 6E) and IL-17 (approximately 10-fold reduction) (Figure 6F), essentially eliminating the major inflammatory drivers of the vasculitic process. As seen in the DAPT experiments, IL-4 expression was low in the controls and Jagged1-Fc had no effect on IL-4 transcript levels (Figure 6G).
NOTCH blockade ameliorates established vascular inflammation
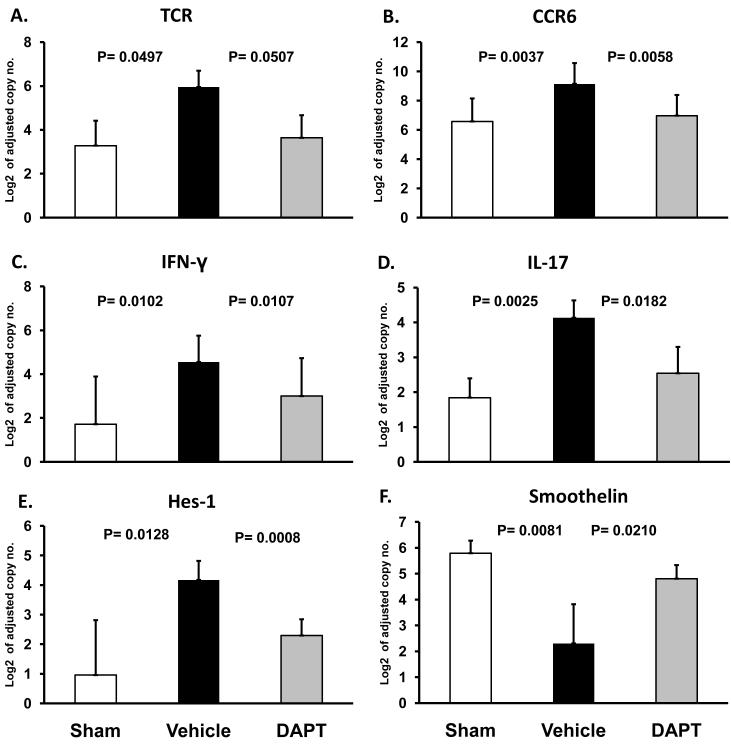
Treatment with either DAPT or Jagged1-Fc protein markedly decreased tissue-infiltrating T cells and suppressed T-cell cytokine production. To assess whether blockade of the NOTCH pathway could be successfully applied after T cells had already established intramural infiltrates, we inhibited NOTCH-derived signals in established vessel wall inflammation. Prior studies have shown that the subset of CCR6+CD4 T cells plays an important role in forming panarteritic infiltrates.11 As vascular smooth muscle cells transition from a contractile to a non-contractile phenotype, they lose production of smoothelin as an indication of damage to the vessel wall.11
Preventing NICD cleavage by administering the γ-secretase inhibitor DAPT 96 h postinduction of vascular inflammation caused a marked reduction in the tissue production of both TCR (Figure 7A); and the T-cell subset marker CCR6 (Figure 7B). Thus, NOTCH inhibitor treatment at the peak of vascular disease evolution was highly effective in thinning the T-cell infiltrate. DAPT therapy essentially eliminated CCR6 transcripts from the lesions (Figure 7B), depleting a T-cell subset implicated in the formation of transmural infiltrates. The suppression of T-cell function was associated with a decline in both IFN-γ (Figure 7C) and IL-17 (Figure 7D). Again, as seen in earlier experiments, DAPT appeared to be more effective in abrogating tissue IL-17 than tissue IFN-γ. Blockade of NOTCH-dependent activation was confirmed by the suppression of the NOTCH target gene Hes-1 post-DAPT injection (Figure 7E). Vascular damage quantified by the levels of smoothelin production was intense in the arteries invaded by the T cells; VSMC literally ceased transcribing this gene diminishing smoothelin levels by almost 10-fold of that in normal tissue (Figure 7F). DAPT was able to partially reverse the smoothelin loss, restricting the reduction of smoothelin transcripts to 2-fold of the undamaged tissue grafts, indicative of protection of VSMC from the inflammatory injury.
Figure 7. NOTCH inhibition attenuates ongoing T-cell inflammatory responses in the vessel wall.
Vascular inflammation was induced in human artery-SCID chimeras by LPS injection and adoptive transfer of PBMC as described in Figure 5. Five days after initiating vessel wall inflammation 1 mg DAPT or vehicle was administered by i.p. injection, with three subsequent doses given 24, 48, and 72 h later. Grafts were explanted on day 20 postimplantation and markers of human CD4 T-cell infiltration were quantified by RT-PCR for TCR (A) and CCR6 (B) transcripts. Inflammatory burden and in situ T-cell activation was evaluated by measuring IFN-γ (C) and IL-17 (D) by RT-PCR. Hes-1 expression (E) in tissue grafts was measured to assess ongoing NOTCH signaling in tissue-infiltrating cells. Smooth muscle cell damage was examined by quantifying the expression of smoothelin (F), a marker of smooth muscle cell viability and function. Data from five independent experiments are shown as mean ± S.D.
Discussion
The NOTCH signaling pathway is mostly recognized for its role in determining cell fate, especially during development and tissue homeostasis.18 The pathway transduces signals between neighboring cells, emphasizing its participation in the 3-dimensional structuring of tissues. Here we report that the NOTCH pathway is of disease relevance in large vessel vasculitis, regulating the activity of vessel-wall infiltrating T cells that orchestrate tissue damage in blood vessels. Blocking NOTCH signaling has profound effects on T-cell dependent immune responses and suppresses the production of proinflammatory cytokines, in particular IL-17. Both early and later stages of vasculitis are sensitive to disrupting NOTCH-NOTCH ligand interactions suggesting that this pathway has potential as a therapeutic target in inflammatory vasculopathies.
In GCA, CD4 T-cell invasiveness, cytotoxicity and in-situ cytokine production lie at the core of the pathogenic process.19 Initiation of vascular inflammation requires DC activation; DC depletion abrogates chronic vasculitis.4 Vasculitic T-cells depend upon continuous instructions provided by DC, which sustain T-cell activation,20 and shape overall disease architecture.11 DC express NOTCH ligands, including Jagged and Delta ligands and differences in DC activation have been associated with the induction of distinct T-cell differentiation trajectories.12, 21 In the current study NOTCH signaling blockade markedly reduce T-cell infiltration into the vessel wall, both during early and late stages of vasculitis. NOTCH blockade particularly affected the density of wall-invading cell populations, essentially depleting T-cells from the infiltrates. This anti-vasculitic effect may either result from impairing T-cell recruitment or survival in the tissue niche. It is unlikely that the NOTCH pathway affects only T-cell migration, as T-cell cytokine production was also profoundly reduced and kinetics of NOTCH receptor induction suggested a delayed role of NOTCH in T-cell activation. The NOTCH pathway has been associated with the regulation of lymphocyte activation and proliferation.22, 23 This may be the mechanism underlying the loss of T-cells from the infiltrates. Alternatively, NOTCH could regulate local T-cell apoptosis,24 a process determining T-cell pool size and density of tissue infiltrates. Finally, NOTCH may interfere with survival signals delivered to wall-infiltrating cells. Thus, it would be important to know where the NOTCH-triggering signals derive from and whether this is unique to the arterial wall.
The contribution of cytokines in GCA is well appreciated, both in the systemic inflammatory response as well as in the blood vessels.25 Tissue cytokine profiles in temporal artery biopsies suggest that local IFN-γ production drives disease progression.3 The recent implication of Th17 cells in the pathogenesis of autoimmune disease26 led us to investigate the expression of both inflammatory cytokines. Up-regulation of inflammatory T-cell cytokines correlated with persistent activation of the NOTCH pathway, both in the tissue lesions and in the blood. Differential effects of NOTCH blockade on IFN-γ versus IL-17 production suggested a formidable role of NOTCH-NOTCH ligand interactions in T-cell differentiation. Independent of the approach taken to suppress canonical NOTCH signaling, IL-17 production appeared more sensitive and IFN-γ more resistant. A selective role of NOTCH-NOTCH ligand interaction in regulating the function of certain T-cell subsets is supported by the finding that regulatory T-cells appear to be unaffected by NOTCH blockade. The outcome of NOTCH signaling is cell-type specific and T-cell cytokine production obviously displays a differential dependence on the NOTCH pathway.
Gaps in the understanding of GCA pathogenesis restrict therapeutic options. Therefore, dissecting the intricacies of the unique signals that regulate and sustain a self-propagating inflammation is warranted. Overall, our in vivo studies assign a role of NOTCH triggering in the initiation of vascular wall inflammation. Disrupting NOTCH activation via GSI-inhibition or using decoy ligands is profoundly immunosuppressive. Therapeutic targeting of the NOTCH signaling pathway, however, may be limited by intestinal toxicity; NOTCH1 and 2 receptors expressed on intestinal epithelium have been connected to secretory goblet cell accumulation and impaired cell proliferation.27 Whether parental GSI formulations could reduce this side effect remains to be explored. The current standard of care in GCA, high doses of glucocorticoids given chronically is associated with serious side effects. A recent study examining the anti-leukemic effects of GSI in T-cell acute lymphoblastic leukemia, a malignancy characterized by activating mutations in the NOTCH receptor gene demonstrated synergistic beneficial effects of glucocorticoids and GSI.28 Moreover, the combination therapy protected mice from gut toxicity. Thus, combining NOTCH signaling inhibition with steroids may allow for improved anti-inflammatory control in GCA and enable steroid sparing, an important need in optimizing the management of large vessel vasculitis.
Clinical Perspective.
Giant cell arteritis (GCA; formerly called temporal arteritis) is an inflammatory vasculopathy that causes aortic wall damage and lumen-stenosing lesions in medium-size arteries. GCA preferentially targets extracranial branches of the aorta resulting in ischemic optic neuropathy, stroke, pulselessness and aortic aneurysm/dissection. If diagnosed and treated promptly, ischemic organ damage, such as blindness can be prevented. The standard-of-care involves high doses of corticosteroids, often given over prolonged periods. Alternative treatment approaches are urgently needed. A critical step in the disease process is the activation of pro-inflammatory T cells in the vessel wall. Here, we searched for receptor-ligand pairs relevant in T cell activation, with the ultimate goal to develop novel anti-inflammatory interventions. We found that vascular lesions of GCA patients abundantly expressed NOTCH and NOTCH ligands; and accumulated NOTCH1+ T cells. The NOTCH signaling pathway has mostly been recognized for its role in determining cell fate, especially during development and tissue homeostasis. NOTCH pathway signaling depends on two proteolytic cleavage processes, including the action of a γ-secretase which is inhibitable by an enzyme blocker. Treatment with the γ-secretase inhibitor or, alternatively, a soluble NOTCH ligand effectively suppressed vascular inflammation in a humanized mouse model in which human T cells form inflammatory lesions in engrafted human vessels. NOTCH signaling blockade suppressed activity of IFN-γ-producing Th1 cells and almost depleted IL-17-producing Th17 cells. Targeting the NOTCH-NOTCH ligand pathway may provide a novel strategy to suppress the activity of vasculitic T cells in large vessel vasculitis.
Supplementary Material
Acknowledgments
Funding Sources This work was funded in part by NIH grants RO1 AR42527, RO1 AI44142, RO1 EY11916, AI57266, PO1 HL 058000, AHA 0715452B and the Vasculitis Foundation.
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kaiser M, Younge B, Bjornsson J, Goronzy JJ, Weyand CM. Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cells. Am J Pathol. 1999;155:765–774. doi: 10.1016/S0002-9440(10)65175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. Ann Intern Med. 2003;139:505–515. doi: 10.7326/0003-4819-139-6-200309160-00015. [DOI] [PubMed] [Google Scholar]
- 3.Weyand CM, Tetzlaff N, Bjornsson J, Brack A, Younge B, Goronzy JJ. Disease patterns and tissue cytokine profiles in giant cell arteritis. Arthritis Rheum. 1997;40:19–26. doi: 10.1002/art.1780400105. [DOI] [PubMed] [Google Scholar]
- 4.Ma-Krupa W, Jeon MS, Spoerl S, Tedder TF, Goronzy JJ, Weyand CM. Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J Exp Med. 2004;199:173–183. doi: 10.1084/jem.20030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma-Krupa W, Kwan M, Goronzy JJ, Weyand CM. Toll-like receptors in giant cell arteritis. Clin Immunol. 2005;115:38–46. doi: 10.1016/j.clim.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Radtke F, Wilson A, Mancini SJ, MacDonald HR. NOTCH regulation of lymphocyte development and function. Nat Immunol. 2004;5:247–253. doi: 10.1038/ni1045. [DOI] [PubMed] [Google Scholar]
- 7.Eagar TN, Tang Q, Wolfe M, He Y, Pear WS, Bluestone JA. NOTCH 1 signaling regulates peripheral T cell activation. Immunity. 2004;20:407–415. doi: 10.1016/s1074-7613(04)00081-0. [DOI] [PubMed] [Google Scholar]
- 8.Weinmaster G. The ins and outs of NOTCH signaling. Mol Cell Neurosci. 1997;9:91–102. doi: 10.1006/mcne.1997.0612. [DOI] [PubMed] [Google Scholar]
- 9.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of NOTCH intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 10.Schroeter EH, Kisslinger JA, Kopan R. NOTCH-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 11.Deng J, Ma-Krupa W, Gewirtz AT, Younge BR, Goronzy JJ, Weyand CM. Toll-like receptors 4 and 5 induce distinct types of vasculitis. Circ Res. 2009;104:488–495. doi: 10.1161/CIRCRESAHA.108.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pryshchep O, Ma-Krupa W, Younge BR, Goronzy JJ, Weyand CM. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation. 2008;118:1276–1284. doi: 10.1161/CIRCULATIONAHA.108.789172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rittner HL, Kaiser M, Brack A, Szweda LI, Goronzy JJ, Weyand CM. Tissue-destructive macrophages in giant cell arteritis. Circ Res. 1999;84:1050–1058. doi: 10.1161/01.res.84.9.1050. [DOI] [PubMed] [Google Scholar]
- 14.Chatelain D, Duhaut P, Loire R, Bosshard S, Pellet H, Piette JC, Sevestre H, Ducroix JP. Small-vessel vasculitis surrounding an uninflamed temporal artery: a new diagnostic criterion for polymyalgia rheumatica? Arthritis and rheumatism. 2008;58:2565–2573. doi: 10.1002/art.23700. [DOI] [PubMed] [Google Scholar]
- 15.Salvarani C, Cantini F, Boiardi L, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med. 2002;347:261–271. doi: 10.1056/NEJMra011913. [DOI] [PubMed] [Google Scholar]
- 16.Weyand CM, Schonberger J, Oppitz U, Hunder NN, Hicok KC, Goronzy JJ. Distinct vascular lesions in giant cell arteritis share identical T cell clonotypes. J Exp Med. 1994;179:951–960. doi: 10.1084/jem.179.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng J, Younge BR, Olshen RA, Goronzy JJ, Weyand CM. Th17 and Th1 T-cell responses in giant cell arteritis. Circulation. 2010;121:906–915. doi: 10.1161/CIRCULATIONAHA.109.872903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Artavanis-Tsakonas S, Rand MD, Lake RJ. NOTCH signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 19.Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003;349:160–169. doi: 10.1056/NEJMra022694. [DOI] [PubMed] [Google Scholar]
- 20.Han JW, Shimada K, Ma-Krupa W, Johnson TL, Nerem RM, Goronzy JJ, Weyand CM. Vessel wall-embedded dendritic cells induce T-cell autoreactivity and initiate vascular inflammation. Circ Res. 2008;102:546–553. doi: 10.1161/CIRCRESAHA.107.161653. [DOI] [PubMed] [Google Scholar]
- 21.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different NOTCH ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 22.Adler SH, Chiffoleau E, Xu L, Dalton NM, Burg JM, Wells AD, Wolfe MS, Turka LA, Pear WS. NOTCH signaling augments T cell responsiveness by enhancing CD25 expression. J Immunol. 2003;171:2896–2903. doi: 10.4049/jimmunol.171.6.2896. [DOI] [PubMed] [Google Scholar]
- 23.Elyaman W, Bradshaw EM, Wang Y, Oukka M, Kivisakk P, Chiba S, Yagita H, Khoury SJ. JAGGED1 and delta1 differentially regulate the outcome of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5990–5998. doi: 10.4049/jimmunol.179.9.5990. [DOI] [PubMed] [Google Scholar]
- 24.Jehn BM, Bielke W, Pear WS, Osborne BA. Cutting edge: protective effects of NOTCH-1 on TCR-induced apoptosis. J Immunol. 1999;162:635–638. [PubMed] [Google Scholar]
- 25.Wagner AD, Bjornsson J, Bartley GB, Goronzy JJ, Weyand CM. Interferon-gamma-producing T cells in giant cell vasculitis represent a minority of tissue-infiltrating cells and are located distant from the site of pathology. Am J Pathol. 1996;148:1925–1933. [PMC free article] [PubMed] [Google Scholar]
- 26.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrando AA. The role of NOTCH1 signaling in T-ALL. Hematology Am Soc Hematol Educ Program. 2009:353–361. doi: 10.1182/asheducation-2009.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Real PJ, Tosello V, Palomero T, Castillo M, Hernando E, de Stanchina E, Sulis ML, Barnes K, Sawai C, Homminga I, Meijerink J, Aifantis I, Basso G, Cordon-Cardo C, Ai W, Ferrando A. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat Med. 2009;15:50–58. doi: 10.1038/nm.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.