Abstract
The L-type Cardiac Calcium Channel (LTCC) plays a prominent role in the electrical and mechanical function of the heart. Mutations in the LTCC have been associated with a number of inherited cardiac arrhythmia syndromes, including Timothy, Brugada and Early Repolarization syndromes. Elucidation of the genetic defects associated with these syndromes has led to a better understanding of molecular and cellular mechanisms and the development of novel therapeutic approaches to dealing with the arrhythmic manifestations. This review provides an overview of the molecular structure and function of the LTCC, the genetic defects in these channels known to contribute to inherited disorders and the underlying molecular and cellular mechanisms contributing to the development of life-threatening arrhythmias.
Keywords: Timothy syndrome, Brugada syndrome, Early repolarization syndrome, ventricular arrhythmias, electrophysiology
Introduction
Transmembrane calcium current (ICa) in the human myocardium is conducted via a macromolecular complex that includes a pore-forming unit and a number of subunits that cooperate to control the entry of calcium ions. ICa is a relatively “new kid on the block” in the arena of inherited arrhythmogenic diseases. Recent studies have identified genetic variants that are associated with electrical abnormalities capable of causing a variety of clinical phenotypes, including Timothy, Brugada and Early Repolarization syndromes. These genetic defects have also guided us towards a better understanding of molecular and cellular mechanisms, which serve as a cornerstone for the development of novel therapies for our patients. In this review we will attempt to provide a comprehensive overview of the, molecular structure and function of the cardiac L-type calcium channel (LTCC), followed by a discussion of inherited disorders associated with genetic mutations in LTCC as well as a discussion of underlying molecular and cellular mechanisms contributing to the development of cardiac arrhythmias.
Molecular diversity and genomic organization of cardiac calcium channels in the heart
Voltage gated calcium channels (VGCC) are macromolecular complexes consisting of an ion conducting protein (the α1 subunit), and additional “accessory” peptides: α2δ, β1–4, and γ subunits. These accessory subunits are not simply innocent bystanders. Indeed they modulate, gating, trafficking and response to neurohormonal stimuli. While the role of α2 and γ subunits is less clear, the β subunit is known to increase the ICa density to enhance single channel open probability and to modulate inactivation;1 it is also reported to activate Ca2+/calmodulin-dependent protein kinase II (CaMKII) leading to Ca2+ overload.2
Classification of calcium channels
Calcium channels were first identified by Fatt and Katz in 19533 and classified according to their biophysical and pharmacological (block by specific toxins) properties in the 1980’s4 (Table 1): neuronal (N-type) channel blocked by ω-conotoxin; resistant (R-type) channel; P/Q-type (P = Purkinje) channel blocked by ω-agatoxins; dihydropyridine-sensitive LTCC responsible for excitation-contraction coupling of in skeletal and cardiac muscle. Transient-type (T-type) calcium channel has been identified in neurons and pace-maker cells (Table 1).
Table 1.
Five Subclasses of calcium channels with distinct electrophysiological and pharmacological properties: L, T, N, P, Q and R.
| Channel type |
Gated By |
Protein | Gene | Tissue Distribution |
Drug Sensitivity |
|---|---|---|---|---|---|
| L | High Voltage | Cav1.1 | CACNA1S | Skeletal muscle | DHP, PAA, BTZ |
| L | High Voltage | Cav1.2 | CACNA1C | Heart, brain, aorta, lung, fibroblast | Dihyropyridines (DHP; nifedipine) Phenylalkylamines (PAA - verapamil) Benzothiazepine (BTZ - diltiazam) |
| L | High Voltage | Cav1.3 | CACNA1D | Brain, endocrine, Atria and ventricle of fetal heart Supraventricular tissues of adult heart |
DHP, PAA, BTZ |
| L | High Voltage | Cav1.4 | CACNA1F | ||
| N | High Voltage | Cav2.2 | CACNA1B | Brain | ω - CgTXGVIA (smaller 100 nM) |
| P/ Q | High Voltage | Cav2.1 | CACNA1A | Brain, heart | ω - Aga-IVA (larger 100 nM) ω - CTX-MVIIC (larger 100 nM) |
| R | Intermediate Voltage | Cav2.3 | CACNA1E | Brain | Ni2+ (smaller 30 mM) |
| T | Low Voltage | Cav3.1 Cav3.2 Cav3.3 |
CACNA1G CACNA1H CACNA1I |
Heart, Brain. Bone (osteoclasts) | Ni2+, Mibefradil |
The molecular architecture of VGCC is complex. There are eleven known alpha subunit (CaV) encoding genes in humans; these are divided into three major classes; CaV1.1 to 1.4 are those conducting the LTCC current expressed in smooth muscle, skeletal muscles and in the central nervous system. CaV 2.1 to 2.3 are responsible for P- N- and R-type Ca2+ current and are expressed in the central nervous system. Finally CaV 3.1 to 3.3 produce the T-type calcium current in the central nervous system, bones and peacemaking cells.
Three types of calcium channels are expressed in the human heart: T-type, P-type, and L-type. T-type calcium channels encompass three different homolog α1 subunits: CaV3.1 (gene: CACNA1G), CaV3.2 (gene: CACNA1H) and CaV3.3 (gene: CACNA1L). CaV3.1 and CaV3.2 are expressed in the sinus node and contribute to peacemaking activity5 but also in ventricular myocytes during fetal life.6 The P-type calcium channels are found in Purkinje cells in cerebellum but also in low levels in the heart, where its expression is altered during heart failure. 7, 8 Cav1.3 is an L-type calcium channel expressed in both atria and ventricle of the fetal heart, but only in supraventricular tissues (SA node, AV node and atria) of the adult heart. 9 Although no mutations of Cav1.3 have as yet been associated with human disease, deletion of Cav1.3 has been reported to cause sinus bradycardia, AV block as well as hearing impairment. 10
Of specific interest to this review, is the L-type calcium channel and more specifically the CaV1.2 channel encoded by the CACNA1c gene, which maps to chromosomal locus 12p13.3. CaV1.2 is the most widely expressed calcium channel in the heart where it plays a prominent role in defining the electrical milieu.11
Genetic diversity of the cardiac calcium channel CACNA1c subunit
CACNA1c has a very complex genomic architecture and transcriptional profile. It spans > 500 kb and undergoes extensive alternative splicing. There are 12 alternative splicing loci that generate 42 different variants. Alternative splicing occurs in the N-terminal, DI-II and DII-DIII linkers, between S5 and S6 in DI and DII, and in the C-terminal region.12 Interestingly some of these splice variants are physiologically expressed and have been associated with a diversity of biophysical properties: (1) depolarizing shift of inactivation for alternatively spliced exons 31–33 encoding the DIV S3–S4 linkers;12 (2) faster inactivation kinetics, strong voltage-dependence, and no Ca2+-dependent inactivation13 for exon 41–42 variants in the C-terminal; (3) dihydropirydine sensitivity for exon 21–22 variants in the DIII-S2 variant13 and for exon 9 in IS6;14 (4) protein kinase C sensitivity for the N-terminal variant resulting from alternative splicing of exon 1–2.15 CACNA1c splice variant relative abundance presents inter-individual variability16 and disease-specific variability. The splice variant containing exon 31 is more abundant in normal human hearts with a switch to the exon 32 variant in failing hearts.17 Preferential deletion of exon 9 and inclusion of exon 33 has been observed in rats with myocardial infarction.18
These observations support the notion that Cav1.2 is responsible for a significant portion of the electrical heterogeneity under physiological and pathophysiological conditions. It follows also that the identification of CACNA1c mutations may bring about interpretation hurdles both at the pathophysiological and clinical level.
Structure-function of Cardiac L-type channels
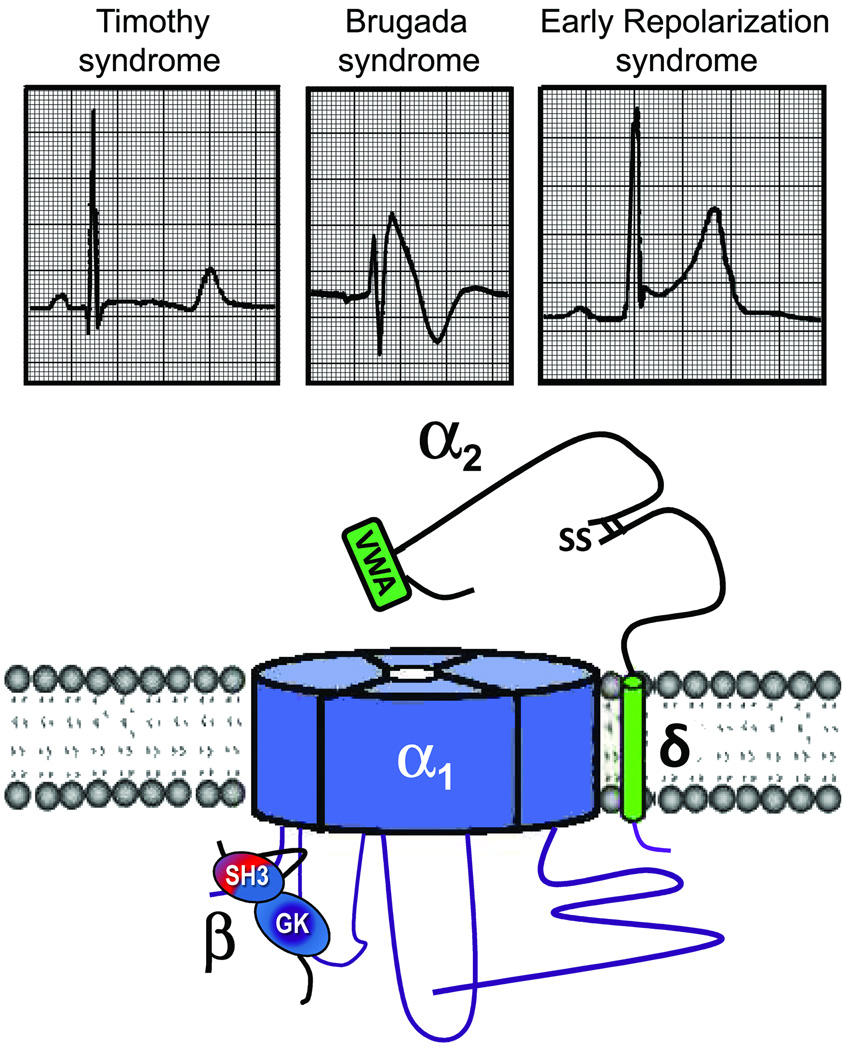
Cardiac calcium channels are composed of a central pore-forming CaV1.2 subunit (α1) and a set of ancillary subunits (α2, δ, and β) (Figure 1). The α1 subunit contains four homologous domains (I–VI) of six transmembrane segments (S1 to S6), each connected by intracellular linkers (I–II, II–III, and III–IV loops) and to the intracellular N- and C-termini. CaV1.2 channels are clustered in the t-tubules in close proximity to the sarcoplasmic reticulum Ca2+ release channels ryanodine receptor 2 (RyR2) to form the so-called “calcium-release units”,19 This spatial interaction allows optimal coupling for the calcium-induced-calcium release process.
Figure 1.
Schematic of L-type calcium channel. SH3-Src homology domain; GK-guanylate kinase like domain; VWA- von Willebrand factor-A domain
ICa activates in response to voltage but the biophysics of the current is not completely understood because of the complexity of molecular and genomic components. ICa activation at physiological extracellular calcium concentration is around–30 mV and it has slower kinetic than INa.20 ICa inactivation has two components, voltage- (VDI) and Ca2+ (CDI)-dependent. The Ca2+ mediated component of inactivation is linked to intracellular concentration of calcium ([Ca2+]i). The interdomain loop I–II of Cav1.2 is important in controlling this process via CaVα1/β interaction. Interestingly, Timothy syndrome mutations (see below), are located in this region. Additional elements that affect inactivation and channel kinetics are in the S6 segments and in the C-terminus.21 Regional and sex-related differences in ICa density have been reported in animal models 22 and may affect the propensity for early afterdepolarization development. Interestingly, higher current density in apex vs. base in females but not in males22 has been postulated to account for the higher propensity of increased risk of cardiac events among females with long QT syndrome (LQTS) type 2(LQT2).23
Genetic disorders associated with cardiac calcium channel mutations
Timothy Syndrome
Cardiologists consider Timothy syndrome (TS) a rare variant of the Long QT syndrome (LQT8) but its complex phenotype involving central nervous system and metabolic abnormalities justifies a separate classification. In 1992, Reichenbach24 reported a case of intrauterine bradycardia secondary to AV block caused by pronounced QT interval prolongation. The male infant also showed hand and feet syndactyly. He died suddenly at 5 months and the authors proposed the clinical phenotype to be a novel clinical entity: the “heart and hand syndrome”.
Phenotype and Natural History
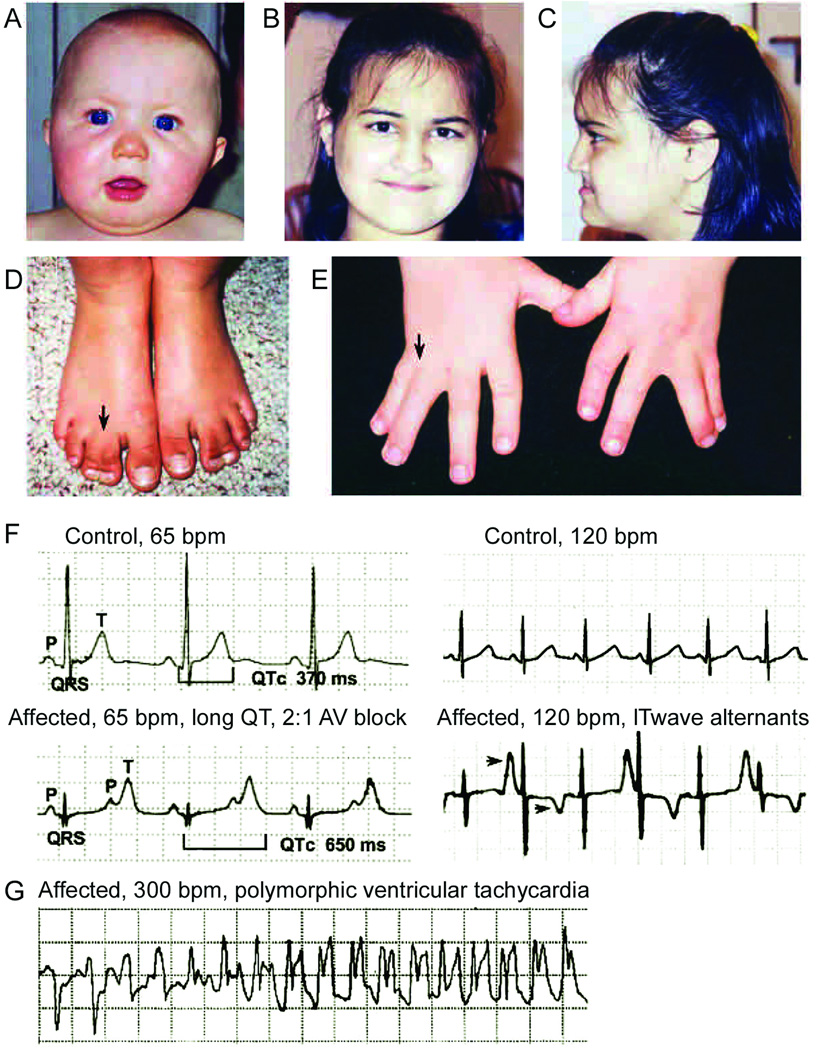
TS is characterized by multisystem dysfunction and developmental defects causing dysmorphic facial features, webbing of the toes and fingers (syndactyly) and autism. 25, 26 The initial cardiac rhythm disturbances often occurs early on in life: fetal bradycardia, marked QT prolongation at birth and 2:1 functional atrioventricular conduction block. In such instances recognition of hand and feet syndactyly (with few exceptions – see below) facilitates the diagnosis (Figure 2).
Figure 2.
Clinical manifestations of Timothy Syndrome (TS). TS is characterized by multisystem dysfunction and developmental defects causing dysmorphic facial features including round face, flat nasal bridge, receding upper jaw, and thin upper lip (A–C) and webbing of the toes and fingers (syndactyly) (D and E). Electrocardiogram (ECG) shows severe QT interval prolongation causing 2:1 atrioventricular block seen as two atrial beats (P-waves) for each ventricular beat (QRS complex). Right panel ECG shows alternating T-wave polarity (arrows), indicating severe cardiac repolarization abnormality (F). Ventricular tachycardia recorded from a TS patient by an implanted automatic defibrillator (G). (from Splawski et al, Cell:119,19,2004, with permission).
QT interval is usually in the upper range of values found in LQTS patients. In our cohort (n=21, one of the largest available), QTc interval range measured at resting electrocardiogram (ECG) is from 510ms to 700ms.25, 27 The shape of repolarization is also recognizable as a hallmark: long ST segment with small T wave (LQT3-like pattern) but also with giant negative T wave in the precordial leads. Macroscopic T wave alternans is often observed during Holter monitoring (Figure 2). TS presentation also includes congenital cardiac defects (60% of patients) and hypertrophic or dilated cardiomyopathy in 25% of cases.27
Autism and autism spectrum disorder is a common manifestation of TS (83% in our series).28 It is well known that CACNA1c is expressed in the central nervous system; recent data suggest it is implicated in brainstem function29 behavioral control such as “fear learning”,30 while population genetics studies have identified an association of specific single nucleotide polymorphisms (SNPs) (e.g. rs7959938 and rs1006737) and the risk of bipolar disorders and schizophrenia.29, 31 Although there is no direct evidence for CACNA1c to cause autism, it is conceivable that, when mutated, it may cause a wide range of neuro-developmental and psychiatric disorders.
Few TS patients have survived to puberty thus far; average survival is 2–3 years; this figure partially explains the low prevalence of the disease, which is almost entirely due to the sporadic mutations with few exceptions (see below).
The onset of cardiac tachyarrhythmia (ventricular tachycardia (VT) or fibrillation (VF)) is the cause of death in 79% of cases. No specific triggers for cardiac arrhythmias are known. However, life-threatening arrhythmias have been frequently reported during anesthesia.25, 26 Non-arrhythmic death is also possible in TS: sepsis possibly due to reduced immunity and intractable hypoglycemia have been anecdotally reported as possible causes of death.27
Genetics of Timothy syndrome
The lack of familial clustering of the disease seemed to be a peculiar feature of the disease. For this reason the initial hypothesis was that of chromosomal rearrangements. However, extensive karyotype investigations did not identify any significant alterations, therefore sporadic mutations or recessive inheritance was postulated. Through careful scrutiny of the ST-T wave morphology we hypothesized the involvement of an inward current and undertook a collaborative genetic screening program in TS patients with Mark Keating’s group. In 2004, we identified the G1216A transition in an alternatively spliced exon (8A), which causes the G406R missense mutation in DI/S6 segment (Figure 3),25 The same nucleotide variation was detected in all patients included in this initial cohort, suggesting an unusual molecular homogeneity. In 2005 Splawski et al. reported two additional cases (G406R and G402S) associated with the typical multifaceted phenotype but without syndactyly.26 One patient was carrier of the G406R mutation while a novel variant, G402S was found in the second case. Interestingly, both variants were present in exon 8 at variance with the first reported G406R occurring in the alternatively spliced exon 8A. The lack of syndactyly was explained by the differential expression of the two alternative exons.
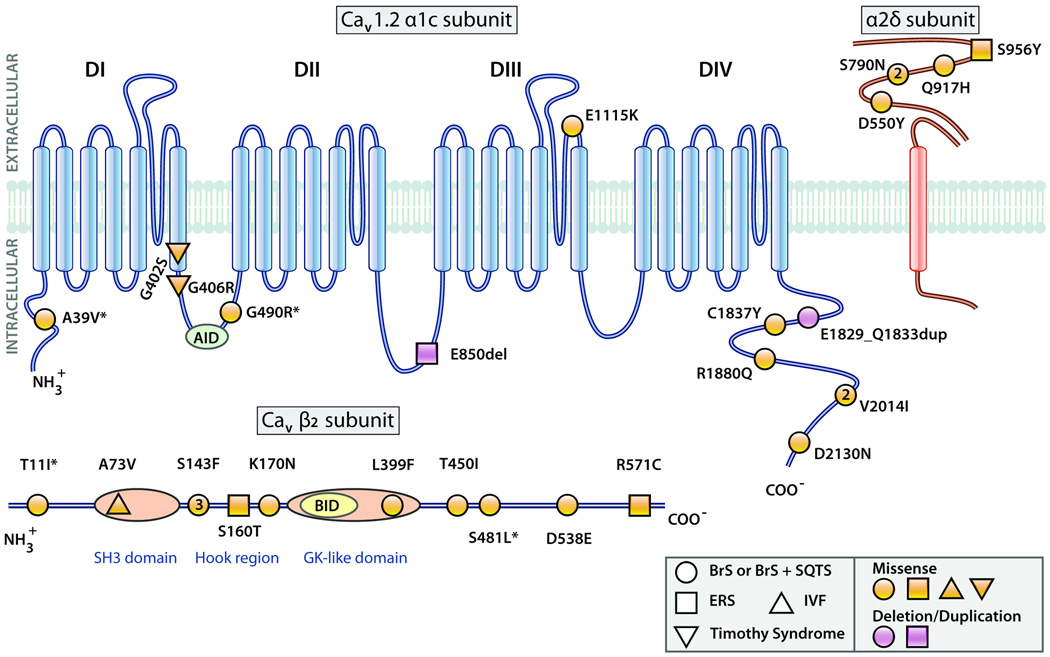
Figure 3.
Predicted topology of the Cav1.2 (α1c) subunit with associated β2 and α2δ subunits showing the location of mutations associated with Brugada syndrome (BrS), Brugada syndrome with shorter than normal QTc intervals (BrS+SQTS), early repolarization syndrome (ERS), Timothy Syndrome and idiopathic ventricular fibrillation (IVF) probands. AID = alpha subunit interaction domain; BID=beta subunit interaction domain. SH3-Src homology domain; GK-guanylate kinase like domain. Larger symbols with numbers denote multiple probands with the same mutation. Modified from 20, with permission. (Illustration Credit: Cosmocyte/Ben Smith).
The rare familial recurrence of TS has been linked to parental mosaicism. A mosaic is defined as an organism carrying cells with variable genotype at one or more loci. Thus apparently healthy parents may harbor a mutation only in the germline (or in few tissues) and be able to generate an affected offspring. If a CACNA1c mutation carrier expresses a low percentage of mutant cells, the clinical manifestation are variable and can be mild and atypical (depending upon the specific tissue expressing the mutant channel).
Mosaicism was initially suggested in the original publication from Reichenback et al.24 and genetically demonstrated by Splawski et al. in 2004 and 2005.25, 26 The demonstration of mosaic CACNA1c mutation carriers has important implications: first of all there is the possibility of incomplete penetrance or mild/atypical manifestations, second phenotypically healthy parents may generate multiple affected offspring due to germline mutations.
Thus, genetic testing should be carried out on DNA extracted from multiple tissues (at least those easily testable: blood, sperm, saliva, skin) even if the proband’s parents are clinically unaffected. Finally, it is important to consider that the combination of syndactyly and QT prolongation may occur in a different inherited arrhythmogenic disorder, such as the Andersen-Tawil syndrome (ATS). This disorder (also referred to as LQT7) has autosomal dominant inheritance and it is characterized by QT prolongation, ventricular arrhythmias, periodic paralysis and facial dimorphisms. Differential diagnosis is important since ATS is usually a benign condition that rarely causes sudden death.32
J Wave Syndromes
Because they share a common arrhythmic platform related to amplification of Ito -mediated J waves, and because of similarities in ECG characteristics, clinical outcomes and risk factors, congenital and acquired forms of Brugada (BrS) and early repolarization (ERS) syndromes have been grouped together under the heading of J wave syndromes.33 Recent studies have implicated loss of function mutations in all three subunits of the cardiac LTCC in the generation and accentuation of electrocardiographic J waves associated with these syndromes, as discussed below.34, 35
Brugada Syndrome
BrS, an inherited cardiac arrhythmia syndrome associated with a relatively high risk of VF, was first described as a new clinical entity in 1992.10 The ECG features of the Brugada patient includes an accentuated J wave displaying a real or apparent right branch bundle block (RBBB) and ST segment elevation in the right precordial leads (V1–V3).36
The ECG characteristics and clinical outcomes of the BrS are influenced by heart rate, autonomic tone as well as other conditions and agents that accentuate the manifestation of the J wave, which is inscribed as a result of a transmural voltage gradient caused by a prominent action potential notch in epicardium but not endocardium.33, 37 Sodium channel blockers like procainamide, pilsicainide, propafenone, and flecainide unmask the prominent J waves via an outward shift in the balance of currents active in the early phases of the action potential.37–39 Overall rate of cardiac arrest / VF in BrS is 8–12%. Few variables have proven to be useful metrics for risk stratification; the presence of a spontaneous coved-type ST segment elevation and history of syncope appear to be clearly associated with risk of events.40 Inducibility with programmed electrical stimulation, QRS fragmentation and the type of underlying mutation are still under debate.40–43
Genetics of Brugada syndrome
BrS has been associated with mutations in eight distinct genes, which encode cardiac ion channels or proteins that modulate ion channel activity. Mutations in SCN5A (Nav1.5, BrS1) have been reported in 11–28% of BrS probands, CACNA1C (Cav1.2, BrS3) in 6.7%, CACNB2b (Cavβ2b, BrS4) in 4.8% and mutations in Glycerol-3-phophate dehydrogenase 1-like enzyme gene (GPD1L, BrS2), SCN1B (β1-subunit of sodium channel, BrS5), KCNE3 (MiRP2; BrS6) and SCN3B (β3-subunit of sodium channel, BrS7) are much more rare.34, 44–50 These genetic defects lead to either a loss of function of sodium (INa) or ICa, or a gain of function of transient outward current (Ito). It is noteworthy that mutations in the calcium channel genes often gives rise to BrS associated with shorter than normal QT interval, in some cases qualifying them for a combined diagnosis of BrS and short QT (BrS+SQT).34
Recent studies have identified CACNA2D1 (CaVa2δ) as a new BrS-susceptibility gene.51 Missense mutations predicting p.S709N and p.D550Y alteration of the a2δ subunit of the LTCC were identified in three BrS probands. Functional expression studies indicate that the p.D550Y mutation coupled with a p.Q917H rare polymorphism in CACNA2D1 reduces ICa to 25% of normal (Barajas et al., unpublished observation). Our most recent yields in a cohort of 205 probands diagnosed with a J wave syndrome indicate that 12.3% BrS and BrS+SQT probands display mutations in α1, β2 or α2δ subunits of LTCC. With inclusion of rare polymorphisms, the yield increased to 17.9%. The genotype of approximately 60–65% of BrS probands remains to be identified.
Although loss-of-function mutations in the LTCC are known to predispose to a phenotype consisting of BrS with an abbreviated QTc, the majority of BrS probands thus far identified with calcium channel mutations present with normal QTc intervals. This apparent paradox has been attributed to the co-presence of genetic variations in other ion channel genes that are known to lead to prolongation of QTc in 86% (12 of 14) of patients displaying characteristics of BrS and a normal QTc.51
Early Repolarization Syndrome
An early repolarization (ER) pattern, consisting of a J point elevation, a notch or slur on the QRS (J wave), and tall/symmetric T waves, is generally found in healthy young males and is traditionally regarded as totally benign.52, 53 The pathogenticity of an ER pattern and its association with potentially life-threatening arrhythmias emerged from observations in the coronary-perfused wedge preparation in 2000.33, 54 Many case reports and experimental studies have long suggested a critical role for the J wave in the pathogenesis of idiopathic ventricular fibrillation (IVF) (see 33 for references). Several recent studies have provided a definitive association between ER and IVF.55–59
Because of its high prevalence in the general population ER cannot be considered a specific marker for sudden cardiac death (SCD), but the high incidence of ER in patients with IVF suggests that ER is a predisposing factor. As will be discussed in the next section, a transient J wave augmentation secondary to a shift of the balance of currents active in the early phases of the action potential due to any one of a number of risk factors may set the stage for the development of VF in patients with ER, thus precipitating the early repolarization syndrome (ERS).
A classification scheme for ERS recently proposed on the basis of available data, associates risk with spatial localization of the ER pattern.33 Type 1 is associated with ER pattern predominantly in the lateral precordial leads; this form is very prevalent among healthy male athletes and is thought to be largely benign. Type 2, displaying an ER pattern predominantly in the inferior or infero-lateral leads, is associated with a moderate level of risk; In a community-based general population study of 10,864 middle-aged subjects, Tikannen et al. reported that J-point elevation of > 0.2 mV in the inferior leads is associated with a markedly elevated risk of death from cardiac arrhythmia (adjusted relative risk, 2.92; 95% CI, 1.45 to 5.89; P = 0.01).56 Type 3, displaying an ER pattern globally in the inferior, lateral and right precordial leads, appears to be associated with the highest level of risk and is often associated with electrical storms.33 BrS represents a fourth variant in which ER is limited to the right precordial leads.
The dynamic nature of J wave manifestation in ERS is well recognized. The amplitude of J waves, which may be barely noticeable during sinus rhythm, may become progressively accentuated with increased vagal tone and bradycardia and still further accentuated following successive extrasystoles and compensatory pauses giving rise to short long short sequences that precipitate VT/VF.33, 58, 60
Genetics of Early repolarization syndrome
Data relative to the genetic and molecular basis for ERS is very limited. Haissaguerre and co-workers were the first to associate a missense mutation in KCNJ8 (S422L) with ERS.61 Functional expression data in support of this as a disease-causing genetic variant was recently provided by Medeiros-Domingo and co-workers.62 However, the gold standard for demonstrating a change in sensitivity to adenosine triphosphate (ATP) involves the study of inside-out patches with the internal membrane exposed to increasing concentrations of ATP. These data unfortunately are still not available. The prospect of a gain of function in IK-ATP as the basis for ERS is supported by the observation that pinacidil, an IK-ATP opener, induces both the electrocardiographic and arrhythmic manifestation of ERS in LV wedge preparations.33
Our recent study designed to identify mutations in the LTCC identified 4 individuals with ERS among 205 J wave syndrome probands with mutations in highly conserved residues of CACNA1C, CACNB2 and CACNA2D1.51 Preliminary expression studies indicate that these mutations are associated with a loss of function of ICa supporting the thesis that all three are ERS-susceptibility genes (Barajas et al., unpublished observation).
Association of Site of Mutation with Channel Dysfunction
The predicted topology of the three subunits of LTCC showing the location of the mutations thus far identified is illustrated in Figure 3. Six of the 9 mutations in Cav1.2 α1 subunit associated with the J wave syndromes were in either the N or C terminus. It is interesting that no mutations were detected in any of the transmembrane regions of Cav1.2. Consistent with this finding is the demonstration by Soldatov and co-workers of voltage-gated mobility of the C-and N-cytoplasmic tails of Cav1.2 and their important regulatory role in voltage- and Ca2+− dependent inactivation.63, 64 In addition, it has been shown that cleavage of the C terminus of native Cav1.2 channels results in a proteolytic fragment that is able to act as a repressor of Cav1.2 promoter activity.65, 66 Thus, mutations in the C terminus could have very significant effects on the regulation of expression level and on function of the Cav1.2 channel. Another mutation of great interest is p.E1115K because it is located in the region of the calcium ion selectivity site, giving rise to multiple SCD in the family.51
Most recently, Navedo et al.67 demonstrated that a cluster of Cav1.2 channels organize to facilitate coordinated openings and closings. Fluorescence resonance energy transfer (FRET) analysis of enhanced green fluorescent protein (EGFP)- and red fluorescent protein (RFP)-tagged Cav1.2 channels suggest that coupled gating between these channels may involve transient interactions between 2–6 adjacent channels of adjacent channels via their C termini. This finding provides further insight into the mechanisms underlying channel dysfunction associated with C terminus mutations.
Molecular and cellular pathophysiology of calcium channel mutations
Timothy Syndrome
Timothy syndrome arises from sporadic missense mutations (G406R and G402R) affecting the I–II linker of Cav1.2, which is believed to act as the inactivation gate. By disrupting inactivation, these mutations lead to a gain of function of ICa, which is responsible for the clinical phenotype.25 Barrett and Tsien found that the TS mutation G406R selectively slows VDI while sparing or slightly speeding the kinetics of CDI.68 CDI did not proceed to completeness but was observed to level off at approximately 50%, consistent with a change in gating modes. Thiel et al.,69 found a VDI loss in the cells infected with the mutant channels when intracellular Ca2+ was buffered. In the presence of normal Ca2+ solution, CaMKII activity was increased and the cells exhibit prolonged action potentials and (ER). Inhibition of CaMKII activity reversed the proarrhythmic phenotypes to normal.69 G406R may create a CaMKII consensus site at Ser-409 and that Ser-409 phosphorylation leads to an increased channel activity (“mode 2” gating). It is unknown however whether or not Ser.409 is a CaMKII target.
J Wave Syndromes
Transmural differences in early phases of the action potential (phases 1 and 2) are responsible for inscription of the electrocardiographic J wave.70, 71 The ventricular epicardium commonly displays action potentials with a prominent Ito -mediated notch or spike and dome. A prominent Ito -mediated action potential notch in ventricular epicardium but not endocardium produces a transmural voltage gradient during ERS that registers as a J wave or J point elevation on the ECG.72
Conditions and agents that influence Ito kinetics or ventricular activation sequence can modify the manifestation of the J wave on the ECG. Because of its slow recovery from inactivation, Ito is increased following slowing of heart rate, resulting in an increase in the magnitude of the J wave.73, 74
Augmentation of net early repolarizing current, due either to a decrease of inward current or an increase of outward current, accentuates the notch leading to augmentation of the J wave or appearance of ST segment elevation. A further increase in net repolarizing current can result in partial or complete loss of the action potential dome, leading to a transmural voltage gradient that manifests as greater ST segment elevation.73–75
Brugada Syndrome
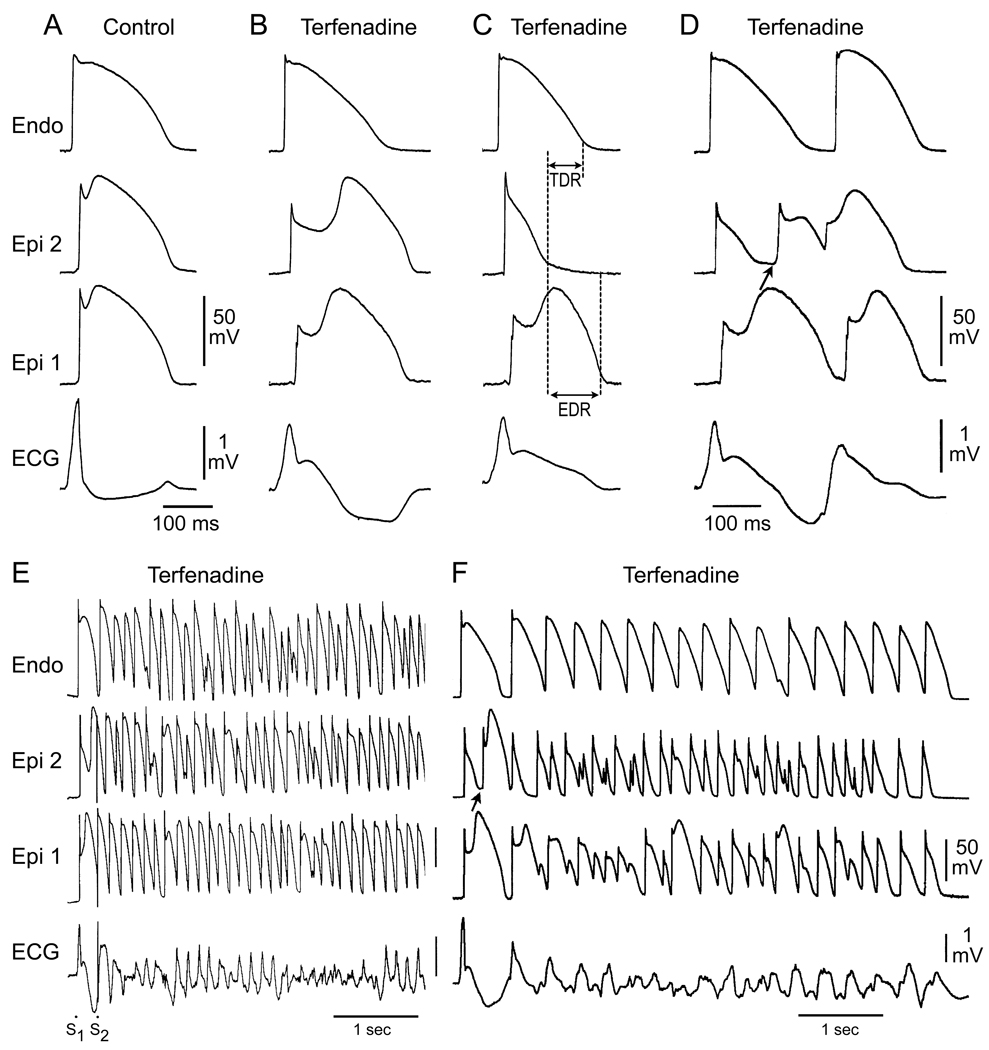
In regions of the myocardium exhibiting a prominent Ito, such as the right ventricular outflow tract epicardium, accentuation of the action potential notch by a reduction of calcium or sodium channel current or an increase in outward current, results in a transmural voltage gradient that leads to coved ST segment elevation (Figure 4B). Under these conditions, there is little in the way of an arrhythmogenic substrate. However, a further outward shift of the currents active during the early phase of the action potential can lead to loss of the action potential dome, thus creating a dispersion of repolarization between epicardium and endocardium as well as within epicardium, between the region where the dome is lost and regions at which it is maintained (Figure 4C).
Figure 4.
Cellular basis for electrocardiographic and arrhythmic manifestation of Brugada Syndrome. Each panel shows transmembrane action potentials from one endocardial (top) and two epicardial sites together with a transmural ECG recorded from a canine coronary-perfused right ventricular wedge preparation. A: Control. B: Combined calcium and sodium channel block with terfenadine (5 µM) accentuates the epicardial action potential notch creating a transmural voltage gradient that manifests as a ST segment elevation or exaggerated J wave in the ECG. C: Continued exposure to terfenadine results in all-or-none repolarization at the end of phase 1 at some epicardial sites but not others, creating a local epicardial dispersion of repolarization (EDR) as well as a transmural dispersion of repolarization (TDR). D: Phase 2 reentry occurs when the epicardial action potential dome propagates from a site where it is maintained to regions where it has been lost giving rise to a closely coupled extrasystole. E: Extrastimulus (S1–S2 = 250 msec) applied to epicardium triggers a polymorphic VT. F: Phase 2 reentrant extrasystole triggers a brief episode of polymorphic VT. (From Antzelevitch and Yan 33, with permission)
When Ito is prominent, as it is in the right ventricular epicardium,74–76 an outward shift of current causes phase 1 of the action potential to progress to more negative potentials at which the ICa fails to activate, leading to an all-or-none repolarization and loss of the dome (Figure 4C). Because loss of the action potential dome is usually heterogeneous, the result is a marked abbreviation of action potential at some sites but not others. The epicardial action potential dome can then propagate from regions where it is maintained to regions where it is lost, giving rise to a very closely coupled extrasystole via a mechanism that we have termed phase 2 reentry (Figure. 4D).77 The extrasystole produced via phase 2 reentry often result in an R-on-T phenomenon precipitating life-threatening arrhythmias (Figures. 4E and F).
Early Repolarization Syndrome
An outward shift of current may extend beyond the action potential notch thus leading to an elevation of the ST segment akin to early repolarization. Activation of the ATP-sensitive potassium current (IK-ATP) or depression of ICa can effect such a change.51 Figure 5 shows an example of ER produced by calcium channel current inhibition. Transmural gradients generated in response to ICa block could manifest in the ECG as a diversity of ER patterns including J point elevation, slurring of the terminal part of the QRS and mild ST segment elevation. The ER pattern could facilitate loss of the dome and lead to the development of ST segment elevation, phase 2 reentry, and VT/VF.
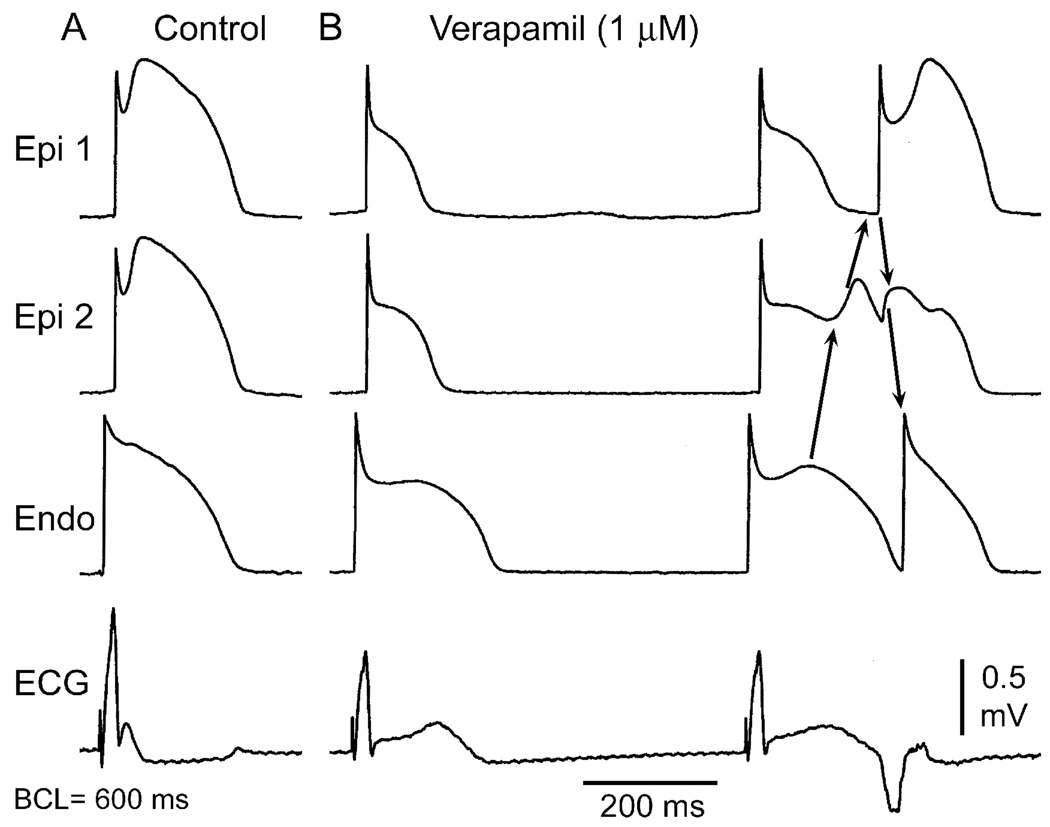
Figure 5.
Verapamil-induced early repolarization and phase 2 reentry mediated extrasystole. Each panel shows simultaneous recordings from two epicardial and one endocardial site of a canine ventricular coronary-perfused wedge preparation together with a pseudo-ECG recorded across the perfusion bath. A: Control B: Recorded in the presence of 1 µM verapamil. Phase 2 reentry gives rise to a closely coupled extrasystole (Courtesy of Jeffrey Fish, DVM).
The ability to recapitulate the ECG and arrhythmic manifestations of ERS using agents that inhibit ICa or augment IK-ATP is consistent with the fact that ERS has been found to be associated with mutations in genes encoding the IK-ATP (KCNJ8) as well calcium channels (CANCA1c, CACNB2, CANCA2D1).
Morrissey and co-workers showed that ventricular Kir6.1 (pore-forming unit of the IK-ATP channel) is more strongly expressed in epicardial ventricular myocytes.78 This heterogeneous transmural distribution of Kir6.1, if present in humans, can contribute to the development of ST segment elevation observed in patients with BrS and ERS carrying mutations in KCNJ8 or SUR2A. The presence of an additional repolarization force during the early phases of the epicardial action potential, due to a gain of function of IK-ATP, can generate an early repolarization pattern in the ECG by causing depression of the epicardial action potential dome.33 The heterogeneous distribution of IK-ATP channels may be unmasked by a reduction in calcium channel activity (due to mutations or calcium channel blocking drugs).The transmural distribution of Ito, may also accentuate the effects of reduced ICa. Moreover, a further outward shift in the balance of current due to augmented vagal influence (IK-ACh activation), bradycardia (greater availability of Ito), or mild ischemia (more IK-ATP), can lead to heterogeneous loss of the action potential dome, thus creating a dispersion of repolarization that can facilitate the development of phase 2 reentry and polymorphic VT.33, 79
Approach to Therapy of Timothy and J waves Syndromes Associated with Calcium Channel Mutations
Timothy Syndrome
Ventricular tachyarrhythmias (VT or VF) is the leading cause of death in TS and should be a primary target for therapy. Although no data are specifically available for the TS cohort, β-blockers are used based on the evidence that they are effective in most LQTS.80 Additional drug therapy (mexiletine, calcium channel blockers) has been proposed in an attempt to abbreviate ventricular repolarization, restore 1:1 conduction, and thus reduce the risk of arrhythmias, but their use has to be considered investigational. Ranolazine has been reported to suppress atrial and ventricular arrhythmias associated with TS in experimental studies as well as in one case report.81, 82 Overall the lack of firm data on effectiveness of drug therapy suggest the use of an implantable cardioverter defibrillator (ICD) in all patients with a confirmed diagnosis and QTc >500ms. Careful monitoring and aggressive therapy of infections (altered immune response) and blood glucose levels is also mandatory.
J Wave Syndromes
The mainstay of therapy for the J wave syndromes is the implantation of an ICD.49, 83–85 However, ICD implantation is problematic in infants and young children. A pharmacologic approach to therapy, based on a rebalancing of currents active during the early phases of the epicardial action potential so as to reduce the magnitude of the action potential notch and/or restore the action potential dome, has been a focus of basic and clinical research. Because the presence of a prominent transient outward current, Ito, is central to the mechanism underlying the J wave syndromes, the most rationale approach to therapy, regardless of the ionic or genetic basis for the disease, is to partially inhibit Ito.86 The only agent on the market with significant Ito blocking properties is quinidine.
Brugada Syndrome
Experimental studies have shown quinidine to be effective in restoring the epicardial action potential dome, thus normalizing the ST segment and preventing phase 2 reentry and polymorphic VT in experimental models of BrS.75 Clinical evidence of the effectiveness of quinidine in normalizing ST segment elevation in patients with the BrS has been reported.87–89 The effects of quinidine to prevent inducible and spontaneous VF has been reported by Belhassen and coworkers.88 A prospective Registry for asymptomatic Brugada patients has been created with the aim of tracking the effectiveness of empiric therapy with quinidine.90 The development of a more cardioselective and Ito-specific blocker would be a most welcome addition to the limited therapeutic armamentarium currently available.
Agents that boost ICa, such as β adrenergic agents like isoproterenol or the phosphodiesterase III inhibitor cilostazol, are useful as well.75, 91, 92 Isoproterenol, sometimes in combination with quinidine, has been shown to be effective in normalizing ST segment elevation in patients with the BrS and in controlling electrical storms, particularly in children.87, 89, 93–95
Early Repolarization Syndrome
Data relative to an approach to therapy of arrhythmic complications of early repolarization syndrome are scarce owing to the recent emergence of this syndrome. Experimental studies suggest an approach to therapy similar to that used in BrS.33 Quinidine and β adrenergic agonists have recently been shown to be effective in suppressing arrhythmic events in patients with ERS.96
Conclusions
The LTCC is widely distributed and plays a critical physiological role in many organs, including the heart. Once thought to be rare, genetic mutations in the subunits of the LTCC are now recognized to be relatively common and to be associated with a wide variety of cardiac arrhythmic syndromes including Timothy, Brugada and early repolarization syndromes as well as other clinical phenotypes. Mutations of LTCC genes were identified only recently. Over the past 6 years, over 25 mutations in the α1, β2 and α2δ subunits have been identified. Although, we have made significant progress in the identification of genetic variations contributing to sudden death syndromes and the ionic and cellular mechanisms responsible for the associated arrhythmias, we are clearly at the tip of the iceberg. With further advances in our understanding of the function of LTCC in health and disease, we hope to develop better therapies for these syndromes.
Acknowledgments
Sources of Funding: Supported by grant HL47678 (CA) from NHLBI, and New York State and Florida Grand Lodges of Free and Accepted Masons.
Non-standard Abbreviations and Acronyms
- ATP
adenosine triphosphate
- ATS
Andersen-Tawil syndrome/LQT7
- BrS
Brugada syndrome
- BrS+SQT
Brugada syndrome and short QT
- [Ca2+]i
intracellular concentration of calcium
- CaMKII
Ca2+-calmodulin-dependent protein kinase II
- CaV
voltage-activated calcium channel
- CaV3.1
gene: CACNA1G
- CaV3.2
gene: CACNA1H
- CaV3.3
gene: CACNA1L
- CDI
Ca2+ dependent
- ECG
electrocardiogram
- EGFP
enhanced green fluorescent protein
- ER
early repolarization
- ERS
early repolarization syndrome
- FRET
fluorescence resonance energy transfer
- ICa
transmembrane calcium current
- ICD
implantable cardioverter defibrillator
- IK-ATP
adenosine triphosphate sensitive potassium current
- IVF
idiopathic ventricular fibrillation
- LTCC
L-type cardiac calcium channel
- LQTS
long QT syndrome
- N-type
neuronal type calcium channel
- P/Q-type
Purkinje type calcium channel
- RBBB
right branch bundle block
- RFP
red fluorescent protein
- SCD
sudden cardiac death
- SNP
single nucleotide polymorphism
- R-type
resistant type calcium channel
- T-type
Transient type calcium
- TS
Timothy syndrome/LQT8
- VDI
voltage dependent
- VF
ventricular fibrillation
- VGCC
voltage gated calcium channels
- VT
ventricular tachycardia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
REFERENCES
- 1.Colecraft HM, Alseikhan B, Takahashi SX, Chaudhuri D, Mittman S, Yegnasubramanian V, Alvania RS, Johns DC, Marban E, Yue DT. Novel functional properties of Ca(2+) channel beta subunits revealed by their expression in adult rat heart cells. J Physiol. 2002;541:435–452. doi: 10.1113/jphysiol.2002.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, Berretta R, Potts ST, Marsh JD, Houser SR. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 3.Fatt P, Katz B. The electrical properties of crustacean muscle fibres. J Physiol. 1953;120:171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleckenstein A. History of calcium antagonists. Circ Res. 1983;52:I3–I6. [PubMed] [Google Scholar]
- 5.Mangoni ME, Traboulsie A, Leoni AL, Couette B, Marger L, Le Quang K, Kupfer E, Cohen-Solal A, Vilar J, Shin HS, Escande D, Charpentier F, Nargeot J, Lory P. Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3.1/alpha1G T-type calcium channels. Circ Res. 2006;98:1422–1430. doi: 10.1161/01.RES.0000225862.14314.49. [DOI] [PubMed] [Google Scholar]
- 6.Bkaily G, Sculptoreanu A, Jacques D, Economos D, Menard D. Apamin, a highly potent fetal L-type Ca2+ current blocker in single heart cells. Am J Physiol. 1992;262:H463–H471. doi: 10.1152/ajpheart.1992.262.2.H463. [DOI] [PubMed] [Google Scholar]
- 7.Barth AS, Kuner R, Buness A, Ruschhaupt M, Merk S, Zwermann L, Kaab S, Kreuzer E, Steinbeck G, Mansmann U, Poustka A, Nabauer M, Sultmann H. Identification of a common gene expression signature in dilated cardiomyopathy across independent microarray studies. J Am Coll Cardiol. 2006;48:1610–1617. doi: 10.1016/j.jacc.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 8.Fournier F, Bourinet E, Nargeot J, Charnet P. Cyclic AMP-dependent regulation of P-type calcium channels expressed in Xenopus oocytes. Pflugers Arch. 1993;423:173–180. doi: 10.1007/BF00374391. [DOI] [PubMed] [Google Scholar]
- 9.Qu Y, Baroudi G, Yue Y, Boutjdir M. Novel molecular mechanism involving alpha1D (Cav1.3) L-type calcium channel in autoimmune-associated sinus bradycardia. Circulation. 2005;111:3034–3041. doi: 10.1161/CIRCULATIONAHA.104.517326. [DOI] [PubMed] [Google Scholar]
- 10.Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 11.Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- 12.Tang ZZ, Liang MC, Lu S, Yu D, Yu CY, Yue DT, Soong TW. Transcript scanning reveals novel and extensive splice variations in human l-type voltage-gated calcium channel, Cav1.2 alpha1 subunit. J Biol Chem. 2004;279:44335–44343. doi: 10.1074/jbc.M407023200. [DOI] [PubMed] [Google Scholar]
- 13.Soldatov NM, Oz M, O'Brien KA, Abernethy DR, Morad M. Molecular determinants of L-type Ca2+ channel inactivation. Segment exchange analysis of the carboxyl-terminal cytoplasmic motif encoded by exons 40–42 of the human alpha1C subunit gene. J Biol Chem. 1998;273:957–963. doi: 10.1074/jbc.273.2.957. [DOI] [PubMed] [Google Scholar]
- 14.Welling A, Ludwig A, Zimmer S, Klugbauer N, Flockerzi V, Hofmann F. Alternatively spliced IS6 segments of the alpha 1C gene determine the tissue-specific dihydropyridine sensitivity of cardiac and vascular smooth muscle L-type Ca2+ channels. Circ Res. 1997;81:526–532. doi: 10.1161/01.res.81.4.526. [DOI] [PubMed] [Google Scholar]
- 15.Blumenstein Y, Kanevsky N, Sahar G, Barzilai R, Ivanina T, Dascal N. A novel long N-terminal isoform of human L-type Ca2+ channel is up-regulated by protein kinase C. J Biol Chem. 2002;277:3419–3423. doi: 10.1074/jbc.C100642200. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Papp AC, Binkley PF, Johnson JA, Sadee W. Highly variable mRNA expression and splicing of L-type voltage-dependent calcium channel alpha subunit 1C in human heart tissues. Pharmacogenet Genomics. 2006;16:735–745. doi: 10.1097/01.fpc.0000230119.34205.8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Chen X, Margulies K, Jeevanandam V, Pollack P, Bailey BA, Houser SR. L-type Ca2+ channel alpha 1c subunit isoform switching in failing human ventricular myocardium. J Mol Cell Cardiol. 2000;32:973–984. doi: 10.1006/jmcc.2000.1138. [DOI] [PubMed] [Google Scholar]
- 18.Liao P, Li G, Yu de J, Yong TF, Wang JJ, Wang J, Soong TW. Molecular alteration of Ca(v)1.2 calcium channel in chronic myocardial infarction. Pflugers Arch. 2009;458:701–711. doi: 10.1007/s00424-009-0652-4. [DOI] [PubMed] [Google Scholar]
- 19.Franzini-Armstrong C, Protasi F, Ramesh V. Shape, size, and distribution of Ca(2+) release units and couplons in skeletal and cardiac muscles. Biophys J. 1999;77:1528–1539. doi: 10.1016/S0006-3495(99)77000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tseng GN, Boyden PA. Multiple types of Ca2+ currents in single canine Purkinje cells. Circ Res. 1989;65:1735–1750. doi: 10.1161/01.res.65.6.1735. [DOI] [PubMed] [Google Scholar]
- 21.Abernethy DR, Soldatov NM. Structure-functional diversity of human L-type Ca2+ channel: perspectives for new pharmacological targets. J Pharmacol.Exp.Ther. 2002;300:724–728. doi: 10.1124/jpet.300.3.724. [DOI] [PubMed] [Google Scholar]
- 22.Sims C, Reisenweber S, Viswanathan PC, Choi BR, Walker WH, Salama G. Sex, age, and regional differences in L-type calcium current are important determinants of arrhythmia phenotype in rabbit hearts with drug-induced long QT type 2. Circ Res. 2008;102:e86–e100. doi: 10.1161/CIRCRESAHA.108.173740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo M, Vicentini A, Spazzolini C, Nastoli J, Bottelli G, Folli R, Cappelletti D. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 24.Reichenbach H, Meister EM, Theile H. The heart-hand syndrome. A new variant of disorders of heart conduction and syndactylia including osseous changes in hands and feet. Kinderarztl Prax. 1992;60:54–56. [PubMed] [Google Scholar]
- 25.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, Sanguinetti MC, Keating MT. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci U S A. 2005;102:8089–8096. doi: 10.1073/pnas.0502506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloise R, Napolitano C, Timothy KW, Pontes Cavalcanti D, Szepesvary E, Drago F, Nastoli J, Splawski I, Keating MT, Priori SG. Clinical profile and risk of sudden death in children with Timothy syndrome. Circulation. 2007;114 suppl II:502–502. [Google Scholar]
- 28.Priori SG, Splawski I, Bloise R, Napolitano C. Timothy syndrome. In: Zipes DP, Jalife P, editors. Cardiac Electrophysiology: From Cell To Bedside. Philadelphia, PA: Elsevier; 2009. pp. 757–761. [Google Scholar]
- 29.Ferreira MA, O'Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, Fan J, Kirov G, Perlis RH, Green EK, Smoller JW, Grozeva D, Stone J, Nikolov I, Chambert K, Hamshere ML, Nimgaonkar VL, Moskvina V, Thase ME, Caesar S, Sachs GS, Franklin J, Gordon-Smith K, Ardlie KG, Gabriel SB, Fraser C, Blumenstiel B, Defelice M, Breen G, Gill M, Morris DW, Elkin A, Muir WJ, McGhee KA, Williamson R, MacIntyre DJ, MacLean AW, St CD, Robinson M, Van Beck M, Pereira AC, Kandaswamy R, McQuillin A, Collier DA, Bass NJ, Young AH, Lawrence J, Ferrier IN, Anjorin A, Farmer A, Curtis D, Scolnick EM, McGuffin P, Daly MJ, Corvin AP, Holmans PA, Blackwood DH, Gurling HM, Owen MJ, Purcell SM, Sklar P, Craddock N. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, Rabah D, Kinet JP, Shin HS. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silvey SV, Stoughton TL, Pearl W, Collazo WA, Belbel RJ. Rupture of the outer partition of aortic dissection during transesophageal echocardiography. Am J Cardiol. 1991;68:286–287. doi: 10.1016/0002-9149(91)90769-h. [DOI] [PubMed] [Google Scholar]
- 32.Sansone V, Griggs RC, Meola G, Ptacek LJ, Barohn R, Iannaccone S, Bryan W, Baker N, Janas SJ, Scott W, Ririe D, Tawil R. Andersen's syndrome: a distinct periodic paralysis. Ann Neurol. 1997;42:305–312. doi: 10.1002/ana.410420306. [DOI] [PubMed] [Google Scholar]
- 33.Antzelevitch C, Yan GX. J wave syndromes. Heart Rhythm. 2010;7:549–558. doi: 10.1016/j.hrthm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antzelevitch C, Pollevick GD, Cordeiro JM, Casis O, Sanguinetti MC, Aizawa Y, Guerchicoff A, Pfeiffer R, Oliva A, Wollnik B, Gelber P, Bonaros EP, Jr, Burashnikov E, Wu Y, Sargent JD, Schickel S, Oberheiden R, Bhatia A, Hsu LF, Haissaguerre M, Schimpf R, Borggrefe M, Wolpert C. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442–449. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cordeiro JM, Marieb M, Pfeiffer R, Calloe K, Burashnikov E, Antzelevitch C. Accelerated inactivation of the L-type calcium current due to a mutation in CACNB2b underlies Brugada syndrome. J Mol Cell Cardiol. 2009;46:695–703. doi: 10.1016/j.yjmcc.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 37.Brugada R, Brugada J, Antzelevitch C, Kirsch GE, Potenza D, Towbin JA, Brugada P. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation. 2000;101:510–515. doi: 10.1161/01.cir.101.5.510. [DOI] [PubMed] [Google Scholar]
- 38.Morita H, Morita ST, Nagase S, Banba K, Nishii N, Tani Y, Watanabe A, Nakamura K, Kusano KF, Emori T, Matsubara H, Hina K, Kita T, Ohe T. Ventricular arrhythmia induced by sodium channel blocker in patients with Brugada syndrome. J Am Coll Cardiol. 2003;42:1624–1631. doi: 10.1016/j.jacc.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu W, Antzelevitch C, Suyama K, Kurita T, Taguchi A, Aihara N, Takaki H, Sunagawa K, Kamakura S. Effect of sodium channel blockers on ST segment, QRS duration, and corrected QT interval in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2000;11:1320–1329. doi: 10.1046/j.1540-8167.2000.01320.x. [DOI] [PubMed] [Google Scholar]
- 40.Fowler SJ, Priori SG. Clinical spectrum of patients with a Brugada ECG. Curr Opin Cardiol. 2009;24:74–81. doi: 10.1097/hco.0b013e32831cb920. [DOI] [PubMed] [Google Scholar]
- 41.Morita H, Kusano KF, Miura D, Nagase S, Nakamura K, Morita ST, Ohe T, Zipes DP, Wu J. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008;118:1697–1704. doi: 10.1161/CIRCULATIONAHA.108.770917. [DOI] [PubMed] [Google Scholar]
- 42.Meregalli PG, Tan HL, Probst V, Koopmann TT, Tanck MW, Bhuiyan ZA, Sacher F, Kyndt F, Schott JJ, Albuisson J, Mabo P, Bezzina CR, Le Marec H, Wilde AA. Type of SCN5A mutation determines clinical severity and degree of conduction slowing in loss-of-function sodium channelopathies. Heart Rhythm. 2009;6:341–348. doi: 10.1016/j.hrthm.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Probst V, Veltmann C, Eckardt L, Meregalli PG, Gaita F, Tan HL, Babuty D, Sacher F, Giustetto C, Schulze-Bahr E, Borggrefe M, Haissaguerre M, Mabo P, Le Marec H, Wolpert C, Wilde AA. Long-term prognosis of patients diagnosed with Brugada syndrome: results from the FINGER Brugada Syndrome Registry. Circulation. 2010;121:635–643. doi: 10.1161/CIRCULATIONAHA.109.887026. [DOI] [PubMed] [Google Scholar]
- 44.Delpon E, Cordeiro JM, Nunez L, Thomsen PE, Guerchicoff A, Pollevick GD, Wu Y, Kanters JK, Larsen CT, Hofman-Bang J, Burashnikov E, Christiansen M, Antzelevitch C. Functional effects of KCNE3 mutation and its role in the development of Brugada syndrome. Circ Arrhythm Electrophysiol. 2008;1:209–218. doi: 10.1161/CIRCEP.107.748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu D, Martinez Hl, Burashnikov E, Springer M, Wu Y, Varro A, Pfeiffer K, Koopmann TT, Cordeiro JM, Guerchicoff A, Pollevick GD, Antzelevitch C. A mutation in the b3 subunit of the cardiac sodium channel associated with Brugada ECG phenotype. Circ Cardiovasc Genet. 2009;3:270–278. doi: 10.1161/CIRCGENETICS.108.829192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kapplinger JD, Tester DJ, Salisbury BA, Carr JL, Harris-Kerr C, Pollevick GD, Wilde AA, Wilde AA, Ackerman MJ. Spectrum and prevalence of mutations from the first 2,500 consecutive unrelated patients referred for the FAMILION long QT syndrome genetic test. Heart Rhythm. 2009;6:1297–1303. doi: 10.1016/j.hrthm.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.London B, Michalec M, Mehdi H, Zhu X, Kerchner L, Sanyal S, Viswanathan PC, Pfahnl AE, Shang LL, Madhusudanan M, Baty CJ, Lagana S, Aleong R, Gutmann R, Ackerman MJ, McNamara DM, Weiss R, Dudley SC., Jr Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation. 2007;116:2260–2268. doi: 10.1161/CIRCULATIONAHA.107.703330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe H, Koopmann TT, Le Scouarnec S, Yang T, Ingram CR, Schott JJ, Demolombe S, Probst V, Anselme F, Escande D, Wiesfeld AC, Pfeufer A, Kaab S, Wichmann HE, Hasdemir C, Aizawa Y, Wilde AA, Roden DM, Bezzina CR. Sodium channel beta1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J Clin Invest. 2008;118:2260–2268. doi: 10.1172/JCI33891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Priori SG, Napolitano C, Gasparini M, Pappone C, Bella PD, Giordano U, Bloise R, Giustetto C, De Nardis R, Grillo M, Ronchetti E, Faggiano G, Nastoli J. Natural history of Brugada syndrome: insights for risk stratification and management. Circulation. 2002;105:1342–1347. doi: 10.1161/hc1102.105288. [DOI] [PubMed] [Google Scholar]
- 50.Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, Potenza D, Moya A, Borggrefe M, Breithardt G, Ortiz-Lopez R, Wang Z, Antzelevitch C, O'Brien RE, Schulze-Bahr E, Keating MT, Towbin JA, Wang Q. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293–296. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 51.Burashnikov E, Pfeiffer R, Barajas-Martinez H, Delpon E, Hu D, Desai M, Borggrefe M, Haissaguerre M, Kanter R, Pollevick GD, Guerchicoff A, Laino R, Marieb M, Nademanee K, Nam GB, Robles R, Schimpf R, Stapleton DH, Viskin S, Winters S, Wolpert C, Zimmern S, Veltmann C, Antzelevitch C. Mutations in the Cardiac L-Type Calcium Channel Associated with Inherited J Wave Syndromes and Sudden Cardiac Death. Heart Rhythm. doi: 10.1016/j.hrthm.2010.08.026. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wasserburger RH, Alt WJ. The normal RS-T segment elevation variant. Am J Cardiol. 1961;8:184–192. doi: 10.1016/0002-9149(61)90204-1. [DOI] [PubMed] [Google Scholar]
- 53.Mehta MC, Jain AC. Early repolarization on scalar electrocardiogram. Am J Med Sci. 1995;309:305–311. doi: 10.1097/00000441-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Gussak I, Antzelevitch C. Early repolarization syndrome: clinical characteristics and possible cellular and ionic mechanisms. J Electrocardiol. 2000;33:299–309. doi: 10.1054/jelc.2000.18106. [DOI] [PubMed] [Google Scholar]
- 55.Sinner MF, Reinhard W, Muller M, Beckmann BM, Martens E, Perz S, Pfeufer A, Winogradow J, Stark K, Meisinger C, Wichmann HE, Peters A, Riegger GA, Steinbeck G, Hengstenberg C, Kaab S. Association of early repolarization pattern on ecg with risk of cardiac and all-cause mortality: a population-based prospective cohort study (MONICA/KORA) PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000314. e1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- 57.Rosso R, Kogan E, Belhassen B, Rozovski U, Scheinman MM, Zeltser D, Halkin A, Steinvil A, Heller K, Glikson M, Katz A, Viskin S. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: incidence and clinical significance. J Am Coll Cardiol. 2008;52:1231–1238. doi: 10.1016/j.jacc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 58.Nam GB, Kim YH, Antzelevitch C. Augmentation of J waves and electrical storms in patients with early repolarization. N Engl J Med. 2008;358:2078–2079. doi: 10.1056/NEJMc0708182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haissaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, de Roy L, Pasquie JL, Nogami A, Babuty D, Yli-Mayry S, De Chillou C, Scanu P, Mabo P, Matsuo S, Probst V, Le Scouarnec S, Defaye P, Schlaepfer J, Rostock T, Lacroix D, Lamaison D, Lavergne T, Aizawa Y, Englund A, Anselme F, O'Neill M, Hocini M, Lim KT, Knecht S, Veenhuyzen GD, Bordachar P, Chauvin M, Jais P, Coureau G, Chene G, Klein GJ, Clementy J. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 60.Nam GB, Ko KH, Kim J, Park KM, Rhee KS, Choi KJ, Kim YH, Antzelevitch C. Mode of onset of ventricular fibrillation in patients with early repolarization pattern vs. Brugada syndrome. Eur Heart J. 2010;31:330–339. doi: 10.1093/eurheartj/ehp423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haissaguerre M, Chatel S, Sacher F, Weerasooriya R, Probst V, Loussouarn G, Horlitz M, Liersch R, Schulze-Bahr E, Wilde A, Kaab S, Koster J, Rudy Y, Le Marec H, Schott JJ. Ventricular fibrillation with prominent early repolarization associated with a rare variant of KCNJ8/KATP channel. J Cardiovasc Electrophysiol. 2009;20:93–98. doi: 10.1111/j.1540-8167.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 62.Medeiros-Domingo A, Tan BH, Crotti L, Tester DJ, Eckhardt L, Cuoretti A, Kroboth SL, Song C, Zhou Q, Kopp D, Schwartz PJ, Makielski JC, Ackerman MJ. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac K(ATP) channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm. 2010;7:1466–1471. doi: 10.1016/j.hrthm.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kobrinsky E, Tiwari S, Maltsev VA, Harry JB, Lakatta E, Abernethy DR, Soldatov NM. Differential role of the alpha1C subunit tails in regulation of the Cav1.2 channel by membrane potential, beta subunits, and Ca2+ ions. J Biol Chem. 2005;280:12474–12485. doi: 10.1074/jbc.M412140200. [DOI] [PubMed] [Google Scholar]
- 64.Kobrinsky E, Schwartz E, Abernethy DR, Soldatov NM. Voltage-gated mobility of the Ca2+ channel cytoplasmic tails and its regulatory role. J Biol Chem. 2003;278:5021–5028. doi: 10.1074/jbc.M211254200. [DOI] [PubMed] [Google Scholar]
- 65.Schroder E, Byse M, Satin J. L-type calcium channel C terminus autoregulates transcription. Circ Res. 2009;104:1373–1381. doi: 10.1161/CIRCRESAHA.108.191387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hulme JT, Yarov-Yarovoy V, Lin TW, Scheuer T, Catterall WA. Autoinhibitory control of the CaV1.2 channel by its proteolytically processed distal C-terminal domain. J Physiol. 2006;576:87–102. doi: 10.1113/jphysiol.2006.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Navedo MF, Cheng EP, Yuan C, Votaw S, Molkentin JD, Scott JD, Santana LF. Increased coupled gating of L-type Ca2+ channels during hypertension and Timothy syndrome. Circ Res. 2010;106:748–756. doi: 10.1161/CIRCRESAHA.109.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barrett CF, Tsien RW. The Timothy syndrome mutation differentially affects voltage- and calcium-dependent inactivation of CaV1.2 L-type calcium channels. Proc Natl Acad Sci U S A. 2008;105:2157–2162. doi: 10.1073/pnas.0710501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thiel WH, Chen B, Hund TJ, Koval OM, Purohit A, Song LS, Mohler PJ, Anderson ME. Proarrhythmic defects in Timothy syndrome require calmodulin kinase II. Circulation. 2008;118:2225–2234. doi: 10.1161/CIRCULATIONAHA.108.788067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sicouri S, Moro S, Litovsky S, Elizari MV, Antzelevitch C. Chronic amiodarone reduces transmural dispersion of repolarization in the canine heart. J Cardiovasc Electrophysiol. 1997;8:1269–1279. doi: 10.1111/j.1540-8167.1997.tb01018.x. [DOI] [PubMed] [Google Scholar]
- 71.Litovsky SH, Antzelevitch C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ Res. 1988;62:116–126. doi: 10.1161/01.res.62.1.116. [DOI] [PubMed] [Google Scholar]
- 72.Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 1996;93:372–379. doi: 10.1161/01.cir.93.2.372. [DOI] [PubMed] [Google Scholar]
- 73.Antzelevitch C, Yan GX. Cellular and ionic mechanisms responsible for the Brugada syndrome. J Electrocardiol. 2000;33 Suppl:33–39. doi: 10.1054/jelc.2000.20321. [DOI] [PubMed] [Google Scholar]
- 74.Yan GX, Lankipalli RS, Burke JF, Musco S, Kowey PR. Ventricular repolarization components on the electrocardiogram: cellular basis and clinical significance. J Am Coll Cardiol. 2003;42:401–409. doi: 10.1016/s0735-1097(03)00713-7. [DOI] [PubMed] [Google Scholar]
- 75.Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 76.Di Diego JM, Sun ZQ, Antzelevitch C. I(to) and action potential notch are smaller in left vs. right canine ventricular epicardium. Am J Physiol. 1996;271:H548–H561. doi: 10.1152/ajpheart.1996.271.2.H548. [DOI] [PubMed] [Google Scholar]
- 77.Krishnan SC, Antzelevitch C. Flecainide-induced arrhythmia in canine ventricular epicardium. Phase 2 reentry? Circulation. 1993;87:562–572. doi: 10.1161/01.cir.87.2.562. [DOI] [PubMed] [Google Scholar]
- 78.Morrissey A, Rosner E, Lanning J, Parachuru L, Dhar Chowdhury P, Han S, Lopez G, Tong X, Yoshida H, Nakamura TY, Artman M, Giblin JP, Tinker A, Coetzee WA. Immunolocalization of KATP channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC Physiol. 2005;5(1) doi: 10.1186/1472-6793-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Antzelevitch C. Brugada syndrome. Pacing Clin Electrophysiol. 2006;29:1130–1159. doi: 10.1111/j.1540-8159.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Priori SG, Napolitano C, Schwartz PJ, Grillo M, Bloise R, Ronchetti E, Moncalvo C, Tulipani C, Veia A, Bottelli G, Nastoli J. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. JAMA. 2004;292:1341–1344. doi: 10.1001/jama.292.11.1341. [DOI] [PubMed] [Google Scholar]
- 81.Shah DP, Baez-Escudero JL, Beshai JF, Burke MC. Ranolazine safely decreases ventricular and atrial fibrillation in Timothy syndrome (LQT8) Pacing Clin Electrophysiol. 2010 doi: 10.1111/j.1540-8159.2010.02913.x. in press. [DOI] [PubMed] [Google Scholar]
- 82.Sicouri S, Timothy KW, Zygmunt AC, Glass A, Goodrow RJ, Bellardinelli L, Antzelevitch C. Cellular basis for the electrocardiographic and arrhythmic manifestations of Timothy syndrome: effects of ranolazin. Heart Rhythm. 2007;4:638–647. doi: 10.1016/j.hrthm.2006.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, Shimizu W, Schulze-Bahr E, Tan H, Wilde A. Brugada Syndrome. Report of the Second Consensus Conference. Endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 84.Brugada J, Brugada R, Brugada P. Pharmacological and device approach to therapy of inherited cardiac diseases associated with cardiac arrhythmias and sudden death. J Electrocardiol. 2000;33 Suppl:41–47. doi: 10.1054/jelc.2000.20322. 41–47. [DOI] [PubMed] [Google Scholar]
- 85.Brugada P, Brugada R, Brugada J, Geelen P. Use of the prophylactic implantable cardioverter defibrillator for patients with normal hearts. Am.J.Cardiol. 1999;83:98D–100D. doi: 10.1016/s0002-9149(98)01009-1. [DOI] [PubMed] [Google Scholar]
- 86.Antzelevitch C, Brugada P, Brugada J, Brugada R, Nadamanee K, Towbin JA. Clinical approaches to tachyarrhythmias. The Brugada syndrome. Armonk, NY: Futura Publishing Company, Inc; 1999. [Google Scholar]
- 87.Alings M, Dekker L, Sadee A, Wilde A. Quinidine induced electrocardiographic normalization in two patients with Brugada syndrome. Pacing Clin Electrophysiol. 2001;24:1420–1422. doi: 10.1046/j.1460-9592.2001.01420.x. [DOI] [PubMed] [Google Scholar]
- 88.Belhassen B, Viskin S. Pharmacologic approach to therapy of Brugada syndrome: quinidine as alternative to ICD therapy? In: Antzelevitch C, Brugada P, Brugada J, Brugada R, editors. The Brugada syndrome from bench to bedside. Oxford, UK: Wiley-Blackwell; 2004. pp. 202–211. [Google Scholar]
- 89.Belhassen B, Viskin S, Antzelevitch C. The Brugada syndrome: is an implantable cardioverter defibrillator the only therapeutic option? Pacing Clin Electrophysiol. 2002;25:1634–1640. doi: 10.1046/j.1460-9592.2002.01634.x. [DOI] [PubMed] [Google Scholar]
- 90.Viskin S, Wilde AA, Tan HL, Antzelevitch C, Shimizu W, Belhassen B. Empiric quinidine therapy for asymptomatic Brugada syndrome: time for a prospective registry. Heart Rhythm. 2009;6:401–404. doi: 10.1016/j.hrthm.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Antzelevitch C. The Brugada syndrome: ionic basis and arrhythmia mechanisms. J Cardiovasc Electrophysiol. 2001;12:268–272. doi: 10.1046/j.1540-8167.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- 92.Tsuchiya T, Ashikaga K, Honda T, Arita M. Prevention of ventricular fibrillation by cilostazol, an oral phosphodiesterase inhibitor, in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 2002;13:698–701. doi: 10.1046/j.1540-8167.2002.00698.x. [DOI] [PubMed] [Google Scholar]
- 93.Shimizu W, Matsuo K, Takagi M, Tanabe Y, Aiba T, Taguchi A, Suyama K, Kurita T, Aihara N, Kamakura S. Body surface distribution and response to drugs of ST segment elevation in Brugada syndrome: clinical implication of eighty-seven-lead body surface potential mapping and its application to twelve-lead electrocardiograms. J Cardiovasc Electrophysiol. 2000;11:396–404. doi: 10.1111/j.1540-8167.2000.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 94.Suzuki H, Torigoe K, Numata O, Yazaki S. Infant case with a malignant form of Brugada syndrome. J Cardiovasc Electrophysiol. 2000;11:1277–1280. doi: 10.1046/j.1540-8167.2000.01277.x. [DOI] [PubMed] [Google Scholar]
- 95.Tanaka H, Kinoshita O, Uchikawa S, Kasai H, Nakamura M, Izawa A, Yokoseki O, Kitabayashi H, Takahashi W, Yazaki Y, Watanabe N, Imamura H, Kubo K. Successful prevention of recurrent ventricular fibrillation by intravenous isoproterenol in a patient with Brugada syndrome. Pacing Clin Electrophysiol. 2001;24:1293–1294. doi: 10.1046/j.1460-9592.2001.01293.x. [DOI] [PubMed] [Google Scholar]
- 96.Haissaguerre M, Sacher F, Nogami A, Komiya N, Bernard A, Probst V, Yli-Mayry S, Defaye P, Aizawa Y, Frank R, Mantovan R, Cappato R, Wolpert C, Leenhardt A, de Roy L, Heidbuchel H, Deisenhofer I, Arentz T, Pasquie JL, Weerasooriya R, Hocini M, Jais P, Derval N, Bordachar P, Clementy J. Characteristics of recurrent ventricular fibrillation associated with inferolateral early repolarization role of drug therapy. J Am Coll Cardiol. 2009;53:612–619. doi: 10.1016/j.jacc.2008.10.044. [DOI] [PubMed] [Google Scholar]