Abstract
Increasing obesity in low- and middle-income countries is well documented in cross-sectional studies. However, few longitudinal studies identify factors that influence individual weight gain patterns over time in relation to the major social and economic changes that now characterize these settings. This study uses data from adult Filipino women participating in the Cebu Longitudinal Health and Nutrition Survey from 1983 to 2005. A sample of 3005 women contributed 1–8 observations each. Longitudinal mixed effects models identified how age and secular weight trends related to underlying effects of urbanization and changing household socioeconomic status (SES) and to proximate individual effects of reproductive history, diet, and occupational physical activity. The 23-y secular trend in weight amounted to nearly 10 kg. Younger women gained more weight than older women (12.4 kg in those < 20 y old in 1983 vs. 4.9 kg in those > 35 y). Periods of more rapid weight gain corresponded to periods of rapid increase in SES and urbanization. Weight was positively related to energy intake, percentage of calories from protein, and more sedentary occupations, but negatively related to months pregnant and lactating and postmenopausal status. These effects all varied with age and over time. The trends contributed to a 6-fold increase in prevalence of overweight and an increasing number of women who have or are likely to develop obesity-related metabolic diseases. The trends are highly relevant for health policy and preventive health measures in the Philippines and other countries now facing the dual burden of over- and undernutrition.
Introduction
Obesity and cardiovascular disease (CVD)6 are now major health problems in low-income as well as middle- and high-income nations. In many developing countries, overweight is more prevalent than underweight (1) and CVD surpasses infectious diseases as the top cause of mortality (2, 3). Worldwide, the most rapid increases in obesity and obesity-related noncommunicable diseases are occurring in Asian populations (4). Increasing overweight in Asians is a particular concern in light of evidence that CVD risk is elevated at a lower BMI among Asians (5, 6). At the same BMI, Asians tend to have a higher percent body fat and more central adiposity (7).
Although obesity trends are well documented in cross-sectional studies, few longitudinal studies have tracked adults over long periods of time in populations undergoing rapid social and economic changes. Having limited longitudinal data hinders identification of individual, household, and community level influences on weight gain and development of obesity in populations experiencing the rapid changes associated with the nutrition transition (8). In addition, longitudinal data across different age groups are needed to inform our understanding of age and secular trends in weight gain and obesity risk. Of particular interest is whether the more recent social, economic, and environmental changes that characterize low- and middle-income countries have different effects in younger and older individuals.
As part of the Cebu Longitudinal Health and Nutrition Survey (CLHNS) (9), we have followed, since 1983–1984, a cohort of women who reside in Metropolitan Cebu in the central Philippines. Metro Cebu, with a population nearing 2 million, shares many similarities with other large cities in developing and transitional countries of Asia. It is one of the fastest growing and rapidly developing regions of the country and has particular relevance for understanding obesity trends. Our prior work with this study documented a >6-fold increase in the prevalence of overweight in the sample of adult childbearing women between 1983 and 2002 (10). Mean real household income nearly doubled among cohort participants from 1983 to 1995, accompanied by related changes in diet and physical activity patterns. At the time of their recruitment into the study, CLHNS participants ranged in age from 15 to 45 y, making this sample well suited for the study of age and secular trends in weight and obesity status. Our objectives were to explore age and secular trends in women’s weight and determine how weight is influenced by biological, behavioral, economic, and environmental factors over time.
Materials and Methods
Study site and sample
Metro Cebu is ecologically diverse, including communities in densely populated urban and peri-urban areas, rural towns, and more isolated mountain and island rural areas. A single-stage cluster sampling procedure was used to randomly select 17 urban and 16 rural Metro Cebu barangays (administrative units that form communities), which included ~28,000 households. Surveys in 1982–1983 located all pregnant women, and those who gave birth from May 1, 1983 to April 30, 1984 were included in the sample. A baseline interview was conducted among 3327 women during pregnancy. Subsequent surveys took place immediately after birth, then every 2 mo for 24 mo. Because the original survey was designed to focus on offspring, only women with singleton, live births were followed in the early postpartum surveys. Subsequently, attempts were made to locate and interview all respondents from the baseline survey who still resided in Metro Cebu. Full follow-up surveys were conducted in 1991, 1994–1995, 1998–1999, 2002, and 2005. From the first 2 y of bimonthly data, we selected 3 time points (4, 12, and 24 mo postpartum) for inclusion in longitudinal models. To maximize sample size, if a woman was missing or pregnant at one of these early surveys, we substituted data from the prior survey (2 mo earlier if she was not pregnant at that time). Such substitutions were made in ~2% of cases. The CLHNS has been reviewed and approved by the University of North Carolina Institutional Review Board, Office of Human Research Ethics.
Data and analysis variables
CLHNS data were collected during in-home interviews. Data relevant to the current analysis are described below.
Age.
Age at the baseline survey was used to define age groups for analysis (<20, 20 to <25, 25 to <30, 30 to <35, and ≥35 y). Age and age-squared were included in longitudinal models to account for the nonlinear association of weight with age.
Anthropometry.
Weight was measured at each survey on portable scales and participants wore light clothing. The WHO definitions of overweight (BMI > 25 kg/m2) and obesity (BMI > 30 kg/m2) were applied.
Diet.
Diet was assessed by 24-h dietary recall except in 1991, when a quantitative FFQ was administered. Energy and nutrient intakes were calculated from the Philippines Food Composition Tables produced by the Food and Nutrition Research Institute of the Philippines. We examined the effects of total energy intake and percentage of energy from each macronutrient.
Occupational physical activity.
Women accounted for usual activities from waking until going to sleep at night, providing a description of each activity and time spent engaged in the activity. This included information about occupational activity, domestic work, and leisure. For this analysis, we focused on occupational activity, because a high percentage of women reported working, most moderate to vigorous physical activity is performed at work, and leisure time activity is uniformly sedentary in this population (11). Each occupation was categorized according to the level of physical demand, and energy expenditure values were assigned for specific occupations common among Filipino women based on field studies conducted by Tuazon et al. (12) supplemented with data from the compendium of physical activity (13). A categorical variable represents, for each survey, the activity level of the woman’s occupation, ranging from sedentary [1.44 metabolic equivalent (METS), including jobs with minimal demand, done while sitting] to more demanding (>4.1 METS, including jobs such as laundress). Not working for pay was included as a separate category to account for the selectivity of working.
Reproductive history.
A complete reproductive history updated at each survey provided information about all pregnancies and duration of breast-feeding for each child. From these data, we estimated, for each survey interval, the total number of months that a woman was pregnant (counting 3 mo for miscarriages and 9 mo for full-term pregnancies), and the total months lactating. A binary variable indicated whether a woman was lactating or not when she was weighed. Menopausal status was prospectively collected starting with the 1991 survey and was represented in the models as a time-varying binary variable indicating whether, at each survey, the woman had experienced menopause or not.
Socioeconomic status.
We represented socioeconomic status (SES) by a summary index indicating the number of selected household assets owned, log of total household income per month (deflated to 1983 values), and the highest grade of education attained by the woman. Household size (number of persons reported to be living in the household at the time of the survey) was also included.
Other environmental variables.
Household microenvironment characteristics (toilet facilities, household and neighborhood cleanliness, and water supply and quality) were combined into a hygiene index, which ranged from 0 (unhygienic) to 9 (clean).
Urbanization.
A multi-component urbanicity index created from community survey data (14) reflected population size and density, community infrastructure, and economic and environment characteristics. An increase in the value of the index over time represents urbanization.
Statistical analysis
We estimated longitudinal mixed models to identify predictors of weight across all survey years. Weight was modeled as a growth curve, using mixed models with fixed and random individual level effects and random slopes. We initially tested whether a 3-level model that also accounted for the nesting of individuals within communities provided a better fit to the data. Because assessment of Bayesian and Akaike information criteria indicated slightly poorer fit with 3-level models, we present results from the simpler, 2-level models.
The longitudinal models used data from all surveys when a woman was not pregnant. We included 3028 women with a mean of 5.8 observations each (range from 1 to 8) for a total of 17,518 observations. We tested a series of models to develop an understanding of age and period effects. Model 1 included age and age-squared, indicator variables representing survey year, and age and age-squared interacted with survey year. The year coefficients describe the period effect and interaction terms describe how period effects differ by age. Model 2 added time-varying indicators of underlying SES, environment, and urbanicity. In addition to providing information about how these variables relate to weight, comparing this model with the basic model tells us whether SES and environmental factors explain some of the period effects. Model 3 added diet, occupational physical activity, and reproductive history variables to model 1. Model 4 included the full set of variables, treating the underlying SES and urbanicity variables as potential confounders. However, the behavioral variables are potential mediators of the relation of underlying SES to weight status. We tested alternate models to assess the role of macronutrient composition. Model fit was better with percentage of energy from protein rather than percentage of energy from fat.
We also estimated a set of models stratified by baseline age group. The groups represent age cohorts, and a comparison of coefficients across age-stratified models provides insight into period-specific effects of covariates. Age and age-squared terms were omitted from the age-stratified models. Models were fitted using Stata’s XTMIXED program (15). Results were considered significant at α < 0.05. Values in the text are means ± SD unless otherwise indicated.
Results
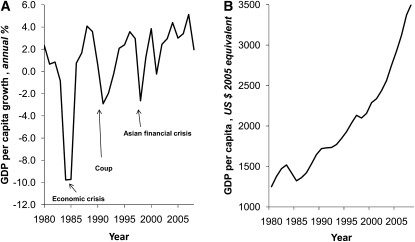
The height of the participants was 150.5 ± 4.9 cm. At entry into the CLHNS, they ranged in age from 15–45 y, mean parity was 2.23 ± 2.20, 19% were pregnant for the first time, 29.2% had less than a 6th grade education, and 12.7% had completed some education beyond high school. SES, diet, reproductive variables, and work-related activity varied substantially over time (Table 1). Changes over time reflected increasing urbanization and household income, improved household hygiene, and acquisition of more assets. The years 1985–1991 and 1998–2002 represent the time periods with the highest annual rates of change in income within the CLHNS. A corresponding increase in gross domestic product per capita occurred in the Philippines at this same time (Fig. 1) (16).
TABLE 1.
Characteristics of CLHNS women across survey years1
| Survey | 1983–1984 | 1984–1985 | 1985–1986 | 1991 | 1994 | 1998 | 2002 | 2005 |
| n | 3055 | 2442 | 2211 | 2218 | 2166 | 1938 | 2072 | 2004 |
| Age, y | 27.1 ± 6.0 | 28.2 ± 6.07 | 29.0 ± 6.0 | 35.7 ± 6.1 | 38.6 ± 6.1 | 42.6 ± 6.1 | 45.8 ± 6.1 | 48.4 ± 6.0 |
| Weight, kg | 46.9 ± 6.9 | 45.7 ± 7.08 | 46.6 ± 7.6 | 52.0 ± 9.5 | 52.6 ± 10.0 | 53.7 ± 10.4 | 55.2 ± 10.8 | 55.2 ± 10.9 |
| BMI, kg/m2 | 20.7 ± 2.6 | 20.2 ± 2.72 | 20.5 ± 2.9 | 22.9 ± 3.7 | 23.2 ± 3.9 | 23.7 ± 4.1 | 24.3 ± 4.3 | 24.4 ± 4.4 |
| BMI > 25 | 0.07 ± 0.25 | 0.06 ± 0.23 | 0.07 ± 0.26 | 0.26 ± 0.44 | 0.30 ± 0.46 | 0.35 ± 0.48 | 0.42 ± 0.49 | 0.43 ± 0.49 |
| Assets score | 2.50 ± 1.93 | 2.53 ± 1.87 | 2.67 ± 1.98 | 3.92 ± 2.36 | 3.93 ± 2.17 | 4.66 ± 2.17 | 5.00 ± 2.06 | 5.23 ± 1.95 |
| Household size, n | 5.69 ± 2.82 | 5.74 ± 2.78 | 5.72 ± 2.80 | 6.83 ± 2.34 | 7.06 ± 2.47 | 6.96 ± 2.54 | 6.97 ± 2.74 | 6.78 ± 2.72 |
| Income, pesos/mo | 2.60 ± 2.80 | 1.98 ± 2.18 | 2.19 ± 2.17 | 3.74 ± 3.19 | 4.84 ± 3.95 | 5.29 ± 4.00 | 5.36 ± 4.51 | 5.34 ± 4.85 |
| Hygiene score | 5.34 ± 1.94 | 5.36 ± 1.90 | 5.46 ± 1.92 | 5.09 ± 0.14 | 5.26 ± 1.89 | 4.97 ± 1.84 | 5.63 ± 1.93 | 6.00 ± 1.69 |
| Urban index | 30.4 ± 12.6 | 29.97 ± 12.8 | 28.65 ± 13.62 | 33.7 ± 14.2 | 35.9 ± 13.2 | 39.0 ± 13.7 | 41.3 ± 4.1 | 40.5 ± 13.6 |
| Energy intake, kJ/d | 6573 ± 3345 | 5878 ± 2864 | 5489 ± 2272 | 7172 ± 2617 | 5757 ± 2625 | 5753 ± 2596 | 5300 ± 2646 | 4769 ± 2119 |
| Protein intake, % energy | 13.2 ± 4.0 | 12.9 ± 3.72 | 12.7 ± 4.0 | 13.0 ± 2.5 | 13.6 ± 4.1 | 13.2 ± 3.9 | 14.7 ± 4.9 | 14.7 ± 4.5 |
| Time pregnant, mo | 0.00 ± 0.00 | 0.29 ± 1.21 | 1.43 ± 2.49 | 11.3 ± 10.3 | 2.5 ± 4.5 | 2.20 ± 4.78 | 1.05 ± 3.09 | 0.43 ± 1.90 |
| Time lactating, mo | 3.43 ± 1.35 | 5.94 ± 3.32 | 4.22 ± 4.74 | 15.73 ± 17.23 | 4.30 ± 8.50 | 4.14 ± 9.51 | 1.15 ± 5.21 | 0.67 ± 4.06 |
| Currently lactating | 0.79 ± 0.40 | 0.66 ± 0.47 | 0.15 ± 0.36 | 0.20 ± 0.40 | 0.13 ± 0.34 | 0.08 ± 0.27 | 0.05 ± 0.22 | 0.03 ± 0.18 |
| Postmenopausal | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.03 ± 0.16 | 0.05 ± 0.23 | 0.14 ± 0.35 | 0.25 ± 0.43 | 0.39 ± 0.49 |
| Not working | 0.37 ± 0.48 | 0.35 ± 0.48 | 0.31 ± 0.46 | 0.23 ± 0.42 | 0.20 ± 0.40 | 0.17 ± 0.38 | 0.16 ± 0.37 | 0.20 ± 0.40 |
| Sedentary work | 0.02 ± 0.14 | 0.02 ± 0.15 | 0.02 ± 0.14 | 0.06 ± 0.24 | 0.08 ± 0.27 | 0.08 ± 0.27 | 0.07 ± 0.25 | 0.06 ± 0.24 |
| Light work | 0.59 ± 0.49 | 0.60 ± 0.49 | 0.62 ± 0.49 | 0.65 ± 0.48 | 0.46 ± 0.50 | 0.49 ± 0.50 | 0.51 ± 0.50 | 0.49 ± 0.50 |
| Moderate work | 0.00 ± 0.04 | 0.01 ± 0.08 | 0.00 ± 0.06 | 0.02 ± 0.14 | 0.17 ± 0.37 | 0.16 ± 0.37 | 0.19 ± 0.39 | 0.17 ± 0.38 |
| Heavy work | 0.02 ± 0.14 | 0.02 ± 0.16 | 0.04 ± 0.20 | 0.03 ± 0.18 | 0.09 ± 0.29 | 0.09 ± 0.29 | 0.08 ± 0.28 | 0.07 ± 0.26 |
Values are mean ± SD.
FIGURE 1.
Rate of change in the Philippines’ GDP per capita (A) and in U.S. dollar equivalents (B) from 1980 to 2008. Data are adapted from the World Bank (16).
Total energy intake declined, but the percentage of energy from protein and fat increased over time. Reproductive stress decreased. Women had fewer pregnancies and spent less time lactating as they got older. Over time, a higher percentage of women worked for pay, but the majority worked in sedentary jobs: only 3% of all jobs reported by women had an energy expenditure of >4.2 METS.
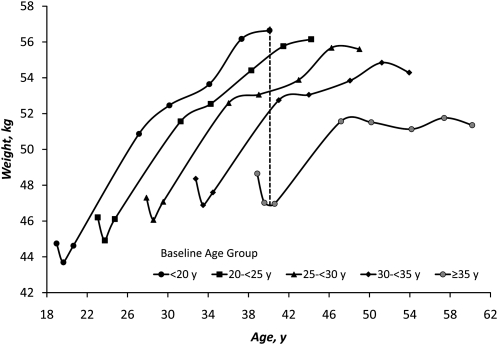
There was a 6-fold increase in the incidence of overweight and obesity (BMI > 25 kg/m2) across the 8 surveys (Table 1). By 2005, 42% of women were overweight or obese. In Figure 2, the mean weight of women in each age group is shown according to mean age of the group in each survey year to show weight trends related to age and year. Each point represents a survey year, and the lines span the 23-y period over which women were observed. Younger women had a lower initial mean weight. Weight declined in the first year and then rebounded in the second year among women in all but the oldest age group. These short-term changes primarily reflected the demands of lactation (17). From 1985 to 1986 when mean weights were the lowest (∼1 y after all gave birth) to 2005, women who were <20 y old at baseline gained 12.4 ± 8.5 kg, and women 35 y and older gained a mean of 4.9 ± 7.3 kg. The largest rate of weight gain occurred between 1986 and 1991, when women gained 0.9 kg/y compared with a mean gain across all years of 0.39 kg/y. A secondary peak in rate of weight gain occurred between 1998 and 2002 (0.55 kg/y).
FIGURE 2.
Age trends in mean nonpregnant weight of CLHNS women according to baseline age groups. Points represent the mean weight of women at the mean age for the age group in each survey year (1984, 1985, 1986, 1991, 1994, 1998, 2002, 2005). The vertical dotted line illustrates the difference in estimated weight for a woman who was 40 y old in 1985 compared with a woman who was aged 40 y in 2005.
The secular trend in weight can be appreciated by looking at age 40 y (Fig. 2), when data were available for all age group cohorts. The oldest cohort at age 40 y (measured in 1985) had a mean weight of ~47 kg, and when the youngest cohort was 40 y old (measured in 2005), they weighed ~9.6 kg more.
Model estimation results
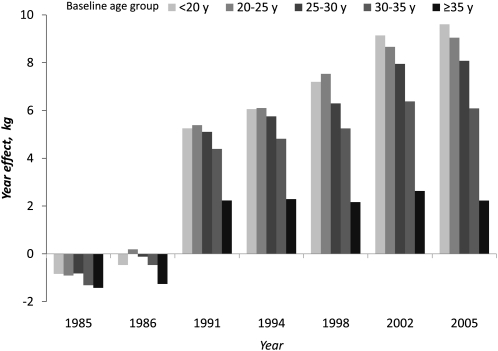
The nonlinear age-related pattern of weight gain ( was confirmed by the longitudinal model results showing significant positive coefficients on age and negative coefficients on age-squared. After accounting for age, year coefficients in the longitudinal model represent the period effect or secular trend in weight. Compared with baseline (4 mo postpartum), y 2 and 3 coefficients (representing 12 and 24 mo postpartum, respectively) were negative, but coefficients were positive and larger in each subsequent year. Significant interactions of year with age and age-squared showed that secular increases in weight differ across the age groups, with the largest increases over time in those who were the youngest at baseline (Table 2, model 1). This is more simply illustrated by comparing the coefficients on year estimated in age-stratified longitudinal models (Fig. 3). The largest period effects were in the youngest age group, who in 2005 weighed nearly 12 kg more than at baseline.
TABLE 2.
Coefficients and 95% CI from mixed effects longitudinal models of CLHNS women's nonpregnant weight across 8 survey years
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
|||||
| Coef. | 95% CI | Coef. | 95% CI | Coef. | 95% CI | Coef. | 95% CI | |
| Height, cm | 0.83 | 0.77; 0.88*** | 0.77 | 0.72; 0.82*** | 0.81 | 0.76; 0.86*** | 0.766 | 0.72; 0.82*** |
| Age | 0.31 | −0.03; 0.66 | 0.19 | −0.15; 0.53 | 0.32 | −0.02; 0.66 | 0.181 | −0.16; 0.52 |
| Age2 | −0.002 | −0.008; 0.004 | 0.000 | −0.005; 0.006 | −0.002 | −0.008; 0.004 | −0.001 | −0.007; 0.005 |
| y 2 | −2.27 | −6.58; 2.04 | −1.23 | −5.48; 3.03 | −1.59 | −5.87; 2.69 | −0.838 | −5.07; 3.39 |
| y 3 | −2.63 | −7.29; 2.02 | −1.35 | −5.94; 3.24 | −1.74 | −6.36; 2.89 | −0.701 | −5.27; 3.87 |
| y 4 | −4.43 | −11.12; 2.26 | −3.37 | −9.97; 3.22 | −0.85 | −7.53; 5.82 | −0.635 | −7.24; 5.97 |
| y 5 | −3.65 | −11.53; 4.24 | −2.74 | −10.50; 5.03 | −0.209 | −8.06; 7.66 | 0.200 | −7.57; 7.97 |
| y 6 | −5.21 | −15.25; 4.82 | −3.30 | −13.19; 6.59 | −1.03 | −11.04; 8.97 | −0.184 | −10.08; 9.71 |
| y 7 | −3.49 | −15.29; 8.31 | −0.45 | −12.07; 11.16 | −0.06 | −11.74; 11.63 | 1.709 | −9.84; 13.25 |
| y 8 | 1.03 | −12.65; 14.71 | 4.91 | −8.56; 18.37 | 0.01 | −13.57; 13.60 | 2.685 | −10.74; 16.11 |
| y 2 × age | 0.10 | −0.20; 0.40 | 0.04 | −0.26; 0.33 | 0.07 | −0.23; 0.36 | 0.023 | −0.27; 0.31 |
| y 3 × age | 0.19 | −0.13; 0.50 | 0.11 | −0.20; 0.42 | 0.15 | −0.16; 0.46 | 0.090 | −0.22; 0.40 |
| y 4 × age | 0.53 | 0.17; 0.89** | 0.46 | 0.11; 0.82** | 0.408 | 0.04; 0.76* | 0.367 | 0.01; 0.72* |
| y 5 × age | 0.53 | 0.14; 0.91** | 0.47 | 0.09; 0.85* | 0.37 | −0.01; 0.76 | 0.361 | −0.02; 0.74 |
| y 6 × age | 0.65 | 0.21; 1.08** | 0.55 | 0.12; 0.98* | 0.47 | 0.03; 0.91* | 0.441 | 0.01; 0.88* |
| y 7 × age | 0.66 | 0.19; 1.12** | 0.51 | 0.05; 0.97* | 0.50 | 0.03; 0.96* | 0.440 | −0.02; 0.90 |
| y 8 × age | 0.48 | −0.02; 0.99 | 0.32 | −0.18; 0.82 | 0.51 | 0.00; 1.01* | 0.427 | −0.07; 0.93 |
| y 2 × age2 | −0.002 | −0.007; 0.003 | −0.001 | −0.006; 0.004 | −0.002 | −0.007; 0.003 | −0.001 | −0.006; 0.004 |
| y 3 × age2 | −0.004 | −0.009; 0.001 | −0.003 | −0.008; 0.002 | −0.003 | −0.009; 0.002 | −0.003 | −0.008; 0.002 |
| y 4 × age2 | −0.008 | −0.014; −0.003*** | −0.008 | −0.013; 0.003*** | −0.007 | −0.012; 0.002** | −0.007 | −0.012; 0.002** |
| y 5 × age2 | −0.009 | −0.014; 0.003*** | −0.009 | −0.014; 0.004*** | −0.006 | −0.012; 0.001*** | −0.007 | −0.012; 0.002** |
| y 6 × age2 | −0.010 | −0.015; 0.005*** | −0.010 | −0.015; 0.005*** | −0.008 | −0.013; 0.002*** | −0.008 | −0.013; 0.003** |
| y 7 × age2 | −0.010 | −0.015; 0.005*** | −0.010 | −0.015; 0.004*** | −0.008 | −0.013; 0.003*** | −0.008 | −0.013; 0.003** |
| y 8 × age2 | −0.008 | −0.013; 0.003*** | −0.008 | −0.013; 0.002** | −0.008 | −0.013; 0.003*** | −0.008 | −0.013; 0.003** |
| Assets score | 0.44 | 0.39; 0.50*** | 0.41 | 0.36; 0.47*** | ||||
| Education, y | 0.04 | −0.03; 0.11 | 0.06 | −0.07; 0.13 | ||||
| Income, pesos/mo | 0.31 | 0.21; 0.41*** | 0.25 | 0.15; 0.35*** | ||||
| Household size, n | −0.17 | −0.20; 0.14*** | −0.15 | −0.18; 0.12*** | ||||
| Hygiene score | 0.11 | 0.06; 0.15*** | 0.09 | 0.04; 0.14*** | ||||
| Urban index | 0.37 | 0.27;.47*** | 0.37 | 0.27; 0.47*** | ||||
| Energy intake, kJ/d | 2.35 | 2.86; 2.85*** | 1.78 | 1.29; 2.3*** | ||||
| Protein intake, % energy | 0.05 | 0.03; 0.07*** | 0.04 | 0.03; 0.06*** | ||||
| Postmenopause | −0.87 | −1.21; 0.53*** | −0.847 | −1.19; 0.51*** | ||||
| Parity | 0.05 | −0.11; 0.20 | 0.321 | 0.16; 0.49*** | ||||
| Lactating | 0.18 | −0.05; 0.40 | 0.208 | −0.02; 0.43 | ||||
| Time lactating, mo | −0.04 | −0.05; 0.03*** | −0.028 | −0.04; 0.02*** | ||||
| Time pregnant, mo | −0.02 | −0.04; 0.00** | −0.006 | −0.02; 0.01 | ||||
| Not working | −0.34 | −0.69; 0.02 | −0.327 | −0.68; 0.03 | ||||
| Light work | 0.04 | −0.31; 0.38 | 0.061 | −0.28; 0.40 | ||||
| Moderate work | −1.36 | −1.78; 0.95*** | −1.166 | −1.58; 0.76*** | ||||
| Heavy work | −1.09 | −1.55; 0.64*** | −0.798 | −1.25; 0.35*** | ||||
| Constant | −84.1 | −93.2; 75.1 | −78.3 | −87.3; 69.4 | −83.3 | −92.3; 74.5*** | −78.1 | −86.9; 69.2*** |
*** < 0.001; **P < 0.01; *P < 0.05.
FIGURE 3.
Secular trends in weight of CLHNS women. Bars represent the difference from baseline (1983–1984) weight, estimated from longitudinal models, stratified by age category.
Effects of SES and urbanicity.
Household income, assets, and hygiene score, and community level urbanization were positively and significantly associated with weight (Table 2, model 2) and household size was negatively associated with weight. The inclusion of these variables attenuated the period effects, as can be seen by comparing model 2 with model 1.
Effects of proximate behaviors.
Higher energy intake and a higher percentage of energy from protein were associated with greater weight, whereas being postmenopausal, experiencing more months pregnant and lactating, and working at jobs with higher energy expenditure (Table 2, model 3) were related to lower weight. Year coefficients were attenuated when these behavioral variables were included in the model. After adjustment for potential confounding effects of SES and urbanicity, the direction of most of these associations remained the same, but coefficients were slightly attenuated (Table 2, model 4). An exception to this is the effect of parity, which was positively related to weight only after adjustment for SES.
Formal tests of age interactions with each exposure variable revealed that, except for hygiene and month pregnant, the effects of all other covariates differed by age. Owing to the complexity of these models and large number of interaction terms, results from stratified models are presented to illustrate the differences (Table 3).
TABLE 3.
Coefficients and 95% CI from mixed effects longitudinal models of CLHNS women's nonpregnant weight across 8 survey years, stratified by baseline age groups
| <20 y |
20–25 y |
25–30 y |
30–35 y |
35 y |
||||||
| Coef. | 95% CI | Coef. | 95% CI | Coef. | 95% CI | Coef. | 95% CI | Coef. | 95% CI | |
| Height, cm | 0.70 | 0.57; 0.83*** | 0.73 | 0.64; 0.81*** | 0.75 | 0.65;0.85*** | 0.86 | 0.72; 0.99*** | 0.93 | 0.76; 1.10*** |
| Assets, score | 0.65 | 0.50; 0.80*** | 0.39 | 0.29; 0.48*** | 0.40 | 0.31;0.50*** | 0.280 | 0.16; 0.40*** | 0.47 | 0.30; 0.63*** |
| Education, y | −0.07 | −0.30; 0.16** | 0.01 | −0.13; 0.15 | −0.01 | −0.15;0.13 | 0.19 | 0.01; 0.37* | 0.22 | 0.01; 0.44* |
| Income, pesos/mo | 0.26 | −0.03; 0.55 | 0.29 | 0.11; 0.47*** | 0.21 | 0.03;0.40* | 0.15 | −0.09; 0.38 | 0.32 | 0.03; 0.62* |
| Household size, n | −0.25 | −0.34; 0.16*** | −0.19 | −0.25; 0.13*** | −0.09 | −0.15; 0.02* | −0.07 | −0.16; 0.02 | −0.06 | −0.17; 0.05 |
| Hygiene, score | −0.01 | −0.15; 0.13 | 0.07 | −0.01; 0.16 | 0.15 | 0.06;0.23*** | 0.07 | −0.05; 0.18 | 0.19 | 0.04; 0.33** |
| Urban index | 0.09 | −0.19; 0.38 | 0.33 | 0.15; 0.52*** | 0.20 | 0.02;0.39* | 0.71 | 0.47; 0.96*** | 0.53 | 0.19; 0.86** |
| Energy intake, kJ/d | 1.17 | −0.25; 2.60 | 1.30 | 0.42; 2.22** | 2.60 | 1.72;3.48*** | 1.72 | 0.59; 2.89** | 1.67 | 0.04; 3.31* |
| Protein intake, % energy | 0.04 | −0.01; 0.09 | 0.06 | 0.03; 0.10*** | 0.03 | 0.00;0.06* | 0.03 | −0.02; 0.07 | 0.03 | −0.02; 0.08 |
| Postmenopausal | −0.18 | −4.63; 4.28 | −0.77 | −2.09; 0.54 | −1.23 | −1.78; 0.68*** | −0.77 | −1.36; 0.18** | −1.11 | −1.83; 0.40** |
| Parity | 1.04 | 0.04; 2.05* | 0.75 | 0.35; 1.15*** | 0.18 | −0.17;0.52 | 0.09 | −0.25; 0.43 | 0.34 | 0.02; 0.66* |
| Lactating | −0.59 | −1.21; 0.03 | 0.34 | −0.05; 0.74** | 0.30 | −0.11;0.71 | 0.75 | 0.18; 1.33** | 0.01 | −0.74; 0.75 |
| Months lactating | −0.03 | −0.05; 0.00 | −0.03 | −0.05; 0.02*** | −0.03 | −0.05; 0.01*** | −0.02 | −0.05; 0.01 | −0.02 | −0.07; 0.02 |
| Months pregnant | −0.01 | −0.06; 0.03 | 0.00 | −0.03; 0.03 | −0.01 | −0.04;0.02 | 0.01 | −0.04; 0.06 | −0.02 | −0.11; 0.06 |
| Not working | −0.30 | −1.56; 0.96 | −0.08 | −0.73; 0.57 | −0.32 | −0.95;0.230 | −0.79 | −1.58; 0.00* | −0.54 | −1.74; 0.66 |
| Light work | −0.24 | −1.48; 1.00 | 0.30 | −0.32; 0.92 | 0.18 | −0.41;0.77 | −0.09 | −0.86; 0.68 | −0.30 | −1.48; 0.88 |
| Moderate work | −0.48 | −1.90; 0.95 | −0.47 | −1.22; 0.28 | −1.53 | −2.25; 0.80*** | −1.83 | −2.75; 0.90*** | −1.60 | −2.95; 0.25* |
| Heavy work | −1.05 | −2.55; 0.44 | −0.39 | −1.24; 0.46 | −0.73 | −1.55;0.08 | −1.28 | −2.31; 0.24* | −0.99 | −2.33; 0.36 |
| y 2 | −0.84 | −1.56; 0.12* | −0.91 | −1.33; 0.49*** | −0.82 | −1.24; 0.41*** | −1.31 | −1.84; 0.78*** | −1.42 | −2.10; 0.75*** |
| Y 3 | −0.47 | −1.34; 0.40 | 0.19 | −0.33; 0.71*** | −0.12 | −0.62;0.38 | −0.47 | −1.12; 0.18 | −1.26 | −2.04; 0.45** |
| y 4 | 5.25 | 4.01; 6.50*** | 5.38 | 4.69; 6.08*** | 5.10 | 4.46;5.74*** | 4.39 | 3.58; 5.20*** | 2.23 | 1.25; 3.22*** |
| y 5 | 6.06 | 5.14; 6.98*** | 6.10 | 5.54; 6.66*** | 5.75 | 5.19;6.31*** | 4.82 | 4.07; 5.56*** | 2.29 | 1.30; 3.28*** |
| y 6 | 7.20 | 6.20; 8.21*** | 7.53 | 6.90; 8.16*** | 6.29 | 5.68;6.91*** | 5.25 | 4.42; 6.08*** | 2.16 | 1.00; 3.32*** |
| y 7 | 9.14 | 8.14; 10.14*** | 8.66 | 8.03; 9.30*** | 7.95 | 7.31;8.59*** | 6.38 | 5.48; 7.28*** | 2.63 | 1.38; 3.88*** |
| y 8 | 9.61 | 8.59; 10.62*** | 9.04 | 8.40; 9.69*** | 8.08 | 7.41;8.75*** | 6.096 | 5.11; 7.07*** | 2.23 | 0.93; 3.52** |
| Constant | −61.9 | −81.4; 42.3*** | −68.75 | −81.2; 56.3*** | −70.8 | −86.0; 55.5*** | −87.8 | −108.1; 67.4*** | −99.0 | −124.7; 73.4*** |
*** < 0.01; **P < 0.05; *P < 0.10.
Higher urbanicity related to higher weight in all but the youngest group. Higher household assets influenced weight to a greater extent in the oldest and youngest cohorts, and education was only influential in the 2 oldest cohorts. Higher income related to higher weight primarily in the oldest cohort. Household size was important for the 3 youngest age groups but was unrelated to weight in older women. The occurrence and magnitude of reproductive stresses varied with age, as did their effect on weight. Menopausal status had no effect on weight of younger women but was substantial in the older cohorts. The effects of current lactation and lactation history were strongest in the middle age cohorts. Larger negative effects of more physically demanding jobs were characteristics of the older age cohorts.
Discussion
Over just 20 y, Cebu women gained a substantial amount of weight and the proportion who were overweight or obese increased 6-fold from <7% at baseline to >40% in 2005. These patterns represent age-related weight increases as well as a strong secular trend. Regardless of age, women were heavier in more recent years. Weight gains were larger and prevalence of overweight increased more markedly in younger women. Women who were <20 y of age at baseline gained ~7.5 kg more from 1986 (after their lactation-related postpartum weight loss) to 2005. In the US CARDIA study, mean 10-y weight increases were 11.9 kg in African American women and 6.9 kg in white women who were 18–30 y old when they entered the study. Greater weight gains were observed in those who were the youngest at the beginning of the survey and the secular trend estimated from a longitudinal model was 0.55 kg/y in whites and 0.96 kg/y in blacks (18)
Evidence from the longitudinal mixed models shows that the magnitude of the secular trend in weight varied by age. Younger women had higher gains related to survey year. This may reflect a greater susceptibility of younger women to environmental variables that promote weight gain or an effect of their quite different health and developmental histories. About one-half of CLHNS women were born in the 1950s and were in their mid 20s to 30s when they entered the study. Those born in prior and subsequent decades experienced quite different conditions in their early childhood years. The higher weight gain in younger Cebu women is consistent with studies in the US (19), Australia (20), and Japan (21).
Our age-stratified models suggest that different variables affect weight to a greater or lesser extent according to age. Urbanization was positively associated with higher weight in all but the youngest group. The youngest women living in urban environments may now be more interested in body image and maintaining thinness (11). Traditional SES measures of assets, education, and income were more strongly related to weight in the oldest and youngest cohorts. The nutrition transition is thought to be the consequence of community level urbanization and modernization along with improvements in household level income and assets. Such changes result in increased intake of fat and processed foods and a decline in energy expenditure related to more time in sedentary activities and lower energy demands of work and transportation (8, 22). During the years of the CLHNS, major economic and social changes occurred in the Philippines. The years of greatest increase in per capita GDP occurred following the first People Power Revolution in 1986 and the subsequent change in national leadership (Fig. 1). During this time, Cebu flourished as a commercial, manufacturing, and trading center and exports grew remarkably. Based on our analysis, this time period corresponded with the highest rate of weight gain in Cebu women. The highest rates of increase in household level income and assets and in urbanization also occurred during this period. These variables related strongly to weight in our longitudinal models and their inclusion attenuated year coefficients, suggesting that improved SES and urbanization were indeed part of the secular trend. Inclusion of more proximate behaviors, which changed along with SES and urbanization, further attenuated the year effects. Of interest, however, is that even after accounting for SES, urbanization, diet, occupational physical activity, and reproductive history, we still observed significant effects of year, suggesting that unmeasured or poorly measured obesigenic, environmental, and behavioral variables underlie the secular trend in weight.
Aside from how this study contributes to our understanding of age and secular trends, we also identified many factors that influence weight gain and thus can serve to inform prevention efforts. In contrast with many studies that examine only a limited number of factors, we simultaneously explored a wide range of socioeconomic and environmental variables as well as diet, physical activity, and reproductive history. Higher body weight was predicted by higher income and assets, consistent with a substantial body of literature showing positive associations of SES and health outcomes in low- and middle-income countries (23, 24). Urbanization was also associated with higher body weight, particularly in older women, consistent with findings from other low- and middle-income countries in Asia (25, 26) and Latin America (24).
Reproductive history was also an important determinant of weight status. While parity-related increases in maternal weight are often observed in well-nourished women (27, 28), depletion of maternal energy reserves may result from limited dietary intake and high levels of reproductive stress in women from lower income countries (27, 29, 30). We observed that while higher parity was related to higher weight, a greater number of months pregnant and lactating in each interval related to lower body weight, consistent with the high energy demands of these reproductive stresses. The occurrence and magnitude of reproductive stresses varied with age, but their effect on weight was similar across age groups. As women aged, more and more experienced menopause, which was associated with lower weight.
Despite declines in total energy intake over time, we observed a positive association of weight with energy intake and of percentage of energy from protein. The former is expected, because larger women have higher energy needs. The positive association of relatively higher protein intakes likely reflects higher meat intake. In separate models (not shown), we also observed a positive association of weight with percentage of dietary energy from fat. Energy balance is the critical determinant of weight gain, so it is important to also consider energy expenditure. A weakness of our analysis is that we did not include measures of all domains of physical activity. We did not account for domestic work and leisure time activity. More time in household chores was associated with lower SES and fewer occupational work hours. Leisure time is marked by an absence of any moderate to vigorous physical activity (11). Occupational activity is an important component of daily energy expenditure, particularly in developing countries (31), and it is sensitive to modernization and socioeconomic development. Improved technology and use of labor-saving devices have lessened the physical demands of many jobs. Our occupational activity measure, while limited, likely captured the important variation in overall energy expenditure in the sample. Overall, women work in increasingly sedentary occupations such as tending small stores. Relatively few women worked at high-METS jobs, but higher energy demands at work were related to lower body weight.
In summary, this study followed a large cohort of adult women over a significant portion of their adult lives, tracking some from just after the birth of their first child into middle adulthood, and others from middle to later adulthood. Strong age and secular trends were observed, resulting in an increasing number of women who have or are likely to develop obesity-related metabolic diseases. These trends are highly relevant for health policy and preventive health measures in the Philippines and other countries that are now faced with a dual burden of over- and undernutrition.
Acknowledgments
S.G. participated in the original design and implementation of the CLHNS and interpretation of research results; L.A. and C.S. designed and conducted data analysis; and L.A. wrote the paper and had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Supported by 1 R01 HL085144 Obesity Development and CVD Risk Factor Clustering in Filipino Women and Offspring.
Abbreviations used: CLHNS, Cebu Longitudinal Health and Nutrition Survey; CVD, cardiovascular disease; METS, metabolic equivalent; SES, socioeconomic status.
Literature Cited
- 1.Mendez MA, Monteiro CA, Popkin BM. Overweight exceeds underweight among women in most developing countries. Am J Clin Nutr. 2005;81:714–21 [DOI] [PubMed] [Google Scholar]
- 2.Mackay J, Mensah GA. The atlas of heart disease and stroke. Geneva: WHO; 2004 [Google Scholar]
- 3.Khor GL. Cardiovascular epidemiology in the Asia-Pacific region. Asia Pac J Clin Nutr. 2001;10:76–80 [DOI] [PubMed] [Google Scholar]
- 4.Popkin BM. The nutrition transition and obesity in the developing world. J Nutr. 2001;131:S871–3 [DOI] [PubMed] [Google Scholar]
- 5.Deurenberg-Yap M, Chew SK, Deurenberg P. Elevated body fat percentage and cardiovascular risks at low body mass index levels among Singaporean Chinese, Malays and Indians. Obes Rev. 2002;3:209–15 [DOI] [PubMed] [Google Scholar]
- 6.Colin Bell A, Adair LS, Popkin BM. Ethnic differences in the association between body mass index and hypertension. Am J Epidemiol. 2002;155:346–53 [DOI] [PubMed] [Google Scholar]
- 7.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–6 [DOI] [PubMed] [Google Scholar]
- 8.Popkin BM. Nutrition in transition: the changing global nutrition challenge. Asia Pac J Clin Nutr. 2001;10 Suppl:S13–8 [PubMed] [Google Scholar]
- 9.Adair LS, Popkin BM, Akin JS, Guilkey DK, Gultiano S, Borja B, Perez L, Kuzawa CW, McDade T, et al. Cohort profile: The Cebu Longitudinal Health and Nutrition Survey. Int J Epid. Epub. 2010 May 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adair LS. Dramatic rise in overweight and obesity in adult Filipino women and risk of hypertension. Obes Res. 2004;12:1335–41 [DOI] [PubMed] [Google Scholar]
- 11.Jennings A. Identifying the determinants of coexisting over and undernutrition in Cebu, Philippines. Chapel Hill (NC): University of North Carolina at Chapel Hill; 2007 [Google Scholar]
- 12.Tuazon MA, van Raaij JM, Hautvast JG, Barba CV. Energy requirements of pregnancy in the Philippines. Lancet. 1987;2:1129–31 [DOI] [PubMed] [Google Scholar]
- 13.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr, Schmitz KH, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504 [DOI] [PubMed] [Google Scholar]
- 14.Dahly DL, Adair LS. Quantifying the urban environment: a scale measure of urbanicity outperforms the urban-rural dichotomy. Soc Sci Med. 2007;64:1407–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.StataCorp Stata Statistical Software, release 11. College Station (TX): StataCorp LP; 2009 [Google Scholar]
- 16.World Bank World development indicators. 2010 [cited 2010 Oct 15]. Available from: http://data.worldbank.org/data-catalog/world-development-indicators
- 17.Adair LS. Postpartum nutritional status of Filipino women. Am J Hum Biol. 1992;4:635–46 [DOI] [PubMed] [Google Scholar]
- 18.Lewis CE, Jacobs DR, Jr, McCreath H, Kiefe CI, Schreiner PJ, Smith DE, Williams OD. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 2000;151:1172–81 [DOI] [PubMed] [Google Scholar]
- 19.Barone BB, Clark JM, Wang NY, Meoni LA, Klag MJ, Brancati FL. Lifetime weight patterns in male physicians: the effects of cohort and selective survival. Obesity (Silver Spring). 2006;14:902–8 [DOI] [PubMed] [Google Scholar]
- 20.Allman-Farinelli MA, Chey T, Merom D, Bowles H, Bauman AE. The effects of age, birth cohort and survey period on leisure-time physical activity by Australian adults: 1990–2005. Br J Nutr. 2009;101:609–17 [DOI] [PubMed] [Google Scholar]
- 21.Matsushita Y, Takahashi Y, Mizoue T, Inoue M, Noda M, Tsugane S, Group JS. Overweight and obesity trends among Japanese adults: a 10-year follow-up of the JPHC Study. Int J Obes (Lond). 2008;32:1861–7 [DOI] [PubMed] [Google Scholar]
- 22.Popkin BM. The nutrition transition and its health implications in lower-income countries. Public Health Nutr. 1998;1:5–21 [DOI] [PubMed] [Google Scholar]
- 23.Monteiro CA, Moura EC, Conde WL, Popkin BM. Socioeconomic status and obesity in adult populations of developing countries: a review. Bull World Health Organ. 2004;82:940–6 [PMC free article] [PubMed] [Google Scholar]
- 24.Pérez-Cueto FJ, Botti AB, Verbeke W. Prevalence of overweight in Bolivia: data on women and adolescents. Obes Rev. 2009;10:373–7 [DOI] [PubMed] [Google Scholar]
- 25.Aekplakorn W, Hogan MC, Chongsuvivatwong V, Tatsanavivat P, Chariyalertsak S, Boonthum A, Tiptaradol S, Lim SS. Trends in obesity and associations with education and urban or rural residence in Thailand. Obesity (Silver Spring). 2007;15:3113–21 [DOI] [PubMed] [Google Scholar]
- 26.Reynolds K, Gu D, Whelton PK, Wu X, Duan X, Mo J, He J, Inter ACG. Prevalence and risk factors of overweight and obesity in China. Obesity (Silver Spring). 2007;15:10–8 [DOI] [PubMed] [Google Scholar]
- 27.Winkvist A, Rasmussen KM, Lissner L. Associations between reproduction and maternal body weight: examining the component parts of a full reproductive cycle. Eur J Clin Nutr. 2003;57:114–27 [DOI] [PubMed] [Google Scholar]
- 28.Gunderson EP. Childbearing and obesity in women: weight before, during, and after pregnancy. Obstet Gynecol Clin North Am. 2009;36:317–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adair LS, Popkin BM. Prolonged lactation contributes to depletion of maternal energy reserves in Filipino women. J Nutr. 1992;122:1643–55 [DOI] [PubMed] [Google Scholar]
- 30.King JC. The risk of maternal nutritional depletion and poor outcomes increases in early or closely spaced pregnancies. J Nutr. 2003;133:S1732–6 [DOI] [PubMed] [Google Scholar]
- 31.Forrest KY, Bunker CH, Kriska AM, Ukoli FA, Huston SL, Markovic N. Physical activity and cardiovascular risk factors in a developing population. Med Sci Sports Exerc. 2001;33:1598–604 [DOI] [PubMed] [Google Scholar]