Abstract
High dietary acid load may be detrimental to bone mineral density (BMD), although sufficient calcium intake might neutralize this effect. In observational studies, the association between BMD and dietary acid load, estimated by net endogenous acid production (NEAP) and potential renal acid load (PRAL), has been inconsistent, and the potential modifying effect of calcium intake has not been assessed. We therefore examined the cross-sectional associations of estimated NEAP and PRAL with BMD in the Framingham Osteoporosis Study. We hypothesized that higher estimated NEAP and PRAL would be associated with lower BMD, but only among those with total calcium intake < 800 mg/d. BMD of the femoral neck and lumbar spine was measured, and estimated NEAP and PRAL were calculated via FFQ among 1069 Framingham Original (1988–1989, 1992–1993; 62% women, mean age 76 y) and 2919 Offspring (1996–2001; 56% women, mean age 60 y) cohort participants. Cohort- and sex-specific ANCOVA was used to calculate multivariable-adjusted mean BMD for estimated NEAP and PRAL quartiles. Assuming no uncontrolled confounding, estimated NEAP, but not PRAL, was inversely associated with femoral neck BMD (P-trend = 0.04) in Original cohort men, whereas neither was associated with lumbar spine BMD. Estimated NEAP and PRAL were not associated with BMD at any site among Original cohort women or Offspring cohort men and women. There were no significant interactions between either estimated NEAP or PRAL and total calcium intake. These results suggest that, with a possible exception of older men, dietary acid load does not have a measureable negative effect on bone health, regardless of total calcium intake.
Introduction
Osteoporosis continues to be a major public health concern, especially among the rapidly expanding U.S. population of older adults. The prospect of escalating burdens of morbidity, mortality, and health care expenditures associated with osteoporosis and related fractures (1–4) highlights the importance of identifying modifiable risk factors for low bone mass in adults. Nutritional risk factors are perhaps among the most amenable to modification, and it has become well recognized that the influence of nutrition on bone mass extends well beyond calcium and vitamin D to include several nutrients (5). The acid-base hypothesis suggests that diets high in acid-forming nutrients elicit the release of calcium from bone as a buffer, which is facilitated by increased bone resorption. It is therefore assumed that chronic exposure to high dietary acid load may contribute to low bone mass. Dietary acid load in human diets can be estimated by calculating the net endogenous acid production (NEAP)8 or the potential renal acid load (PRAL) of the diet using information on dietary intakes. Several epidemiologic studies have used these measures to investigate the association of dietary acid load and bone mineral density (BMD), with mixed and inconsistent results (6–11).
The apparent inconsistencies in the existing literature may be due to the conflicting roles for protein in the etiology of bone loss. Protein is an essential building block for bone tissue and also stimulates production of insulin-like growth factor 1 (12, 13), which regulates osteoblast function to help maintain bone mass (14). Indeed, the beneficial effects of dietary protein are supported by several population-based observational studies, which were recently summarized in a systematic review and meta-analysis indicating that in middle-aged and older adults, greater dietary protein intake is associated with higher BMD at several bone sites; pooled correlation coefficient (r) ranged from 0.11 for femur BMD to 0.15 for total body BMD (15). Yet protein is a primary contributor to dietary acid load and, under the acid-base hypothesis, may have detrimental effects on BMD (16). Therefore, the benefits of dietary protein intake for BMD may be suppressed by a consequent acidic environment.
Dietary calcium intake may play a key role in the balance between the potential opposing effects of dietary acid load and protein on bone mass (17). To counter the hypothesized detrimental effect of a high acid load, increased calcium intake may offset calcium lost from bone. Thus, increased dietary acid load may be detrimental to bone only when there is inadequate calcium intake. Previous studies of dietary acid load and BMD have not adequately addressed the potential modifying effect of dietary calcium intake.
Our objective in this study was to determine the cross-sectional association of diet-dependent acid load with BMD of the femoral neck and lumbar spine among community-dwelling, middle-aged and older men and women participating in the Framingham Osteoporosis Study. We hypothesized that higher acid load, defined by estimated NEAP and PRAL, would be associated with lower BMD, but only among those with low calcium intake.
Materials and Methods
Study participants.
The Framingham Study began in 1948 with the primary goal of determining risk factors for heart disease. The Framingham Original Cohort was comprised of 5209 men and women ranging in age from 28 to 62 y, recruited from a two-thirds sample of all Framingham, Massachusetts residences and have been followed biennially for over 60 y with extensive physical examinations, comprehensive questionnaires, anthropometric measurements, blood chemistries, and assessments of risk factors by trained clinical personnel (18). In 1971 the Framingham Offspring Study was initiated with the enrollment of 5124 adult children (and their spouses) of the Original cohort who have been similarly followed approximately every 4 y (19). The Framingham Osteoporosis Study first measured BMD of the hip and spine in 1273 Original cohort participants during either 1988–1989 or 1992–1993 and in 3036 Offspring participants from 1996 to 2001. Participants in the current study included the 1069 Original cohort and 2919 Offspring who had valid FFQ data collected concurrently with their baseline BMD measures. The appropriate institutional review boards at Hebrew SeniorLife, Tufts University, and Boston University approved this study and informed consent was obtained from all participants.
Dietary assessment.
A 126-item semiquantitative FFQ that has been validated for several nutrients (20) was used to assess usual dietary intakes (average intake per day) for the preceding 12 mo, as previously described (21). Participants who reported energy intakes < 2.5 or > 16.7 MJ/d or who left >12 food items blank were considered to have invalid questionnaires and were not included in the study population.
Dietary acid load.
Estimated NEAP was calculated based on the method of Frassetto et al. (22), which calculates the diet’s net acid load from dietary intakes of protein and potassium using the following equation, which was developed using a combination of chemical analysis of experimentally controlled diets and diet composition tables:
with protein and potassium standardized to 10.46 MJ. This measure is based primarily on the dietary protein:potassium ratio and does not account for other nutrients that influence dietary acid load. Thus, it may be limited in estimating the acid load of the overall diet. Therefore, PRAL was calculated as another estimate of dietary acid load using the following equation from Remer and Manz (23):
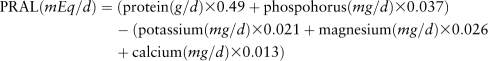 |
PRAL was calculated 2 ways: using calcium intake from the diet only and using total calcium intake, which included calcium from supplements. Because results using the 2 estimates of PRAL were similar, only results based on using calcium intake from the diet are presented.
BMD.
BMD of the proximal right femoral neck and the lumbar spine (average BMD of L2–L4) was measured in g/cm2 by using a Lunar dual-photon absorptiometer (DP3, Lunar Radiation) during the Original Cohort 1988–1989 examinations, and a dual-energy X-ray absorptiometry densitometer (DPX-L, Lunar Radiation) for all other assessments. We have previously shown high correlations between dual-photon and dual-energy X-ray absorptiometry (24). However, because of a small but consistent shift in BMD values between the 2 technologies, femoral BMD obtained on the DP3 were adjusted for the change in equipment to DPX-L technology by using published corrections (24). The right femur was scanned at each exam unless there was a history of previous fracture or hip joint replacement, in which case the left side was scanned. We used standard positioning as recommended by the manufacturer. The CV for repeat scans over time using the DP3 were 2.6 and 2.2% and for the DPX-L were 1.7 and 0.9% for the femoral neck and lumbar spine, respectively.
Other variables.
Additional covariates were obtained from data collected concurrently with BMD measures and included sex, age, height, weight, smoking status (current smoker: yes/no), physical activity, average daily intakes of total energy (MJ), alcohol (g), caffeine (mg), total calcium (mg) and dietary vitamin D from sources other than supplements (μg), current vitamin D supplement use (yes/no), and, in women, menopause status (postmenopausal: yes/no) and current estrogen use (yes/no). Weight (in light clothing without shoes) was measured to the nearest pound with the use of a standard balance beam scale and converted to kilograms. Height without shoes was measured to the nearest one-quarter inch and converted to meters. Smoking status was assessed as whether the participant regularly smoked cigarettes over the year prior to the BMD exam. Physical activity was assessed in the Original cohort using a physical activity index, which is a weighted sum of hours spent on strenuous, moderate, and light activity, as well as at rest (25, 26). In the Offspring, physical activity was measured using the Physical Activity Scale for the Elderly, a validated questionnaire of self-reported activity over the past 7 d (27). Usual intakes of total energy, alcohol, caffeine, total calcium, and dietary vitamin D, as well as vitamin D supplement use over the previous year were obtained using FFQ data. Menopause status was assessed at the time of BMD measurement and defined as having no menstrual periods for more than 1 y or currently using postmenopausal estrogens (oral, patch, or cream). Estrogen use was classified as either current use or no use of hormone replacement therapy at the time of the BMD assessment.
Statistical analysis.
Most previous studies of the relations of estimated NEAP and PRAL with bone health have considered women only (6, 7, 9, 28) and 1 study suggests that the association may differ by gender (8). Thus, to facilitate comparison of our results to those of other studies, all analyses for this study were conducted for men and women separately. Furthermore, given that the Offspring cohort consists primarily of children of the Original cohort, dietary intakes and BMD values within the combined cohorts are likely not independent. Therefore, cohort-specific analyses were conducted.
Prior to all statistical analyses, estimated NEAP and PRAL were adjusted for total energy intake using the residual method (29) and sex- and cohort-specific quartiles of energy-adjusted estimated NEAP and PRAL were created. To evaluate the relation between dietary acid load and BMD, ANCOVA was used to test for a linear trend in least squares-adjusted mean femoral neck and lumbar spine BMD across estimated NEAP and PRAL quartiles. Models were adjusted for age, height, weight, total energy intake, physical activity, smoking, intakes of alcohol and caffeine, dietary vitamin D intake, vitamin D supplement use, and, in women, menopause status and estrogen use.
To determine whether calcium intake modified the relation of dietary acid load with BMD, we tested for statistical interactions of estimated NEAP and PRAL quartiles, modeled as single ordinal variables, with total calcium intake, categorized as <800 mg/d or ≥800 mg/d, adjusting for the previously listed covariates. P-interaction values < 0.05 were considered as suggestive that the association between dietary acid load and BMD differed according to calcium category. All statistical analyses were conducted using SAS/STAT software version 9.1 (SAS Institute).
Results
Original cohort participants were older, had a lower proportion of women using estrogen replacement therapy, lower protein intake and dietary acid load, and lower BMD compared with the Offspring cohort (Table 1). At the time of their respective BMD assessments, Offspring cohort participants had greater mean total calcium intake compared with Original cohort participants. Yet whereas similar proportions of men in both cohorts had total calcium intake < 800 mg/d (64 vs. 58%), a higher proportion of Original cohort women had intakes < 800 mg/d compared with Offspring participants (60 vs. 34%). All Original cohort women were postmenopausal, whereas 14% of Offspring women had not yet reached menopause. Within both cohorts, men had higher total energy intake, protein intake, dietary acid load, and BMD at both the femoral neck and lumbar spine compared with the women.
TABLE 1.
Descriptive statistics for members of the Framingham Original and Offspring Cohort study participants with valid FFQ and BMD1
| Original cohort |
Offspring cohort |
|||
| Men | Women | Men | Women | |
| n | 410 | 659 | 1280 | 1639 |
| Age, y | 75 ± 5 | 77 ± 5 | 61 ± 9 | 60 ± 9 |
| Range | 68–92 | 67–96 | 35–86 | 29–86 |
| Height, m | 1.70 ± 0.08 | 1.57 ± 0.08 | 1.75 ± 0.08 | 1.60 ± 0.05 |
| Weight, kg | 78.9 ± 12.7 | 64.4 ± 12.2 | 88.8 ± 15.0 | 71.2 ± 15.0 |
| % Menopausal | — | 100 | — | 86 |
| % Current estrogen user | — | 5 | — | 31 |
| Physical activity2 | 34 ± 6 | 33 ± 5 | 156 ± 87 | 134 ± 71 |
| % Current smoker | 9 | 12 | 11 | 13 |
| Daily intakes from FFQ | ||||
| Alcohol, g | 13 ± 19 | 6 ± 11 | 14 ± 18 | 7 ± 11 |
| Caffeine, mg | 192 ± 163 | 197 ± 164 | 259 ± 208 | 217 ± 182 |
| Dietary vitamin D, μg | 5.75 ± 3.98 | 5.25 ± 3.48 | 5.48 ± 3.38 | 5.38 ± 3.18 |
| % Vitamin D supplement user | 22.9 | 30.4 | 39.9 | 53.2 |
| Total calcium, mg | 755 ± 388 | 812 ± 445 | 847 ± 439 | 1110 ± 572 |
| % < 800 mg | 64 | 60 | 58 | 34 |
| Total protein g | 70 ± 25 | 67 ± 25 | 81 ± 28 | 77 ± 26 |
| Potassium, g | 2.99 ± 1.02 | 2.94 ± 1.03 | 3.05 ± 9.97 | 3.02 ± 1.01 |
| Phosphorus, g | 1.13 ± 4.30 | 1076 ± 409 | 1.27 ± 4.52 | 1.21 ± 4.32 |
| Magnesium, mg | 298 ± 111 | 288 ± 111 | 324 ± 115 | 316 ± 114 |
| Total energy, MJ | 7.82 ± 0.61 | 6.94 ± 2.35 | 8.12 ± 2.65 | 7.27 ± 2.31 |
| Dietary acid load, mEq/d | ||||
| Estimated NEAP | 40 ± 13 | 39 ± 12 | 47 ± 15 | 45 ± 14 |
| PRALdiet | −3.6 ± 12 | −5.5 ± 12 | 3.9 ± 14 | 1.0 ± 14 |
| BMD, g/cm2 | ||||
| Femoral neck | 0.873 ± 0.14 | 0.720 ± 0.12 | 0.971 ± 0.14 | 0.869 ± 0.14 |
| Lumbar spine (L2-L4) | 1.329 ± 0.23 | 1.067 ± 0.20 | 1.319 ± 0.20 | 1.153 ± 0.20 |
Values are means ± SD unless otherwise noted.
Original cohort, physical activity index; offspring, Physical Activity Scale for the Elderly.
There was a significant inverse association between multivariable-adjusted mean femoral neck BMD and estimated NEAP quartile (P-trend = 0.04) for the Original cohort men, indicating lower BMD with greater dietary acid load (Table 2). Mean femoral neck BMD in both quartiles 3 and 4 was ∼5% lower than that in the lowest quartile (P < 0.05), suggesting a potential threshold effect. Femoral neck BMD was not associated with estimated NEAP among Original cohort women nor was lumbar spine BMD for either the men or the women.
TABLE 2.
Total protein and calcium intakes, range of estimated NEAP, and multivariable-adjusted femoral neck and lumbar spine BMD for quartiles of estimated NEAP in Framingham Original Cohort men and women1,2
| NEAP quartile |
|||||
| 1 | 2 | 3 | 4 | P-trend | |
| Men | |||||
| n | 93 | 96 | 94 | 88 | |
| Total protein,1g/d | 57 ± 20 | 67 ± 22 | 75 ± 24 | 79 ± 25 | |
| Total calcium,1mg/d | 717 ± 377 | 756 ± 398 | 819 ± 411 | 696 ± 348 | |
| Estimated NEAP range, mEq/d | 8.32, 31.24 | 31.41, 39.28 | 39.35, 47.65 | 47.68, 83.60 | |
| Femoral neck BMD,22 | 0.885 ± 0.018 | 0.853 ± 0.017 | 0.844 ± 0.017 | 0.842 ± 0.017 | 0.04 |
| L2-L4 BMD,2g/cm2 | 1.276 ± 0.035 | 1.282 ± 0.034 | 1.298 ± 0.034 | 1.251 ± 0.035 | 0.56 |
| Women | |||||
| n | 148 | 148 | 145 | 146 | |
| Total protein,1g/d | 56 ± 20 | 67 ± 22 | 73 ± 26 | 72 ± 26 | |
| Total calcium,1mg/d | 786 ± 397 | 907 ± 490 | 870 ± 472 | 680 ± 339 | |
| Estimated NEAP range, mEq/d | 1.64, 30.63 | 30.70, 37.43 | 37.59, 45.83 | 45.84, 104.7 | |
| Femoral neck BMD,2g/cm2 | 0.716 ± 0.010 | 0.715 ± 0.010 | 0.717 ± 0.010 | 0.737 ± 0.010 | 0.06 |
| L2-L4 BMD,2g/cm2 | 1.103 ± 0.019 | 1.073 ± 0.020 | 1.088 ± 0.020 | 1.079 ± 0.020 | 0.46 |
Values are means ± SD.
Values are least-squares means ± SE. Means adjusted for age, height, weight, total energy, physical activity, smoking, alcohol, caffeine, dietary vitamin D, vitamin D supplement use, and, in women, estrogen use.
When dietary acid load was estimated as PRAL among Original cohort participants, the trend with femoral neck BMD among the men was no longer significant (P = 0.08), likely due to higher BMD in the quartile 4 relative to quartile 3 (Table 3). Similar to the estimated NEAP results, femoral neck BMD in women and lumbar spine BMD for both genders was not significantly associated with PRAL. In the Offspring cohort, dietary acid load, whether measured as estimated NEAP or PRAL, was not associated with BMD at either the femoral neck or lumbar spine (Tables 4, 5).
TABLE 3.
Total protein and calcium intakes, range of estimated PRAL, and multivariable-adjusted femoral neck and lumbar spine BMD for quartiles of estimated PRAL in Framingham Original Cohort men and women1,2
| PRAL quartile |
|||||
| 1 | 2 | 3 | 4 | P-trend | |
| Men | |||||
| n | 92 | 96 | 98 | 85 | |
| Total protein1, g/d | 62 ± 21 | 64 ± 22 | 69 ± 24 | 83 ± 24 | |
| Total calcium1, mg/d | 762 ± 393 | 704 ± 351 | 739 ± 380 | 791 ± 423 | |
| Estimated PRAL range, mEq/d | −67.34, −10.73 | −10.54, −2.52 | −2.50, 5.12 | 5.16, 25.76 | |
| Femoral neck BMD2, g/cm2 | 0.889 ± 0.018 | 0.853 ± 0.017 | 0.831 ± 0.016 | 0.859 ± 0.018 | 0.08 |
| L2-L4 BMD2, g/cm2 | 1.292 ± 0.035 | 1.308 ± (0.034 | 1.236 ± 0.032 | 1.295 ± 0.036 | 0.69 |
| Women | |||||
| n | 149 | 148 | 147 | 143 | |
| Total protein1, g/d | 64 ± 23 | 63 ± 22 | 64 ± 25 | 77 ± 25 | |
| Total calcium1, mg/d | 855 ± 431 | 830 ± 443 | 801 ± 478 | 755 ± 386 | |
| Estimated PRAL range, mEq/d | −49.20, −12.90 | −12.85, −5.22 | −5.15, 2.41 | 2.42, 40.97 | |
| Femoral neck BMD2, g/cm2 | 0.716 ± 0.010 | 0.720 ± 0.010 | 0.722 ± 0.010 | 0.728 ± 0.010 | 0.31 |
| L2-L4 BMD2, g/cm2 | 1.110 ± 0.020 | 1.072 ± 0.019 | 1.079 ± 0.021 | 1.087 ± 0.020 | 0.35 |
Values are means ± SD.
Values are least-squares means ± SE. Means adjusted for age, height, weight, total energy, physical activity, smoking, alcohol, caffeine, dietary vitamin D, vitamin D supplement use, and in women, estrogen use.
TABLE 4.
Total protein and calcium intakes, range of estimated NEAP and multivariable-adjusted femoral neck and lumbar spine BMD for quartiles of estimated NEAP in Framingham Offspring men and women1,2
| NEAP Quartile |
|||||
| 1 | 2 | 3 | 4 | P-trend | |
| Men | |||||
| n | 316 | 316 | 319 | 318 | |
| Total protein,1g/d | 69 ± 23 | 81 ± 27 | 83 ± 27 | 90 ± 30 | |
| Total calcium,1mg/d | 893 ± 442 | 884 ± 432 | 835 ± 453 | 780 ± 423 | |
| Estimated NEAP range, mEq/d | 11.53, 37.87 | 37.90, 45.68 | 45.71, 55.12 | 55.14, 140.1 | |
| Femoral neck BMD,2g/cm2 | 0.972 ± 0.007 | 0.970 ± 0.007 | 0.965 ± 0.007 | 0.975 ± 0.007 | 0.86 |
| L2-L4 BMD,2g/cm2 | 1.315 ± 0.012 | 1.313 ± 0.011 | 1.310 ± 0.011 | 1.338 ± 0.011 | 0.17 |
| Women | |||||
| n | 399 | 404 | 403 | 405 | |
| Total protein,1g/d | 66 ± 23 | 76 ± 25 | 79 ± 24 | 85 ± 28 | |
| Total calcium,1mg/d | 1200 ± 602 | 1190 ± 602 | 1070 ± 516 | 1000 ± 544 | |
| Estimated NEAP range, mEq/d | 6.99, 35.43 | 35.44, 43.78 | 43.82, 53.04 | 53.07, 142.0 | |
| Femoral neck BMD,2g/cm2 | 0.867 ± 0.006 | 0.877 ± 0.006 | 0.865 ± 0.006 | 0.866 ± 0.006 | 0.60 |
| L2-L4 BMD,2g/cm2 | 1.148 ± 0.009 | 1.160 ± 0.008 | 1.147 ± 0.008 | 1.157 ± 0.009 | 0.66 |
Values are means ± SD.
Values are least-squares means ± SE. Means adjusted for age, height, weight, total energy, physical activity, smoking, alcohol, caffeine, dietary vitamin D, vitamin D supplement use, and in women, menopause status and estrogen use.
TABLE 5.
Total protein and calcium intakes, range of estimated PRAL and multivariable-adjusted femoral neck and lumbar spine BMD for quartiles of estimated PRAL in Framingham Offspring men and women1,2
| PRAL quartile |
|||||
| 1 | 2 | 3 | 4 | P-trend | |
| Men | |||||
| n | 317 | 315 | 319 | 318 | |
| Total protein,1g/d | 76 ± 23 | 74 ± 26 | 78 ± 27 | 95 ± 31 | |
| Total calcium,1mg/d | 914 ± 438 | 807 ± 389 | 792 ± 419 | 878 ± 495 | |
| Estimated PRAL range, mEq/d | −66.51, −3.96 | −3.94, 4.63 | 4.66, 12.27 | 12.27, 59.65 | |
| Femoral neck BMD,2g/cm2 | 0.970 ± 0.007 | 0.972 ± 0.007 | 0.970 ± 0.007 | 0.970 ± 0.007 | 0.93 |
| L2-L4 BMD,2g/cm2 | 1.304 ± 0.011 | 1.324 ± 0.011 | 1.320 ± 0.011 | 1.327 ± 0.011 | 0.21 |
| Women | |||||
| n | 400 | 406 | 404 | 401 | |
| Total protein,1g/d | 73 ± 24 | 71 ± 24 | 74 ± 24 | 89 ± 27 | |
| Total calcium,1mg/d | 1230 ± 569 | 1110 ± 573 | 1020 ± 533 | 1100 ± 597 | |
| Estimated PRAL range, mEq/d | −59.91, −6.89 | −6.78, 1.35 | 1.35, 9.65 | 9.70, 129.8 | |
| Femoral neck BMD,2g/cm2 | 0.875 ± 0.006 | 0.871 ± 0.006 | 0.864 ± 0.006 | 0.865 ± 0.006 | 0.14 |
| L2-L4 BMD,2g/cm2 | 1.157 ± 0.009 | 1.154 ± 0.008 | 1.143 ± 0.008 | 1.159 ± 0.009 | 0.91 |
Values are means ± SD.
Values are least-squares means ± SE. Means adjusted for age, height, weight, total energy, physical activity, smoking, alcohol, caffeine, dietary vitamin D, vitamin D supplement use, and, in women, menopause status and estrogen use.
For both estimated NEAP and PRAL, there was no evidence of statistical interaction between dietary acid load and total calcium intake in relation to BMD at either the femoral neck or lumbar spine for any of the cohort/gender groups (P-interaction range, 0.09–0.93).
Discussion
In our study of older and middle-aged men and women, dietary acid load as measured by estimated NEAP was inversely associated with femoral neck BMD among older men. PRAL, however, was not associated with femoral neck BMD, and neither estimated NEAP nor PRAL was related to lumbar spine BMD in this group of older men. Dietary acid load was not associated with BMD at any site among older women or among middle-aged men and women. Furthermore, total calcium intake did not modify the relation between dietary acid load and BMD. To our knowledge, ours is the first study to suggest that greater dietary acid load is associated with lower BMD among elderly men.
The primary basis for the acid-base hypothesis for osteoporosis is that dietary proteins, particularly those from animal sources, are the main contributors to endogenous acid load (23) and that long-term exposure to diets high in proteins lead to an acidic environment in the body, which in turn induces release of calcium from bone and subsequent bone loss. Several dietary intervention studies examining the influence of acid-base intake on urinary acid and calcium excretion have been summarized in a meta-analysis by Fenton et al. (30), showing a direct relation between net acid excretion and urinary calcium excretion. This would seem to support the acid-base hypothesis, but only under the assumption that the source of increase in excreted calcium is indeed bone. Fenton et al. (31) examined this supposition in another recent meta-analysis of calcium balance studies and concluded that the increased urinary calcium excretion induced by a high-acid diet is not due to reduced calcium retention but more likely to increased calcium absorption. Furthermore, their meta-analysis revealed no evidence of a relation between net acid excretion and N-telopeptides, a marker of bone metabolism. Thus, the notion of bone being called upon as a buffer to mild metabolic acidosis has been challenged.
Although our results suggest that increased dietary acid load is associated with lower BMD among older men, we did not observe a similar association among the primarily middle-aged men in the Offspring cohort. These discordant results may be attributed to differences in the proportion of animal-based protein, the primary source of acid-forming nutrients in the diet. Both estimated NEAP and PRAL consider the contribution of total protein intake to dietary acid load and do not account for source of protein. Nevertheless, although Offspring cohort men had more acidic overall diets, their proportion of total protein intake from animal sources (mean 69%, range 22–94%) was similar to that of the Original cohort men (mean 65%, range 29–90%). Alternatively, our ability to observe an association among the Original cohort men may be due to bone loss occurring more rapidly in the older compared with younger men; thus, any association may be more easily identifiable in this group. Additionally, because this was an observational study, it is possible that our significant finding among older men could be attributed to confounding by factors that were not measured and adjusted for in our analyses.
Apart from our isolated finding among older men, our results are primarily in line with the above mentioned meta-analyses that dispute the acid-base hypothesis of osteoporosis. Although estimated NEAP was negatively associated with femoral neck BMD in the elderly men, the association was not significant when dietary acid load was measured using PRAL. Neither estimated NEAP nor PRAL were associated with lumbar spine BMD among these older men, nor were they associated with BMD at any site among the Offspring cohort of mainly middle-aged men. Although only 2 other previous investigations of dietary acid load and BMD have included men, neither found any associations (8, 10).
Furthermore, our results suggest that higher dietary acid load is not associated with lower BMD among middle-aged and older women. Similar results were recently published by Pedone et al. (11), who found no association between PRAL and BMD of the tibia measured by peripheral quantitative computed tomography among women 60 y of age and older. In contrast, however, other cross-sectional observational studies have noted negative associations between acidic dietary profiles and BMD in women (6–10). Results have, however, been inconsistent. For example, 2 separate analyses of women participating in the Aberdeen Prospective Osteoporosis Screening Study found that higher estimated NEAP was associated with lower BMD at the hip (femoral neck, Ward’s area, trochanter) and lumbar spine among premenopausal but not postmenopausal women (6, 7). Rahbar et al. (10) reported that estimated NEAP was negatively associated only with femoral neck BMD among premenopausal women but among postmenopausal women was associated only with wrist and lumbar spine BMD. Furthermore, in a study of elderly women, Wynn et al. (9) found a negative association between estimated NEAP and heel BMD, as estimated by quantitative ultrasound, but only among those with a history of fracture. The patterns of association in various subgroups of individuals such as premenopausal women, and significant associations at only one bone site and not another call into question the importance of the acid-base hypothesis to bone health.
There is some evidence that calcium intake could play a part in determining whether the benefits of a high-protein diet are realized or if the resulting high-acid load is detrimental to bone. A recent randomized trial of a calcium and vitamin D supplements among older men and women found that protein intake was not associated with 3-y bone loss in the control group, whereas higher protein intake was associated with an increase in total body and femoral neck BMD in the treated group (32). This study suggests that with sufficient calcium intake, increased protein intake may be beneficial for bone. With insufficient calcium intake, the acid-base hypothesis would predict that a high dietary acid load due to high-protein intake would be detrimental for bone. We did not, however, observe significant interactions between dietary acid load and total calcium intake, indicating that a high dietary acid load may not be detrimental to bone health, even when calcium intake is low. This is in agreement with both Bonjour and Kerstetter (33, 34), who suggest that a diet high in acid-forming nutrients is unlikely to produce so severe a state of acidosis as to overwhelm the body’s first-line buffering mechanisms and require calcium mobilization from bone.
Estimates of dietary acid load in our Framingham Study participants are consistent with those previously reported from other population-based cohorts. Using data from NHANES III, Sebastian et al. (35) calculated the estimated NEAP of the average American diet as 48 mEq/d, which is similar to the Offspring cohort participants in our study (men: 47; women: 45), and slightly more acidic than the older Original cohort participants (men: 40; women: 39). The PRAL values for our study participants were also similar to those reported in population-based studies of dietary acid load and BMD that were conducted in the US and Europe (7–9, 11, 28). Furthermore, the protein intakes observed in our study represent a typical range commonly consumed by adult men and women in the population (36) as opposed to the more extreme protein intakes that may have been examined in laboratory settings. Thus, the mostly null associations between dietary acid load and BMD that we observed do not reflect the effects of high-protein intake but rather those intakes found in the population. Our primarily null findings could, perhaps, be explained by the potentially opposing effects of dietary protein on bone. Both estimated NEAP and PRAL use total protein intake as a surrogate for sulfur amino acid production, and it has been shown that intakes of total protein and animal protein are highly correlated with renal net acid excretion measured in urine (22). Yet these measures of acid load cannot separate the detrimental effects of acid production from the anabolic effects of protein. Thus, any negative association with acid load may be masked by a positive association with protein, and vice versa, resulting in an overall lack of association. Thorpe et al. (28) recently reported that in separate regression models, total protein and sulfur were not associated with lumbar spine BMD, but when included in the same model there was a positive association for protein and a negative association for sulfur. The investigators concluded that the effects of protein or sulfur are essentially nullified when the other is not accounted for, which could lead to erroneous null findings in studies of dietary acid load and bone that use estimated NEAP or PRAL, and thereby recommend that studies directly estimate intakes of protein and sulfur amino acids rather than depend on estimated NEAP and PRAL. Analyses in the Framingham cohorts along these lines are currently in progress.
Some limitations of our study are worth mentioning. The cross-sectional design of our study cannot address the potential causal relation between dietary acid load and BMD. Furthermore, measurements of estimated NEAP and PRAL at a single time point may not reflect the long-term status of dietary acid load, which may be important for studies of bone health, because it is chronic exposure to a high acid load that is likely to have effects on BMD. Additionally, estimated NEAP and PRAL are calculated based on algorithms using self-reported dietary intakes of only a small number of nutrients and may thus be limited in their accuracy for estimating dietary acid load. It has, however, been shown that these algorithms do provide reasonable estimates of NEAP and are more feasible than the optimal method of collecting 24-h urine samples, for use in large population-based cohorts that include older individuals (37). Finally, the generalizability of our results is limited to older and middle-aged Caucasian men and women. Our study also has some important strengths. To our knowledge, ours is the first investigation to assess the potential modifying effect of calcium intake on the relation between dietary acid load and BMD. Furthermore, The Framingham Study cohorts include large numbers of men, as well as substantial numbers of both middle-aged and older community-dwelling adults.
In conclusion, despite the observed association between estimated NEAP and femoral neck BMD among older men, this study suggests that increased dietary acid load is not associated with lower BMD among middle-aged men and women or older women, even when total calcium intake is low. Our results add to the evidence that challenges the importance of the acid-base hypothesis of osteoporosis and supports recommendations that adults receive adequate protein intake to promote bone health. However, because the observational studies that have examined dietary acid load and BMD, including our own, have been almost exclusively cross-sectional, we cannot rule out a causal relationship between dietary acid load and bone loss. Additional longitudinal studies are needed to further explore this potential modifiable risk factor for osteoporosis.
Acknowledgments
R.R.M., K.L.T., K.E.B., L.A.C., D.P.K., and M.T.H. designed research; N.Q. analyzed data; R.R.M., K.E.B., K.L.T., V.A.C., L.A.C., D.P.K., and M.T.H. wrote the paper; and R.R.M. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR 053205), the National Institute on Aging (AR/AG 041398), and the National Heart, Lung and Blood Institute’s Framingham Heart Study (N01-HC-25195). From the Framingham Heart Study of the National Heart Lung and Blood Institute of the NIH and Boston University School of Medicine.
Abbreviations used: BMD, bone mineral density; NEAP, net endogenous acid production; PRAL, potential renal acid load.
Literature Cited
- 1.Chrischilles EA, Butler CD, Davis CS, Wallace RB. A model of lifetime osteoporosis impact. Arch Intern Med. 1991;151:2026–32 [PubMed] [Google Scholar]
- 2.Leibson CL, Tosteson AN, Gabriel SE, Ransom JE, Melton LJ. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50:1644–50 [DOI] [PubMed] [Google Scholar]
- 3.Ray NF, Chan JK, Thamer M, Melton LJ III. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24–35 [DOI] [PubMed] [Google Scholar]
- 4.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–75 [DOI] [PubMed] [Google Scholar]
- 5.Tucker KL. Osteoporosis prevention and nutrition. Curr Osteoporos Rep. 2009;7:111–7 [DOI] [PubMed] [Google Scholar]
- 6.New SA, MacDonald HM, Campbell MK, Martin JC, Garton MJ, Robins SP, Reid DM. Lower estimates of net endogenous non-carbonic acid production are positively associated with indexes of bone health in premenopausal and perimenopausal women. Am J Clin Nutr. 2004;79:131–8 [DOI] [PubMed] [Google Scholar]
- 7.Macdonald HM, New SA, Fraser WD, Campbell MK, Reid DM. Low dietary potassium intakes and high dietary estimates of net endogenous acid production are associated with low bone mineral density in premenopausal women and increased markers of bone resorption in postmenopausal women. Am J Clin Nutr. 2005;81:923–33 [DOI] [PubMed] [Google Scholar]
- 8.Welch AA, Bingham SA, Reeve J, Khaw KT. More acidic dietary acid-base load is associated with reduced calcaneal broadband ultrasound attenuation in women but not in men: results from the EPIC-Norfolk cohort study. Am J Clin Nutr. 2007;85:1134–41 [DOI] [PubMed] [Google Scholar]
- 9.Wynn E, Lanham-New SA, Krieg MA, Whittamore DR, Burckhardt P. Low estimates of dietary acid load are positively associated with bone ultrasound in women older than 75 years of age with a lifetime fracture. J Nutr. 2008;138:1349–54 [DOI] [PubMed] [Google Scholar]
- 10.Rahbar A, Larijani B, Nabipour I, Mohamadi MM, Mirzaee K, Amiri Z. Relationship among dietary estimates of net endogenous acid production, bone mineral density and biochemical markers of bone turnover in an Iranian general population. Bone. 2009;45:876–81 [DOI] [PubMed] [Google Scholar]
- 11.Pedone C, Napoli N, Pozzilli P, Lauretani F, Bandinelli S, Ferrucci L, Antonelli-Incalzi R. Quality of diet and potential renal acid load as risk factors for reduced bone density in elderly women. Bone. 2010;46:1063–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schurch MA, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP. Protein supplements increase serum insulin-like growth factor-I levels and attenuate proximal femur bone loss in patients with recent hip fracture. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:801–9 [DOI] [PubMed] [Google Scholar]
- 13.Ammann P, Bourrin S, Bonjour JP, Meyer JM, Rizzoli R. Protein undernutrition-induced bone loss is associated with decreased IGF-I levels and estrogen deficiency. J Bone Miner Res. 2000;15:683–90 [DOI] [PubMed] [Google Scholar]
- 14.Canalis E, McCarthy TL, Centrella M. Growth factors and cytokines in bone cell metabolism. Annu Rev Med. 1991;42:17–24 [DOI] [PubMed] [Google Scholar]
- 15.Darling AL, Millward DJ, Torgerson DJ, Hewitt CE, Lanham-New SA. Dietary protein and bone health: a systematic review and meta-analysis. Am J Clin Nutr. 2009;90:1674–92 [DOI] [PubMed] [Google Scholar]
- 16.Barzel US, Massey LK. Excess dietary protein can adversely affect bone. J Nutr. 1998;128:1051–3 [DOI] [PubMed] [Google Scholar]
- 17.Dawson-Hughes B. Interaction of dietary calcium and protein in bone health in humans. J Nutr. 2003;133:S852–4 [DOI] [PubMed] [Google Scholar]
- 18.Dawber TR, Meadors GF, Moore FEJ. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health. 1951;41:279–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–90 [DOI] [PubMed] [Google Scholar]
- 20.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 21.Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:2504–12 [DOI] [PubMed] [Google Scholar]
- 22.Frassetto LA, Todd KM, Morris RC, Jr, Sebastian A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr. 1998;68:576–83 [DOI] [PubMed] [Google Scholar]
- 23.Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc. 1995;95:791–7 [DOI] [PubMed] [Google Scholar]
- 24.Kiel DP, Mercier CA, Dawson-Hughes B, Cali C, Hannan MT, Anderson JJ. The effects of analytic software and scan analysis technique on the comparison of dual X-ray absorptiometry with dual photon absorptiometry of the hip in the elderly. J Bone Miner Res. 1995;10:1130–6 [DOI] [PubMed] [Google Scholar]
- 25.Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study. Arch Intern Med. 1979;139:857–61 [PubMed] [Google Scholar]
- 26.Hannan MT, Felson DT, Anderson JJ, Naimark A. Habitual physical activity is not associated with knee osteoarthritis: the Framingham Study. J Rheumatol. 1993;20:704–9 [PubMed] [Google Scholar]
- 27.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–51 [DOI] [PubMed] [Google Scholar]
- 28.Thorpe M, Mojtahedi MC, Chapman-Novakofski K, McAuley E, Evans EM. A positive association of lumbar spine bone mineral density with dietary protein is suppressed by a negative association with protein sulfur. J Nutr. 2008;138:80–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27 [DOI] [PubMed] [Google Scholar]
- 30.Fenton TR, Eliasziw M, Lyon AW, Tough SC, Hanley DA. Meta-analysis of the quantity of calcium excretion associated with the net acid excretion of the modern diet under the acid-ash diet hypothesis. Am J Clin Nutr. 2008;88:1159–66 [DOI] [PubMed] [Google Scholar]
- 31.Fenton TR, Lyon AW, Eliasziw M, Tough SC, Hanley DA. Meta-analysis of the effect of the acid-ash hypothesis of osteoporosis on calcium balance. J Bone Miner Res. 2009;24:1835–40 [DOI] [PubMed] [Google Scholar]
- 32.Dawson-Hughes B, Harris SS. Calcium intake influences the association of protein intake with rates of bone loss in elderly men and women. Am J Clin Nutr. 2002;75:773–9 [DOI] [PubMed] [Google Scholar]
- 33.Bonjour JP. Dietary protein: an essential nutrient for bone health. J Am Coll Nutr. 2005;24:S526–36 [DOI] [PubMed] [Google Scholar]
- 34.Kerstetter JE. Dietary protein and bone: a new approach to an old question. Am J Clin Nutr. 2009;90:1451–2 [DOI] [PubMed] [Google Scholar]
- 35.Sebastian A, Frassetto LA, Sellmeyer DE, Merriam RL, Morris RC., Jr Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors. Am J Clin Nutr. 2002;76:1308–16 [DOI] [PubMed] [Google Scholar]
- 36.USDA, Agricultural Research Service Nutrient intakes from food: mean amounts consumed per individual, by gender and age. What We Eat in America, NHANES 2007–2008. 2010. [cited 2010 Dec]. Available from: http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/0708/tables_1–36_2007–2008.pdf
- 37.Frassetto LA, Lanham-New SA, Macdonald HM, Remer T, Sebastian A, Tucker KL, Tylavsky FA. Standardizing terminology for estimating the diet-dependent net acid load to the metabolic system. J Nutr. 2007;137:1491–2 [DOI] [PubMed] [Google Scholar]


