Abstract
High vitamin A (VA) intakes have been correlated with increased risk of bone fracture. Over 50% of the U.S. adult population reports use of dietary supplements, which can result in VA intakes > 200% of the RDA. In this study, 2 experiments were designed to determine the effect of dietary VA on cortical and trabecular bone properties and resistance to ex vivo fracture. In Expt. 1, we investigated whether orally administered VA accumulates in bone. Seven-week-old rats were treated daily with VA (6 mg/d for 14 d). Total retinol increased in both the tibia and femur (P < 0.01). In Expt. 2, we conducted a longitudinal study in which rats were fed 1 of 3 levels of dietary VA (marginal, adequate, and supplemented, equal to 0.35, 4, and 50 μg retinol/g diet, respectively) from weaning until the ages of 2–3 mo (young), 8–10 mo (middle-age), and 18–20 mo (old). Tibial trabecular and cortical bone structure, bone mineral density, and resistance to fracture were measured using micro-computed tomography and material testing system analysis, respectively. The VA-marginal diet affected measures of cortical bone dimension, suggesting bone remodeling was altered. VA supplementation increased medullary area and decreased cortical thickness in young rats (P < 0.05), but these changes were not present during aging. VA supplementation did not affect resistance to fracture or bone mineral content in old rats. From these results, we conclude that VA-marginal status affects trabecular bone more than cortical bone, and VA supplementation at a moderate level over the lifetime is unlikely to increase the risk of age-related bone fracture in rats.
Introduction
Vitamin A (VA)6 is a fat-soluble dietary component required for vision, cellular differentiation, proliferation, normal growth, and bone development (1,2). The principal biologically active metabolite of VA, retinoic acid, acts through retinoid-specific receptors to modulate gene expression (1). Both VA deficiency and toxicity (hypervitaminosis A) result in skeletal abnormalities. Spontaneous fractures are often noted as a well known sign of hypervitaminosis A (3). However, hypervitaminosis A has most often been studied in the context of short-term animal studies using high doses of VA or case studies of humans who inadvertently ingested very high doses of VA, generally over a short period of time. The consequences of marginal intakes, or, conversely, of moderately high but nontoxic intakes of VA over the lifetime are not understood.
For humans, the RDA for adult males and females is 900 and 700 μg retinol activity equivalents (RAE)/d, respectively, and the tolerable upper intake level is 3000 μg/d of preformed retinol (4). According to data from the 1999–2000 NHANES, 52% of U.S adults report using a dietary supplement (5). Epidemiological studies report total dietary retinol intakes as high as 8771 μg/d RAE (6) and mean intakes of 1250 μg/d RAE are common (7–12).
As a fat-soluble vitamin, VA is absorbed and transported with TG and cholesterol in chylomicrons. After chylomicron TG undergo lipolysis in capillaries, chylomicron remnants then bind, primarily, to the hepatic apoprotein receptor, LDL receptor-related protein, LRP (2,12), and deliver most of their VA to the liver. However, bone marrow osteoblasts express LRP5 and have been reported to be the second largest clearance site for chylomicron remnants (13–16). Studies in rabbits using 3H-retinol as a tracer showed that a portion of newly absorbed chylomicron VA resided, at least transiently, in bone marrow in the postprandial period (13–16). Other studies have indicated uptake of chylomicron lipids by osteoblasts (15). Thus, the hypothesis that dietary VA accumulates in bone is plausible, and if VA weakens bone, it could lead over time to reduced bone strength.
Many nutrients affect the integrity of bone (17). Information in the literature is mixed regarding the response of bone to alterations in dietary VA. Studies of both self-reported high VA intake and experimental dosing have reported a variety of outcomes ranging from no interaction, a detrimental interaction, to a beneficial result (6–9,16,18–30). Elevated VA achieved through the use of supplements might be associated with negative consequences related to the risk of bone fracture and decreases in mineralization, although this too is controversial (10,20,25). Animal studies on VA and bone have typically been conducted over a short time period and have investigated only the period of rapid growth and/or have used pharmacologic doses of VA or synthetic retinoids known to be more toxic than VA (3,31,32). In the present study, we conducted 2 experiments to determine, first, whether VA accumulates in long bones after an acute exposure to high-dose VA and second, whether a range of intake of dietary VA, when consumed over the lifetime, affects the properties of cortical and trabecular bone and the bone’s ability to resist fracture. For the latter objectives, we used the tibia of rats that had been fed VA-marginal, -adequate, or -supplemented diets from weaning until the ages of 2–3, 8–10, or 18–20 mo (33,34), thus allowing an assessment of the effects of diet on each measured variable at 3 times that are representative of the periods of bone growth (young rats), maintenance (middle-aged rats), and age-related bone loss (old rats), respectively.
Materials and Methods
Rats and diets.
Approval for animal studies was obtained from the Institutional Animal Use and Care Committee of the Pennsylvania State University. Animals were housed under a normal 12-h-light/-dark cycle in a climate controlled environment and had free access to food and water. In Expt. 1 (acute treatment), young male and female Sprague-Dawley rats obtained through mating within our facility were used to determine whole bone VA (measured as total retinol) content. In preparation, rat dams were fed a VA-deficient diet based on the AIN-93 Growth diet, prepared by Research Diets; this diet has been shown to reduce VA storage in nursing pups (35,36). After weaning at 25 d of age, the young rats continued to receive the same diet, while they were randomly assigned to also receive either a daily oral supplement of all-trans retinyl palmitate [n = 5; 6 mg of retinol as retinyl palmitate (Sigma-Aldrich) dispersed in 100 μL canola oil daily] for 14 d or the same volume of oil alone as a control (n = 4). After treatment for 14 d, the rats were euthanized by carbon dioxide asphyxiation.
In Expt. 2 we evaluated the effects of VA status over the life span, from young to middle age to old age, on bone properties. This study utilized the tibia of male Lewis rats that had been obtained in a previously reported study (33,34). Briefly, rat pups were weaned as described above and randomly assigned in a 3 × 3 design to an AIN-93 purified diet, modified to contain 1 of 3 levels of VA (33,34,37): 0.35 (VA-marginal), 4.0 (VA-adequate level, designated as control), or 50 mg (VA-supplemented) retinol equivalents/kg diet. Diets were prepared by Dyets, Inc. and the VA was added as retinyl palmitate. The diets were continuously fed until the rats were 2–3, 8–10, or 18–20 mo old.
At the time of euthanasia and bone collection, the right tibia from each rat was removed and cleaned of excess tissue while submersed in ice-cold PBS. After thoroughly cleaning, they were stored individually at −20°C in sealed Whirl-pak containers until they were analyzed in the present Expt. 2. The number of rats for which tibiae (one tibia per rat) were available for analysis equaled, for young rats, n = 6, 9, 6; for middle-aged rats, 7, 6, 9; and for old rats, 8, 7, 6 for the VA-marginal, control, and VA-supplemented diets, respectively. Body weight was determined for the rats used in these analyses. For some analyses, e.g., to test breaking strength, a few bones that had broken were not suitable for analysis. The final n per assay is included in each figure.
Retinoid analysis.
Whole bone total VA was determined as total retinol after extraction of the right tibia and femur from each VA-treated rat in Expt. 1. The wet weight was determined, followed by Folch extraction of total lipids (38). The washed Folch extract was evaporated under argon, saponified, and analyzed for total retinol as previously described (39). Plasma from the same rats was similarly analyzed. The extracts were dried and then reconstituted in methanol for analysis of total retinol content by reverse-phase HPLC at a wavelength of 325 nm (39).
Bone dimensional property measurements.
In Expt. 2, bone dimensions, trabecular and cortical measurements, and mechanical testing were determined on tibia that had been stored as described above. Samples were rehydrated by complete submersion in 10 mL of sterile PBS at 4°C overnight. Preliminary studies using freshly collected tibias from VA-adequate rats were conducted to ensure that results were similar for the stored bones. For caliper measurements, the operator was unaware of each sample’s treatment group to prevent bias. Caliper (Traceable Digital Calipers, VWR) measurements were taken for total length, epiphyseal, saggital, and coronal widths to detect changes in bone size or shape.
Micro-computed tomography (μCT) was used to reconstruct trabecular structure and measure periosteal and endosteal radii, cortical thickness, bone mineral density (BMD), number of trabeculae, trabeculae thickness, and bone fraction. The proximal epiphysis was scanned using a desktop μCT system (μCT 40; Scanco Medical) at 15-μm voxel resolution with medium energy level (55 kVp and 145 μA) and 200-ms integration time.
A 5-mm section at the proximal metaphysis of the right tibia was scanned for histomorphological analyses by using the IPL scripts (Scanco Medical). An additional section was scanned for mid-diaphyseal shaft cross-sectional analysis using a purpose-written MATLAB program (version 6.5; MathWorks), as described previously (40). A group of 4 bone samples of similar length was scanned in a custom-built 30-mm cartridge made of poly methyl methacrylate to scan the same region of the bones.
Material testing system analysis.
After scanning, tibias were stored in PBS for no longer than 48 h at 4°C. Tibial 3-point flexural bending tests were conducted using a material testing apparatus (MiniBionix 858; MTS) with a 16-mm support span attached to a 22.7-g (50-lb) load cell (40), and crosshead speed was set at 2 mm/min. Bones were consistently oriented with the load applied on the posterior side at the midpoint of the midshaft, producing compression on the posterior surface and tension on the anterior surface. Load-displacement curve was obtained to determine force and displacement at yield and ultimate points of each tibia. These data along with the cross-sectional data at the midshaft were used to determine material properties. Stress (σ) and flexural modulus (E) were calculated by using ε = FLc/4I and E = FL3/48dI, respectively (40,41), where (F) was yield/ultimate loads in Newtons, (L) was the length of the support span in millimeters, (c) was the distance from the centroid to the periosteal tensile surface in millimeters, (I) was cross-sectional area moment of inertia about the medilateral axis of the tibia in millimeters, and (d) was the displacement at the yield point in millimeters.
Ashing.
After breaking, bones were collected for analysis. The samples were weighed to the 5th decimal place under standardized conditions, dried in a vacuum oven at 200°C for 24 h, cooled, reweighed to determine dry weight, and then ashed in a muffle furnace at 800°C for 24 h (40). Calculations were made according to the following formulas: water (%) = 100 (wet weight – dry weight)/wet weight; organic (%) = 100 (dry weight – ash weight)/wet weight; ash (%) = 100 (ash weight)/wet weight; tissue mineral (%) = 100 (ash weight)/dry weight.
Statistical analysis.
Data are presented as the mean ± SEM unless otherwise noted. Results in Expt. 1 were analyzed by 1-way ANOVA. Results in Expt. 2 were analyzed by 2-way ANOVA and P-values for the main effects of diet and age and their interaction are reported. The effect of the level of dietary VA within each age group was further analyzed by least squares means test (SuperAnova). Values of P < 0.05 were considered significant.
Results
Plasma and bone retinoid content after acute VA treatment in young rats.
In Expt. 1, plasma total retinol was significantly elevated in rats treated orally with VA for 14 d compared with oil-treated controls (Fig. 1A).
FIGURE 1.
Plasma retinol (A) and long-bone total retinol (B) content in tibia and femur of VA- and placebo (oil) treated rats. Values are means ± SEM, n = 4 (control) or 5 (VA treated) per bone. Means without a common letter differ, P < 0.05.
In the same rats, bone VA was significantly increased in both tibia and femur (Fig. 1B). The concentration was 25% higher in the tibia than in the femur of VA-treated rats. Short-term treatment with VA did not affect body or bone weights. Thus, Expt. 1 showed that ingestion of supplementary VA can increase the retinol content of bone and supported the use of the tibia for analysis of the effects of VA on bone properties.
Dietary VA intake and body weight during aging.
In Expt. 2, we analyzed various bone variables in the tibia of male Lewis rats that had been fed VA-marginal, control, or -supplemented diets until the ages of 2–3, 8–10, or 18–20-mo, respectively. Age was a main factor (P < 0.0001; Table 1), as expected for a model of growth and aging (33,34), whereas diet had no effect on body weight until old age, when VA-marginal and -supplemented rats differed from each other, but neither differed from the control group.
TABLE 1.
Body weight and bone dimensions in tibia of young, middle-aged, and old rats fed diets differing in VA concentration for 2–3, 8–10, or 18–20 mo12
| Age | Diet | n | Body weight, g | Medullary area, 2 | Endosteal radius,3mm | Periosteal radius,3mm | Cortical thickness,3mm |
| Young | Mar | 6 | 378 ± 6.5d | 2.59 ± 0.14d,e | 0.89 ± 0.03d,e | 1.50 ± 0.02e | 0.49 ± 0.01c |
| Con | 9 | 389 ± 5.4d | 1.54 ± 0.08f | 0.69 ± 0.02f | 1.38 ± 0.01f | 0.58 ± 0.02a | |
| Sup | 6 | 425 ± 43.7d | 2.33 ± 0.11e | 0.85 ± 0.02e | 1.45 ± 0.02e | 0.49 ± 0.01c | |
| Middle | Mar | 7 | 584 ± 10.2c | 3.14 ± 0.16d | 0.94 ± 0.02c,d | 1.74 ± 0.01b,c | 0.60 ± 0.01a |
| Con | 8 | 612 ± 18.0c | 2.69 ± 0.11d,e | 0.94 ± 0.02c,d | 1.70 ± 0.02c,d | 0.61 ± 0.01a | |
| Sup | 9 | 641 ± 14.7c | 2.48 ± 0.07e | 0.89 ± 0.01d,e | 1.65 ± 0.01d | 0.61 ± 0.01a | |
| Old | Mar | 8 | 792 ± 25.8b | 5.02 ± 0.18a | 1.25 ± 0.03a | 1.92 ± 0.03a | 0.52 ± 0.01b,c |
| Con | 7 | 846 ± 30.5a,b | 4.34 ± 0.25b | 1.14 ± 0.04b | 1.81 ± 0.03b | 0.53 ± 0.01b,c | |
| Sup | 6 | 901 ± 16.7a | 3.74 ± 0.16c | 1.09 ± 0.03b,c | 1.74 ± 0.03c | 0.55 ± 0.02b | |
| Two-way ANOVA P-values | |||||||
| Diet | 0.011 | 0.0001 | 0.0001 | 0.0001 | 0.004 | ||
| Age | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | ||
| Interaction | 0.52 | 0.0003 | 0.0003 | 0.013 | 0.0007 | ||
Values are means ± SEM. Means in a column without a common letter differ, P < 0.05.
Mar, marginal; Con, control; Sup, supplemented diet.
Illustrations of measurements can be found in Supplemental Figure 2.
Mid-diaphyseal bone medullary area, cortical thickness, and endosteal and periosteal radii.
The mid-diaphyseal medullary area of the tibia, cortical thickness, endosteal radii, and periosteal radii were determined as indicators of bone growth and shape change (Table 1). Age was a factor for each variable (P < 0.0001). The medullary area of the tibia was smaller in VA-supplemented compared with VA-marginal rats (middle age and old) and control rats (old group only; P < 0.05) (Table 1). Age but not diet was a significant factor for tibial length and epiphyseal, coronal, and saggital width (Supplemental Fig. 1).
To determine whether the change in medullary area was due to alterations in the endosteal (inner) or periosteal (outer) bone surfaces, the radius from the centroid to each of the locations above was determined using MATLAB software (Table 1; schematic of measurements in Supplemental Fig. 2). There was an age-related increase in endosteal radius in old compared with young and middle-age rats (P < 0.05). Endosteal radius was larger in VA-marginal than supplemented rats (middle-age and old) (P < 0.05) (Table 1). To ensure that these differences were not compounded with a change in bone shape, the periosteal radius was also measured (Table 1). By middle and old age, periosteal radius was greater in VA-marginal compared with supplemented rats (P < 0.05), similar to medullary area and endosteal radius. Thus, bone dimensions tended to change in proportion to one another during aging, with VA-marginal > control > supplemented for old rats for medullary area and periosteal radius, and VA-marginal > control supplemented rats for endosteal radius.
Because there was a difference in endosteal and/or periosteal radii with diet during aging, we also determined mid-diaphyseal cortical thickness. Mid-diaphyseal cortical bone thickness differed with age (P < 0.0001), being greatest in middle-age rats. Differences due to diet were observed between the young control group and both the VA-marginal and VA-supplemented groups (P < 0.05) but not in other age groups.
Trabeculi number, thickness, and BMD.
Trabecular number decreased with age, as expected (Table 2). The tibia of old VA-supplemented rats contained more trabeculae per area compared with old VA-marginal rats (P < 0.05). Conversely, trabecular thickness increased with age (Table 2). Trabeculae were thicker in middle-aged and old VA-marginal rats compared with control and VA-supplemented rats of the same ages (P < 0.05). Trabecular BMD, determined by μCT, also increased with age and showed small but significant differences with intake of VA, similar to trabecular thickness (Table 2).
TABLE 2.
Trabecular measurements in tibia of young, middle-aged, and old rats fed diets differing in VA concentration for 2–3, 8–10 or 18–20 mo12
| Age | Diet | n | Trabeculae, 2 | Trabeculae thickness, nm | Trabecular BMD,3mg mineral/mm3 |
| Young | Mar | 6 | 3.7 ± 0.02a | 74 ± 2c | 657 ± 7d |
| Con | 9 | 3.4 ± 0.11b | 71 ± 3c | 666 ± 7d | |
| Sup | 6 | 3.6 ± 0.25a,b | 69 ± 4c | 664 ± 10d | |
| Middle | Mar | 5 | 2.1 ± 0.05c | 147 ± 1a | 838 ± 4b |
| Con | 6 | 2.4 ± 0.13c | 129 ± 2b | 807 ± 4c | |
| Sup | 7 | 2.5 ± 0.12c | 130 ± 4b | 813 ± 8c | |
| Old | Mar | 8 | 1.3 ± 0.05e | 144 ± 5a | 887 ± 5a |
| Con | 7 | 1.4 ± 0.07d,e | 119 ± 7b | 845 ± 10b | |
| Sup | 6 | 1.8 ± 0.20c,d | 131 ± 6b | 849 ± 5b | |
| Two-way ANOVA P-values | |||||
| Diet | 0.043 | 0.0001 | 0.0007 | ||
| Age | 0.0001 | 0.0001 | 0.0001 | ||
| Interaction | 0.032 | 0.05 | 0.004 | ||
Values are means ± SEM. Means in a column without a common letter differ, P < 0.05.
Mar, marginal; Con, control; Sup, supplemented diet.
Illustrations from representative samples can be found in Supplemental Figure 4.
Resistance of bone to mechanical loading.
The posterior surface of the tibia was loaded using 3-point bending analysis until failure to determine stiffness, stress, load at yield point, and ultimate load (Supplemental Fig. 3). Neither age nor dietary VA affected the yield point, ultimate load, or stress of bone when mechanically loaded (Supplemental Table 1). Stiffness was lower in middle-age VA-supplemented compared with VA-marginal rats (P < 0.05) but did not differ with VA in old rats.
Whole bone mineral content.
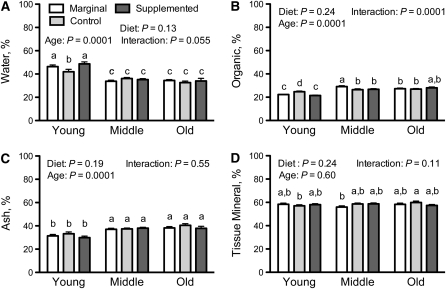
Bones were weighed and ashed to determine the percentages of water, organic matrix, and ash, and the percentage of mineralized tissue was calculated (Fig. 2). Water percentage (Fig. 2A) was higher in young rats and slightly lower in the young VA-control group compared with VA-marginal and -supplemented groups (P < 0.05). Conversely, organic material was slightly higher in the young VA-control group (Fig. 2B). Neither ash (Fig. 2C) nor mineralized tissue (Fig. 2D) differed by age or dietary VA.
FIGURE 2.
Bone composition of tibia of young, middle-aged, and old rats fed diets differing in VA concentration for 2–3, 8–10, or 18–20 mo. (A) Percent water, (B) percent organic, (C) percent mineral or ash based on initial wet weight of the bone, and (D) percent mineralized tissue based on dry weight of bone. Values are means ± SEM; for young rats, n = 6, 9, 6; for middle-aged rats, n = 7, 6, 9; and for old rats, n = 8, 7, 6, for VA-marginal, control, and -supplemented diets, respectively. Results for 2-way ANOVA are shown for each panel. Means without a common letter differ, P < 0.05.
Discussion
There is essentially no information on VA dose-dependent changes on bone variables over the lifetime. In the present study, we evaluated tibia from rats that had been fed 3 graded doses of VA, which were chosen to resemble the range of VA intakes of most humans. Our conditions thus ranged from VA-marginal to VA-supplemented, but we avoided both frank VA deficiency and toxicity (33,34,42,43). To our knowledge, this was a unique study design and it provided an opportunity to assess various parameters of bone quality, including bone dimensions over time as animals grew and aged, bone mineral properties, bone strength as measured ex vivo, and bone composition. First, to be sure that dietary VA reaches bone, we conducted a short-term experiment with a high daily dose of VA. The results (Fig. 1) demonstrated that VA does travel to both the femur and tibia and that bone VA increases when VA intake is elevated. The tibia was a more active site of VA accumulation than the femur, with higher retinol concentration per bone weight, which is a reasonable finding, because it has been shown that the tibia has a higher luminal adipose content than the femur (44) and retinol is known to accumulate in adipose tissue. This study did not identify cell types containing VA, but other studies have shown that chylomicron lipids, including fat-soluble vitamins, are taken up by osteoblasts (15,45) and multiple cells in bone could have contributed to the accumulation of VA. These results also supported the use of the tibia for bone analyses in our long-term study (Expt. 2).
In Expt. 2, by μCT analysis, some aspects of bone dimensions were altered by dietary VA in young rats (Table 1). Differences in VA intake (both marginal and supplemented) resulted in a reduction in cortical bone thickness and an increase in lumen size in young rats (Table 1). These results are consistent with current literature. It is noteworthy that young rats have been used in most previous studies on VA and bone (28). Although it may seem surprising that both VA-marginal and VA-supplemented young rats differed in a similar way from the VA-adequate group, there are other situations, e.g. fetal development, where similar outcomes have resulted from both VA deficiency and VA excess (46). Therefore, our VA-marginal and -supplemented diets may have resulted in too little and too much VA, respectively, for optimal bone formation during the phase of rapid growth. However, the long-term picture obtained by studying middle-age and old rats provides a new and unique look at the in vivo interaction of VA and bone. Despite the differences observed over a short period in young rats, there was no difference between the VA-supplemented compared with the control group for endosteal or periosteal radii, or cortical thickness, in middle-age and old rats (Table 1). Thus, with time the remodeling of the bone appears to have recovered in the VA-supplemented rats. On the other hand, the radial dimensions measured remained higher in VA-marginal compared with VA-supplemented rats. VA is known to be an important mediating factor in proper bone development (1,2). Overall, these results suggest that the tibia was somewhat wider with a larger cavity in young VA-marginal rats, which could imply that VA was limiting with respect to bone osteoid formation and mineralization early in life. However, these effects seem to have been temporary, because age-dependent but not diet-dependent differences in cortical thickness were observed in middle-age and old rats (Table 1). Although there was no lasting effect of VA-marginal diet on cortical thickness, the overall bone shape as evaluated by endosteal and periosteal radii exhibited modest changes in middle-age rats that were lasting and significant by old age (Table 1). Both VA-marginal and VA-supplemented rats had mid-diaphyseal measurements that differed from those of control rats of the same age. These results indicate a difference in the shape of VA-supplemented rat bones by the time they reach middle age, which did not appear to be detrimental to the quality of the bone, because, as discussed below, all diet groups had similar responses to mechanical testing and similar mineralization.
The trabecular properties we measured changed with age and VA. The number and thickness of trabeculae as well as trabecular BMD showed significant differences in the VA-marginal group (Table 1). These results were consistent in both the middle-age and old groups and the variance in these measurements was quite small. These results suggest that further studies of the effect of VA and its metabolites, such as retinoic acid, on trabecular formation and maintenance would be of interest. An increase in the number of trabeculae in old VA-supplemented rats (Table 1), was not expected. However, this increase in trabeculae might be explained by the differences in weight between the VA-marginal and VA-supplemented rats, or other unknown mechanisms, because the increase was normalized when trabeculae number was adjusted for body weight. As seen in representative μCT reconstructions (Supplemental Fig. 4), long-term VA supplementation appears to have exerted a protective effect on trabecular bone loss associated with aging, while marginal VA status resulted in increased trabecular thickness and a slight increase in trabecular BMD. This suggests the potential for a biphasic response of bone to VA treatment, as has been suggested from the results of human epidemiological studies (6–9,11,16,18–30). Overall, our data suggest that dietary VA more dramatically affects trabecular bone than cortical bone in the tibia of rats. Although we were able to study only the tibia, other bone sites would also be of interest.
In our long-term study, multiple bone variables were evaluated, including mechanical and material properties testing of bone tissue and determination of bone composition. Diet did not affect the mechanical properties of the tibia tested by the 3-point bending test (Table 2) or bone composition as shown in Figure 2. These results imply that VA supplementation, at levels in excess of requirements but not high enough to produce hypervitaminosis A (33,34,42) did not have detrimental effects on bone loading capacity or strength. The larger variation within treatment groups for the materials testing analyses (Table 2) combined with the smaller sample size due to fewer bones being suitable for this analysis are limitations for our materials testing analyses. Although the expected outcome that VA supplementation would cause alterations in the mechanical properties of bone was not observed, the observance of no difference of VA-supplemented rats compared with control rats is also a potentially important point, because there is currently controversy regarding VA intakes above the RDA in human populations at risk for fracture.
Although we have illustrated our data without correction for body weight, we also analyzed our results after correcting for body weight, because weight can affect mechanical factors such as increased loading associated with changes to bone tissue, and increased weight can encourage mineralization and alter bone microarchitecture (47–49). The effects we reported for VA were similar both without and after normalization for body weight. Thus, the data presented reflect diet-dependent effects that are not due to age-related changes in body mass.
In summary, our study has revealed the potential for a VA-marginal diet to affect bone formation and architecture, especially of trabecular bone. Conversely, we did not observe differences in mechanical properties due to elevated dietary VA throughout the life time. The latter is regarded as an important finding, because VA has been regarded as a potential risk factor for reduced BMD and increased fracture risk in older humans. Our data show that higher intakes of VA, similar to those that have been proposed to reduce BMD and increase fracture risk in epidemiological studies (5–11,20), may not sufficiently alter bone properties related to strength to be detected in breaking strength assays. Our results more closely resemble the observational study reported by Sowers and Wallace (19), which investigated the relationship among current VA supplement use, serum retinol levels, radial bone mass, and fracture history in 246 postmenopausal women, 55–80 y of age. Although >36% of these women used a VA supplement, with 8% in excess of 2000 μg/d retinol, after controlling for relevant factors there was no significant relationship between VA supplement use or serum retinol and radial bone mass or fractures. In short-term studies, Kawahara et al. (50) found no relationship between VA supplementation and bone turnover in men, and a recent large case-control study of patients treated with retinoid analogs that are in use for therapy did not observe increased risk of any hip, forearm, or spine fractures (51). Thus, the long-held notion that elevated dietary intake of VA is detrimental to bones requires further assessment. Animal models provide an ethical way to assess bone health with intakes of VA, which resemble those below and above levels currently recommended for human populations. Further investigations are needed to better understand VA signaling in bone tissue that affects bone modeling, microarchitecture, and mechanical properties across the lifetime, including growth, maintenance, and aging.
Supplementary Material
Acknowledgments
We thank Dr. Neil A. Sharkey and Dr. Dena Lang for their helpful advice and for making available facilities in the Biomechanics Laboratory, Department of Kinesiology. A.E.W. and A.C.R. designed the research; A.E.W. and N.O. conducted the research and analyzed the data; A.E.W. and A.C.R. wrote the paper; and A.C.R. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Supported in part by NIH AG-09839 (A.C.R.), the Graduate Program in Nutrition, Pennsylvania State University, and funds from the Howard Heinz Endowment.
Supplemental Table 1 and Supplemental Figures 1–4 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: BMD, bone mineral density; µCT, micro-computed tomography; RAE, retinol activity equivalent; VA, vitamin A.
Literature Cited
- 1.Evans TR, Kaye SB. Retinoids: present role and future potential. Br J Cancer. 1999;80:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross AC, Harrison EH. Vitamin A. Zempleni J, Rucker RR, McCormick DM, Suttie JW, Handbook of vitamins. 4th ed. Boca Raton (FL): CRC Press; 2007. p. 1–39 [Google Scholar]
- 3.Subcommittee on Vitamin Tolerance, Committee on Animal Nutrition, Council NR Vitamin tolerance of animals. Washington, DC: National Academy Press; 1987 [Google Scholar]
- 4.Genaro Pde S, Martini LA. Vitamin A supplementation and risk of skeletal fracture. Nutr Rev. 2004;62:65–7 [DOI] [PubMed] [Google Scholar]
- 5.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol. 2004;160:339–49 [DOI] [PubMed] [Google Scholar]
- 6.Lim LS, Harnack LJ, Lazovich D, Folsom AR. Vitamin A intake and the risk of hip fracture in postmenopausal women: the Iowa Women's Health Study. Osteoporos Int. 2004;15:552–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sowers MR, Wallace RB, Lemke JH. Correlates of mid-radius bone density among postmenopausal women: a community study. Am J Clin Nutr. 1985;41:1045–53 [DOI] [PubMed] [Google Scholar]
- 8.Yano K, Heilbrun LK, Wasnich RD, Hankin JH, Vogel JM. The relationship between diet and bone mineral content of multiple skeletal sites in elderly Japanese-American men and women living in Hawaii. Am J Clin Nutr. 1985;42:877–88 [DOI] [PubMed] [Google Scholar]
- 9.Michaelsson K, Lithell H, Vessby B, Melhus H. Serum retinol levels and the risk of fracture. N Engl J Med. 2003;348:287–94 [DOI] [PubMed] [Google Scholar]
- 10.Promislow JH, Goodman-Gruen D, Slymen DJ, Barrett-Connor E. Protein consumption and bone mineral density in the elderly: the Rancho Bernardo Study. Am J Epidemiol. 2002;155:636–44 [DOI] [PubMed] [Google Scholar]
- 11.Penniston KL, Weng N, Binkley N, Tanumihardjo SA. Serum retinyl esters are not elevated in postmenopausal women with and without osteoporosis whose preformed vitamin A intakes are high. Am J Clin Nutr. 2006;84:1350–6 [DOI] [PubMed] [Google Scholar]
- 12.Ross AC. Vitamin A and carotenoids. Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, Modern nutrition in health and disease. 10th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. p. 351–75 [Google Scholar]
- 13.Hussain MM, Mahley RW, Boyles JK, Fainaru M, Brecht WJ, Lindquist PA. Chylomicron-chylomicron remnant clearance by liver and bone marrow in rabbits. Factors that modify tissue-specific uptake. J Biol Chem. 1989;264:9571–82 [PubMed] [Google Scholar]
- 14.Hussain MM, Mahley RW, Boyles JK, Lindquist PA, Brecht WJ, Innerarity TL. Chylomicron metabolism. Chylomicron uptake by bone marrow in different animal species. J Biol Chem. 1989;264:17931–8 [PubMed] [Google Scholar]
- 15.Niemeier A, Niedzielska D, Secer R, Schilling A, Merkel M, Enrich C, Rensen PC, Heeren J. Uptake of postprandial lipoproteins into bone in vivo: impact on osteoblast function. Bone. 2008;43:230–7 [DOI] [PubMed] [Google Scholar]
- 16.Twining SS, Schulte DP, Wilson PM, Fish BL, Moulder JE. Retinol is sequestered in the bone marrow of vitamin A-deficient rats. J Nutr. 1996;126:1618–26 [DOI] [PubMed] [Google Scholar]
- 17.Morgan SL. Nutrition and bone: it is more than calcium and vitamin D. Womens Health (Lond Engl). 2009;5:727–37 [DOI] [PubMed] [Google Scholar]
- 18.Freudenheim JL, Johnson NE, Smith EL. Relationships between usual nutrient intake and bone-mineral content of women 35–65 years of age: longitudinal and cross-sectional analysis. Am J Clin Nutr. 1986;44:863–76 [DOI] [PubMed] [Google Scholar]
- 19.Sowers MF, Wallace RB. Retinol, supplemental vitamin A and bone status. J Clin Epidemiol. 1990;43:693–9 [DOI] [PubMed] [Google Scholar]
- 20.Houtkooper LB, Ritenbaugh C, Aickin M, Lohman TG, Going SB, Weber JL, Greaves KA, Boyden TW, Pamenter RW, Hall MC. Nutrients, body composition and exercise are related to change in bone mineral density in premenopausal women. J Nutr. 1995;125:1229–37 [DOI] [PubMed] [Google Scholar]
- 21.Melhus H, Michaelsson K, Kindmark A, Bergstrom R, Holmberg L, Mallmin H, Wolk A, Ljunghall S. Excessive dietary intake of vitamin A is associated with reduced bone mineral density and increased risk for hip fracture. Ann Intern Med. 1998;129:770–8 [DOI] [PubMed] [Google Scholar]
- 22.Johansson S, Lind PM, Hakansson H, Oxlund H, Orberg J, Melhus H. Subclinical hypervitaminosis A causes fragile bones in rats. Bone. 2002;31:685–9 [DOI] [PubMed] [Google Scholar]
- 23.Kaptoge S, Welch A, McTaggart A, Mulligan A, Dalzell N, Day NE, Bingham S, Khaw KT, Reeve J. Effects of dietary nutrients and food groups on bone loss from the proximal femur in men and women in the 7th and 8th decades of age. Osteoporos Int. 2003;14:418–28 [DOI] [PubMed] [Google Scholar]
- 24.Michaelsson K, Melhus H, Bellocco R, Wolk A. Dietary calcium and vitamin D intake in relation to osteoporotic fracture risk. Bone. 2003;32:694–703 [DOI] [PubMed] [Google Scholar]
- 25.Macdonald HM, New SA, Golden MH, Campbell MK, Reid DM. Nutritional associations with bone loss during the menopausal transition: evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. Am J Clin Nutr. 2004;79:155–65 [DOI] [PubMed] [Google Scholar]
- 26.Kneissel M, Studer A, Cortesi R, Susa M. Retinoid-induced bone thinning is caused by subperiosteal osteoclast activity in adult rodents. Bone. 2005;36:202–14 [DOI] [PubMed] [Google Scholar]
- 27.Barker ME, McCloskey E, Saha S, Gossiel F, Charlesworth D, Powers HJ, Blumsohn A. Serum retinoids and beta-carotene as predictors of hip and other fractures in elderly women. J Bone Miner Res. 2005;20:913–20 [DOI] [PubMed] [Google Scholar]
- 28.Lind PM, Johansson S, Ronn M, Melhus H. Subclinical hypervitaminosis A in rat: measurements of bone mineral density (BMD) do not reveal adverse skeletal changes. Chem Biol Interact. 2006;159:73–80 [DOI] [PubMed] [Google Scholar]
- 29.Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr. 2006;83:191–201 [DOI] [PubMed] [Google Scholar]
- 30.Caire-Juvera G, Ritenbaugh C, Wactawski-Wende J, Snetselaar LG, Chen Z. Vitamin A and retinol intakes and the risk of fractures among participants of the Women's Health Initiative Observational Study. Am J Clin Nutr. 2009;89:323–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cashin CH, Lewis EJ. Evaluation of hypervitaminosis A in the rat by measurement of tibial bone breaking strain. J Pharmacol Methods. 1984;11:91–5 [DOI] [PubMed] [Google Scholar]
- 32.Subcommittee on Laboratory Animal Nutrition, Committee on Animal Nutrition Board on Agriculture, National Research Council Nutrient requirements of laboratory animals. Nutrient requirements of laboratory animals. 4th rev ed. Washington, DC: National Academy Press; 1995 [Google Scholar]
- 33.Dawson HD, Li NQ, DeCicco KL, Nibert JA, Ross AC. Chronic marginal vitamin A status reduces natural killer cell number and function in aging Lewis rats. J Nutr. 1999;129:1510–7 [DOI] [PubMed] [Google Scholar]
- 34.Dawson HD, Ross AC. Chronic marginal vitamin A status affects the distribution and function of T cells and natural T cells in aging Lewis rats. J Nutr. 1999;129:1782–90 [DOI] [PubMed] [Google Scholar]
- 35.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51 PubMed [DOI] [PubMed] [Google Scholar]
- 36.Reeves PG, Rossow KL, Lindlauf J. Development and testing of the AIN-93 purified diets for rodents: results on growth, kidney calcification and bone mineralization in rats and mice. J Nutr. 1993;123:1923–31 [DOI] [PubMed] [Google Scholar]
- 37.Ross AC. Diet in vitamin A research. Methods Mol Biol. 2010;652:295–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509 [PubMed] [Google Scholar]
- 39.Ross AC. Separation and quantitation of retinyl esters and retinol by high-performance liquid chromatography. Methods Enzymol. 1986;123:68–74 [DOI] [PubMed] [Google Scholar]
- 40.Shahnazari M, Lang DH, Fosmire GJ, Sharkey NA, Mitchell AD, Leach RM. Strontium administration in young chickens improves bone volume and architecture but does not enhance bone structural and material strength. Calcif Tissue Int. 2007;80:160–6 [DOI] [PubMed] [Google Scholar]
- 41.Martin RB, Burr DB, Sharkey NA. Mechanical properties of bone. Skeletal tissue mechanics. New York: Springer; 1998. p. 127–80 [Google Scholar]
- 42.Dawson HD, Yamamoto Y, Zolfaghari R, Rosales FJ, Dietz J, Shimada T, Li N, Ross AC. Regulation of hepatic vitamin A storage in a rat model of controlled vitamin A status during aging. J Nutr. 2000;130:1280–6 [DOI] [PubMed] [Google Scholar]
- 43.Zolfaghari R, Ross AC. Chronic vitamin A intake affects the expression of mRNA for apolipoprotein A-I, but not for nuclear retinoid receptors, in liver of young and aging Lewis rats. Arch Biochem Biophys. 1995;323:258–64 [DOI] [PubMed] [Google Scholar]
- 44.Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98:251–66 [DOI] [PubMed] [Google Scholar]
- 45.Niemeier A, Kassem M, Toedter K, Wendt D, Ruether W, Beisiegel U, Heeren J. Expression of LRP1 by human osteoblasts: a mechanism for the delivery of lipoproteins and vitamin K1 to bone. J Bone Miner Res. 2005;20:283–93 [DOI] [PubMed] [Google Scholar]
- 46.Clagett-Dame M, DeLuca HF. The role of vitamin A in mammalian reproduction and embryonic development. Annu Rev Nutr. 2002;22:347–81 [DOI] [PubMed] [Google Scholar]
- 47.Frost HM. The Utah paradigm of skeletal physiology: an overview of its insights for bone, cartilage and collagenous tissue organs. J Bone Miner Metab. 2000;18:305–16 [DOI] [PubMed] [Google Scholar]
- 48.Frost HM. From Wolff's law to the Utah paradigm: insights about bone physiology and its clinical applications. Anat Rec. 2001;262:398–419 [DOI] [PubMed] [Google Scholar]
- 49.Frost HM. Bone's mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol. 2003;275:1081–101 [DOI] [PubMed] [Google Scholar]
- 50.Kawahara TN, Krueger DC, Engelke JA, Harke JM, Binkley NC. Short-term vitamin A supplementation does not affect bone turnover in men. J Nutr. 2002;132:1169–72 [DOI] [PubMed] [Google Scholar]
- 51.Vestergaard P, Rejnmark L, Mosekilde L. High-dose treatment with vitamin A analogues and risk of fractures. Arch Dermatol. 2010;146:478–82 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.