Abstract
The farnesoid X receptor (FXR) is a nuclear receptor that plays key roles in hepatoprotection by maintaining the homeostasis of liver metabolism. FXR null mice display strong hepatic inflammation and develop spontaneous liver tumors. In this report, we demonstrate that FXR is a negative modulator of NF-κB-mediated hepatic inflammation. Activation of FXR by its agonist ligands inhibited the expression of inflammatory mediators in response to the NF-κB activation in both HepG2 cells and primary hepatocytes cultured in vitro. In vivo, compared to the wild-type controls, FXR−/−mice displayed elevated mRNA levels of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), interferon-γ-inducible protein 10 (IP-10), and interferon-γ (IFN-γ) in response to lipopolysacchride (LPS). Examination of FXR−/− livers showed massive necroses and inflammation after treatment with LPS at a dose that does not induce significant liver damage and inflammation in wild-type mice. Moreover, transfection of a constitutively active FXR expression construct repressed the iNOS, COX-2, IP-10 and IFN-γ mRNA levels induced by LPS administration. FXR activation had no negative effects on NF-κB-activated anti-apoptotic genes, suggesting that FXR selectively inhibits the NF-κB-mediated hepatic inflammatory response but maintains or even enhances the cell survival response. On the other hand, NF-κB activation suppressed FXR-mediated gene expression both in vitro and in vivo, indicating a negative crosstalk between the FXR and NF-κB signaling pathways. Our findings reveal that FXR is a negative mediator of hepatic inflammation, which may contribute to the critical roles of FXR in hepatoprotection and suppression of hepatocarcinogenesis.
Keywords: FXR, Nuclear receptor, Inflammation, NF-κB, Hepatoprotection
Introduction
Inflammatory responses play important roles in pathological conditions of the liver in both humans and experimental animals (1, 2). Therefore, the precise control of inflammation is essential for the prevention of chronic inflammatory disorders, as well as for inhibiting the exacerbation or progression of diseases, including many types of cancers (3). Hepatocellular carcinoma (HCC) often occurs as a secondary condition to chronic hepatitis and it is a prototypical inflammation-associated cancer (4). Recently, several reports have linked the NF-κB signaling pathway to HCC, thereby providing insight into the molecular mechanism by which inflammation affects HCC development (5).
NF-κB has received considerable attention as a key regulator of inflammation because activated NF-κB is frequently detected in various inflammatory diseases and tumours (4). The activation of NF-κB, which down-regulates the transcriptional activity of multiple steroid/nuclear receptors (6), is one of the critical cellular responses to acute infections and inflammations (3, 7). One of the pivotal functions of NF-κB is rapid activation in response to lipopolysacchride (LPS) or pro-inflammatory cytokines, which is an evolutionally conserved, defensive mechanism against infections. Recent animal studies provide strong, direct genetic evidence that the classical, IKK-dependent NF-κB-activation pathway is a crucial mediator of tumor promotion (4, 8). The classic NF-κB consists of a p65 (RelA) and p50 heterodimer that is activated in response to various stimuli, including LPS, TNF-α, double-stranded RNA, and ultra-violet radiation. Functional crosstalk between NF-κB and several other nuclear receptors, such as the nuclear steroid and xenobiotic receptor, estrogen receptor, and androgen receptor, has been reported and suggested to have different physiological significance in xenobiotic or lipid metabolisms and inflammation (2, 6).
Nuclear receptors (NRs) are ligand-activated transcription factors that have central roles in nearly every aspect of development and adult physiology (9), and several NRs play important roles in regulating inflammatory responses(10). For example, peroxisome proliferator-activated receptors (PPARs) and liver X receptors (LXRs) have been reported to be molecular links between lipid metabolism and inflammatory responses (11, 12). Recently, it was shown that the nuclear steroid and xenobiotic receptor SXR and NF-κB mutually suppress each other (6).
Another nuclear receptor, farnesoid X receptor (FXR), is a bile acid receptor that is essential for bile acid (BA) homeostasis, as well as for normal lipid and glucose metabolism (13–15). FXR binds as a heterodimer with retinoid X receptor (RXR) and coordinates the expression of genes involved in BA production, efflux, and influx, as well as detoxification in the liver, which suggests that a key function of FXR is for the maintenance of bile acid homeostasis and reduction of BA toxicity (16). We recently showed that FXR−/− mice displayed prominent liver injury and inflammation, and developed spontaneous liver tumors as they aged (17). A similar finding indicated that the expression of inflammatory genes in the liver was elevated in FXR null mice (14). We hypothesized that FXR may directly modulate liver inflammation.
Here we report a novel role for FXR in the control of liver inflammation that of antagonizing the NF-κB signaling pathway. Our results identify FXR as a potential regulator of hepatic inflammation and suggest that FXR ligands may be used to treat liver inflammatory diseases and prevent hepatocarcinogenesis. On the other hand, activated NF-κB repressed the FXR signaling pathway, suggesting negative crosstalk between the FXR and NF-κB signaling pathways.
Materials and Methods
Reagents and plasmids
CDCA, LPS (from E. coli 0111:B4) and TPA were purchased from Sigma Chemical (St. Louis, MO). GW4064 and 6ECDCA were provided by Dr. Barry M. Forman. TNF-α was purchased from R&D Systems, Inc. The phFXR, phRXR expression vectors and FXR-dependent reporter (EcRE-LUC) were created in our lab. The p65 expression vector and the phRL-TK vector were kindly provided by Xufeng Chen (City of Hope, Duarte, CA) and Akio Kruoda (City of Hope, Duarte, CA), respectively. Estrogen receptor-α (ERα) reporter plasmid and ERα expression plasmid were provided by Dr. Barry M. Forman. The NF-κB-dependent reporter (NF-κBx3-LUC) was kindly provided by Dr. Peter Tontonoz (UCLA, Los Angeles, CA) and Dr. Bruce Blumberg (UCLA, Los Angeles, CA).
Cell culture and transient transfection
Human hepatoblastoma cells (HepG2) were seeded into 6-well plates (1×106 cells/well) and grown in complete culture medium [high glucose DMEM (with L-glutamine) supplied with 10% (vol/vol) inactivated fetal calf serum and 1% (vol/vol) antibiotics-antimycotics] as described previously (18). The following day, cells were treated with GW4064 (2μM) or 6ECDCA (3μM). Eighteen hours after treatment, the cells were treated with TPA (50 nM), LPS (1 ug/mL) or TNF-α (10 ng/mL) and then collected for RNA isolation after a 6 h incubation.
Transient transfection of HepG2 cells was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Twenty-four hours after transfection, cells were pre-treated with GW4064 (2μM) or 6ECDCA (3μM) for, unless stated otherwise, 18 h. Then cells were treated with/without LPS or TPA. Following a 6 h incubation, cells were harvested and the luciferase activity was determined by using a dual-luciferase reporter assay system in accordance with the manufacturer’s instructions (Promega, Madison, WI). Luciferase activities were normalized by co-transfection of the control thymidine kinase-driven Renilla luciferase plasmid, phRL-TK. Data are expressed as relative fold activation to that of non-stimulated (−) sets.
Primary mouse hepatocyte culture
Primary hepatocytes from 8-week-old mice were prepared as described previously (19–21). Cells were treated with GW4064 (2μM) and 6ECDCA (3μM). Eighteen hours after treatment, the cells were treated with LPS (20 μg/ml), TPA (150 nM) or TNF-α (10 ng/ml) and then collected for RNA isolation after a 6 h incubation.
RNA isolation and quantitative real-time polymerase chain reaction
Total RNA isolation from HepG2 cells, primary mouse hepatocytes, and mouse livers and quantitative real-time polymerase chain reaction (PCR) were performed as described previously (17). Amplification of β-actin was used as an internal reference. Primers sequences are available on request.
Animals
Eight-week-old mice, unless stated otherwise, were used in this work. The wild-type and FXR−/− mice (gift from Dr. Frank Gonzalez at National Institute of Health) (22) were maintained in a pathogen-free animal facility under a standard 12:12-hour light/dark cycle. Mice were fed standard rodent chow and water ad libitum. Eight-week-old female wild-type and FXR−/− mice were fasted overnight and then injected intraperitoneally (i.p.) with a single dose of LPS (20 mg/kg) or PBS, followed by feeding water ad libitum. Six hours after the injection, mice were killed, and blood and livers were removed for further analysis. All procedures followed the NIH guidelines for the care and use of laboratory animals.
Adenovirus
Adenovirus that expressed VP16 (the transactivation domain of herpes simplex virus) alone (Ad-VP16) or murine FXRα2 fused to VP16 (FXRα2-VP16; constitutively active) was provided by Dr. Peter A. Edwards (University of California, Los Angeles, CA). Adenovirus was amplified in HEK293 cells and purified with an Adeno-X Virus Purification Kit (Clontech Laboratories, Inc, Mountain View, CA). Wild-type or FXR−/−mice were injected in the tail vein with 1×109 plaque-forming units per mouse of either Ad-VP16 or FXRα2-VP16 (4 mice per group). After 7 days, mice were fasted overnight and then injected i.p. with a single dose of LPS (30 mg/kg) or PBS. After 6 hours, mice were killed and livers were removed for further analysis.
Analysis of alanine transaminase (ALT) activity and liver histology
ALT activity analysis, H&E and terminal nucleotidyl transferase mediated nick end labeling (TUNEL) staining were performed as previously described (17).
Immunoblot analysis
The nuclear extracts of mouse liver and HepG2 cells were prepared as reported by Najima et al. (23) and Bontemps et al. (24), respectively. Immunoblot analysis was performed as described previously (18). The samples were blotted using p65 (Cell Signaling) or Lamin B1 (Santa Cruz Biotechnology) antibodies.
Gel-shift assay
Nuclear extracts of HepG2 cells were prepared as described in section “Immunoblot analysis”. EMSAs (gel shift assays) were performed as described previously (25, 26). The following oligonucleotide was used for the gel shift assay: NF-κB-binding site; 5′-tcgagggctggggattccccat-3′.
Statistics
All data represent at least three independent experiments and are expressed as the mean ± SD. The Student’s t test was used to calculate P values. P less than 0.05 was considered significant.
Results
FXR−/− mouse primary hepatocytes are sensitive to activation of NF-κB
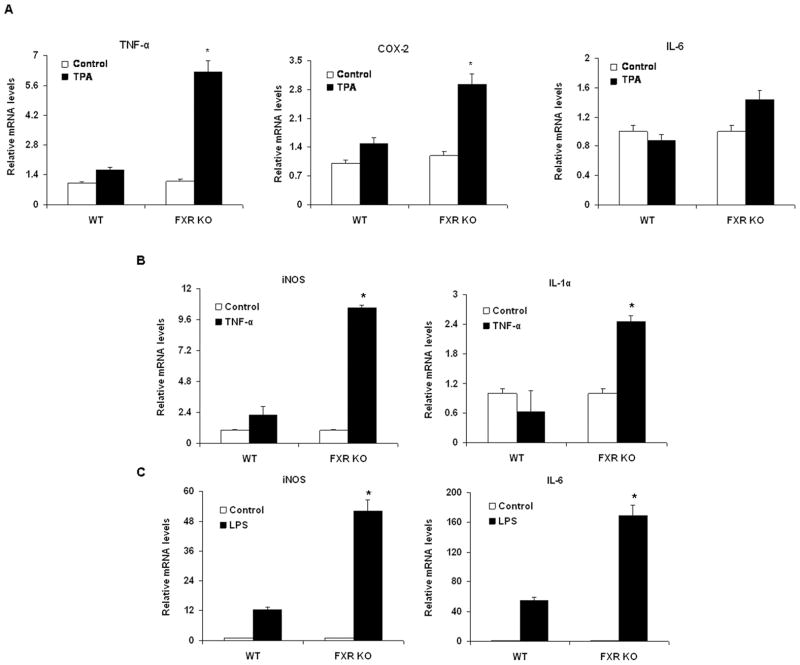
Because some nuclear receptors such as pregnane X receptor (PXR) and LXR regulate the inflammatory response by repressing NF-κB signaling, we hypothesized that FXR−/−mice are more sensitive than wild-type mice to inflammation mediated by NF-κB. We first compared the mRNA levels of pro-inflammatory genes in primary hepatocytes from wild-type and FXR−/− mice after activating the NF-κB pathway with 12-o-tetradecanoyl-phorbol-13-acetate (TPA), tumor necrosis factor-α (TNF-α) or LPS. Primary FXR−/−hepatocytes that were treated with TPA expressed higher levels of TNF-α and cyclooxygenase-2 (COX-2) mRNA than did untreated primary FXR−/− hepatocytes. This induction was considerably reduced in wild-type primary hepatocytes (Fig. 1A). TPA-treated primary hepatocytes from FXR−/− mice expressed higher levels of interleukin-6 (IL-6) mRNA than non-treated FXR−/− hepatocytes, but this change in expression was not observed in wild-type controls (Fig. 1A). We also compared the expression of pro-inflammatory genes in primary hepatocytes from FXR−/− and wild-type mice after treatment with TNF-α (Fig. 1B) and LPS (Fig. 1C). Induction of hepatic inducible nitric oxide synthase (iNOS), interleukin-1α (IL-1α) and IL-6 expression in response to TNF-α or LPS was greatly higher in FXR−/− primary hepatocytes than in wild-type hepatocytes. These results suggest that FXR−/− primary hepatocytes are more sensitive than wild-type hepatocytes to NF-κB activation.
Figure 1. Induction of pro-inflammatory genes in mouse primary hepatocytes in response to NF-κB activation.
Quantitative real-time PCR analysis of the levels of expression of pro-inflammatory genes in wild-type (WT) or FXR−/− (FXR KO) mouse primary hepatocytes treated with vehicle (DMSO or water), TPA (150 nM) (A), TNF-α (10 ng/ml) (B) or LPS (20 μg/ml) (C) for 6 h. *P < .05 compared to the control group (n=3).
Inhibition of NF-κB-regulated pro-inflammatory genes by FXR agonists
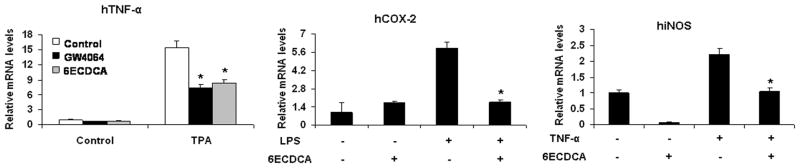
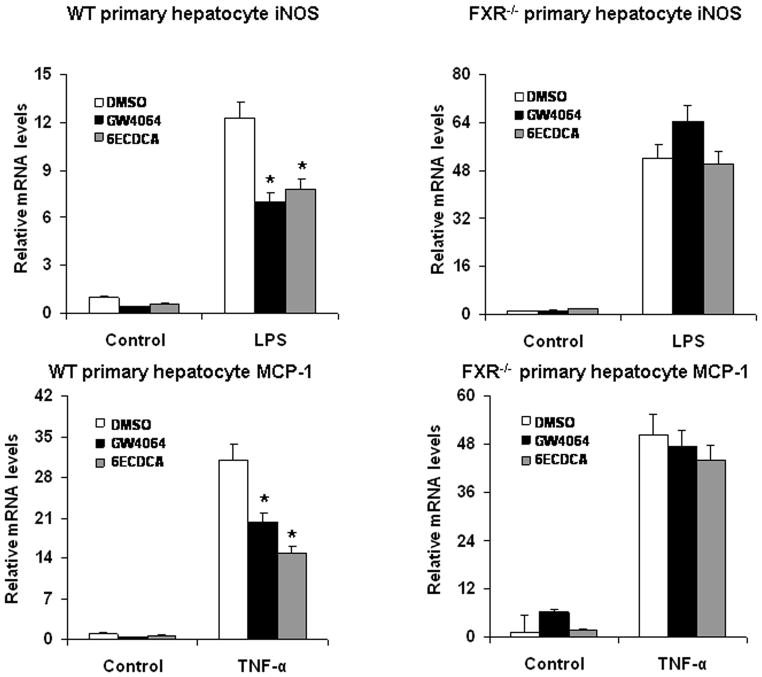
In our previous work, it has been indicated that the effects of FXR agonists in HepG2 cells are through FXR (18). To investigate whether activation of FXR has effects on the NF-κB pathway, we tested the influence of FXR agonists on the expression of TNF-α, COX-2 and iNOS in HepG2 cells. Cells that were pretreated with the FXR agonists GW4064 and 6ECDCA showed greatly less TPA-induced expression of TNF-α mRNA than did non-pretreated cells (Fig. 2). A similar inhibition of expression of COX-2 and iNOS by 6ECDCA was observed in response to stimulation with LPS or TNF-α, respectively (Fig. 2). To confirm that these effects were mediated by FXR, we also tested the influence of FXR agonists on the expression of pro-inflammatory gene in response to NF-κB activation in primary hepatocytes from wild-type and FXR−/− mice. Inhibition of LPS-induced iNOS expression by the FXR agonists GW4064 and 6ECDCA was preserved in primary hepatocytes from wild-type mice, but was abolished in FXR−/−hepatocytes (Fig. 3). GW4064 and 6ECDCA also repressed the TNF-α-induced expression of the NF-κB target gene MCP-1 in wild-type hepatocytes, but not in FXR−/−hepatocytes (Fig. 3). Our results indicate that FXR activation represses the expression of NF-κB-regulated genes in HepG2 cells and mouse primary hepatocytes. Similar phenomenon on FXR activation by its synthetic ligands (GW4064 and 6ECDCA) downregulating iNOS and COX-2 was observed by Li et al. in vascular smooth muscle cells (27).
Figure 2. FXR ligands suppress the induction of pro-inflammatory genes induced by TPA, LPS or TNF-α in HepG2 cells.
Quantitative real-time PCR analysis of the levels of expression of pro-inflammatory genes in HepG2 cells that were pretreated with vehicle (DMSO), GW4064 (2 μM) or 6ECDCA (3 μM) for 18 h before treatment with TPA (50 nM), LPS (1 ug/ml) or TNF-α (10 ng/ml) for 6 h. *P < .05 compared to TPA, LPS or TNF-α alone treatment groups, respectively (n=3).
Figure 3. FXR ligands suppress the induction of iNOS and MCP-1 in primary hepatocytes.
Quantitative real-time PCR analysis of the expression of iNOS and MCP-1 in wild-type and FXR−/− mouse primary hepatocytes that were pretreated with vehicle (DMSO), GW4064 (2 μM) or 6ECDCA (3 μM) for 18 h before treatment with LPS (20 μg/ml) or TNF-α (10 ng/ml) for 6 h. *P < .05 compared to LPS or TNF-α alone treatment groups, respectively (n=3).
Activation of FXR antagonizes NF-κB signaling
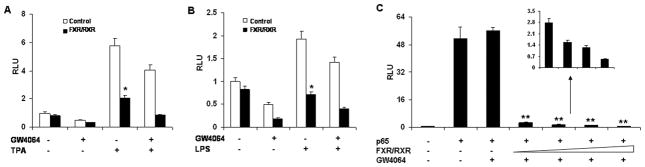
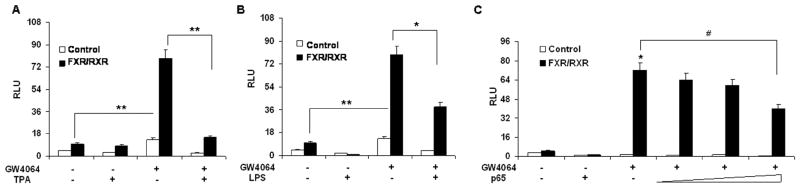
Because FXR agonists such as GW4064 and 6ECDCA inhibited the expression of NF-κB target genes, we next tested whether FXR agonists inhibited NF-κB activity at the level of gene transcription. We co-transfected HepG2 cells with an NF-κB reporter plasmid and the control plasmid phRL-TK, and assessed the effects of GW4064 on the regulation of NF-κB reporter activity. Treatment with known NF-κB pathway activators such as TPA and LPS resulted in 6-fold and 2-fold greater NF-κB reporter activity, respectively (Fig. 4A,B). NF-κB activity induced by TPA or LPS was inhibited by GW4064 treatment. Transfection of these cells with FXR/RXR inhibited NF-κB activity in the absence of ligand, suggesting that FXR may suppress NF-κB activity without addition of exogenous ligand due to the fact that HepG2 cells may synthesize bile acid to activate FXR as reported previously (28). However, addition of GW4064 further enhanced this repression (Fig. 4A,B). Furthermore, to eliminate the possibility that the compounds were affecting other pathways, we used p65 overexpression to activate the NF-κB reporter (6). Overexpression of p65 significantly activated the NF-κB reporter (Fig. 4C). NF-κB activity was inhibited by GW4064 in the presence of FXR/RXR, but GW4064 treatment had no significant effect on NF-κB activity in the absence of the FXR/RXR expression vectors. The observed inhibition was proportional to the amounts of FXR/RXR vectors (Fig. 4C). The results were confirmed by transfections of a shorter incubation (1 h) with GW4064 and FXR expression alone (Supplementary Fig. 1A-D). The results of FXR expression alone suggest that FXR alone (without RXR) may suppress NF-κB activity (Supplementary Fig. 1B-D). To evaluate the specificity of effects of FXR on NF-κB report activity, a negative control transfection was also performed using ERα reporter plasmid (Supplementary Fig. 1E). These results indicate that activation of FXR can antagonize NF-κB activity at the level of gene transcription.
Figure 4. Activation of FXR antagonizes the NF-κB transactivity.
(A, B) Relative luciferase activities of HepG2 cells that were co-transfected with the NF-κB reporter plasmids, the control plasmid phRL-TK, and/or FXR/RXR expression plasmids. Cells were pre-treated with GW4064 or vehicle (DMSO) for 18 h before treatment with TPA (50nM) or LPS (1 μg/ml) for 6 h. *P < .05 compared to the groups of TPA or LPS treatment without transfection of FXR/RXR plasmids (n=3). (C) Relative luciferase activities of HepG2 cells that were co-transfected with the NF-κB reporter plasmids, the control plasmid phRL-TK, and increasing amounts of FXR/RXR at 0.5:1, 1:1, 2:1 or 3:1 ratios with the p65 expression plasmid. Cells were treated with GW4064 or vehicle (DMSO) for 24 h. **P < .005 compared to the group of p65 overexpression with GW4064 treatment (n=3).
Anti-inflammatory activity of FXR in vivo
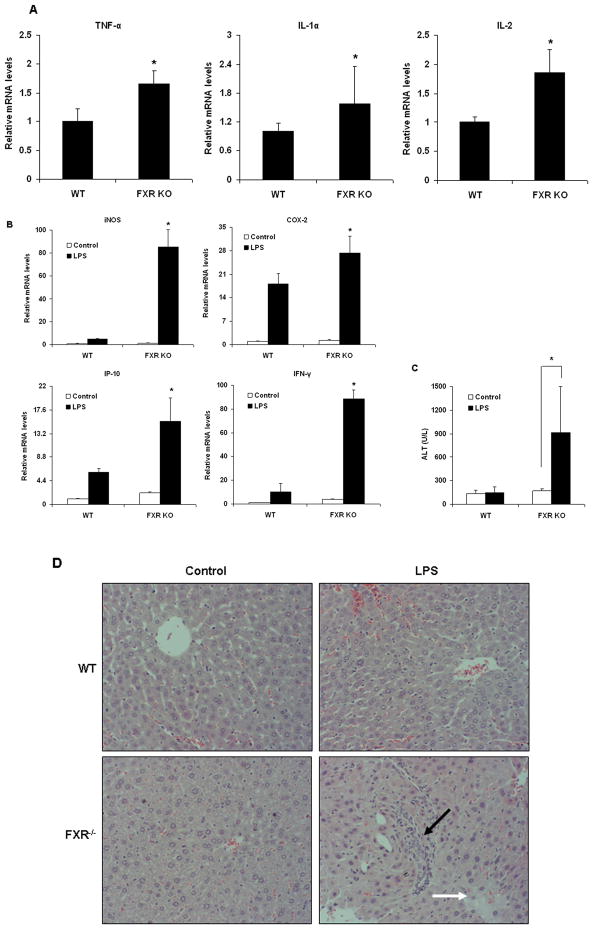
Yang et al. (17) reported that FXR−/− livers from 9–12-month-old mice displayed prominent liver injury and inflammation although there were no obvious tumors. We tested expression of some pro-inflammatory NF-κB target genes in wild-type and FXR−/−livers from 12-month-old mice. TNF-α, IL-1α and IL-2 were up-regulated in FXR−/− livers as compared with wild-type livers (Fig. 5A). To further address whether FXR may modulate inflammatory gene expression in vivo, we compared the induction of inflammatory genes by LPS in wild-type and FXR−/− mice (n=6). Induction of hepatic iNOS, COX-2, IP-10 and IFN-γ expression in response to LPS was significantly greater in FXR−/− mice compared with wild-type (Fig. 5B). The difference in expression of iNOS and IFN-γ between FXR−/− and wild-type mice was considerably greater than difference in expression of COX-2 and IP-10, suggesting that certain inflammatory genes are more sensitive to the loss of FXR signaling in vivo.
Figure 5. FXR has anti-inflammatory activity in vivo.
(A) Quantitative real time PCR analysis of the expression of pro-inflammatory genes in livers from 12-month-old wild-type (WT) or FXR−/− (FXR KO) mice (n=4). *P < .05 compared to the WT group. (B) Quantitative real time PCR analysis of the expression of pro-inflammatory genes in livers from wild-type (WT) and FXR−/− (FXR KO) mice that were treated with a single dose of LPS (20 mg/kg) or PBS (as controls) (n=5). *P < .05 compared to the control group of FXR−/−.(C) ALT levels in wild-type (WT) and FXR−/− (FXR KO) mice that were treated with either vehicle (PBS) or 20 mg/kg LPS (n=5). *P < .05 compared to the control group of FXR−/−. (D) Representative H&E staining of liver sections from wild-type and FXR−/− livers (200×). Black arrow indicates infiltrated inflammatory cells; white arrow indicates regions of necrosis. (E) Representative TUNEL staining of sections from wild-type (WT) and FXR−/− livers (200×) and statistical analysis of the number of TUNEL-positive cells per total number of cells. The number of cells in at least 20 microscopic fields was counted. *P < .05 compared to the control group of FXR−/− (n=5). (F, G) Quantitative real-time PCR analysis of expression of FXR target genes and pro-inflammatory genes in livers from wild-type (F) or FXR−/− (G) mice that were transfected with adenovirus expressing VP16 alone (ConAd) or adenovirus expressing constitutively active murine FXRα2 (FXRAd) (n=4). The mice received a single dose of LPS (30 mg/kg) or PBS as control. *P < .05 compared to the corresponding control groups.
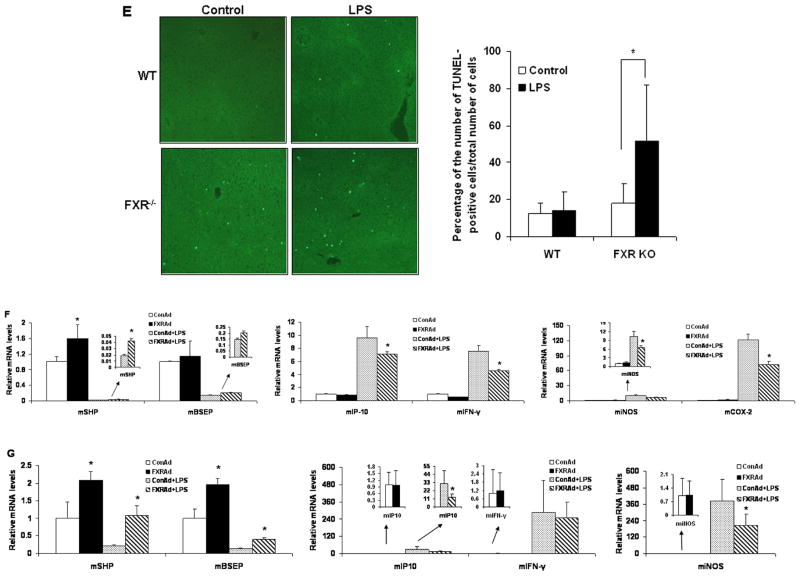
The levels of alanine transaminase (ALT), a marker of liver damage, were also significantly increased by treatment with LPS in FXR−/− mice compared with wild-type mice (Fig. 5C). Examination of liver pathology showed that massive necroses and inflammation were present in FXR−/− mice, but not in wild-type controls after injection of LPS (Fig. 5D). The liver injury induced by LPS was further confirmed by TUNEL assays. Considerable TUNEL-positive staining was detected in the livers of FXR−/− mice, but not in wild-type mice, after administration of LPS (Fig. 5E). The results suggest that FXR−/− mice are more sensitive to inflammatory stimuli.
To better understand the physiological role of hepatic FXR in the suppression of inflammation, we injected wild-type mice with adenovirus that expressed VP16 (Ad-VP16), the transactivation domain of herpes simplex virus, or murine FXRα2 fused to VP16 (Ad-FXRα2-VP16). FXR-VP16 is constitutively active in the absence of FXR ligands (15). Hepatic expression of FXRα2-VP16 led to the induction of the FXR target genes small heterodimer partner (SHP) and bile salt export pump (BSEP) (Fig. 5F). In contrast, hepatic expression of FXRα2-VP16 suppressed the LPS-induced expression of IP-10, IFN-γ, iNOS and COX-2 (Fig. 5F). The results were confirmed by hepatic expression of FXRα2-VP16 in FXR−/− mice (Fig. 5G). Collectively, these results demonstrate that FXR is a negative regulator of the hepatic inflammation in vivo.
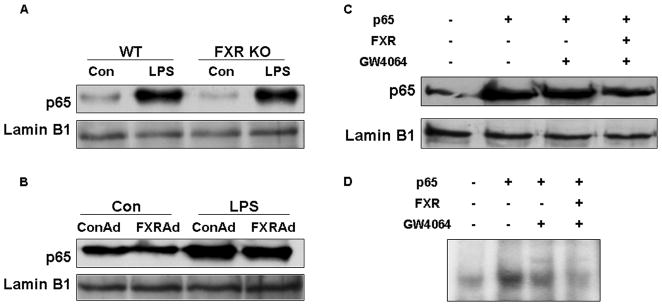
FXR activation suppressed NF-κB transcriptional activity by decreasing the binding between NF-κB and DNA sequences
The nuclear p65 levels in mouse livers and HepG2 cells were shown in Fig. 6A-C. It can be seen that LPS administration increased nuclear p65 levels in both wild-type and FXR−/−mice. However, there was no difference of nuclear p65 levels between wild-type and FXR−/− mice after LPS administration. Hepatic expression of FXRα2-VP16 did not reduce nuclear p65 induced by LPS (Fig. 6B). Similarly, FXR activation did not change the levels of nuclear p65 induced by p65 overexpression in HepG2 cells (Fig. 6C). Both results in vitro and in vivo indicate that FXR activation did not change the translocation of p65. The binding of NF-κB to DNA sequences was then tested by EMSA assay using nuclear extracts from HepG2 cells. FXR activation clearly reduced the binding activity of NF-κB to DNA sequences induced by p65 overexpression (Fig. 6D). These results suggest that FXR activation may suppress NF-κB transcriptional activity by decreasing the binding between NF-κB and DNA sequences.
Figure 6. FXR activation suppressed NF-κB transcriptional activity by decreasing the binding between NF-κB and DNA sequences.
(A) Immunoblot analysis for p65 and lamin B1 from nuclear protein pools in livers of wild-type or FXR−/− mice that were treated with a single dose of LPS (20 mg/kg) or PBS (as controls) (n=5). Lamin B1 was served as a loading control. Con, control; WT, wild-type; FXR KO, FXR knockout. (B) Immunoblot analysis for p65 and lamin B1 from nuclear protein pools in livers of wild-type mice that were transfected with adenovirus expressing VP16 alone (ConAd) or adenovirus expressing constitutively active murine FXRα2 (FXRAd) (n=4). The mice received a single dose of LPS (30 mg/kg) or PBS as control. Con, control. (C, D) HepG2 cell nuclear extracts were used in immunoblot analysis for p65 and lamin B1 (C) and a gel mobility shift assay with NF-κB binding site as a probe (D). HepG2 cells were transfected with p65 expression plasmid or control plasmid with or without co-transfection of FXR plasmid. After 24-h transfection, cells were treatment with 2μM GW4064 or DMSO (control) for 24 h. Finally, cells were collected and then nuclear proteins were extracted for immunoblot ananlysis or gel shift assay.
Activation of FXR dose not suppress the expression of NF-κB anti-apoptosis target genes
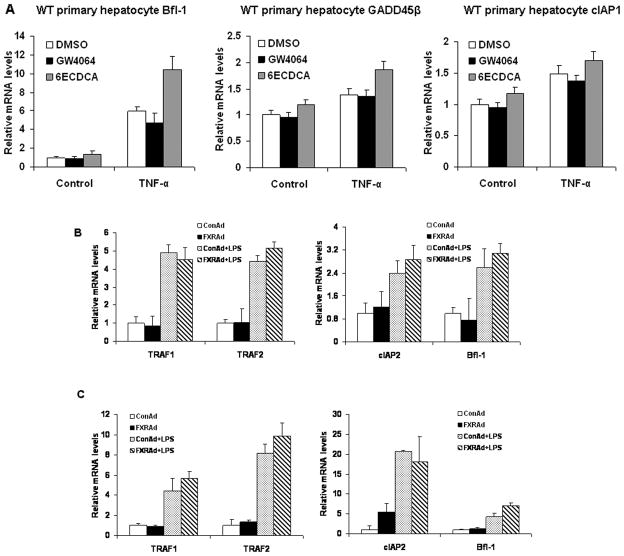
In addition to its roles in regulating pro-inflammatory genes, another major function of NF-κB is to regulate many anti-apoptotic genes, including members of Bcl-2 family such as Bcl-xL and Bfl-1/A1 (29), as well as the cellular inhibitors of apoptosis, cIAP1 and cIAP2, TRAF1, TRAF2, and GADD45β (30, 31). To determine the effects of FXR activation on the anti-apoptotic genes that are activated by NF-κB, we measured the mRNA levels of NF-κB-activated anti-apoptotic genes after FXR agonist treatment. Neither GW4064 nor 6ECDCA suppressed the expression of Bfl-1, GADD45β, and cIAP1 induced by TNF-α in mouse primary hepatocytes (Fig. 7A). Similarly, hepatic expression of FXRα2-VP16 did not suppress the LPS-induced expression of TRAF1, TRAF2, cIAP2 and Bfl-1 mRNA in vivo (Fig. 7B). The results were confirmed by hepatic expression of FXRα2-VP16 in FXR−/− mice (Fig. 7C). These results suggest that FXR activation selectively inhibited NF-κB target genes for hepatic inflammation but not anti-apoptotic genes.
Figure 7. FXR activation has no effect on NF-κB anti-apoptotic target genes.
(A) Quantitative real-time PCR analysis of the expression of Bfl-1, GADD45β, and cIAP1 in wild-type mouse primary hepatocytes that were pretreated with vehicle (DMSO), GW4064 (2 μM) or 6ECDCA (3 μM) for 18 h before treatment with TNF-α (10 ng/ml) for 6 h. (B, C) Quantitative real-time PCR analysis of the expression of Traf1, Traf2, cIAP2 and Bfl-1 in livers from wild-type (B) or FXR−/− (C) mice that were transfected with ConAd or FXRAd (n=4). The mice received a single dose of LPS (30 mg/kg) or PBS as control.
Suppression of FXR signaling by NF-κB activation
Because nuclear receptor signaling pathways are repressed in the inflammatory response (2, 6, 32), we investigated whether NF-κB activators inhibited the expression of FXR-mediated target genes in vitro and in vivo. The results show that NF-κB activation suppressed the expressions of FXR and its target genes SHP and BSEP (Supplementary Fig. 2), which was consistent with the report of Kim et al. (33). In addition, NF-κB activation induced by TNF-α repressed FXR activation induced by its ligand GW4064 in hepatocytes of wild-type mice (Supplementary Fig. 2B). To further investigate the effects of activation of NF-κB on the transcriptional activity of FXR, we co-transfected HepG2 cells with an FXR reporter (EcRE-LUC) and FXR/RXR expression plasmids. Treatment with GW4064 dramatically induced FXR reporter activity in the presence of FXR/RXR (Fig. 8). TPA and LPS strongly repressed GW4064-induced FXR reporter activity (Fig. 8A,B). Repression of FXR reporter activity by TPA was more prominent than repression induced by LPS, possibly due to the additional inhibition of TPA on expression of FXR and RXR mRNA (Supplementary Fig. 2A). The transactivation of NF-κB by overexpression of p65 also repressed GW4064-induced FXR reporter activity in a dose-dependent manner (Fig. 8C), suggesting that activation of NF-κB reciprocally antagonizes FXR activity.
Figure 8. Activation of NF-κB by TPA, LPS and p65 overexpression antagonizes FXR transactivation.
(A, B) Relative luciferase activities of HepG2 cells that were co-transfected with the FXR reporter plasmid EcRE-LUC, the control plasmid phRL-TK, and/or FXR/RXR expression plasmids, and pre-treated with GW4064 or vehicle (DMSO) for 18 h before treatment with TPA (50 nM) (A) or LPS (1 μg/mL) (B) for 6 hours. *P < .05, **P < .005 (n=3); (C) Relative luciferase activities of HepG2 cells were co-transfected with the FXR reporter plasmid EcRE-LUC, the control plasmid phRL-TK, and increasing amounts of a p65 expression plasmid at 0.5:1, 1:1 or 3:1 ratios with FXR/RXR expression plasmids, and then treated with GW4064 or vehicle (DMSO) for 24 h. *P < .05 compared to the control group; #P < .05 (n=3).
Discussion
The known functions of FXR in the liver have recently expanded rapidly from initial roles in regulating liver metabolism to also participating in liver regeneration and hepatocarcinogenesis (17, 34). The novel roles of FXR in promoting liver regeneration and protecting against hepatocarcinogenesis are consistent with FXR’s previous roles in defending against bile acid toxicity. In contrast to its well established mechanism in regulating BA homeostasis, little is known about how FXR functions in liver regeneration and carcinogenesis. Our results suggest that one potential role for FXR in protecting against hepatocarcinogenesis is by modulating NF-κB-mediated hepatic inflammatory responses. FXR activation strongly suppresses the activity of NF-κB in cell culture experiments in vitro. This is further supported by both primary hepatocyte and animal studies in vivo. However, FXR does not suppress the NF-κB-activated anti-apoptotic genes. This differential effect of FXR is consistent with its key role as a hepatocyte protector. There are several implications of modulation of NF-κB by FXR in liver function: 1) Hydrophobic bile acids induce hepatic inflammation (35) and bile acids such as deoxycholic acid can activate NF-κB by increasing the binding of NF-κB to DNA (36, 37) during pathological conditions of the liver, such as cholestasis (35). Therefore, FXR may decrease bile acid-induced hepatoxicity through its anti-inflammation function. 2) Gallbladder mucosal inflammation is an important event in cholesterol gallstone disease, and the gallbladder epithelia of FXR−/− mice under lithogenic conditions showed increased inflammation as compared to wild-type mice (38). Our findings may be directly related to Moschetta’s studies demonstrating that activation of FXR by GW4064 prevents the development of cholesterol gallstone disease in a mouse model (38). 3) NF-κB-mediated hepatic inflammation may to contribute to liver insulin resistance and FXR−/−mice exhibit insulin resistance (15, 39). Our results may provide an explanation for the increased insulin resistance in FXR−/− mice. 4) Hepatic inflammation is closely linked to hepatocarcinogenesis (40, 41) and FXR−/− mice also display intense liver inflammation prior to developing spontaneous liver tumours. The mutual suppression between FXR and NF-κB may be an important mechanism for preventing tumorigenesis.
Our results indicate that FXR does not affect p65 nuclear translocation. However, FXR may suppress p65 transactivity by decreasing its DNA binding activity. Previous reports have shown that another nuclear receptor, glucocorticoid receptor (GR), antagonizes the action of NF-κB through direct physical protein-protein interactions (42–44). Recently, Pascual et al. (45) reported that sumoylation of PPAR-γ is a potential mechanism involved in the inhibition of NF-κB by PPAR-γ ligands. The sumoylation-dependent pathway is also involved in the regulation of pro-inflammatory genes by LXR (46). Whether this is a common mechanism for FXR remains to be tested.
On the other hand, infections and inflammatory responses have long been observed to suppress hepatic gene expression (2, 6). NF-κB is the central transcriptional regulator of the hepatic inflammatory responses, and it may provide a link between inflammation and the suppression of NR signalling in metabolic function by antagonizing the activities of a number of nuclear receptors, including GR, the aryl hydrocarbon receptor, and SXR (6, 44, 47). We can now expand this list to include FXR. We observed two effects of suppression of FXR signalling by NF-κB activation. First, we observed decreased expression of FXR as shown by the marked reduction of FXR and RXR mRNA after injection of LPS into mice. This is consistent with the report from Kim et al. (33). Second, we observed direct antagonizing FXR transactivity by NF-κB activation as shown by the suppression of FXR target genes by TNF-α, which was not associated with changes in the expression of FXR and RXR mRNA (Supplementary Fig. 2B). This was further confirmed by the observation that LPS (which did not alter FXR or RXR mRNA levels in HepG2 cells), TPA or overexpression of p65 dramatically repressed FXR reporter activity. Gu et al. (2) reported that the p65 subunit of NF-κB directly interacts with the DNA-binding domain of RXRα and may prevent its binding to the consensus DNA sequences. Because RXR is a dimerization partner of FXR, it is possible that NF-κB suppresses FXR activity by reducing the number of FXR/RXR complexes.
We noted that activation of FXR repressed specific sets of NF-κB target genes but not all the target genes in response to the NF-κB activators that we used in this study (TPA, LPS and TNF-α). This phenomenon has also been observed for other nuclear receptors. Ogawa et al. (48) demonstrated that a cohort of genes was sensitive to GR-mediated repression when induced by LPS but was GR resistant when induced by poly I:C. Similar results were obtained in LXR- and PPAR-γ-mediated repression for inflammatory genes (46). One possibility is that the transrepression programs that are mediated by nuclear receptors are regulated in a signal-specific manner. In addition, the transrepression pathways themselves may be subject to further regulation and can be overridden by specific signals in a gene-specific manner (46). It will be interesting to define the mechanism by which FXR activation inhibits NF-κB in a gene-specific manner.
In summary, our results reveal that FXR is a negative mediator of liver inflammation and that there is reciprocal suppression between FXR and NF-κB signaling pathways. These findings support the role of FXR as a central hepatoprotector, and suggest that FXR agonist ligands offer possible therapies to prevent and treat hepatitis, liver fibrosis and hepatocarcinogenesis.
Supplementary Material
Acknowledgments
Financial support: This work was supported by the Sidney Kimmel Foundation for Cancer Research (W.H.) and P30 CA033572-24 developmental fund from the City of Hope Cancer Center Core Grant.
We thank Keely Walker for proofreading the manuscript; Xufeng Chen, Akio Kruoda, Dr. Peter Tontonoz and Dr. Bruce Blumberg for plasmids; Dr. Peter A. Edwards for adenovirus that expressed VP16 alone (Ad-VP16) or murine FXRα2 fused to VP16.
Abbreviations
- 6ECDCA
6α-ethylchenodeoxycholic acid
- FXR
farnesoid X receptor
- LXR
liver X receptors
- NR
nuclear receptor
- PXR
pregnane X receptor
- RXR
retinoid X receptor
- TPA
12-o-tetradecanoyl-phorbol-13-acetate
Footnotes
Conflicts of interest: None to report.
References
- 1.Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355–363. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- 2.Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, Gallo MA, et al. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem. 2006;281:17882–17889. doi: 10.1074/jbc.M601302200. [DOI] [PubMed] [Google Scholar]
- 3.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 4.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 5.Elsharkawy AM, Mann DA. Nuclear factor-kappaB and the hepatic inflammation-fibrosis-cancer axis. Hepatology. 2007;46:590–597. doi: 10.1002/hep.21802. [DOI] [PubMed] [Google Scholar]
- 6.Zhou C, Tabb MM, Nelson EL, Grun F, Verma S, Sadatrafiei A, Lin M, et al. Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest. 2006;116:2280–2289. doi: 10.1172/JCI26283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Zhang X, Farrar WL, Yang X. Transcriptional crosstalk between nuclear receptors and cytokine signal transduction pathways in immunity. Cell Mol Immunol. 2004;1:416–424. [PubMed] [Google Scholar]
- 11.Ricote M, Huang JT, Welch JS, Glass CK. The peroxisome proliferator-activated receptor(PPARgamma) as a regulator of monocyte/macrophage function. J Leukoc Biol. 1999;66:733–739. doi: 10.1002/jlb.66.5.733. [DOI] [PubMed] [Google Scholar]
- 12.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 14.Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, et al. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res. 2007;48:2664–2672. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang YD, Chen WD, Huang W. FXR, a target for different diseases. Histol Histopathol. 2008;23:621–627. doi: 10.14670/HH-23.621. [DOI] [PubMed] [Google Scholar]
- 17.Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–867. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- 18.Wang YD, Yang F, Chen WD, Huang X, Lai L, Forman BM, Huang W. Farnesoid X Receptor (FXR) Protects Liver Cells from Apoptosis Induced by Serum Deprivation in Vitro and Fasting in Vivo. Mol Endocrinol. 2008 doi: 10.1210/me.2007-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang W, Zhang J, Wei P, Schrader WT, Moore DD. Meclizine is an agonist ligand for mouse constitutive androstane receptor (CAR) and an inverse agonist for human CAR. Mol Endocrinol. 2004;18:2402–2408. doi: 10.1210/me.2004-0046. [DOI] [PubMed] [Google Scholar]
- 20.Huang W, Zhang J, Moore DD. A traditional herbal medicine enhances bilirubin clearance by activating the nuclear receptor CAR. J Clin Invest. 2004;113:137–143. doi: 10.1172/JCI200418385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiao L, Studer E, Leach K, McKinstry R, Gupta S, Decker R, Kukreja R, et al. Deoxycholic acid (DCA) causes ligand-independent activation of epidermal growth factor receptor (EGFR) and FAS receptor in primary hepatocytes: inhibition of EGFR/mitogen-activated protein kinase-signaling module enhances DCA-induced apoptosis. Mol Biol Cell. 2001;12:2629–2645. doi: 10.1091/mbc.12.9.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 23.Najima Y, Yahagi N, Takeuchi Y, Matsuzaka T, Sekiya M, Nakagawa Y, Amemiya-Kudo M, et al. High mobility group protein-B1 interacts with sterol regulatory element-binding proteins to enhance their DNA binding. J Biol Chem. 2005;280:27523–27532. doi: 10.1074/jbc.m414549200. [DOI] [PubMed] [Google Scholar]
- 24.Bontemps Y, Vuillermoz B, Antonicelli F, Perreau C, Danan JL, Maquart FX, Wegrowski Y. Specific protein-1 is a universal regulator of UDP-glucose dehydrogenase expression: its positive involvement in transforming growth factor-beta signaling and inhibition in hypoxia. J Biol Chem. 2003;278:21566–21575. doi: 10.1074/jbc.M209366200. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto K, Matsumoto S, Yamada M, Satoh T, Mori M. Liver X receptor-alpha gene expression is positively regulated by thyroid hormone. Endocrinology. 2007;148:4667–4675. doi: 10.1210/en.2007-0150. [DOI] [PubMed] [Google Scholar]
- 26.Urizar NL, Dowhan DH, Moore DD. The farnesoid X-activated receptor mediates bile acid activation of phospholipid transfer protein gene expression. J Biol Chem. 2000;275:39313–39317. doi: 10.1074/jbc.M007998200. [DOI] [PubMed] [Google Scholar]
- 27.Li YT, Swales KE, Thomas GJ, Warner TD, Bishop-Bailey D. Farnesoid x receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler Thromb Vasc Biol. 2007;27:2606–2611. doi: 10.1161/ATVBAHA.107.152694. [DOI] [PubMed] [Google Scholar]
- 28.Schuetz EG, Strom S, Yasuda K, Lecureur V, Assem M, Brimer C, Lamba J, et al. Disrupted bile acid homeostasis reveals an unexpected interaction among nuclear hormone receptors, transporters, and cytochrome P450. J Biol Chem. 2001;276:39411–39418. doi: 10.1074/jbc.M106340200. [DOI] [PubMed] [Google Scholar]
- 29.Zong WX, Edelstein LC, Chen C, Bash J, Gelinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 1999;13:382–387. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 31.Kucharczak J, Simmons MJ, Fan Y, Gelinas C. To be, or not to be: NF-kappaB is the answer--role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene. 2003;22:8961–8982. doi: 10.1038/sj.onc.1207230. [DOI] [PubMed] [Google Scholar]
- 32.Venteclef N, Smith JC, Goodwin B, Delerive P. Liver receptor homolog 1 is a negative regulator of the hepatic acute-phase response. Mol Cell Biol. 2006;26:6799–6807. doi: 10.1128/MCB.00579-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim MS, Shigenaga J, Moser A, Feingold K, Grunfeld C. Repression of farnesoid X receptor during the acute phase response. J Biol Chem. 2003;278:8988–8995. doi: 10.1074/jbc.M212633200. [DOI] [PubMed] [Google Scholar]
- 34.Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, et al. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312:233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- 35.Li MK, Crawford JM. The pathology of cholestasis. Semin Liver Dis. 2004;24:21–42. doi: 10.1055/s-2004-823099. [DOI] [PubMed] [Google Scholar]
- 36.Shah SA, Volkov Y, Arfin Q, Abdel-Latif MM, Kelleher D. Ursodeoxycholic acid inhibits interleukin 1 beta [corrected] and deoxycholic acid-induced activation of NF-kappaB and AP-1 in human colon cancer cells. Int J Cancer. 2006;118:532–539. doi: 10.1002/ijc.21365. [DOI] [PubMed] [Google Scholar]
- 37.Jenkins GJ, Harries K, Doak SH, Wilmes A, Griffiths AP, Baxter JN, Parry JM. The bile acid deoxycholic acid (DCA) at neutral pH activates NF-kappaB and induces IL-8 expression in oesophageal cells in vitro. Carcinogenesis. 2004;25:317–323. doi: 10.1093/carcin/bgh032. [DOI] [PubMed] [Google Scholar]
- 38.Moschetta A, Bookout AL, Mangelsdorf DJ. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat Med. 2004;10:1352–1358. doi: 10.1038/nm1138. [DOI] [PubMed] [Google Scholar]
- 39.Baek JH, Jang JE, Kang CM, Chung HY, Kim ND, Kim KW. Hypoxia-induced VEGF enhances tumor survivability via suppression of serum deprivation-induced apoptosis. Oncogene. 2000;19:4621–4631. doi: 10.1038/sj.onc.1203814. [DOI] [PubMed] [Google Scholar]
- 40.Rogers AB, Fox JG. Inflammation and Cancer. I. Rodent models of infectious gastrointestinal and liver cancer. Am J Physiol Gastrointest Liver Physiol. 2004;286:G361–366. doi: 10.1152/ajpgi.00499.2003. [DOI] [PubMed] [Google Scholar]
- 41.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 42.Karin M, Chang L. AP-1--glucocorticoid receptor crosstalk taken to a higher level. J Endocrinol. 2001;169:447–451. doi: 10.1677/joe.0.1690447. [DOI] [PubMed] [Google Scholar]
- 43.Herrlich P. Cross-talk between glucocorticoid receptor and AP-1. Oncogene. 2001;20:2465–2475. doi: 10.1038/sj.onc.1204388. [DOI] [PubMed] [Google Scholar]
- 44.Nissen RM, Yamamoto KR. The glucocorticoid receptor inhibits NFkappaB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 2000;14:2314–2329. doi: 10.1101/gad.827900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghisletti S, Huang W, Ogawa S, Pascual G, Lin ME, Willson TM, Rosenfeld MG, et al. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ke S, Rabson AB, Germino JF, Gallo MA, Tian Y. Mechanism of suppression of cytochrome P-450 1A1 expression by tumor necrosis factor-alpha and lipopolysaccharide. J Biol Chem. 2001;276:39638–39644. doi: 10.1074/jbc.M106286200. [DOI] [PubMed] [Google Scholar]
- 48.Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, Hoffmann A, et al. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122:707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.