Abstract
3, 4-Didehydroretinol (DR) metabolism was previously followed in vitamin A (VA)-replete lactating sows. This study followed DR appearance and clearance after dosage in serum and milk during 2 lactation cycles in sows (n = 8) fed VA-free feed for 3 gestation-lactation cycles. During lactations 2 and 3, 35 μmol 3, 4-didehydroretinyl acetate was given orally after overnight food deprivation. Blood and milk were collected at 0, 1.5, 3, 5, 7, 9, 16, 24, 36, 48, 60, and 72 h; livers were obtained at kill. Samples were analyzed for DR, retinol (R), and 3, 4-didehydroretinyl esters. During lactations 2 and 3, the 5-h serum DR:R ratios were 0.028 ± 0.017 and 0.069 ± 0.042, respectively, and serum R concentrations were 0.75 ± 0.23 and 0.86 ± 0.37 μmol/L, respectively. The DR:R ratio and serum R were 0.018 ± 0.013 and 0.94 ± 0.12 μmol/L, respectively, in VA-replete sows from the same herd. After lactation 3, liver VA was 0.23 ± 0.05 μmol/g, indicating low-normal VA status. Serum DR area-under-the curve from 0 to 48 h increased as liver stores decreased. Thirteen to 23% of DR dose was secreted into milk, consistent with VA-replete sows. Milk DR concentrations were greater during lactation 3 than 2. Peak concentration occurred earlier and the half-life was shorter for milk DR in the more VA-depleted sows. The milk and serum DR:R were correlated from 3 to 9 h (r = 0.70; P < 0.0001) and increased as VA stores decreased regardless of serum R concentration. Milk DR:R may replace serum measurements during lactation.
Introduction
Vitamin A (VA)6 deficiency (VAD) is a global health concern known to suppress immune function, complicate pregnancy, and cause blindness (1). Controlling VAD by 2015 is a critical step in achieving the 4th Millennium Development Goal, which aims to reduce the under 5-y mortality rate by at least two-thirds (2). One effective method for improving both maternal and infant VA status is postpartum supplementation (3). More sustainable long-term approaches include increasing consumption of VA-containing foods through dietary diversification (4, 5) and biofortification of staple crops (6, 7). To determine efficacy of VA interventions, accurate assessment of status before and after an intervention is necessary. Reliable assessment of VA status in individuals and populations is not trivial and often requires sophisticated methodology and adequate resources (8, 9).
Various assessment methods for VA status and their utility have been reviewed (8–11). Liver reserves are the gold standard, because liver contains the majority of total body VA stores unless overall status is deficient. Liver biopsies, however, are neither ethical nor feasible in field studies. Serum retinol (R) concentrations are commonly used but are not always sensitive to interventions or changes in liver reserves (9, 12). The modified relative dose response (MRDR) test qualitatively assesses deficient to normal liver VA reserves (4, 5, 10–17). The test works by administering an oral dose of 3, 4-didehydroretinyl acetate, which circulates in the blood as 3, 4-didehydroretinol (DR). A blood sample is taken 4–6 h after dosage to determine the ratio of DR:R, also called the MRDR value. A MRDR value ≥ 0.060 usually signifies low hepatic VA reserves. If initial DR:R was high, the value will decrease with VA treatment to indicate if adequate status is achieved.
Breast milk VA concentrations offer a unique opportunity to assess the status of lactating women and infer that of nursing infants (10, 11). Breast milk collection is less invasive and usually easier and more culturally acceptable than blood collection. The purpose of this study was to determine whether the DR:R in milk can be used to measure VA status when hepatic reserves are low and to further evaluate the use of DR as a tracer for R (17). Previous work showed that the milk DR:R ratio in VA-replete lactating sows was positively correlated with that in serum (r = 0.64; P < 0.0001) but 10 times higher (15). In the current study, milk DR:R was monitored in lactating sows fed a VA-free feed during 3 gestation-lactation cycles and compared with that in VA-replete sows from a prior study (PL1), which were from the same herd and of similar genetic background (15). The metabolism and kinetics of newly ingested DR during the second lactation (L2) and third lactation (L3) were followed for 72 h.
Materials and Methods
Animals and diet.
Approval for this study was obtained from the University of Wisconsin-Madison Research Animal Resources Center. A total of 15 sows (crossbreeds of Large White and Landrace) were housed at the Swine Research and Teaching Center in Arlington, WI, and the University of Wisconsin-Madison Livestock Laboratory and bred by artificial insemination. When pregnancy was confirmed, the sows were switched from a standard feed (18) to a feed containing no preformed VA for a total of 3 gestation-lactation cycles (Table 1). The typical gestation for a sow is 115 d and piglets are weaned at ∼1 mo. All sows were given 2 kg feed/d during gestation and were allowed ad libitum access during lactation. A typical maize-based, preformed VA-free feed was fed during the first 2 gestation-lactation cycles (∼16 mo). At the beginning of L2, 8 sows were randomly selected for the long-term experiment presented herein. After L2, these same sows were switched to a wheat-based, preformed VA-free feed (Table 1) for the 3rd gestation-lactation cycle. One sow did not conceive during the 3rd cycle; therefore, 7 sows comprised L3. Approximately 10 d after parturition, jugular catheters were placed into the sows without anesthetic, as described previously (15). The sows continued on the wheat-based feed until kill. Baseline characteristics were obtained before treatment (Table 2). Although the mean day of lactation for dosage differed by 4 d between L2 and L3, the ranges overlapped, i.e. 8–13 d for L2 and 11–17 d for L3. All milk sampled was mature and piglets were exclusively milk fed.
TABLE 1.
Nutrient composition of feeds for depleting VA stores in lactating sows
| Ingredient | L2 | L3 |
| g/kg feed | ||
| Corn | 480 | – |
| Barley | 300 | – |
| Wheat grain | – | 794 |
| Soybean meal, 48% crude protein | 138 | 126 |
| Dicalcium phosphate | 19.5 | 17.2 |
| Limestone, ground | 7.0 | 8.3 |
| Fat1 | 30.0 | 30.0 |
| Iodized sodium chloride | 5.0 | 5.0 |
| UW vitamin and mineral mix2 | 20.0 | 20.0 |
MaxFat (Maxco), a blend of animal and vegetable fats.
A vitamin and mineral premix was developed that consisted of 50% corn, 20% vitamin mix (Teklad, Madison, WI) [in g/kg vitamin mix: vitamin D (as cholecalciferol), 0.26; E (as dl-α-tocopheryl acetate), 72.0; vitamin B complex, 5.40; biotin, 7.50; folic acid 0.50; niacin, 5.53; pantothenic acid, 12.87; riboflavin, 7.84; vitamin B-12, 13.64; corn, 874.5], and 30% University of Wisconsin Mineral Mix (Mineral Mix resulted in the following minerals in mg/kg final feed: iron, 57; selenium, 0.3; zinc, 135; iodine 0.45; copper, 2.3).
TABLE 2.
Baseline characteristics of lactating sows at each lactation cycle1
| Characteristic | L2, n = 8 | L3, n = 7 |
| Age at experiment start, y | 2.1 ± 0.3 | 2.5 ± 0.3 |
| Parities, n | 3.5 ± 0.5 | 4.4 ± 0.5 |
| Offspring, n | 12.3 ± 3.6 | 13.9 ± 5.2 |
| Time of lactation,2d | 9.3 ± 1.8 | 13.6 ± 1.9* |
Data are means ± SD. *Different from L2, < 0.05 (paired t test).
n = 7 for L2 and L3.
Dose preparation, administration, and sample collection.
3, 4-Didehydroretinyl acetate was synthesized using previously published methods (13) and stored at −30°C until use. After an overnight fast, sows were orally dosed with 35 μmol 3, 4-didehydroretinyl acetate dissolved in 4.7 mL corn oil by syringe. The sows were observed to ascertain that the entire dose was consumed. During L2 and L3, blood (10 mL) and milk (~40 mL) samples were collected in the same manner as described previously (15) at 0 (predose), 1.5, 3, 5, 7, 9, 16, 24, 36, 48, 60, and 72 h (postdose). Before collection, piglets were partitioned from the sows. Sows were injected with 40 IU oxytocin; after letdown, fore and hind milk were collected from 1 or more teats. One mo after L3, whole livers were collected at kill (n = 7). Samples were stored on dry ice until transferred to a −80°C freezer.
Serum, milk, and liver DR and R analyses.
Samples were analyzed by published procedures under gold fluorescent lights to minimize photo-oxidation and isomerization of VA (15, 16, 18–20). To determine serum DR and R, 200 μL serum was prepared using a standardized method (16). Modifications were made to published methods to determine milk DR and R (15, 18–20). C23-apo-carotenol was added as an internal standard to 500 μL milk, which was then saponified for 1.25 h and extracted with hexanes. Analysis was performed by HPLC with the detector set at 335 nm, which is midway between λmax of 325 nm for R and 350 nm for DR. Milk fat content was determined by a modified Folch method (15, 21). Liver R and retinyl esters were analyzed using published procedures (15). Two samples were also analyzed at 350 nm to confirm that no DR or 3, 4-didehydroretinyl esters remained in the liver at time of kill. All analyses included HPLC-purified external and internal standards for quality control.
Statistical analysis.
Statistical analyses were performed with SAS software (version 9.1; SAS Institute). A repeated-measures ANOVA with fixed effects was applied using SAS PROC MIXED to determine the differences between lactation cycles. Significant differences between sampling times were determined by using least squares mean (LSM) differences. Repeated-measures models were fitted to investigate 8 variables over time: DR, R, and DR:R in serum and milk and DR and R/g milk fat. Results were considered significant at P ≤ 0.05. Significant lactation period by time interactions were further evaluated. Because peak milk DR:R occurs ~2 h later than that in serum, values were also correlated using a lag model. VA indicators were compared using unpaired t tests for PL1 with L2 and L3 and paired t tests between L2 and L3.
Noncompartmental analysis was performed using WINNONLIN (version 4.1, Pharsight). The elimination rate constant (λz) in 1/h and half-life (t1/2) in h were estimated via linear regression of time compared with log concentration. Trapezoidal area under the curve (AUC) to 48 h was performed to compare results with previously published data in VA-replete PL1 sows (15). AUC0-∞ was done by adding Ct/λz to AUC0-t, where Ct is serum or milk DR concentration at the last sampled time point, t. Other parameters determined from observed data were the maximum concentration (Cmax), time of Cmax (Tmax), volume distribution (V), and clearance (Cl).
Results
Serum and milk DR:R ratios.
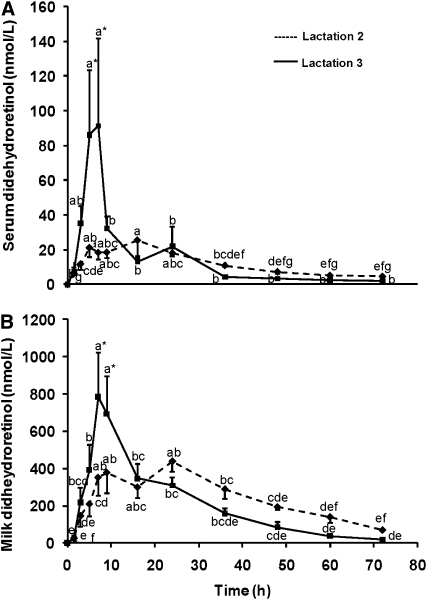
DR:R in serum and milk followed similar patterns (Fig. 1). Serum DR:R changed with time in both L2 and L3 (P < 0.0001 for each) and the lactation cycle effect was marginal (P < 0.10) (Fig. 1A). A significant lactation by time interaction was found for serum DR:R (P = 0.0002). Serum DR:R increased with the length of time the sows consumed VA-free feed. Serum DR:R was greater during L3 than L2, specifically at 7 and 9 h (P < 0.05), which is consistent with a decrease in VA status. Milk DR:R changed with time (P = 0.017) and was driven by substantial changes within L2 and L3 (P = 0.0002 and P < 0.0001, respectively) (Fig. 1B). Milk DR:R ratios were 10-times higher than and directly correlated with the ratios in serum at each time point throughout the 72-h period (r = 0.36; P < 0.0001). A stronger correlation was found between 3 and 9 h, the more sensitive response times for potential field use (r = 0.70; P < 0.0001). Because the time of the peak milk DR:R ratio lags behind that in serum, the correlation model was offset by one time point at biologically sensitive times (between 3 and 9 h and 5 and 16 h for serum and milk, respectively), which improved the correlation (r = 0.77; P < 0.0001).
FIGURE 1.
Time course of the DR:R ratios in serum (A) and milk (B) from lactating sows given 35 μmol 3, 4-didehydroretinyl acetate orally. The sows were fed a VA-free feed for 2 (n = 8) or 3 (n = 7) gestation-lactation cycles. The lactation by time interaction was significant for the serum DR:R ratio (P = 0.0002) but not the milk DR:R ratio (P = 0.62). The serum DR:R ratio changed with time during both L2 and L3 (P < 0.0001). The milk DR:R ratio changed with time (P = 0.017). Values are means ± SEM. Means without a common letter differ within a single curve, P ≤ 0.05 (repeated-measures ANOVA with fixed effects followed by LSM differences). *Different between lactation cycles, P ≤ 0.05. Note: scales differ for milk and serum DR:R ratio values.
Serum and milk DR and R.
Serum DR concentrations (Fig. 2) were affected by lactation cycle (P = 0.05) and time (P < 0.0001) and an interaction occurred (P = 0.014). Serum DR concentrations changed with time during L2 and L3 (P < 0.0001 and P = 0.01, respectively) and concentrations within L3 were higher than in L2 at 7 and 9 h (P < 0.05) (Fig. 2A). 3, 4-Didehydroretinyl esters were not detected in the serum. Milk DR concentrations were not affected by lactation (P = 0.41) but were affected by time (P < 0.0001) and an interaction occurred (P = 0.012). The milk DR concentrations were higher during L3 than in L2 at 7 and 9 h (P < 0.05) (Fig. 2B). Milk DR/g fat changed with time (P < 0.0001) (Supplemental Fig. 1A).
FIGURE 2.
Time course of DR concentrations in serum (A) and milk (B) in lactating sows after oral administration of 35 μmol 3, 4-didehydroretinyl acetate. The sows were fed a VA-free feed for 2 (n = 8) or 3 (n = 7) lactation cycles. The lactation by time interaction was significant for DR concentrations in serum and milk (P = 0.014 and = 0.012, respectively) driven by changes with time (P < 0.0001). Values are means ± SEM. Means without a common letter differ within a single curve, P < 0.05 (repeated-measures ANOVA test with fixed effects followed by LSM differences). *Different between lactation cycles, P ≤ 0.05.
For serum and milk, R concentrations were higher during L3 than in L2. A lactation by time interaction occurred for serum R concentration (P = 0.0093). The serum R concentration changed with time during L2 (P < 0.0001). The milk R concentration was affected by lactation (P = 0.028) and time of sampling (P < 0.0001) and the lactation by time interaction approached significance (P = 0.053). Milk R concentrations increased over time (P < 0.0001) and were significantly higher during L3 than in L2 at 0 through 7 h. Correcting milk R for fat mitigated the interaction and resulted in an effect of lactation cycle (P = 0.044) and time (P = 0.036) (Supplemental Fig. 1B).
Kinetic parameters.
The calculated Tmax for milk DR concentration and amount of DR/g fat lagged behind the serum Tmax (Table 3). Comparing the noncompartmental parameters between L2 and L3, the calculated Tmax was earlier in L3 for the serum DR concentration (P < 0.05). The calculated Cmax, Tmax, elimination rate constant (λz), and t1/2 were different for DR in milk between lactations (P < 0.05), whereas only λz was higher in L3 for the amount of DR/g milk fat (P < 0.05). The AUC0–48h of serum and milk DR concentrations and the amount of DR/g fat were computed and extrapolated to infinity (Table 3). L2 and L3 were compared with PL1 VA-replete sows (15) from the same herd. In comparing PL1 to L2, the calculated Cmax, AUC0–48h, AUC0-∞, V, and Cl were different for serum DR (P < 0.05). For milk DR during PL1 and L2, the calculated V and λz were different (P < 0.05). Comparing PL1 to L3, the calculated AUC0–48h, V, and Cl were higher in L3 for serum DR (P < 0.05). Comparing milk DR in PL1 to L3 sows, the calculated Cmax, V, and λz were higher in L3, and V and λz were higher for DR/g milk fat (P < 0.05), resulting in a shorter t1/2 for both milk DR and DR/g milk fat.
TABLE 3.
Pharmacokinetic data and AUC from 0 to 48 h and 0 to ∞ after 35 μmol 3, 4-didehydroretinyl acetate was administered to PL1 sows and sows on a VA-free feed for L2 or L3 gestation-lactation cycles1
| Tmax2 | Cmax | λz | t1/2h | V | Cl | AUC0–48h | AUC0-∞ | |
| Serum DR | h | nmol/L | 1/h | h | L | L/h | nmol/L ⋅ h | |
| PL13 | 7.5 ± 1.9 | 7.0 ± 2.5b | 0.04 ± 0.01 | 17 ± 4.5 | 9290 ± 9940a,A,A | 250 ± 117a,A | 366 ± 193b | 570 ± 275b |
| L2 | 14 ± 8.1 | 35 ± 23a | 0.03 ± 0.01 | 27 ± 19 | 1270 ± 428b | 38 ± 17b | 743 ± 258a | 1050 ± 433a |
| L3 | 6.4 ± 1.5* | 110 ± 130 | 0.04 ± 0.01 | 23 ± 11 | 1350 ± 770B | 44 ± 25B | 963 ± 662 | 1090 ± 693 |
| Milk DR | nmol/L | |||||||
| PL1 | 16 ± 9.8 | 290 ± 95B | 0.01 ± 0.006b,B | 72 ± 51a,A | NA | NA | 9790 ± 4830 | 26,800 ± 21,200 |
| L2 | 20 ± 13 | 490 ± 260 | 0.04 ± 0.01a | 19 ± 6.6b | NA | NA | 14,000 ± 6590 | 19,200 ± 6710 |
| L3 | 8.6 ± 7.0* | 870 ± 540*,A | 0.09 ± 0.03A,* | 8.6 ± 2.8*,B | NA | NA | 14,000 ± 6520 | 15,300 ± 6990 |
| Milk DR | nmol/g fat | nmol/g fat ⋅ h | ||||||
| PL1 | 8.5 ± 1.0 | 4.6 ± 1.5 | 0.02 ± 0.006B | 30 ± 6.9A | NA | NA | 118 ± 57 | 212 ± 136B |
| L2 | 17 ± 12 | 8.5 ± 5.9 | 0.03 ± 0.02 | 48 ± 65 | NA | NA | 164 ± 94 | 328 ± 239 |
| L3 | 10 ± 6.6 | 14 ± 8.6 | 0.08 ± 0.01*,A | 4.8 ± 1.8B | NA | NA | 212 ± 110 | 235 ± 116A |
Values are means ± SD, = 4, 8, and 7 for PL1, L2, and L3, respectively. Labeled PL1 and L2 means without a common lowercase letter differ, P < 0.05; labeled PL1 and L3 means without a common uppercase letter differ, P < 0.05 (unpaired t test). *Different from L2, P < 0.05 (paired t test).
NA, not applicable.
PL1 were a separate group of VA-replete lactating sows from previous work (15). L2 and L3 were the same sows, but fed a VA-deficient feed; data were collected during L2 and L3.
To determine the irreversible loss of DR tracer in milk over the first 48 h, a time course was generated by estimating DR concentration in milk every 0.5 h. Assuming a sow produces 7.5–13.6 kg milk/d (22) with milk density equal to ~1 kg/L, 13–23% of the DR dose was estimated to be irreversibly lost in L2 milk and 12.5–22% was lost during L3 over the 48-h period, which is comparable to 10–20% for PL1 (15). The amount of DR tracer in serum was calculated at Cmax by assuming a sow has 9 L serum during lactation (23). Only 0.88% of the 35-μmol DR tracer was estimated to be circulating in serum in L2, but this rose to 2.7% during L3, which is consistent with the difference in VA status between the cycles.
Liver VA reserves compared with indicators.
Liver VA reserves (n = 7) after L3 were 0.23 ± 0.05 μmol R/g. Liver values, as well as other VA indicators, were compared among the sows of the PL1 (15), L2, and L3 (Table 4). The 5-h serum DR:R ratio in the VA-depleted sows during L3 was 3.8-times higher than that in the VA-replete PL1 sows (P = 0.0097), which is consistent with the difference in liver reserves. Serum and milk R concentrations did not differ between the PL1 and L3 groups. Milk R concentrations in the L2 group were lower than those in the PL1 group and those during L3. The milk DR:R ratio at 7 h was higher in L3 than that in PL1 (P = 0.037).
TABLE 4.
Comparison of VA status indicators in 3 groups of lactating sows on VA-replete or VA-free feed1
| Indicator | Deficiency cutoffs | VA-replete sows (PL1), n = 5 | VA-depleted sows (L2), n = 8 | VA-depleted sows (L3), n = 7 |
| Liver reserves, μmol R/g | <0.07 | 0.73 ± 0.21a | — | 0.23 ± 0.05b |
| Serum DR:R ratio | >0.0602 | 0.018 ± 0.013b | 0.028 ± 0.017 | 0.069 ± 0.042a,* |
| Serum R, μmol/L | <1.053,4 | 0.94 ± 0.12 | 0.75 ± 0.23 | 0.86 ± 0.37 |
| Milk R, μmol/L | <1.053 | 1.07 ± 0.34a | 0.76 ± 0.17b | 1.23 ± 0.33* |
| Milk 5-h DR:R ratio | Unknown | 0.14 ± 0.12 | 0.30 ± 0.32 | 0.35 ± 0.32 |
| Milk 7-h DR:R ratio | Unknown | 0.20 ± 0.16b | 0.47 ± 0.42 | 0.60 ± 0.49a |
Values are means ± SD. Means in a row with different superscript letters are different using a one-tailed test (unpaired t test, P < 0.05). *Significantly higher value between L2 and L3 in the same sows (paired t test P < 0.05).
The relationship of liver reserves and common indicators is in (9).
Published cutoffs for the DR:R ratio, serum R (0.7 mol/L), and milk R are found in (24).
A higher proposed value is 1.05 mol/L is found in (25).
Discussion
Accurate assessment tools that are minimally invasive, require small sample volumes, and are sensitive to changes in VA status from an intervention are essential (8, 9). Although many factors influence the concentration of breast milk R [e.g. stage of lactation, time of day, last feeding episode, and milk fat content (26)], making it less than ideal as an individual indicator, breast milk is a unique population indicator. It represents the mother’s VA status and current dietary intake, and inference to the nursing infant’s VA status is possible. Collection of breast milk for VA analysis is less invasive than serum and does not require further processing at field stations, resulting in shorter preparation time (8, 27). Increasing dietary VA enhances the R concentration in mammary tissue and milk (28, 29).
In this study, the milk DR:R ratio was investigated as an indicator of VA status over 2 lactation cycles during VA depletion and compared with VA-replete sows. The milk DR:R ratio during L2 was biphasic, with peaks at 9 and 24 h, possibly corresponding to chylomicron and retinol-binding protein (RBP) delivery, respectively. Biphasic peaks were not observed during L3, possibly due to saturation of the mammary gland from chylomicron delivery. Peak DR concentration in milk was higher and occurred earlier during L3 than during L2 at 7 and 9 h. The early peak DR concentration during L3 may have masked the DR delivered through RBP at subsequent times, which is consistent with findings using an RBP-independent tracer in the same model system (30). More dietary VA is deposited into milk during VAD (28, 29); therefore, a higher milk DR:R ratio would reflect a larger portion of newly ingested VA being shunted to the mammary gland during VAD. Although the milk DR:R ratio peaked later than serum and may not have been as sensitive as that measured in serum, its application to lactating women in response to interventions should be explored.
The serum R concentration is used to assess VA status [abnormal < 0.7 μmol/L (24)], but did not have utility in distinguishing the difference in VA status among the sows in these studies. Liver reserves and the serum DR:R ratio, on the other hand, reflected a difference in VA status between PL1 and L3 sows. Assessment methods need to be sensitive to detect marginal liver reserves (26). The maximum serum DR concentration and AUC0–48h increased as liver reserves decreased. The amount of DR tracer in the blood was 3 times higher in L3 than in L2, consistent with the more depleted VA stores. Measuring serum R concentration alone may not reflect changes in VA status (12, 31), and WHO recommends that another indicator be used in conjunction with serum R to assess population risk for VAD (24).
Diet changes may affect the utilization rate of VA, shifting serum R concentration. That is, as VA intake decreases, the VA disposal rate is altered to conserve body stores. When switching from a feed with VA to one without VA in piglets, serum R decreased, possibly indicating a mechanism to form a new physiological set point. In the current study, the sows had lower serum R concentrations in L2 than in L3 despite lower liver stores in L3, but this may be due in part to a change in the feed between L2 and L3. Other explanations for the increase in serum R concentrations in L3 are the mobilization of R from the liver to meet tissue needs or more recycling because of sustained depletion. Serum R concentrations may also have decreased due to stress at L2 because of initial contact with the sows. Serum R increased during L2, which may have been due to displacement of R with the DR from the dose in the liver or stress recovery. Another possibility may be a response to the newly ingested intake of VA in the form of DR.
The sensitivity of the MRDR test during lactation has been validated (14, 15). The serum DR:R ratio was directly correlated with the milk DR:R ratio in this VA depletion model and in VA-replete sows (15). Milk:plasma ratios are often used to estimate the dose of a maternal drug delivered to an infant (32). As shown in this study, serum and milk DR:R ratio values peaked at 7 and 9 h, respectively, and the values at these times did not differ from each other in the same fluid. The irreversible loss of DR tracer into the milk was similar to the 10–20% determined during PL1 (15). The milk DR Cmax was higher during L3 at an earlier Tmax, indicating that the DR was quickly released into the milk, which is expected when VA stores are decreased. The milk DR t1/2 in L3 was also much shorter, demonstrating quick delivery to the nursing piglets.
VA indicators need to be compared along a range of liver reserves to determine their utility to measure VA status along the continuum. The 5-h serum and 7-h milk DR:R ratios in the VA-depleted sows were higher than the values in the VA-replete sows, reflecting the difference in liver reserves and illustrating a lag. Collection times for this milk response are currently being evaluated in several groups of women. The milk DR:R ratio was correlated with that in serum at biologically sensitive response times. DR trafficking in women of varying VA status is needed to determine the feasibility of using the milk DR:R ratio as a potential unique indicator of VA status during lactation.
Supplementary Material
Acknowledgments
We are grateful for the continued support of Thomas Crenshaw, professor of animal science, for his expertise in all aspects of swine care and Peter Crump, University of Wisconsin-Madison College of Agriculture and Life Sciences Statistical Consulting Service, for statistical analysis consultation. We also thank Jamie Reichert, program manager, for valuable assistance during sow and piglet procedures. R.L.S. was responsible for conducting the research, serum and liver analysis, input into statistical analysis, and wrote the paper; P.H. assisted with the analysis of data; A.R.V. and J.P.M. provided hands-on care of the swine and provided input into the paper; and S.A.T. designed the research, provided input into data analysis, and revised the paper. All authors read and approved the final manuscript.
Footnotes
Supported by USDA National Research Initiative (NRI) 2003-35200-13754, USDA NRI 2007-35200-17729, and NIH–National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) 61973. Presented as a poster: Surles RL, Valentine AR, Mills JP, Tanumihardjo SA. Application of 3,4-didehydroretinol for the modified relative dose response test and as a vitamin A tracer during lactation in a sow model. In: Consequences and control of micronutrient deficiencies: science, policy, and programs: defining the issues. Micronutrient Forum, Istanbul, Turkey, April 2007; p. 80 (Abstract T45).
Supplemental Figure 1 is available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: AUC, area under the curve; Cmax, maximum concentration; DR, 3, 4-didehydroretinol; L2, second lactation period; L3, third lactation period; LSM, least squares mean; MRDR, modified relative dose response; PL1, prior lactaction 1; R, retinol; RBP, retinol-binding protein; Tmax, time of maximum concentration; V, volume distribution; VA, vitamin A; VAD, vitamin A deficiency.
Literature Cited
- 1.Ross SA, McCaffery PJ, Drager UC, De Luca LM. Retinoids in embryonal development. Physiol Rev. 2000;80:1021–54 [DOI] [PubMed] [Google Scholar]
- 2.The United Nations Population Fund Health and the links to nutrition: maternal health is key [cited 2010 Jan 26]. Available from: http://www.unfpa.org/news/news.cfm?ID=441&Language=1
- 3.Ross DA. Recommendations for vitamin A supplementation. J Nutr. 2002;131:S2902–6 [DOI] [PubMed] [Google Scholar]
- 4.van Jaarsveld PJ, Faber M, Tanumihardjo SA, Nestel P, Lombard CJ, Benade AJ. β-Carotene-rich orange-fleshed sweet potato improves the vitamin A status of primary school children assessed with the modified-relative-dose-response test. Am J Clin Nutr. 2005;81:1080–7 [DOI] [PubMed] [Google Scholar]
- 5.Tchum SK, Newton S, Tanumihardjo SA, Arthur FKN, Tetteh A, Owusu-Agyei S. Evaluation of a green leafy vegetable intervention in Ghanaian postpartum mothers. Afr J Food Agric Nutr Dev. 2009;9:1294–308 [Google Scholar]
- 6.Howe JA, Tanumihardjo SA. Carotenoid-biofortified maize maintains adequate vitamin A status in Mongolian gerbils. J Nutr. 2006;136:2562–7 [DOI] [PubMed] [Google Scholar]
- 7.Tanumihardjo SA, Bouis H, Hotz C, Meenakshi JV, McClafferty B. Biofortification of staple crops: an emerging strategy to combat hidden hunger. Comp Rev Food Sci Food Safety. 2008;7:329–34 [Google Scholar]
- 8.Tanumihardjo SA. Assessing vitamin A status: past, present and future. J Nutr. 2004;134:S290–3 [DOI] [PubMed] [Google Scholar]
- 9.Vitamin A Tracer Task Force Appropriate uses of vitamin A tracer (stable isotope) methodology. Washington, DC: ILSI Human Nutrition Institute; 2004 [Google Scholar]
- 10.Rice AL, Stoltzfus RJ, de Francisco A, Chakraborty J, Kjolhede CL, Wahed MA. Maternal vitamin A or beta-carotene supplementation in lactating Bangladeshi women benefits mothers and infants but does not prevent subclinical deficiency. J Nutr. 1999;129:356–65 [DOI] [PubMed] [Google Scholar]
- 11.Rice AL, Stoltzfus RJ, de Francisco A, Kjolhede CL. Evaluation of serum retinol, the modified-relative-dose-response ratio, and breast-milk vitamin A as indicators of response to postpartum maternal vitamin A supplementation. Am J Clin Nutr. 2000;71:799–806 [DOI] [PubMed] [Google Scholar]
- 12.Tanumihardjo SA, Permaesih D. Muherdiyantiningsih, Rustan E, Rusmil K, Fatah AC, Wilbur S, Muhilal, Karyadi D, et al. Vitamin A status of Indonesian children infected with Ascaris lumbricoides after dosing with vitamin A supplements and albendazole. J Nutr. 1996;126:451–7 [DOI] [PubMed] [Google Scholar]
- 13.Tanumihardjo SA, Barua AB, Olson JA. Use of 3, 4-didehydroretinol to assess vitamin A status in rats. Int J Vitam Nutr Res. 1987;57:127–32 [PubMed] [Google Scholar]
- 14.Tanumihardjo SA. Muherdiyantiningsih, Permaesih D, Komala, Muhilal, Karyadi D, Olson JA. Daily supplements of vitamin A (8.4 μmol; 8000 IU) improve the vitamin A status of lactating Indonesian women. Am J Clin Nutr. 1996;63:32–5 [DOI] [PubMed] [Google Scholar]
- 15.Surles RL, Li J, Tanumihardjo SA. The modified-relative-dose-response values in serum and milk are positively correlated over time in lactating sows with adequate vitamin A status. J Nutr. 2006;136:939–45 [DOI] [PubMed] [Google Scholar]
- 16.Valentine AR, Tanumihardjo SA. Adjustments to the modified relative dose response (MRDR) test for assessment of vitamin A status minimize the blood volume used in piglets. J Nutr. 2004;134:1186–92 [DOI] [PubMed] [Google Scholar]
- 17.Escaron AL, Green MH, Tanumihardjo SA. Plasma turnover of 3, 4-didehydroretinol (vitamin A2) increases in vitamin A-deficient rats fed low versus high dietary fat. J Lipid Res. 2009;50:694–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penniston KL, Valentine AR, Tanumihardjo SA. A theoretical increase in infants’ hepatic vitamin A is realized using a supplemented lactating sow model. J Nutr. 2003;133:1139–42 [DOI] [PubMed] [Google Scholar]
- 19.Tanumihardjo SA, Penniston KL. Simplified methodology to determine breast milk retinol concentrations. J Lipid Res. 2002;43:350–5 [PubMed] [Google Scholar]
- 20.Tanumihardjo SA, Howe JA. Twice the amount of α-carotene isolated from carrots is as effective as β-carotene in maintaining the vitamin A status of Mongolian gerbils. J Nutr. 2005;135:2622–4 [DOI] [PubMed] [Google Scholar]
- 21.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509 [PubMed] [Google Scholar]
- 22.Boyd RD, Kensinger RS. Metabolic precursors for milk synthesis. Verstegen MWA, Moughan PJ, Schrama JW, The lactating sow. Wageningen (The Netherlands): Wageningen Pers; 1998. p. 71–95 [Google Scholar]
- 23.Anderson DM, Elsley FWH, McDonald I. Blood volume changes during pregnancy and lactation of sows. Q J Exp Physiol Cogn Med Sci. 1970;55:293–300 [DOI] [PubMed] [Google Scholar]
- 24.WHO Indicators for assessing vitamin A deficiency and their application in monitoring and evaluating intervention programmes. Geneva: WHO/NUT/96.10.;1996 [Google Scholar]
- 25.de Pee S, Dary O. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr. 2002;132:S2895–901 [DOI] [PubMed] [Google Scholar]
- 26.Stoltzfus RJ, Underwood BA. Breast-milk vitamin A as an indicator of the vitamin A status of women and infants. Bull World Health Organ. 1995;73:703–11 [PMC free article] [PubMed] [Google Scholar]
- 27.Haskell MJ, Brown KH. Maternal vitamin A nutriture and the vitamin A content of human milk. J Mammary Gland Biol Neoplasia. 1999;4:243–57 [DOI] [PubMed] [Google Scholar]
- 28.Green MH, Green JB, Akohoue SA, Kelley SK. Vitamin A intake affects the contribution of chylomicrons vs. retinol-binding protein to milk vitamin A in lactating rats. J Nutr. 2001;131:1279–82 [DOI] [PubMed] [Google Scholar]
- 29.Ross AC, Pasatiempo AMG, Green MH. Chylomicron margination, lipolysis, and vitamin A uptake in the lactating rat mammary gland: implications for milk retinoid content. Exp Biol Med (Maywood). 2004;229:46–55 [DOI] [PubMed] [Google Scholar]
- 30.Dever JT, Surles RL, Davis CR, Tanumihardjo SA. α-Retinol is distributed through serum retinol-binding protein-independent mechanisms in the lactating sow-nursing piglet dyad. J Nutr. 2011;141:42–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanumihardjo SA, Permaesih D. Muhilal. Vitamin A status and hemoglobin concentrations are improved in Indonesian children with vitamin A and deworming interventions. Eur J Clin Nutr. 2004;58:1223–30 [DOI] [PubMed] [Google Scholar]
- 32.Wilson JT, Brown RD, Hinson JL, Dailey JW. Pharmacokinetic pitfalls in the estimation of the breast milk/plasma ratio for drugs. Annu Rev Pharmacol Toxicol. 1985;25:667–89 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.