Abstract
Nonheme food ferritin (FTN) iron minerals, nonheme iron complexes, and heme iron contribute to the balance between food iron absorption and body iron homeostasis. Iron absorption depends on membrane transporter proteins DMT1, PCP/HCP1, ferroportin (FPN), TRF2, and matriptase 2. Mutations in DMT1 and matriptase-2 cause iron deficiency; mutations in FPN, HFE, and TRF2 cause iron excess. Intracellular iron homeostasis depends on coordinated regulation of iron trafficking and storage proteins encoded in iron responsive element (IRE)-mRNA. The noncoding IRE-mRNA structures bind protein repressors, IRP1 or 2, during iron deficiency. Integration of the IRE-RNA in translation regulators (near the cap) or turnover elements (after the coding region) increases iron uptake (DMT1/TRF1) or decreases iron storage/efflux (FTN/FPN) when IRP binds. An antioxidant response element in FTN DNA binds Bach1, a heme-sensitive transcription factor that coordinates expression among antioxidant response proteins like FTN, thioredoxin reductase, and quinone reductase. FTN, an antioxidant because Fe2+ and O2 (reactive oxygen species generators) are consumed to make iron mineral, is also a nutritional iron concentrate that is an efficiently absorbed, nonheme source of iron from whole legumes. FTN protein cages contain thousands of mineralized iron atoms and enter cells by receptor-mediated endocytosis, an absorption mechanism distinct from transport of nonheme iron salts (ferrous sulfate), iron chelators (ferric-EDTA), or heme. Recognition of 2 nutritional nonheme iron sources, small and large (FTN), will aid the solution of iron deficiency, a major public health problem, and the development of new policies on iron nutrition.
Introduction
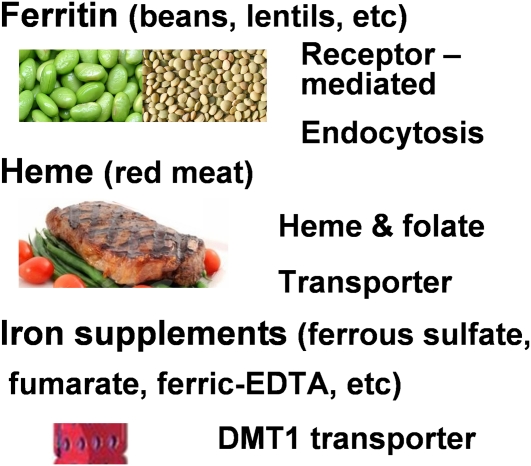
Iron deficiency remains a major public health problem in the 21st century, more than one-half a millennium since medieval physicians diagnosed and treated the condition. Recognition of the functional impact of the iron deficiency has been extended from diminished red cell formation to many other aspects of physiology, including cognition (1). However, continuous development of new pharmacological sources to correct iron deficiency (ferric carboxymaltose and ferumoxytol) (2) and the identification of gene mutations related to iron deficiencies (3) have failed to solve the problem in either the general population or those with confounding medical conditions, such as chronic kidney disease (2). Given the persistence of iron deficits in so many people, the importance of understanding and comparing iron absorption mechanisms for different chemical species of iron is very high (4). The 3 major chemical forms of nutritional iron (Fig. 1) are the nonheme ferritin (FTN)4 iron mineral (legumes), heme (meat), and nonheme, low molecular weight (as in iron salts and chelates); each has a different iron absorption mechanism. Iron from the different dietary nonheme and heme sources feeds into a common regulatory pathway to a common signal that is thought to be ferrous ion, which regulates the FTN feedback loop.
FIGURE 1.
Food iron has multiple, different structures but inside enterocytes enters an iron pool for FPN export. The 3 best-known biochemical types of nutrition iron and abundant food sources are shown. Each is absorbed by a different mechanism: iron in heme (meat): PCP/HCP1 (SLC46A) transporter (9, 33), FTN, nonheme iron, mineralized ferric oxide in a protein, abundant in whole legume seeds: clathrin-dependent, receptor-mediated endocytosis (10), and nonheme iron in salts or chelators from supplements, which is absorbed by the DMT1 (SLC11A) transporter (7, 8).
Iron homeostasis is extensively reviewed from the perspective of the regulation of genes encoding proteins that contribute to cellular iron traffic and the recently discovered peptide hormone, hepcidin, which controls the activity of the iron efflux protein ferroportin (3, 5–8). Because renal excretion of iron is relatively small, compared to other metals and vitamins, the intestine is the key to maintaining iron homeostasis. In this brief review, iron homeostasis will be related to the different chemical species of nutritional heme and nonheme iron. I discuss homeostatic responses to abnormal iron metabolism and of the absorption of different species of dietary iron, i.e. heme, nonheme iron supplements, and nonheme iron biominerals (FTN).
Homeostatic responses to abnormal iron metabolism.
The role of the intestine in iron homeostasis is illustrated by 3 genetic mutations in humans that cause abnormal iron absorption. First, an iron deficiency anemia is caused by mutation in DMT1 (proton coupled transporter of divalent ions) (7). However, the absorption of heme (meat) and FTN (legume) iron depends on different mechanisms of absorption (9, 10), which are not changed by the DMT1 mutation. In fact, the anemia from DMT1 mutation is associated with increased liver iron (8), because DMT1 has at least one other function in addition to transporting ferrous iron into the enterocyte. DMT1 also distributes iron from the surface within the red cell cytoplasm. As a result, DMT1 mutations lead to ineffective erythropoiesis and accumulations of iron in liver. The role of DMT1 in red cell formation is now thought to be a major cause of the anemia when DMT1 is mutated (7, 8).
Hereditary hemochromatosis (HH) is a second disease that is caused by changes in iron absorption. One of the HH mutations in the HH protein (HFE) has spread so quickly that it may have had a positive selective effect when dietary iron was limiting (11). HH can be caused by mutation in ferroportin (FPN), the iron efflux protein on the basolateral side of the intestine, by mutation in the liver iron uptake proteins (12), or by a decrease in serum hepcidin, the peptide hormone that inhibits FPN activity. Such HH mutations include the liver proteins transferrin receptor 2 (TFR2) and hemojuvelin, as well as HFE, the hemochromatosis protein (12). In HH, interference with the FPN and hepcidin regulatory interactions results in unregulated release of absorbed intestinal iron into the blood.
A mutation in TMPRSS6 (Matriptase-2) causes a 3rd type of abnormal iron absorption. Matriptase-2 is a transmembrane protease on the surface of liver. When mutated increased serum hepcidin with decreased FPN activity and iron release from the intestine into the blood results in an iron-refractory, iron deficiency anemia (3). Other diseases related to altered iron distribution/metabolism include restless leg syndrome, but the molecular defect is not yet clear (13).
Within intestinal cells themselves and other cells, iron homeostasis depends on the coordinated activity of a group of proteins for iron uptake: DMT1 (ferrous ion and ferric ions after reduction) and TFR1, the generic transferrin receptor; FTN, the iron concentration/storage protein in cytoplasm, mitochondria, and sometimes lysosomes; and FPN, the iron export protein (14, 15). Intracellular iron chaperones and transporters are only partly identified. DMT1, TFR1, FTN, and FPN genes are regulated by transcription (mRNA synthesis) pathways during differentiation and development, as are many homeostatic genes. When cell iron concentrations change, the protein concentrations of FTN, FPN, DMT1, and TRF1 also change, mediated by a second iron response mechanism targeted to mRNA. RNA may be the target of iron regulation, because the response rate is faster than DNA transcription and iron signaling can occur in the cytoplasm. Mature mRNA encoding the iron-regulated proteins are stored in the cytoplasm in a repressed or inactive form (16) until the iron level changes. The repressor proteins, called iron regulatory proteins IRP1 and IRP2, bind specifically to noncoding RNA structures in DMT1, TFR1, FTN, and FPN1, each called an iron responsive element (IRE). The IRE sequences/structures are similar enough to bind IRP1 and IRP2; IRPs bind IRE-RNA or iron-sulfur clusters. However, the IRE in each individual mRNA has distinctive base pairs and an internal bulge structure to create a set of RNA/protein interactions for each IRE-mRNA and a quantitatively varied response to iron.
Iron homeostasis proteins.
IRE-RNA/IRP binding has 2 different effects depending on the structure and location of the IRE-RNA relative to the coding region, i.e. before or after ribosome binding. IRP binding to mRNA encoding iron uptake proteins, DMT1 and TFR1, protects the iron regulatory structures and prevents mRNA degradation. In such mRNA, the mRNA turnover element is downstream from the coding region; IRP binding stabilizes the concentration of the mRNA, thus increasing mRNA translation and protein synthesis. IRE-RNA/IRP binding to FTN and FPN mRNA, where the IRE is between the cap and the coding region, inhibits ribosome binding and protein synthesis/translation.
The IRP/IRE-RNA complex is most stable when cellular iron concentrations are low, which facilitates DMT1 and TFR1 synthesis and iron uptake into cells, while diminishing FTN and FPN synthesis to minimize iron storage and export. On the other hand, when intracellular iron is high and the IRP dissociation from the IRE-RNA increases, DMT1 and TRF1 mRNA are degraded, decreasing TFR and DMT1 and iron flow into the cell. And at the same time, ribosomes bind to the free (no repressor) FTN and FPN mRNA to synthesize FTN for mineralizing the extra iron and FPN for exporting the iron out of the cell. Because there are many copies of an mRNA, a small number of mRNA molecules will be free of IRP and will be translated, even when iron concentrations are low. The more tightly an IRE-mRNA binds IRP, the lower will be the translation when iron is low, because a much larger fraction of the mRNA molecules will be repressed. For that reason, translation of FTN mRNA, which has an IRE that binds IRP 10 times more tightly than mitochondrial aconitase, is much more inhibited by low iron than mitochondrial aconitase, an IRE-RNA with different, conserved base pairs than FTN (17, 18). Interestingly, although many of these observations were made in the 1990s, only when the crystal structure of IRP/IRE-RNA was recently obtained (19) could a mechanism be envisioned for the IRE-RNA/IRP dissociation. In the crystal structure, some surfaces of the IRE-RNA are exposed; complete coverage of the IRE-RNA by the IRP was the model before the crystal structure data. Some of the exposed RNA sites are, in experimental conditions, near Mg2+ or Cu2+-1,10-phenanthroline binding sites. Such data suggested that excess Fe2+ itself might bind to the RNA and free the IRE-RNA to be translated. In a recent study, Fe2+ did increase the number of free IRE-RNA and IRP molecules (17). The difference in IRE-RNA conformation free in solution and bound to IRP in the crystals and the effect of Fe2+ on the IRE-RNA/IRP complex show that IRE-RNA is an Fe2+-sensing riboswitch.
The regulation of FTN synthesis differs from that of other IRE-RNA–encoded iron trafficking proteins, because FTN DNA has a regulatory element, the antioxidant response element (ARE), that coordinates FTN synthesis with that of other antioxidant response proteins such as thioredoxin reductase and NADPH-quinone reductase. The ARE genes are repressed by Bach1, a heme-binding transcription repressor (20–22). In fact, the FTN DNA-ARE is very sensitive to peroxides, whereas the FTN RNA-IRE is very sensitive to iron. In combination, the FTN DNA-ARE and FTN RNA-IRE have a synergistic effect on FTN synthesis. The FTN antioxidant response is trapping Fe2+ and O2/H2O2, released from damaged proteins during oxidative stress, during iron biomineral synthesis inside the FTN protein nanocage. The concept of the FTN feedback loop, with iron and oxygen signals, is introduced in (23). In the FTN feedback loop, oxygen and iron signals induce FTN gene (DNA-ARE) transcription and/or FTN mRNA (IRE) translation, which results in the synthesis of more FTN protein. Then, the gene product, FTN protein, consumes iron and oxygen to make biomineral and shuts down the loop until the iron/oxygen signals again increase.
FTN is a central component in iron homeostasis and a dietary source of concentrated iron (4, 24–27) as well as a very complex protein (28, 29). Members of the FTN family are found in archaea, bacteria, plants, and animals. The importance of FTN is illustrated by the embryonic lethality of gene deletion (30). FTN is a multifunctional protein with a very unusual protein structure (Fig. 2). First, multiple subunits fold and self-assemble into a nanocage with ion channels that connect the outer cage surface to the interior mineral cavity and to the catalytic sites. Second, 2 Fe2+ and O2 react at the catalytic sites to begin mineral synthesis, forming the same diferric peroxo intermediate found in di-iron oxygenases. In eukaryotic FTN, the active sites are deeply buried; channels, which connect the active sites to the cavity, facilitate mineral buildup, because the channels exits are clustered so that emerging mineral nuclei can rapidly react with each other (31). It is the FTN mineral, containing thousands of iron atoms, that is the natural, nutritional iron concentrate.
FIGURE 2.
FTN protein with caged nanominerals enters polarized cells intact. (A) A eukaryotic FTN protein cage with a single subunit shown in green; the panel is original and based on the author’s own work in (31) using PDB file 1MFR. (B) Soybean FTN shown entering the apical side of cultured, polarized Caco-2 cells incubated in transwell plates and incubated for 30 min at 37°C with 5 nmol/L SoyFTN and a Z projection obtained from integration of the confocal microscopy slices using with a fluorescent tagged antisoybean FTN antiserum (left) or Oregon-green 488 tagged FTN (FTN) (right). The panels are original and based on the author’s own work in (10); FTN absorption is a clathrin-dependent endocytic process (10).
Absorption of dietary iron.
How receptors and transporters on the apical surface of the intestine recognize iron in the different chemical shapes and sizes found in digested foods is only partly understood; iron varies from a coordinated complex with a flat aromatic ring (heme) to inorganic iron ions (nonheme iron) to large, protein-caged iron minerals (FTN), which are also nonheme iron. Large iron complexes, such as iron dextran, which also contain mineralized iron similar to small FTN particles (32), are usually administered by injection and are not considered here. Currently, the iron absorbed from the intestine can be divided into 3 categories: heme, nonheme small iron salts or complexes, and nonheme iron minerals (FTN).
Heme iron is iron-protoporphyrin IX, a small and ancient metal iron cage that is absorbed intact. Released after digestion of the protein in meat (myoglobin), heme is transported into the intestinal cells by PCFT/HCP1, a member of the solute carrier family, named SLC46A1; the SLC group of integral membrane proteins has over 300 members and 47 families. PCFT/HCP1 was independently discovered to be the folate transporter mutated in hereditary folate malabsorption (33). Heme oxygenase, an integral membrane protein of the endoplasmic reticulum, releases the iron from the absorbed heme; how absorbed heme and the enzyme make contact in the cell is not known, but intracellular heme transporters have been identified (34). Whether there is another gene specific for heme iron or hemoglobin fragment absorption, or whether all heme absorption is shared with folate, is unknown.
Nonheme iron absorption from small iron complexes or salts, such as those found in most iron supplements, are taken up by DMT1 (nramp2), SLC11A, another proton-coupled divalent metal ion transporter, which is highly conserved in bacteria, plants, and animals and which imports iron from supplements such as ferrous sulfate, ferrous gluconate, etc. The DMT1 transporter recognizes natural, divalent metal ions such as Fe2+ and Mn2+, as well as toxic ions such as Cd2+ and Pd2+, moving all of them into intestinal cells from the gut lumen. Whether recognition involves removal of the water from the ion, which will change the size and recognition (35), is not known. DMT1 structure studies have just begun (36). For Fe3+ to enter the intestinal cell from nonheme iron supplements such as salts or chelators such as EDTA or citrate, the Fe3+ needs to bind to a reductase such as dcytb (37) after dissociating from the chelator in the case of EDTA or citrate and, after reduction, be released as hydrated Fe2+.
Nonheme iron absorption from coated, solid iron minerals (FTN) has begun to be studied relatively more recently than the other nutritional forms of iron. The stable protein cage around the solid iron mineral is a naturally coated iron concentrate used to provide iron at key stages of development such as germinating seeds and newborn mammals. When tested in rats and humans, absorption of iron from FTN was high, whether the FTN was in high-phytate soybeans grown to radiolabel FTN iron mineral endogenously (24, 25, 38), or in purified FTN demineralized and remineralized in vitro with radiolabeled iron. Because animal FTN minerals have almost no phosphate and plant FTN minerals have a high phosphate content, soy FTN was reconstituted with a Fe:P = 4:1 (26, 27, 39). The slow turnover of iron in minerals makes it impossible to add iron tracers to a FTN-rich food and achieve equilibration of the trace with iron in FTN mineral in a reasonable experimental time.
FTN protein is relatively stable to proteolysis (40) and is absorbed by phytate-resistant (24, 41), clathrin-dependent receptor endocytosis, mediated by a high affinity receptor that has not yet been identified (Fig. 2); iron from the absorbed exogenous FTN enters the cellular iron pool and the protein cage is degraded (10). Neither FeSO4 nor hemoglobin compete with absorption of ferritin iron in humans and iron from exogenous ferritin is transported across rat intestine ex vivo (E.C. Theil, H. Chen, M.T. Nunez, F. Pizarro, and K. Schumann, unpublished data). FTN iron or iron ions, found in many supplements and absorbed by DMT-1, are clearly 2 distinct chemical species of nutritional nonheme iron that are recognized and absorbed differently by the apical surface of the intestine.
Conclusions
Recognition of 3 different forms of dietary iron, with 3 different absorption mechanisms [heme, nonheme iron in small absorbable iron complexes, and nonheme iron in coated, solid iron minerals (FTN)] increases the types of nutritional iron sources to consider when developing supplements for different conditions and for dietary recommendations themselves, especially because consumption of heme iron is changing. FTN is enriched in whole lentils, soybeans, garbanzos/chickpeas, and many other beans (25, 42). Different absorption mechanisms of nonheme iron absorption from FTN and small iron complexes provide a new way to think about iron absorption and iron nutrition (43–45). Increased consumption of FTN iron, Nature’s iron concentrate, should have an impact on the centuries-old and vexingly unsolved problems of iron deficiency.
Acknowledgments
This paper is dedicated to the memory of John L. Beard, iron nutrition expert extraordinaire, who introduced me to iron nutritional studies during his sabbatical. Our collaborations were fruitful and our friendship enriching. The sole author had responsibility for all parts of the manuscript.
Footnotes
Published in a supplement to The Journal of Nutrition. Presented at the symposium, “Iron Works…The John Beard Memorial Symposium”, held in State College, PA, November 2, 2009. The symposium was organized by the Department of Nutritional Sciences as a tribute to Dr. Beard’s contribution to improving our understanding of iron metabolism. Its contents are solely the responsibility of the authors. The Supplement Coordinator for this supplement was Jere D. Haas, Cornell University. Supplement Coordinator disclosures: Jere D. Haas had no relationships to disclose. The supplement is the responsibility of the Guest Editor, to whom the Editor of The Journal of Nutrition has delegated supervision of both technical conformity to the published regulations of The Journal of Nutrition and general oversight of the scientific merit of each article. The Guest Editor for this supplement was Mary Cogswell, Centers for Disease Control. Guest Editor disclosure: Mary Cogswell had no relationships to disclose. Publication costs for this supplement were defrayed in part by the payment of page charges. This publication must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of The Journal of Nutrition.
Supported by NIH grants DK20251 and HL56169.
Abbreviations used: ARE, DNA antioxidant response element; HH, hereditary hemochromatosis; IRE, (mRNA) iron regulatory response element; SLC, a group of more than 300 genes encoding proteins that are solute carriers in cells.
Literature Cited
- 1.Kordas K. Iron, lead, and children’s behavior and cognition. Annu Rev Nutr. 2010;30:123–48 [DOI] [PubMed] [Google Scholar]
- 2.Macdougall IC. Iron supplementation in the non-dialysis chronic kidney disease (ND-CKD) patient: oral or intravenous? Curr Med Res Opin. 2010;26:473–82 [DOI] [PubMed] [Google Scholar]
- 3.Finberg KE, Heeney MM, Campagna DR, Aydinok Y, Pearson HA, Hartman KR, Mayo MM, Samuel SM, Strouse JJ, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40:569–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theil EC. Iron, ferritin and nutrition. Annu Rev Nutr. 2004;24:327–43 [DOI] [PubMed] [Google Scholar]
- 5.Ganz T. Cellular iron: ferroportin is the only way out. Cell Metab. 2005;1:155–7 [DOI] [PubMed] [Google Scholar]
- 6.Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol. 2006;2:406–14 [DOI] [PubMed] [Google Scholar]
- 7.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85 [DOI] [PubMed] [Google Scholar]
- 8.Wessling-Resnick M. Iron homeostasis and the inflammatory response. Annu Rev Nutr. 2010;30:105–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laftah AH, Latunde-Dada GO, Fakih S, Hider RC, Simpson RJ, McKie AT. Haem and folate transport by proton-coupled folate transporter/haem carrier protein 1 (SLC46A1). Br J Nutr. 2009;101:1150–6 [DOI] [PubMed] [Google Scholar]
- 10.San Martin CD, Garri C, Pizarro F, Walter T, Theil EC, Núñez MT. Caco-2 intestinal epithelial cells absorb soybean ferritin by mu2 (AP2)-dependent endocytosis. J Nutr. 2008;138:659–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Distante S, Robson KJ, Graham-Campbell J, Arnaiz-Villena A, Brissot P, Worwood M. The origin and spread of the HFE-C282Y haemochromatosis mutation. Hum Genet. 2004;115:269–79 [DOI] [PubMed] [Google Scholar]
- 12.Gao J, Chen J, De Domenico I, Koeller DM, Harding CO, Fleming RE, Koeberl DD, Enns CA. Hepatocyte-targeted HFE and TFR2 control hepcidin expression in mice. Blood. 2010;115:3374–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trenkwalder C, Hening WA, Montagna P, Oertel WH, Allen RP, Walters AS, Costa J, Stiasny-Kolster K, Sampaio C. Treatment of restless legs syndrome: an evidence-based review and implications for clinical practice. Mov Disord. 2008;23:2267–302 [DOI] [PubMed] [Google Scholar]
- 14.Muckenthaler MU, Galy B, Hentze MW. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu Rev Nutr. 2008;28:197–213 [DOI] [PubMed] [Google Scholar]
- 15.Leipuviene R, Theil EC. The family of iron responsive RNA structures regulated by changes in cellular iron and oxygen. Cell Mol Life Sci. 2007;64:2945–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shull GE, Theil EC. Regulation of ferritin mRNA: a possible gene-sparing phenomenon. Induction of ferritin synthesis by iron in liver as well as red cells combines high translational efficiency with increased utilization of preformed ferritin mRNA. J Biol Chem. 1983;258:7921–3 [PubMed] [Google Scholar]
- 17.Khan MA, Walden WE, Goss DJ, Theil EC. Direct Fe2+ sensing by iron-responsive messenger RNA:repressor complexes weakens binding. J Biol Chem. 2009;284:30122–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goforth JB, Anderson SA, Nizzi CP, Eisenstein RS. Multiple determinants within iron-responsive elements dictate iron regulatory protein binding and regulatory hierarchy. RNA. 2010;16:154–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walden WE, Selezneva AI, Dupuy J, Volbeda A, Fontecilla-Camps JC, Theil EC, Volz K. Structure of dual function iron regulatory protein 1 complexed with ferritin IRE-RNA. Science. 2006;314:1903–8 [DOI] [PubMed] [Google Scholar]
- 20.Hintze KJ, Theil EC. DNA and mRNA elements with complementary responses to hemin, antioxidant inducers, and iron control ferritin-L expression. Proc Natl Acad Sci USA. 2005;102:15048–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwasaki K, Mackenzie EL, Hailemariam K, Sakamoto K, Tsuji Y. Hemin-mediated regulation of an antioxidant-responsive element of the human ferritin H gene and role of Ref-1 during erythroid differentiation of K562 cells. Mol Cell Biol. 2006;26:2845–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hintze KJ, Katoh Y, Igarashi K, Theil EC. Bach1 repression of ferritin and thioredoxin reductase1 is heme-sensitive in cells and in vitro and coordinates expression with heme oxygenase1, beta-globin, and NADP(H) quinone (oxido) reductase1. J Biol Chem. 2007;282:34365–71 [DOI] [PubMed] [Google Scholar]
- 23.Theil EC, Goss DJ. Living with iron (and oxygen): questions and answers about iron homeostasis. Chem Rev. 2009;109:4568–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beard JL, Burton JW, Theil EC. Purified ferritin and soybean meal can be sources of iron for treating iron deficiency in rats. J Nutr. 1996;126:154–60 [DOI] [PubMed] [Google Scholar]
- 25.Murray-Kolb LE, Welch R, Theil EC, Beard JL. Women with low iron stores absorb iron from soybeans. Am J Clin Nutr. 2003;77:180–4 [DOI] [PubMed] [Google Scholar]
- 26.Davila-Hicks P, Theil EC, Lonnerdal B. Iron in ferritin or in salts (ferrous sulfate) is equally bioavailable in nonanemic women. Am J Clin Nutr. 2004;80:936–40 [DOI] [PubMed] [Google Scholar]
- 27.Lonnerdal B, Bryant A, Liu X, Theil EC. Iron absorption from soybean ferritin in nonanemic women. Am J Clin Nutr. 2006;83:103–7 [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Theil EC. Ferritin: dynamic management of biological iron and oxygen chemistry. Acc Chem Res. 2005;38:167–75 [DOI] [PubMed] [Google Scholar]
- 29.Lewin A, Moore GR, Le Brun NE. Formation of protein-coated iron minerals. Dalton Trans. 2005;3597–610 [DOI] [PubMed] [Google Scholar]
- 30.Ferreira C, Bucchini D, Martin ME, Levi S, Arosio P, Grandchamp B, Beaumont C. Early embryonic lethality of H ferritin gene deletion in mice. J Biol Chem. 2000;275:3021–4 [DOI] [PubMed] [Google Scholar]
- 31.Turano P, Lalli D, Felli I, Theil E, Bertini I. NMR reveals pathway for ferric mineral precursors to the central cavity of ferritin. Proc Natl Acad Sci USA. 2010;107:545–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theil EC, Sayers DE, Brown MA. Similarity of the structure of ferritin and iron. dextran (imferon) determined by extended X-ray absorption fine structure analysis. J Biol Chem. 1979;254:8132–4 [PubMed] [Google Scholar]
- 33.Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, et al. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917–28 [DOI] [PubMed] [Google Scholar]
- 34.Severance S, Hamza I. Trafficking of heme and porphyrins in metazoa. Chem Rev. 2009;109:4596–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Courville P, Chaloupka R, Cellier MF. Recent progress in structure-function analyses of Nramp proton-dependent metal-ion transporters. Biochem Cell Biol. 2006;84:960–78 [DOI] [PubMed] [Google Scholar]
- 36.Xiao S, Li J, Wang Y, Wang C, Xue R, Wang S, Li F. Identification of an "alpha-helix-extended segment-alpha-helix" conformation of the sixth transmembrane domain in DMT1. Biochim Biophys Acta. 2010;1798:1556–64 [DOI] [PubMed] [Google Scholar]
- 37.McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, et al. An iron regulated ferric reductase associated with the absorption of dietary iron. Science. 2001;291:1755–9 [DOI] [PubMed] [Google Scholar]
- 38.Burton JW, Harlow C, Theil EC. Evidence for reutilization of nodule iron in soybean seed development. J Plant Nutr. 1998;21:913–27 [Google Scholar]
- 39.Waldo GS, Wright E, Wang ZH, Briat JF, Theil EC, Sayers DE. Formation of the ferritin iron mineral occurs in plastids: an x-ray absorption spectroscopy (EXAFS) study. Plant Physiol. 1995;109:797–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crichton RR. Studies on the structure of ferritin and apoferritin from horse spleen. II. Chymotrypsin, subtilisin, cathepsin D and pepsin digestion of ferritin and apoferritin. Biochim Biophys Acta. 1971;229:75–82 [DOI] [PubMed] [Google Scholar]
- 41.Kalgaonkar S, Lonnerdal B. Effects of dietary factors on iron uptake from ferritin by Caco-2 cells. J Nutr Biochem. 2008;19:33–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lukac RJ, Aluru MR, Reddy MB. Quantification of ferritin from staple food crops. J Agric Food Chem. 2009;57:2155–61 [DOI] [PubMed] [Google Scholar]
- 43.Lonnerdal B. Soybean ferritin: implications for iron status of vegetarians. Am J Clin Nutr. 2009;89:S1680–5 [DOI] [PubMed] [Google Scholar]
- 44.Theil EC, Burton JW, Beard JL. A sustainable solution for dietary iron deficiency through plant biotechnology and breeding to increase seed ferritin control. Eur J Clin Nutr. 1997;51 Suppl 4:S28–31 [PubMed] [Google Scholar]
- 45.Beard J. One person’s view of iron deficiency, development, and cognitive function. Am J Clin Nutr. 1995;62:709–10 [DOI] [PubMed] [Google Scholar]