Abstract
Although methylenetetrahydrofolate reductase (MTHFR) genetic variants are associated with plasma homocysteine (Hcy) and cardiovascular disease (CVD), little is known whether dietary fatty acid intake modulates these associations. The goal was to examine the interaction of MTHFR variants with dietary fatty acids influencing plasma Hcy in 995 Boston Puerto Rican adults. We found that plasma Hcy concentration was negatively correlated with (n-3) PUFA intake (r = −0.117; P = 0.022), and the ratio of (n-3):(n-6) PUFA in the diet (r = −0.122; P = 0.009). Further, 2 functional MTHFR variants, 1298A>C and 677C>T, which are not in linkage disequilibrium in this population, were significantly associated with hypertension (OR = 1.72, P = 0.024, and OR = 1.60, P = 0.002, respectively). In addition, the 1298A>C variant was significantly associated with CVD (OR = 3.32; P = 0.030). Importantly, this variant exhibited significant interactions with intakes of total and (n-6) PUFA and the (n-3):(n-6) PUFA ratio of the diet. The plasma Hcy concentration of carriers of risk allele 1298C was greater than that of noncarriers only when participants had consumed a high-PUFA diet (>7.8% energy) but was not greater when they had low intake of PUFA (≤7.8% energy). In addition, participants with combined genotypes of both SNP (677 TT with 1298 AC or CC) who consumed high levels of (n-3) PUFA (>0.66% energy) had lower plasma Hcy compared with those who had the same genotype and consumed low levels of (n-3) PUFA (≤0.66% energy). Our study suggests that dietary PUFA intake modulates the effect of 2 MTHFR variants on plasma Hcy in Boston Puerto Rican adults.
Introduction
During the past several decades, hyperhomocysteinemia (HHcy)8 has been suggested as an important risk factor for cardiovascular diseases (CVD) (1–3). Moderately elevated plasma Hcy concentration tends to be seen in patients with coronary and peripheral vascular diseases compared with the general population (4–6). The major causes of HHcy include impairment of renal function, deficiencies of plasma folate, vitamin B-12, and vitamin B-6, and dietary and genetic factors (7, 8). Recently, we investigated the relationship between (n-3) PUFA and plasma Hcy in Chinese and Australian populations and provided evidence that increased (n-3) PUFA concentrations in plasma phospholipids and platelet phospholipids were associated with a protective effect on CVD and lower plasma Hcy concentrations (9, 10). Human intervention studies also demonstrated that dietary (n-3) PUFA can decrease plasma Hcy (11–13). To understand how fatty acids regulate Hcy metabolism, we conducted a feeding study in rats and found that tuna oil and salmon oil rich in (n-3) PUFA regulate both gene expression and enzyme activity of constituents of Hcy metabolism (14). However, the question remains how dietary PUFA intake regulates Hcy metabolism in humans. Methylenetetrahydrofolate reductase (MTHFR) catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, an important enzymatic process in folate metabolism and in remethylation of Hcy into methionine. In humans, 2 putative functional variants at MTHFR, 677C>T and 1298A>C, are known to be associated with HHcy (15, 16). The homozygous MTHFR 677TT genotype results in a thermolabile enzyme with reduced activity and consequentially decreased concentration of plasma folate and increased plasma Hcy concentration (17, 18). Similarly, 1298C also yields decreased MTHFR activity (19). MTHFR 1298CC genotypes are associated with increased risk of hypertension and higher Hcy in essential hypertensive participants (15). Although the determinants of Hcy and the relationship between MTHFR variants and plasma Hcy have been most extensively evaluated with B vitamins and folate, the interaction of fatty acids with MTHFR polymorphisms on plasma Hcy concentration remains inadequately described. Considering the relationship between (n-3) PUFA and the critical enzymes involved in Hcy metabolism, the goal of the present study was to test the hypothesis that fatty acid intake modulates the effects of MTHFR variants on Hcy metabolism.
The population of Puerto Rican adults living in the Boston, Massachusetts metropolitan area has a disproportionate health burden, including high prevalence of hypertension and CVD (20). Thus, we examined the association between MTHFR variants and hypertension and CVD and assessed the interaction between dietary fatty acids and MTHFR variants on plasma Hcy in this population.
Methods
Study design and participants.
The current study was conducted in a nested fashion within the ongoing Boston Puerto Rican Health Study(BPRHS), described in detail elsewhere (21). Briefly, areas of high Puerto Rican density in the Boston metropolitan area were identified from the year 2000 census and 1 Puerto Rican adult from households with at least 1 Puerto Rican between 45 and 75 y of age was randomly selected for participation. Interviews were conducted in the home and, in addition to a host of health-related and anthropometric data, detailed data were collected on dietary intake using a questionnaire previously adapted from the NCI/Block FFQ and validated for this population (22). Fasting blood samples were collected in the volunteer’s home the morning following the health interviews. Approval for the Boston Puerto Rican Health Study was obtained from the Institutional Review Board of the New England Medical Center and Tufts University Health Sciences and the current nested study was declared exempt from such requirements due to the use of de-identified samples and data (NIH exemption category 4).
Genetic analysis.
DNA was isolated from blood samples using QIAamp DNA Blood Mini kits according to the manufacturer’s instructions (QIAGEN). MTHFR 1298A>C (rs1801131) and MTHFR 677C>T (rs1801133) were genotyped by using TaqMan SNP genotyping kits with ABI PRISM 7900HT Sequence Detection system (Applied Biosystems) (23).
Measurement of anthropometric and plasma biochemical parameters.
Anthropometric variables, including height and weight, were measured by standard techniques. BMI was calculated as weight (kg)/height (m)2. Blood samples were collected by venipuncture from all participants while they were fasting. Plasma total Hcy was measured using HPLC with fluorescence detection as previously described (24). Plasma pyridoxal phosphate (PLP) was determined using the radioenzymatic method of Camp et al. (25). Plasma folate and vitamin B-12 were measured using Immulite Chemiluminescent kits according to the manufacturer’s instructions (Diagnostic Products /Siemens). Hypertension was identified as 1 of the following: 1) a positive response to the question “Have you ever been told by a physician that you had high blood pressure/hypertension?”; 2) reported use of blood pressure medication; or 3) high systolic (≥140 mmHg) or diastolic (≥90 mmHg) blood pressure. CVD was defined as a positive response to the question “Have you ever been told by a physician that you had heart disease” or to similar questions on heart attack or stroke or reported use of CVD medication. Smokers or drinkers were defined as a positive response to the question “Do you currently smoke/drink?” Thus, past smokers or drinkers were not considered as a smoker or drinker. Using the American Diabetes Association criteria (26), participants were classified as having type 2 diabetes when the fasting plasma glucose concentration was ≥126 mg/dL (≥7.0 mmol/L) or use of insulin or diabetes medication was reported. Physical activity was estimated as a physical activity score based on the Paffenbarger questionnaire (27).
Dietary assessment.
Dietary intake was assessed using a FFQ that was designed for and tested in this population (17). Dietary data were linked to the Minnesota Nutrient Data system (1999, version 25) for nutrient analysis. Fatty acid intakes were expressed as percentages of total energy intake and were included in analyses as both continuous and categorical variables. To construct categorical variables, intakes were classified into 2 groups according to the median intake of the population.
Population admixture.
Population admixture was calculated using STRUCTURE 2.2 based on 100 single nucleotide polymorphisms (SNP) selected as ancestry informative markers specifically for Puerto Rican populations (20, 28). Using the estimated admixture of each participant, we adjusted for population admixture for all genotype-associated analyses.
Statistical analyses.
Data analyses were performed using SPSS version 12 (SPSS) or SAS 9.1. All continuous dependent variables that were not normally distributed were Box-Cox transformed (29) prior to statistical analysis. Gender differences in demographic, anthropometric, and biochemical characteristics were examined using a t test. Correlations between dietary fatty acid compositions and plasma Hcy were estimated as a Pearson correlation coefficient after adjustment for potential confounding factors and exclusion of outliers for (n-3) PUFA (>1.35% energy) and (n-6) PUFA intake (>13.5% energy) using the simplest statistical outlier detection techniques (informal box plots), as described by Kentala et al. (30). Men and women were first examined separately for any gender effect. To ensure adequate statistical power, men and women were analyzed together when there was no gender-specific influence on phenotypes. Chi-square tests were conducted to examine whether genotype frequencies of the selected SNP were in Hardy-Weinberg equilibrium. The relationships among MTHFR genotypes, dietary intakes, and anthropometric measures were assessed using linear regression models. The interactions between dietary fatty acid intakes and genotypes were tested in a multivariate interaction model while controlling for potential confounders, including age, sex, population admixture, diabetes status, tobacco and alcohol use, dietary energy, and plasma folate, vitamin B-12, and PLP concentrations. The population medians for total SFA, MUFA, PUFA, and (n-3) and (n-6) PUFA intakes were used as cutoffs to dichotomize these variables. Differences between groups were considered significant at P ≤ 0.05.
Results
Clinical characteristics of populations and genetic variants at MTHFR.
Information about demographic, biochemical, dietary intake, and genotypic data are provided in Table 1. Men had significantly higher plasma Hcy and lower plasma folate than women. No gender differences were observed in dietary fatty acids or plasma PLP or vitamin B-12. Genotype frequencies did not deviate from Hardy-Weinberg equilibrium expectation. Allele frequencies of the minor alleles of MTHFR 1298A>C (rs1801131) and MTHFR 677C>T (rs1801133) were 0.358 and 0.242, respectively. Both SNP were independent of each other, i.e. not in linkage disequilibrium (r2 = 0.002; P = 0.95).
TABLE 1.
Demographic, anthropometric, biochemical, and genotype data in BPRHS participants1
| Characteristics | Men | Women |
| n | 292 | 702 |
| Age, y | 57.6 ± 7.6 | 57.8 ± 7.2 |
| BMI, 2 | 29.7 ± 5.1 | 33.1 ± 6.9* |
| Alcohol, g/d | 9.2 ± 3.4 | 1.5 ± 0.5* |
| Smoker, n (%) | 80 (31.1) | 126 (19.8)* |
| Drinker, n (%) | 132 (51.4) | 219 (34.5)* |
| Energy intake,2kcal/d | 2696 ± 1321 | 2175 ± 1115* |
| Total fat, % of energy | 31.9 ± 5.4 | 30.7 ± 5.2* |
| Total SFA, % of energy | 9.7 ± 2.5 | 9.3 ± 2.2 |
| Total MUFA, % of energy | 11.6 ± 2.1 | 11.2 ± 2.1 |
| Total PUFA, % of energy | 7.9 ± 1.7 | 7.6 ± 1.8 |
| (n-3) PUFA, % of energy | 0.68 ± 0.17 | 0.67 ± 0.16 |
| (n-6) PUFA, % of energy | 6.9 ± 1.7 | 7.2 ± 1.6 |
| Plasma folate,3ng/mL | 17.7 ± 8.7 | 20.1 ± 9.4* |
| Plasma vitamin B-12,4pg/mL | 526.6 ± 276.1 | 549.6 ± 284.3 |
| Plasma PLP, nmol/L | 61.4 ± 60.3 | 59.2 ± 63.3 |
| Plasma Hcy, μmol/L | 10.7 ± 6.2 | 8.8 ± 4.2* |
| Hypertension, n (%) | 153 (59.8) | 390 (61.4) |
| CVD, n (%) | 61 (23.8) | 132 (20.8) |
| MTHFR 1298 A>C, n (%) | ||
| AA | 120 (50.2) | 320 (54.9) |
| C carriers | 119 (49.8) | 262 (45.1) |
| MTHFR 677 C>T, n (%) | ||
| CC | 90 (37.8) | 271 (44.8) |
| T carriers | 148 (62.2) | 333 (55.2) |
Values are mean ± SD, or (%). *Different from men, P<0.01.
1 kcal = 4.184 kJ.
1 ng/mL = 2.3 nmol/L.
1 pg/mL = 0.74 pmol/L.
Correlations between dietary fatty acid compositions and plasma Hcy.
Plasma Hcy concentration was negatively correlated with (n-3) PUFA expressed as total energy intake (r = −0.12; P = 0.022), and with the ratio of (n-3):(n-6) PUFA (r = −0.12; P = 0.009), after adjustment for potential confounding factors (Supplemental Table 1). However, plasma Hcy was not correlated with the intakes of other fatty acids.
Association between MTHFR genotype and hypertension and CVD.
MTHFR 677C>T showed a significant association with hypertension (OR = 1.60 for TT vs. CC, P = 0.009; OR = 1.60 for TT+CT vs. CC, P = 0.002, respectively) (Table 2). Participants homozygous for the minor allele (TT) or carriers for T (TT+CT) had a 60% higher likelihood of hypertension than did homozygotes (CC), but this variant was not associated with CVD. The second variant, 1298A>C, was also associated with hypertension (OR = 1.72 for CC vs. AA; P = 0.024). In addition, 1298A>C variants displayed a significant association with CVD (OR = 3.32 for CC vs AA; P = 0.030). Minor allele (1298C) homozygotes were more than 3-fold as likely to have CVD than homozygotes for the major allele (AA).
TABLE 2.
Association between MTHFR variants and hypertension and CVD in BPRHS participants
| SNP name | Genotype | n1 | OR | Hypertension 95% Interval | P2 | OR | CVD 95% Interval | P2 |
| 677 C>T | TT vs CC | 137 vs 486 | 1.60 | 0.99–2.59 | 0.009 | 1.07 | 0.62–1.84 | 0.89 |
| TT vs CT | 137 vs 502 | 1.01 | 0.62–1.61 | 1.13 | 0.67–1.92 | |||
| TT+CT vs CC | 639 vs 486 | 1.60 | 1.19–2.15 | 0.002 | 1.03 | 0.73–1.45 | 0.86 | |
| 1298 A >C | CC vs AA | 66 vs 598 | 1.72 | 0.94–9.14 | 0.024 | 3.32 | 1.26–8.74 | 0.030 |
| CC vs AC | 66 vs 439 | 2.23 | 1.20–4.14 | 2.61 | 0.98–6.95 |
n = sample size.
P were calculated by logistic regression models and adjusted for sex, smoking, drinking, BMI, age, diabetes, population admixture, plasma folate, vitamin B-12, and PLP.
Association between 2 MTHFR variants and plasma Hcy.
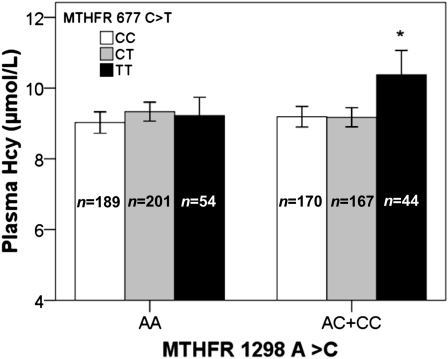
MTHFR 1298A>C was significantly associated with plasma Hcy (Table 3). Plasma Hcy concentrations were higher in participants homozygous for the 1298C risk allele compared with carriers of the 1298A allele (P = 0.011). In contrast, participants homozygous for 1298C had significantly lower plasma folate compared with carriers of 1298A. MTHFR 677C>T was marginally associated with plasma Hcy under a recessive model (i.e. CC+CT vs. TT). Homozygotes for the risk allele of 677T had higher plasma Hcy compared with carriers of 677C (P = 0.050; Table 3). In addition, we evaluated the combined effect of the 2 MTHFR 677C>T and 1298A>C polymorphisms on Hcy concentration. Participants homozygous for 677T, when carrying 1298C, had higher plasma Hcy concentrations than all other genotypes (Fig. 1).
TABLE 3.
Association between the MTHFR variants and plasma homocysteine and B vitamin group in BPRHS participants1
| Hcy and B vitamin group |
MTHFR 1298 A>C |
P-value |
MTHFR 677 C>T |
P-value |
||||||||
| AA, n = 498 | AC, n = 369 | CC, n = 53 | P2 | P3 | P4 | CC, n = 404 | CT, n = 424 | TT, n = 117 | P2 | P3 | P4 | |
| Hcy, μmol/L | 9.4 ± 5.5 | 9.2 ± 4.2 | 10.9 ± 4.8 | 0.011 | 0.003 | 0.62 | 9.1 ± 4.0 | 9.2 ± 3.7 | 9.7 ± 4.1 | 0.43 | 0.05 | 0.49 |
| Folate,5 ng/mL | 19.6 ± 0.4 | 19.3 ± 0.5 | 17.3 ± 1.3 | 0.84 | 0.029 | 0.39 | 19.7 ± 9.3 | 19.4 ± 9.0 | 19.1 ± 10.4 | 0.26 | 0.23 | 0.64 |
| Vitamin B-12,6pg/mL | 527.4 ± 12.2 | 557.5 ± 16.8 | 539.2 ± 39.9 | 0.06 | <0.001 | 0.15 | 537.3 ± 272.5 | 532.6 ± 275.5 | 605.6 ± 335.4 | 0.33 | 0.35 | 0.59 |
| PLP, nmol/L | 59.7 ± 3.1 | 59.0 ± 2.9 | 69.0 ± 11.6 | 0.65 | <0.001 | 0.90 | 57.6 ± 56.1 | 61.8 ± 71.4 | 60.5 ± 51.5 | 0.58 | <0.001 | 0.36 |
Values are mean ± SD. Plasma Hcy data were transformed before analysis.
P for additive model.
P for recessive model (MTHFR 1298A>C: AA+AC vs. CC; MTHFR 677 C>T: CC+CT vs. TT).
P for dominant model (MTHFR 1298A>C: AA vs. AC+CC; MTHFR 677 C>T: CC vs. CT+TT).
1 ng/mL = 2.3 nmol/L.
1 pg/mL = 0.74 pmol/L.
FIGURE 1.
The combined effect of MTHFR 677C>T and 1298A>C variants on plasma homocysteine. Mean plasma Hcy was estimated and plotted based on the combined genotypes of 2 SNP, MTHFR 677C>T and 1298A>C, after adjustment for potential confounders (age, sex, smoking, drinking, BMI, diabetes, population admixture, plasma folate, plasma vitamin B-12, plasma PLP, and total energy). The sample size of each genotype is given inside each bar. *Different from all other groups, P < 0.05. Values are adjusted means ± SEM.
Interaction of MTHFR variants with dietary fat intakes on plasma Hcy.
We observed an interaction between total PUFA consumption and MTHFR 1298A>C on plasma Hcy (P = 0.003) (Table 4). Plasma Hcy concentrations in participants who carried the 1298C allele were higher than those of noncarriers (P = 0.039) when they had high daily PUFA intakes (>7.8% energy) but were not significantly (P = 0.27) different when they had low daily PUFA intakes (≤7.8% energy).
TABLE 4.
Interaction between MTHFR variants and dietary fatty acids on plasma Hcy in BPRHS participants
| Dietary fatty acids | Total energy,1% |
MTHFR 1298 A>C |
MTHFR 677 C>T |
||||||
| AA (n) | AC+CC (n) | P-trend | P-interaction | CC (n) | CT+TT (n) | P-trend | P-interaction | ||
| Total PUFA | ≤7.8 | 9.5 ± 0.322 (26) | 9.2 ± 0.312 (92) | 0.273 | 0.0033 | 9.1 ± 0.312 (92) | 9.4 ± 0.222 (36) | 0.563 | 0.483 |
| >7.8 | 9.0 ± 0.32 (27) | 9.6 ± 0.31 (95) | 0.039 | 9.1 ± 0.31 (77) | 9.2 ± 0.32 (53) | 0.35 | |||
| Total MUFA | ≤11.4 | 9.5 ± 0.32 (25) | 9.2 ± 0.21 (94) | 0.15 | 0.38 | 9.0 ± 0.31 (87) | 9.5 ± 0.32 (38) | 0.53 | 0.72 |
| >11.4 | 8.8 ± 0.32 (24) | 9.2 ± 0.21 (90) | 0.29 | 9.4 ± 0.31 (80) | 9.1 ± 0.32 (50) | 0.48 | |||
| Total SFA | ≤9.3 | 9.6 ± 0.32 (26) | 9.5 ± 0.21 (96) | 0.84 | 0.71 | 9.3 ± 0.21 (87) | 9.5 ± 0.32 (39) | 0.31 | 0.68 |
| >9.3 | 8.9 ± 0.32 (25) | 9.0 ± 0.31 (94) | 0.64 | 8.9 ± 0.31 (82) | 9.2 ± 0.32 (49) | 0.76 | |||
| (n-6) PUFA | ≤7.1 | 9.3 ± 0.22 (24) | 9.1 ± 0.31 (95) | 0.21 | 0.005 | 8.9 ± 0.31 (86) | 9.4 ± 0.32 (39) | 0.16 | 0.23 |
| >7.1 | 9.1 ± 0.32 (27) | 9.6 ± 0.21 (92) | 0.032 | 9.3 ± 0.31 (80) | 9.4 ± 0.32 (50) | 0.78 | |||
| (n-3) PUFA | ≤0.66 | 9.5 ± 0.32 (22) | 9.4 ± 0.32 (09) | 0.11 | 0.67 | 9.3 ± 0.31 (95) | 9.3 ± 0.32 (44) | 0.89 | 0.76 |
| >0.66 | 9.4 ± 0.32 (29) | 9.3 ± 0.31 (83) | 0.21 | 8.8 ± 0.21 (74) | 9.3 ± 0.32 (44) | 0.031 | |||
| (n-3):(n-6) | ≤0.09 | 9.2 ± 0.32 (26) | 9.8 ± 0.21 (84) | 0.75 | 0.59 | 9.6 ± 0.31 (86) | 9.4 ± 0.32 (30) | 0.20 | 0.027 |
| >0.09 | 9.2 ± 0.32 (25) | 8.8 ± 0.32 (03) | 0.45 | 8.5 ± 0.31 (83) | 9.4 ± 0.32 (58) | 0.001 | |||
Dichotomized values for fatty acids were adjusted for the total energy.
Data in this column are mean ± SEM. Plasma Hcy data were transformed before analysis.
Data in this column are adjusted for age, sex, BMI, smoking, drinking, population admixture, diabetes, dietary energy, plasma folate, plasma vitamin B-12, and plasma PLP.
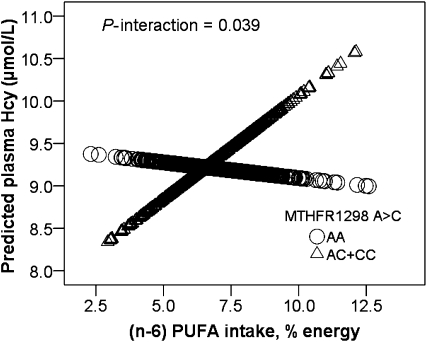
We next examined the interaction between PUFA and MTHFR genotype by splitting total PUFA into (n-3) and (n-6) PUFA. (n-6) PUFA normalized to total energy intake was dichotomized into high (>7.1% energy) and low (≤7.1% energy) daily (n-6) PUFA intakes based on the median intake of the population. After adjusting for potential confounders (including age, sex, tobacco smoking, alcohol drinking, population admixture, diabetes, dietary energy, plasma folate, plasma vitamin B-12, and plasma PLP), we observed that MTHFR 1298A>C displayed a significant interaction with dietary (n-6) PUFA, influencing the plasma Hcy concentration (P = 0.039) (Fig. 2). When daily intake of (n-6) PUFA was high (>7.1% energy), carriers of the 1298C allele had higher plasma Hcy than 1298AA homozygotes (P = 0.032), whereas under a low (≤7.1% energy) daily intake of (n-6) PUFA, the 1298C carriers did not differ in plasma Hcy concentrations from the AA homozygotes (P = 0.21) (Table 4).
FIGURE 2.
Interaction between MTHFR 1298A>C genotype and (n-6) PUFA intake on plasma homocysteine. MTHFR: Predicted values were calculated from regression models containing (n-6) PUFA intake, MTHFR 1298A>C genotype, their interaction terms, and potential confounders (age, sex, smoking, drinking, BMI, diabetes, population admixture, plasma folate, plasma vitamin B-12, plasma PLP, and total energy). The sample sizes of each genotype of MTHFR 1298A>C are as follows: n = 98 (AA), n = 22 (AC+CC). The P-interaction between MTHFR 1298A>C genotype and (n-6) PUFA intake (percent energy) as a continuous variable is 0.039. Values are adjusted means ± SEM.
Using the same statistical models, we also found an interaction between the dietary (n-3):(n-6) PUFA ratio and the MTHFR 677C>T SNP (P = 0.027). Plasma Hcy concentrations in participants who harbored the 677T allele were higher than those of noncarriers (P = 0.001) when the diet had a high (n-3):(n-6) ratio (>0.09% energy), whereas carriers and noncarriers did not differ when the (n-3):(n-6) consumption ratio was low (≤0.09% energy) (P = 0.20). We did not observe any significant interactions on Hcy between these 2 SNP and total MUFA, total SFA, and total fat intake (Table 4).
Analysis of combining the 2 MTHFR SNP identified a significant interaction with dietary (n-3) PUFA intake on plasma Hcy (P = 0.024) (Supplemental Fig. 1). Participants who carried 2 risk alleles (677TT + 1298AC or CC) and consumed high (n-3) PUFA (>0.66% energy) had significantly lower Hcy than those consuming low (n-3) PUFA (≤0.66% energy). No other significant interaction between individual SNP and (n-3) PUFA was observed.
Discussion
HHcy has been associated with CVD and is thus considered an important risk factor for this disease (1, 2, 31). The mechanism underlying this correlation, however, is not clearly understood. Although in the past 2 decades many studies have demonstrated the protective effects of fatty acids on CVD (32–34), correlations between plasma/platelet phospholipid fatty acids and Hcy in humans (12) and rats (14) have been reported. These studies suggested that fatty acids are central to Hcy metabolism (12, 13). Increased consumption of dietary (n-3) PUFA increases the concentration of (n-3) PUFA in plasma phospholipids. This is associated with a protective effect on CVD and lowers plasma Hcy concentrations (9). An increased ratio of (n-3):(n-6) PUFA in platelet phospholipids was also associated with decreased thrombotic risks, such as plasma Hcy, in middle-aged and geriatric hyperlipemia patients in Hangzhou, China (10). In the present study, we confirmed that plasma Hcy concentrations were negatively correlated with dietary (n-3) PUFA and with the ratio of (n-3):(n-6) PUFA. These correlations, however, remain weak even after removal of the outliers, a result perhaps of the confounding factors and the low (n-3) PUFA intake (<0.7% energy) in this population.
A possible mechanism underlying the correlation between PUFA and plasma Hcy concentration is taken from the observation that fatty acids modulate expression of a gene encoding an enzyme(s) involved in the metabolism of plasma Hcy (9). In animal studies, expression of Mthfr, encoding an enzyme central to Hcy metabolism, was modulated by (n-3) PUFA (14). In the present study and others (14, 17), MTHFR variants are associated with plasma Hcy concentrations. Furthermore, in this population, we also observed that carriers of MTHFR 677T or 1289C had an increased risk of hypertension and that 1289C was associated with an elevated risk of CVD. Importantly, we found that dietary PUFA consumption significantly interacted with MTHFR variants (677C>T and 1298A>C) on plasma Hcy. Persons carrying risk allele 1298C had higher plasma Hcy than the noncarriers (AA) only when consuming a high-PUFA diet (>7.8% energy) (Table 4) but did not when consuming low concentrations of PUFA (≤7.8% energy). Additionally, participants with combined genotypes of both SNP (677TT with 1298 AC or CC) who consumed higher (n-3) PUFA tended to exhibit low Hcy. Thus, combined with our previous results (14), this finding strengthens support for a regulatory role by PUFA on Hcy metabolism acting through MTHFR. Although (n-3) PUFA regulates expression of MTHFR in cell culture (data not shown), we observed only weak interactions between the 2 MTHFR variants and (n-3) PUFA intake. This may be the consequence of low (n-3) PUFA intake (0.7% energy) in this population. In the typical Western diet, consumption of (n-6) PUFA is ∼20- to 25-fold greater than that of (n-3) PUFA (35). Persons consuming a vegetarian diet only, with little saturated fat, have a low (n-3):(n-6) PUFA ratio in plasma and can still develop CVD as elderly persons (36). The predominance of (n-6) PUFA in the typical diet results from the abundance of linoleic acid [18:2(n-6)], which is high in soy, corn, safflower, and sunflower oils. In contrast, there is lower intake of the (n-3) homolog of linoleic acid, α-linolenic acid [18:3(n-3)], which is present in leafy green vegetables and in flaxseed and canola oils (37). The indiscriminate recommendation to substitute (n-6) PUFA for saturated fats to lower serum cholesterol concentrations could also contribute to excessive intake of (n-6) PUFA in the current Western diet (38). High (n-6) PUFA, or a low ratio of (n-3):(n-6) PUFA intake, may increase plasma Hcy concentrations in carriers of the 1298C or 677T allele, and, importantly, this may contribute to increased risk for hypertension and CVD in these participants.
MTHFR 677C>T has been shown to be functional in that the 677T allele shows reduced MTHFR enzyme activity (39). This is also the most common genetic variant associated with HHcy (40, 41). In this population, however, we observed only a weak association between 677C>T and Hcy concentration and an association with CVD that did not reach significance (P = 0.86). This may be due to the high plasma folate concentrations in this population, which consumes a diet high in rice fortified with folic acid. The MTHFR 1298C allele decreased MTHFR activity and increased plasma Hcy (15, 39, 42). We have confirmed this association in CVD patients, observing that MTHFR 1298C increased the risk of hypertension and CVD. Inconsistent association between MTHFR variants and CVD, however, was observed in other populations (43). The discrepancy of such observations could result from differences in LD patterns at the MTHFR locus and dissimilar dietary structure in diverse populations. Such factors may affect the interaction between MTHFR genotype and dietary PUFA intake on Hcy. The LD between 677C>T and 1298A>C is strong (r2 = 0.19; D’=0.91) in the HapMap European population (http://hapmap.ncbi.nlm.nih.gov/). In marked contrast, this Puerto Rican population shows that these 2 variants are genetically independent. In addition, substantial evidence establishes that this population is genetically different from European populations. For example, using 100 ancestry informative markers, we have estimated the ancestry of this population on average to be 57.2% European, 27.4% African, and 15.4% Native American (16). This could be 1 important factor that contributes to the inconsistencies between populations. Other population characteristics, such as dietary or nondietary environmental factors, and a small sample size are all likely to also contribute. Indeed, the observation of a strong interaction between the 2 variants analyzed here and PUFA intake could be another important factor contributing to the discrepancy between different study populations.
In summary, dietary fatty acid intake modulates the effect of MTHFR genotypes on plasma Hcy in Boston Puerto Ricans. MTHFR 677T increases the risk of hypertension and MTHFR 1298C increases the risk of both hypertension and CVD in Boston Puerto Rican adults.
Supplementary Material
Acknowledgments
C-Q.L., D.L., and T.H. designed research; T.H., Y-C.L., and J.W.C. conducted research; T.H. and C-Q.L. analyzed data; T.H., C.E.S., L.D.P., D.L., and C-Q.L. wrote the paper; and T.H. and C-Q.L. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by the China Scholarship Council, the NIH, National Institute on Aging grant no. 5P01AG023394-02, NIH/National Heart, Lung and Blood Institute grant nos. HL54776 and HL078885, contracts 53-K06–5-10 and 58–1950-9–001 from the USDA Research Service and the National Natural Science Foundation of China (no. 30972464).
Supplemental Table 1 and Figure 1 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: BPRHS, Boston Puerto Rican Health Study; CVD, cardiovascular disease; Hcy, homocysteine; HHcy, hyperhomocysteinemia; LD, linkage disequilibrium; PLP, pyridoxal phosphate; SNP, single nucleotide polymorphism.
Literature Cited
- 1.McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–28 [PMC free article] [PubMed] [Google Scholar]
- 2.Huang T, Yuan G, Zhang Z, Zou Z, Li D. Cardiovascular pathogenesis in hyperhomocysteinemia. Asia Pac J Clin Nutr. 2008;17:8–16 [PubMed] [Google Scholar]
- 3.Refsum H, Nurk E, Smith AD, Ueland PM, Gjesdal CG, Bjelland I, Tverdal A, Tell GS, Nygard O, et al. The Hordaland Homocysteine Study: a community-based study of homocysteine, its determinants, and associations with disease. J Nutr. 2006;136:S1731–40 [DOI] [PubMed] [Google Scholar]
- 4.Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324:1149–55 [DOI] [PubMed] [Google Scholar]
- 5.Harker LA, Slichter SJ, Scott CR, Ross R. Homocystinemia. Vascular injury and arterial thrombosis. N Engl J Med. 1974;291:537–43 [DOI] [PubMed] [Google Scholar]
- 6.Brude IR, Finstad HS, Seljeflot I, Drevon CA, Solvoll K, Sandstad B, Hjermann I, Arnesen H, Nenseter MS. Plasma homocysteine concentration related to diet, endothelial function and mononuclear cell gene expression among male hyperlipidaemic smokers. Eur J Clin Invest. 1999;29:100–8 [DOI] [PubMed] [Google Scholar]
- 7.Yeh YY, Yeh SM. Homocysteine-lowering action is another potential cardiovascular protective factor of aged garlic extract. J Nutr. 2006;136:S745–9 [DOI] [PubMed] [Google Scholar]
- 8.McMahon JA, Skeaff CM, Williams SM, Green TJ. Lowering homocysteine with B vitamins has no effect on blood pressure in older adults. J Nutr. 2007;137:1183–7 [DOI] [PubMed] [Google Scholar]
- 9.Li D, Mann NJ, Sinclair AJ. A significant inverse relationship between concentrations of plasma homocysteine and phospholipid docosahexaenoic acid in healthy male subjects. Lipids. 2006;41:85–9 [DOI] [PubMed] [Google Scholar]
- 10.Li D, Yu XM, Xie HB, Zhang YH, Wang Q, Zhou XQ, Yu P, Wang LJ. Platelet phospholipid n-3 PUFA negatively associated with plasma homocysteine in middle-aged and geriatric hyperlipaemia patients. Prostaglandins Leukot Essent Fatty Acids. 2007;76:293–7 [DOI] [PubMed] [Google Scholar]
- 11.Piolot A, Blache D, Boulet L, Fortin LJ, Dubreuil D, Marcoux C, Davignon J, Lussier-Cacan S. Effect of fish oil on LDL oxidation and plasma homocysteine concentrations in health. J Lab Clin Med. 2003;141:41–9 [DOI] [PubMed] [Google Scholar]
- 12.Zeman M, Zak A, Vecka M, Tvrzicka E, Pisarikova A, Stankova B. N-3 fatty acid supplementation decreases plasma homocysteine in diabetic dyslipidemia treated with statin-fibrate combination. J Nutr Biochem. 2006;17:379–84 [DOI] [PubMed] [Google Scholar]
- 13.Pooya S, Jalali MD, Jazayery AD, Saedisomeolia A, Eshraghian MR, Toorang F. The efficacy of omega-3 fatty acid supplementation on plasma homocysteine and malondialdehyde levels of type 2 diabetic patients. Nutr Metab Cardiovasc Dis. 2010;20:326–31 [DOI] [PubMed] [Google Scholar]
- 14.Huang T, Wahlqvist ML, Li D. Docosahexaenoic acid decreases plasma homocysteine via regulating enzyme activity and mRNA expression involved in methionine metabolism. Nutrition. 2010;26:112–9 [DOI] [PubMed] [Google Scholar]
- 15.Markan S, Sachdeva M, Sehrawat BS, Kumari S, Jain S, Khullar M. MTHFR 677 CT/MTHFR 1298 CC genotypes are associated with increased risk of hypertension in Indians. Mol Cell Biochem. 2007;302:125–31 [DOI] [PubMed] [Google Scholar]
- 16.Lim U, Peng K, Shane B, Stover PJ, Litonjua AA, Weiss ST, Gaziano JM, Strawderman RL, Raiszadeh F, et al. Polymorphisms in cytoplasmic serine hydroxymethyltransferase and methylenetetrahydrofolate reductase affect the risk of cardiovascular disease in men. J Nutr. 2005;135:1989–94 [DOI] [PubMed] [Google Scholar]
- 17.Zetterberg H, Zafiropoulos A, Spandidos DA, Rymo L, Blennow K. Gene-gene interaction between fetal MTHFR 677C>T and transcobalamin 776C>G polymorphisms in human spontaneous abortion. Hum Reprod. 2003;18:1948–50 [DOI] [PubMed] [Google Scholar]
- 18.Fodinger M, Buchmayer H, Heinz G, Papagiannopoulos M, Kletzmayr J, Perschl A, Vychytil A, Horl WH, Sunder-Plassmann G. Association of two MTHFR polymorphisms with total homocysteine plasma levels in dialysis patients. Am J Kidney Dis. 2001;38:77–84 [DOI] [PubMed] [Google Scholar]
- 19.van Rooij IA, Vermeij-Keers C, Kluijtmans LA, Ocke MC, Zielhuis GA, Goorhuis-Brouwer SM, van der Biezen JJ, Kuijpers-Jagtman AM, Steegers-Theunissen RP. Does the interaction between maternal folate intake and the methylenetetrahydrofolate reductase polymorphisms affect the risk of cleft lip with or without cleft palate? Am J Epidemiol. 2003;157:583–91 [DOI] [PubMed] [Google Scholar]
- 20.Lai CQ, Tucker KL, Choudhry S, Parnell LD, Mattei J, Garcia-Bailo B, Beckman K, Burchard EG, Ordovas JM. Population admixture associated with disease prevalence in the Boston Puerto Rican health study. Hum Genet. 2009;125:199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tucker KL. Stress and nutrition in relation to excess development of chronic disease in Puerto Rican adults living in the Northeastern USA. J Med Invest. 2005;52 Suppl:252–8 [DOI] [PubMed] [Google Scholar]
- 22.Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol. 1998;148:507–18 [DOI] [PubMed] [Google Scholar]
- 23.Lai CQ, Tucker KL, Parnell LD, Adiconis X, Garcia-Bailo B, Griffith J, Meydani M, Ordovas JM. PPARGC1A variation associated with DNA damage, diabetes, and cardiovascular diseases: the Boston Puerto Rican Health Study. Diabetes. 2008;57:809–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr A. 1987;422:43–52 [DOI] [PubMed] [Google Scholar]
- 25.Camp VM, Chipponi J, Faraj BA. Radioenzymatic assay for direct measurement of plasma pyridoxal 5'- phosphate. Clin Chem. 1983;29:642–4 [PubMed] [Google Scholar]
- 26.American Diabetes Association Standards of medical care in diabetes. Diabetes Care. 2007;28 Suppl:4–36 [Google Scholar]
- 27.Lee IM, Paffenbarger RS., Jr Physical activity and stroke incidence: the Harvard Alumni Health Study. Stroke. 1998;29:2049–54 [DOI] [PubMed] [Google Scholar]
- 28.Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Box GEP, Cox DR. An analysis of transformations. J Roy Stat Soc. 1964; Series B:41 [Google Scholar]
- 30.Kentala E, Viikki K, Laurikkala J, Juhola M. Impact and management of confounding values and outliers in a neurotologic expert system. Ann N Y Acad Sci. 2001;942:472. [DOI] [PubMed] [Google Scholar]
- 31.Dangour AD, Breeze E, Clarke R, Shetty PS, Uauy R, Fletcher AE. Plasma homocysteine, but not folate or vitamin B-12, predicts mortality in older people in the United Kingdom. J Nutr. 2008;138:1121–8 [DOI] [PubMed] [Google Scholar]
- 32.Calder PC. n-3 Fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin Sci (Lond). 2004;107:1–11 [DOI] [PubMed] [Google Scholar]
- 33.Dewailly E, Blanchet C, Lemieux S, Sauve L, Gingras S, Ayotte P, Holub BJ. n-3 Fatty acids and cardiovascular disease risk factors among the Inuit of Nunavik. Am J Clin Nutr. 2001;74:464–73 [DOI] [PubMed] [Google Scholar]
- 34.Kinsella JE, Lokesh B, Stone RA. Dietary n-3 polyunsaturated fatty acids and amelioration of cardiovascular disease: possible mechanisms. Am J Clin Nutr. 1990;52:1–28 [DOI] [PubMed] [Google Scholar]
- 35.Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–63 [DOI] [PubMed] [Google Scholar]
- 36.Li D, Sinclair A, Wilson A, Nakkote S, Kelly F, Abedin L, Mann N, Turner A. Effect of dietary alpha-linolenic acid on thrombotic risk factors in vegetarian men. Am J Clin Nutr. 1999;69:872–82 [DOI] [PubMed] [Google Scholar]
- 37.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:S343–8 [DOI] [PubMed] [Google Scholar]
- 38.Simopoulos AP. Essential fatty acids in health and chronic disease. Am J Clin Nutr. 1999;70:S560–9 [DOI] [PubMed] [Google Scholar]
- 39.van der Put NM, Gabreels F, Stevens EM, Smeitink JA, Trijbels FJ, Eskes TK, van den Heuvel LP, Blom HJ. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet. 1998;62:1044–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–3 [DOI] [PubMed] [Google Scholar]
- 41.Weisberg I, Tran P, Christensen B, Sibani S, Rozen R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. 1998;64:169–72 [DOI] [PubMed] [Google Scholar]
- 42.Kumar J, Das SK, Sharma P, Karthikeyan G, Ramakrishnan L, Sengupta S. Homocysteine levels are associated with MTHFR A1298C polymorphism in Indian population. J Hum Genet. 2005;50:655–63 [DOI] [PubMed] [Google Scholar]
- 43.Ye H, Yan JT, Shao JM, Zhang F, Hong ML, Wang DW. [A case-control study on the relationship between stroke and plasma homocysteine level and the mutation of MTHFR gene.] Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:958–61 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.