Abstract
Intestinal T-cell and natural killer (NK)–cell lymphomas are clinically aggressive and can be challenging to diagnose in small endoscopic biopsies. We describe 8 patients in whom atypical NK-cell lymphoproliferative lesions mimicked NK- or T-cell lymphoma. The patients (2 men; 6 women; ages 27-68 years) presented with vague gastrointestinal symptoms with lesions involving stomach, duodenum, small intestine, and colon. At endoscopy, the lesions exhibited superficial ulceration, edema, and hemorrhage. Biopsies revealed a mucosal infiltrate of atypical cells with an NK-cell phenotype (CD56+/TIA-1+/Granzyme B+/cCD3+), which displaced but did not invade the glandular epithelium. Epstein-Barr virus–encoded RNA in situ hybridization was negative, and T-cell receptor-γ gene rearrangement showed no evidence of a clonal process. Based on an original diagnosis of lymphoma, 3 patients received aggressive chemotherapy followed by autologous bone marrow transplantation in 2. Five patients were followed without treatment. However, no patient developed progressive disease or died of lymphoma (median follow-up, 30 months). Repeat endoscopies in 6 of 8 patients showed persistence or recurrence of superficial gastrointestinal lesions. This unique entity mimics intestinal and NK-/T-cell lymphomas on endoscopic biopsies and can result in erroneous diagnosis, leading to aggressive chemotherapy. We propose the term “NK-cell enteropathy” for this syndrome of as yet unknown etiology.
Introduction
Extranodal natural killer (NK)-/T-cell lymphomas are postulated to originate from activated NK cells, although some cases have been shown to have a true T-cell derivation.1 Distinctive morphologic features of extranodal NK-/T-cell lymphoma are an angiocentric and angiodestructive growth pattern with frequent necrosis and apoptosis. These lymphomas are almost always associated with Epstein-Barr virus (EBV) and have not been linked with celiac disease.2–4 The nasal cavity and upper aerodigestive tract are the most common sites of origin, but involvement of the gastrointestinal tract is common and the gastrointestinal tract may be the primary site of presentation.5 The differential diagnosis of gastrointestinal NK-cell and T-cell lymphomas includes enteropathy-associated T-cell lymphoma (EATL; commonly associated with active or latent celiac disease), γδ T-cell lymphoma, and more rarely anaplastic large cell lymphoma, anaplastic lymphoma kinase–positive.6–10 All of these lymphomas are clinically aggressive diseases approached with systemic chemotherapy. However, there are 2 case reports in the literature of relapsing but indolent clonal T-cell proliferations in the gastrointestinal tract.11,12
NK cells, a subset of lymphocytes, are an important element of innate immunity. These cells exhibit cytotoxic function and participate in control of various viruses and tumors. As innate sentinels, NK cells are distributed in peripheral blood (10%-15% of lymphocytes), lymphoid tissue, spleen, and extranodal sites. NK cells do not bear T-cell receptors or cell surface immunoglobulins.13 These cells are identified in gastrointestinal mucosa with a primary role in innate immunity.14 However, recently, it has been shown that NK cells also have a cytokine-producing effector function and can act as regulatory cells during inflammation and influence subsequent adaptive immune responses.15 In event of acute inflammation or autoimmune reactions, localization of NK cells at various anatomic sites, including skin and gastrointestinal tract, has been documented.14,16 Relevant to their normal distribution, neoplastic lesions of NK cells are primarily seen at extranodal sites, including the gastrointestinal tract.5,17 These lymphomas show distinct geographic and ethnic distribution, constituting up to 3% of all non-Hodgkin lymphomas in Asia, but are uncommon in the United States and Europe.5
We previously reported a case in which an atypical NK-cell proliferation with multiple ulcerative lesions in the gastrointestinal tract mimicked NK-cell lymphoma.18 That patient had antigliadin antibodies but lacked other evidence of celiac disease. Since then, we have encountered in our consultation practice 7 additional patients with similar clinicopathologic findings. This report reviews the features of these 8 patients, which we propose, represent an as yet unrecognized clinical and pathologic syndrome. In all patients, atypical and persistent NK-cell proliferations mimicked NK-cell lymphoma, resulting in unnecessary invasive investigations; 3 patients received aggressive chemotherapy with or without bone marrow transplantation based on a presumptive diagnosis of an NK-cell malignancy.
Methods
In each case, an endoscopic biopsy led to a pathologic consultation by the Hematopathology Section, National Cancer Institute, National Institutes of Health, Bethesda, MD between 2006 and 2010. Clinical information, endoscopic findings, and other relevant laboratory data were provided along with the biopsy material. The Institutional Review Board of the National Cancer Institute approved this study. All biopsy specimens were fixed in 10% buffered formalin, embedded in paraffin, sectioned (4 μm), and stained with hematoxylin and eosin. Additional studies were performed at the National Institutes of Health. Immunohistochemical stains were performed using an automated immunostainer (Ventana Medical Systems) according to the company's protocols, as previously described.19 Tissue sections were incubated with antibodies specific for Ki-67 (MIB-1), CD20 (L26), CD3 (F7.2.38), CD8 (144B), CD30 (Ki-1), CD68 (KP1), CD5 (CD5/54/F6), CD7 (CBC.37), and EBV-LMP-1 (CS1-4) (Dako North America); CD4 (4B12) and CD56 (1B6) (Novocastra); PAX-5 (Transduction Labs); CD138 (B-B4) (Serotec); CD10 (56C6; Lab Vision); TIA-1 (2G9A10F5) (Beckman Coulter); Granzyme B (BrB7; Monosan); and CD43(L60 (BD Biosciences). In situ hybridization for EBV-encoded RNA was conducted on formalin-fixed paraffin sections using a fluorescein isothiocyanate–labeled oligonucleotide probe supplied by Ventana on an automated stainer (Ventana-Benchmark).20 Visualization was achieved using the ISH iView system with alkaline phosphatase and nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate substrate, with Fast red as contrast. Multicolor flow cytometry was performed in selective patients, using a panel of T cell- and NK cell-associated fluorochrome-conjugated antibodies.21 Molecular analysis to assess rearrangement of the T-cell receptor-γ chain gene was performed using polymerase chain reaction on formalin-fixed, paraffin-embedded tissue, according to standard laboratory protocols in Clinical Laboratory Improvement Amendments-approved laboratories using the published methods of either McCarthy et al22 or Trainor et al.23 Both methods have a sensitivity of more than 90% for the detection of a clonal T-cell process.
Results
Clinical features
Clinical features are summarized in Table 1. The patients included 2 men and 6 women (male/female ratio = 1:3; between the ages of 27 and 68 years; mean, 47 years; median, 46 years). Seven patients were of white origin, whereas one patient had Hispanic ethnicity. Seven patients presented with vague gastrointestinal symptoms, including abdominal pain, constipation, diverticulosis, and reflux. One patient was asymptomatic and underwent elective colonoscopy because of a family history of colon cancer (case 1). There was no prior history of celiac disease, inflammatory bowel disease, or malabsorption. None of these patients had clinical evidence of any lymphadenopathy or organomegaly. Endoscopic examinations showed multiple small lesions (mucosal hemorrhages, target lesions, or superficial bleeding ulcers 1-2 cm; Figure 1). In 4 patients, the lesions were identified in a single gastrointestinal site (4 of 8; 50%) involving stomach (1) and colon (3), respectively. The other 4 had involvement of multiple gastrointestinal sites (stomach, duodenum, small intestine, and colon). An extensive imaging workup (including computed tomography scan, magnetic resonance imaging, positron emission tomography scan) was performed in all patients and showed no evidence of lymphadenopathy, organomegaly, or masses. Two patients had biopsies of tonsil and lymph node, respectively (patients 1, 5).
Table 1.
Demographics, clinical presentation, endoscopic findings, and follow-up
| Patient no. | Age, y/sex | Clinical presentation | Gastrointestinal site(s) | Endoscopic findings | Follow-up (months) |
|---|---|---|---|---|---|
| 1* | 31/M | Asymptomatic, elective colonoscopy | Stomach, small intestine, colon | Superficial erythematous lesions | A/P (84) |
| 2 | 27/F | Abdominal pain, hematochezia | Stomach | Multiple, superficial ulcers | A/P (23) |
| 3 | 29/F | Constipation, rectal bleeding | Colon | Ulcerative lesions | A† (31) |
| 4 | 53/M | Asymptomatic | Stomach, duodenum | Gastric lesions | A/P (30) |
| 5 | 46/F | Chronic vague abdominal symptoms | Duodenum, colon | Superficial ulcers | A/P (36)‡ |
| 6 | 64/F | Abdominal pain, diverticulosis | Colon | Multiple erosions/ulcers | A/P (22) |
| 7 | 61/F | Vague abdominal symptoms, history of polyps | Duodenum, colon | Multiple ulcerations | A/P (120)‡ |
| 8 | 68/F | Abdominal pain, diarrhea, diverticulosis | Colon | Ulcerations | A (22)‡ |
A/P indicates alive with persistent disease but without progression; and A, alive.
Index case, previously reported.
Lost to follow-up.
These patients received chemotherapy for an initial presumptive diagnosis of lymphoma.
Figure 1.
Endoscopic images of NK-cell enteropathy. (A-B) Colonic lesions. The colonic lesions generally appeared as ulcers with surrounding erythema and edema (A), but some lesions had a more nodular or polypoid appearance (B). (C-D) Gastric and duodenal lesions. The main lesions noted were raised ulcer-like lesions surrounded by erythema and edema (duodenal lesion; C); a few small punctuate deep ulcers in the stomach (D) were also noted and had similar pathologic appearance as the other NK-cell lesions throughout the gastrointestinal tract. Images were processed with Adobe Photoshop.
Follow-up and clinical management
All patients were closely followed by regular imaging studies and clinical evaluation. During the follow-up period (22-120 months; median, 30 months), continued endoscopic evaluation and repeat biopsies revealed persistence of lesions in the large bowel and other gastrointestinal sites in 6 patients. However, no progression was noted in terms of size or extent of involvement. One patient refused repeat endoscopy (patient 3) but has had persistence of vague gastrointestinal symptoms. One patient has not undergone repeat endoscopy after chemotherapy for an initial suspected diagnosis of lymphoma (patient 8). In 1 patient (patient 1), implementation of a gluten-free and lactose-free diet led to a significant improvement clinically, with the lesions reduced in number and in size, but histologic features of celiac disease were not identified. Moreover, recurrence was noted on the restricted diet with persistent lesions observed during annual endoscopic evaluations and biopsies performed over a 7-year period.
In 3 patients (patients 5, 7, and 8), chemotherapy with the CHOP (cyclophosphamide, adriamycin, vincristine, and prednisone) regimen was initiated; and in 2 patients (patients 5, 8), this was followed by autologous bone marrow transplantation because of the presumed aggressive nature of the disease. These 3 patients are well and asymptomatic after 22 to 120 months of follow-up. However, 2 of the 3 who underwent repeat endoscopy have had recurrence of the gastrointestinal lesions confirmed by biopsy. No further therapy was instituted in these patients because of reassessment of the findings after pathologic consultation with the authors.
Histologic findings
The gastrointestinal biopsies showed similar morphologic features among all patients. There was expansion of the lamina propria by a relatively well-circumscribed but confluent infiltrate of intermediate to large-sized cells with irregular nuclei, inconspicuous nucleoli, finely clumped chromatin, and a moderate amount of pale cytoplasm (Figure 2). In early-phase lesions, the mucosal glands were displaced because of dense atypical cellular infiltrate; however, in advanced stages, sheets of atypical cells with destruction of mucosal glands were noted. There was in general an absence of epitheliotropism identified in glandular epithelium. No angiocentricity or angiodestructive pattern of growth was seen in any patient. Focal infiltration of the submucosa was seen rarely, but in most instances the muscularis mucosa, if observed, was intact. Apart from areas of mucosal ulceration, necrosis was absent, but focal apoptotic bodies were present. Focal superficial hemorrhage was observed in conjunction with some of the infiltrates. A rim of small mature lymphocytes (mainly B cells) and a polymorphous infiltrate of eosinophils, plasma cells, and histiocytes surrounded the atypical infiltrates or was present in the base. In some cases, the adjacent mucosa contained lymphoid follicles. No villous atrophy or crypt hyperplasia was identified in any sample. Peripheral blood and bone marrow specimens obtained (6 of 8 patients) around the time of the gastrointestinal biopsies showed neither atypical cells nor increase in large granular lymphocytes. In 2 patients, additional biopsies of tonsil and lymph node were performed (patients 1, 5). These were histologically unremarkable.
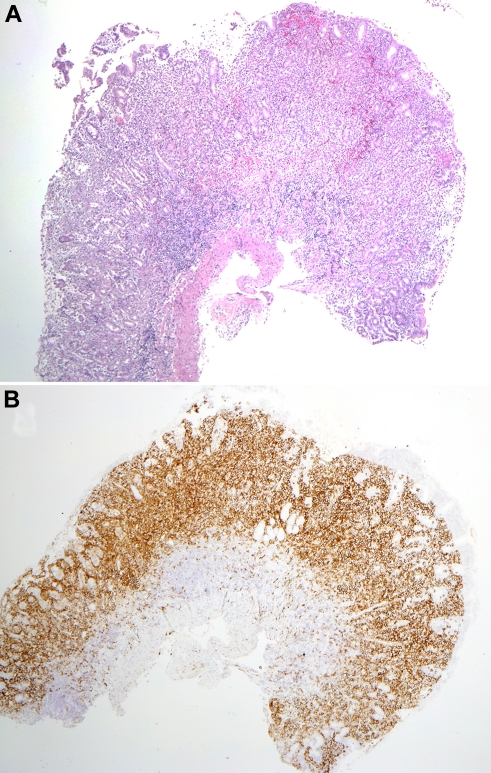
Figure 2.
Mucosal biopsy findings. (A) Biopsy of the gastric antrum shows diffuse infiltration of the lamina propria by the atypical cells. The muscularis mucosa is intact, without infiltration of the submucosa. (B) A CD56 stain of the same biopsy highlights the atypical infiltrate. Photomicrographic images were acquired with a Nikon Eclipse 50i microscope equipped with an Olympus DP71 camera and software. Final image preparation was performed with Adobe Photoshop CS3 extended Version 10.0.1. Magnification: 2×/0.1 NA Plan Apo lens.
Immunohistochemical and molecular results
The atypical cells in the gastrointestinal biopsies expressed CD56, CD7, cytoplasmic CD3, TIA-1, and/or Granzyme B, but not CD5, CD4, CD8, CD10, CD20, CD30, EBV-latent membrane protein (LMP), PAX-5, CD138, or CD68 (Figure 3; Table 2). CD2 was negative in 3 cases and focally positive in one. This immunophenotype suggested an NK-cell origin of the atypical cells. The proliferative fraction as determined by MIB-1 or Ki-67 was low (average ∼ 25%). EBV-encoded RNA was not detected in any patient, by in situ hybridization technique using EBV-encoded RNA probe. Patient 1 had a biopsy of tonsil showing few clusters of CD56+/ CD3+ lymphoid cells in a background of reactive hyperplasia, not thought to be indicative of lymphoma. Flow cytometry was performed on lymphoid cells isolated from the biopsy specimens in cases 1 and 5. The cells failed to express surface CD3 but were positive for cytoplasmic CD3 and strongly positive for CD56 and CD7. The cells were negative for CD4, CD8, and CD5. Because of limited material available in these endoscopic biopsies, flow cytometry was not performed on additional cases. Polymerase chain reaction studies for clonal rearrangement of the T-cell receptor-γ gene were performed in each case and failed to identify evidence of a clonal process.
Figure 3.
Histologic and immunohistochemical features of NK-cell enteropathy. Representative cases are shown involving stomach (A-D), duodenum (E), and colon (F). (A) The atypical cells infiltrate the mucosa, separating and displacing the glands. (B) Epitheliotropism is largely absent, but the cells show cytologic atypia, with irregular nuclei and eosinophilic, granular cytoplasm. (C) NK cells are strongly positive for CD56 and diffusely infiltrate the mucosa, with rare cells infiltrating the glandular epithelium. The infiltrate does not extend to the submucosa. (D) The cells express cytoplasmic CD3 (D), and immunohistochemical stains highlight the cytologic atypia. (E) CD56 identifies atypical NK cells diffusely infiltrating and expanding duodenal villi. (F) Atypical cells surround colonic glands with focal areas of superficial hemorrhage. (C-E) Immunohistochemistry with avidin-biotin-peroxidase complex immunoperoxidase technique and hematoxylin counterstain. Photomicrographic images were acquired with a Nikon Eclipse 50i microscope equipped with an Olympus DP71 camera and software. Final image preparation was performed with Adobe Photoshop CS3 extended Version 10.0.1. Original magnifications: panel A, 4×/0.2 NA; panel B, 40×/0.95 NA; panel C, 10×/0.45 NA; panel D, 100×/1.40 NA; panel E, 10×/0.45 NA; panel F, 20×/0.75 NA.
Table 2.
Immunophenotypic and molecular studies
| Patient no. | cCD3 | CD56 | TIA/GRZB | CD7 | CD5 | CD4/CD8 | CD20 | CD43 | EBER | TCR |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | + | + | − | − | − | + | − | Polyclonal |
| 2 | + | + | NA | + | − | − | − | + | − | Polyclonal |
| 3 | + | + | + | + | − | − | − | + | − | Polyclonal |
| 4 | + | + | + | + | − | − | − | + | − | Polyclonal |
| 5 | + | + | + | + | − | − | − | NA | − | Polyclonal |
| 6 | + | + | + | + | − | − | − | + | − | Polyclonal |
| 7 | + | + | + | + | − | − | − | + | − | Oligoclonal |
| 8 | + | + | + | NA | − | − | − | + | − | Polyclonal |
cCD3 indicates cytoplasmic CD3; GRZB, Granzyme B; EBER, Epstein-Barr virus-encoded RNA; TCR, T-cell receptor-γ gene rearrangement by polymerase chain reaction; +, positive; −, negative; and NA, not applicable.
Discussion
In this report, we describe a unique series of 8 patients, who presented with vague abdominal symptoms resulting from persistent, multiple, mucosal involvement of the gastrointestinal tract by an extensive atypical NK-cell lymphoproliferative process. Among all patients, a diagnosis of “NK-cell lymphoma” was suspected at initial review because of the strong expression of CD56 and cytoplasmic CD3, in the absence of clonal T-cell receptor-γ gene rearrangement by PCR analysis. Negative studies for EBV essentially eliminated the diagnosis of extranodal NK-/T-cell lymphoma.1 All patients were extensively investigated by invasive procedures for an aggressive lymphoma, but lesions were limited to the gastrointestinal tract. Three patients ultimately received high-dose chemotherapy with or without bone marrow transplantation because of a presumptive diagnosis of lymphoma. The etiology of this process is unknown; but based on the protracted but indolent course in all patients, we think it is benign, although clonality cannot be determined in these NK cell-derived lesions. Cytogenetic studies were attempted in case 1 but were unsuccessful because of limited cell recovery and the absence of sufficient metaphases. In the future, additional cytogenetic or molecular studies would be of interest to investigate the presence of genetic aberrations. Based on our current data, we propose the term “NK-cell enteropathy” for this novel condition.
This series is unique in several aspects. Atypical but indolent NK-cell proliferations at extranodal sites have not been documented in the literature and are not recognized as a form of neoplasia in the new World Health Organization classification of hematopoietic and lymphoid tissue.24 We previously reported the first patient (patient 1) as an atypical NK-cell lymphoproliferative lesion in gastrointestinal tract.18 Since then, we have encountered 7 additional patients with a remarkably similar clinical syndrome and pathologic features. Recognition and detailed description of this entity are critically important to prevent misdiagnosis as an aggressive disease, such as NK-cell or T-cell lymphoma.
The differential diagnosis at the time of biopsy included EATL. Two forms of EATL are recognized in the World Health Organization classification,25 referred to as type I and type II, also termed the monomorphic variant.8,26 Both are characteristically associated with celiac disease and are of cytotoxic T-cell origin. However, the type II form also can occur sporadically and more closely mimics NK-cell enteropathy. The cells are CD56+, and generally round and moderate in cell size.8 In addition, type II EATL is usually CD8+, which was negative in all cases in the present series. In contrast to NK-cell enteropathy, the infiltrate shows marked epitheliotropism, which was largely absent. The absence of clonal T-cell gene rearrangement further precludes a diagnosis of EATL in the present cases.
Celiac disease is an intestinal inflammatory disorder induced by dietary gluten in genetically susceptible persons, with extensive expansion of intraepithelial cytotoxic T lymphocytes (CTLs). Genetic reprogramming of these CTLs leads first to oligoclonal expansion, gluten-independent tissue damage, and latter uncontrolled CTL proliferation leading to malignant lymphoma.27 It has been suggested that EATL is a stepwise transformation of CTLs into NK-like T cells by the underlying immunopathology.28,29 These observations might lead to speculation that NK-cell enteropathy may represent a precursor lesion of aggressive NK-cell lymphomas. Indolent NK-cell proliferations, progressing to aggressive NK-cell malignancies after a long follow-up, are rare but are described in 2 case reports.30,31 However, in our case series, persistence of the gastrointestinal lesions without evidence of clinical or pathologic progression argues against this eventuality.
NK cells (CD16/CD56+) are a subset of lymphocytes associated with innate immunity and cytotoxic function against viruses and tumor cells, with primary distribution in peripheral blood, lymphoid tissue, and spleen.15 Less is known regarding the presence and function of NK cells in epithelial tissues. NK cells have been detected in the gut, where their prime function is to provide innate immunity.14 A subset of NK cells in gastrointestinal mucosa is devoid of cytotoxic function and has low levels of TIA-1 and Granzyme B.32 Because the NK-cell infiltrates in all of our patients had relatively high expression of Granzyme B and/or TIA-1, it appears that functionally the NK cells in these lesions were primed for the cytotoxic function, most probably responding to local inflammation, autoimmunity, or viruses.
It has been recently reported that NK cells can mediate hapten-specific recall responses, independent of B cells and T cells, in a model of contact hypersensitivity.33 In humans, NK cells have been shown to home to inflamed skin in various conditions, such as vernal keratoconjunctivitis,34 atopic dermatitis,35 psoriasis,36 and lichen planus.16 Among our series, the presence of persistent lesions in gastrointestinal tract with minimal or no involvement at other anatomic sites indicates that the NK-cell proliferation was primarily a localized phenomenon in the gastrointestinal tract, most probably secondary to local inflammation or immune reaction. The exact stimulus remains to be determined.
Since submission of this manuscript, Takeuchi et al described a virtually identical process to NK-cell enteropathy involving the stomach in 10 patients from Japan.37 As in our cases, lymphoma was suspected histologically, but no patient had progressive disease, and spontaneous regression was observed in most cases. Two patients had gastrectomies for presumptive diagnoses of malignancy. Interestingly, 3 of the patients had a history of gastric cancer, which prompted gastroscopy. Takeuchi et al proposed the term “lymphomatoid gastropathy” for their cases and did not report lesions in the small or large bowel.37 However, it is not clear to what extent these patients were investigated for intestinal disease.
In intestinal immunology, it is important to distinguish between NK cells and natural killer T cells. NK T cells represent a minor subset of T lymphocytes that share cell-surface proteins with conventional T cells and NK cells. Being an essential component of intestinal mucosal immunity, NK T cells are present primarily as intraepithelial lymphocytes but also in lamina propria.38 The role of NK T cells in intestinal immunity is complex and paradoxical (promotion or suppression) probably because of the existence of functionally distinct subsets. These subsets can be CD4+, CD8+, CD4+/CD8+, and a minor subset (1%-2%) negative for both CD4 and CD8. Distinction by routine immunohistochemistry between true NK cells and this minor subset of NK T cells (CD4−/CD8−) is currently not possible; however, expression of CD56 is unique to NK cells and has not been reported for NK T cells.39 This finding suggests that the cellular proliferation in our patients is of NK-cell derivation.
In conclusion, we describe a unique case series characterized by atypical NK-cell proliferative lesions involving the gastrointestinal mucosa in adult patients who presented clinically with vague or nonspecific abdominal symptoms. The pathologic features on endoscopic biopsies showed a diffuse and destructive atypical NK-cell proliferation, which was initially misdiagnosed as aggressive NK-cell lymphoma. This resulted in unnecessary extensive and invasive investigations, and in 3 patients led to treatment with combination chemotherapy with or without bone marrow transplantation. It is extremely critical to recognize this lesion as a distinctive clinicopathologic entity, to prevent misinterpretation, which can lead to harmful consequences for the patients. We propose the term “NK-cell enteropathy” for this condition to allow identification of other cases and investigation of its pathogenesis. A close partnership between the pathologist and clinician is required to recognize this condition, with a careful clinical history and description of the endoscopic findings.
Acknowledgments
The authors thank the pathologists (Drs Jeffrey Chang, Carol Adair, Violette Ghali, Steven Kussick, Patrick Treseler, and Sara Duesterhoeft), gastroenterologists (Drs Ray Verm, Bhavani Moparty, Babak Mohajer, Allen Savoy, Kevin S. Palumbo, and Michael Rifkins), and oncologists (Drs Douglas A. Stewart, Johan Lategan, and Douglas Gladstone) who contributed these unique cases for consultation to the National Cancer Institute, National Institutes of Health, and provided clinical and follow-up information, and Dr Mark Raffeld and the Specialized Diagnostics Unit of the Laboratory of Pathology, National Cancer Institute for technical support.
This work was supported by the intramural research budget of the Center for Cancer Research, National Cancer Institute.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.S.J. described this entity and identified all patients; A.M. and E.S.J. collected data and wrote the manuscript; S.P. reviewed pathology material and contributed to the manuscript; and P.L.B., W.H.W., and J.A.F. provided pathologic and clinical information and contributed to the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elaine S. Jaffe, Hematopathology Section, Laboratory of Pathology, 10 Center Dr, Bldg 10, Rm 2B42, National Institutes of Health, Bethesda, MD 20892-1500; e-mail: elainejaffe@nih.gov.
References
- 1.Chan JKC, Quintanilla-Martinez L, Ferry JA, Peh SC. Extranodal NK-/T-cell lymphoma, nasal type. In: Swerdlow S, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: International Agency for Research on Cancer Press; 2008. pp. 285–288. [Google Scholar]
- 2.Meijer JW, Mulder CJ, Goerres MG, Boot H, Schweizer JJ. Coeliac disease and (extra)intestinal T-cell lymphomas: definition, diagnosis and treatment. Scand J Gastroenterol Suppl. 2004;241:78–84. doi: 10.1080/00855920410014605. [DOI] [PubMed] [Google Scholar]
- 3.van de Water JM, Cillessen SA, Visser OJ, Verbeek WH, Meijer CJ, Mulder CJ. Enteropathy associated T-cell lymphoma and its precursor lesions. Best Pract Res Clin Gastroenterol. 2010;24(1):43–56. doi: 10.1016/j.bpg.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Kwong YL, Chan AC, Liang R, et al. CD56+ NK lymphomas: clinicopathological features and prognosis. Br J Haematol. 1997;97(4):821–829. doi: 10.1046/j.1365-2141.1997.1462962.x. [DOI] [PubMed] [Google Scholar]
- 5.Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113(17):3931–3937. doi: 10.1182/blood-2008-10-185256. [DOI] [PubMed] [Google Scholar]
- 6.Jaffe ES. Pathobiology of peripheral T-cell lymphomas. Hematology Am Soc Hematol Educ Program. 2006:317–322. doi: 10.1182/asheducation-2006.1.317. [DOI] [PubMed] [Google Scholar]
- 7.Bagdi E, Diss TC, Munson P, Isaacson PG. Mucosal intra-epithelial lymphocytes in enteropathy-associated T-cell lymphoma, ulcerative jejunitis, and refractory celiac disease constitute a neoplastic population. Blood. 1999;94(1):260–264. [PubMed] [Google Scholar]
- 8.Chott A, Haedicke W, Mosberger I, et al. Most CD56+ intestinal lymphomas are CD8+CD5-T-cell lymphomas of monomorphic small to medium size histology. Am J Pathol. 1998;153(5):1483–1490. doi: 10.1016/S0002-9440(10)65736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gale J, Simmonds PD, Mead GM, Sweetenham JW, Wright DH. Enteropathy-type intestinal T-cell lymphoma: clinical features and treatment of 31 patients in a single center. J Clin Oncol. 2000;18(4):795–803. doi: 10.1200/JCO.2000.18.4.795. [DOI] [PubMed] [Google Scholar]
- 10.Arnulf B, Copie-Bergman C, Delfau-Larue MH, et al. Nonhepatosplenic gammadelta T-cell lymphoma: a subset of cytotoxic lymphomas with mucosal or skin localization. Blood. 1998;91(5):1723–1731. [PubMed] [Google Scholar]
- 11.Egawa N, Fukayama M, Kawaguchi K, et al. Relapsing oral and colonic ulcers with monoclonal T-cell infiltration: a low grade mucosal T-lymphoproliferative disease of the digestive tract. Cancer. 1995;75(7):1728–1733. doi: 10.1002/1097-0142(19950401)75:7<1728::aid-cncr2820750727>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Ranheim EA, Jones C, Zehnder JL, Warnke R, Yuen A. Spontaneously relapsing clonal, mucosal cytotoxic T-cell lymphoproliferative disorder: case report and review of the literature. Am J Surg Pathol. 2000;24(2):296–301. doi: 10.1097/00000478-200002000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tagliabue A, Befus AD, Clark DA, Bienenstock J. Characteristics of natural killer cells in the murine intestinal epithelium and lamina propria. J Exp Med. 1982;155(6):1785–1796. doi: 10.1084/jem.155.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 16.Parolini S, Santoro A, Marcenaro E, et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood. 2007;109(9):3625–3632. doi: 10.1182/blood-2006-08-038844. [DOI] [PubMed] [Google Scholar]
- 17.Ng SB, Lai KW, Murugaya S, et al. Nasal-type extranodal natural killer/T-cell lymphomas: a clinicopathologic and genotypic study of 42 cases in Singapore. Mod Pathol. 2004;17(9):1097–1107. doi: 10.1038/modpathol.3800157. [DOI] [PubMed] [Google Scholar]
- 18.Vega F, Chang CC, Schwartz MR, et al. Atypical NK-cell proliferation of the gastrointestinal tract in a patient with antigliadin antibodies but not celiac disease. Am J Surg Pathol. 2006;30(4):539–544. doi: 10.1097/00000478-200604000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Krenacs L, Smyth MJ, Bagdi E, et al. The serine protease granzyme M is preferentially expressed in NK-cell, gamma delta T-cell, and intestinal T-cell lymphomas: evidence of origin from lymphocytes involved in innate immunity. Blood. 2003;101(9):3590–3593. doi: 10.1182/blood-2002-09-2908. [DOI] [PubMed] [Google Scholar]
- 20.Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES. EBV positive mucocutaneous ulcer: a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34(3):405–417. doi: 10.1097/PAS.0b013e3181cf8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morice WG, Kurtin PJ, Leibson PJ, Tefferi A, Hanson CA. Demonstration of aberrant T-cell and natural killer-cell antigen expression in all cases of granular lymphocytic leukaemia. Br J Haematol. 2003;120(6):1026–1036. doi: 10.1046/j.1365-2141.2003.04201.x. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy KP, Sloane JP, Kabarowski JH, Matutes E, Wiedemann LM. A simplified method of detection of clonal rearrangements of the T-cell receptor-gamma chain gene. Diagn Mol Pathol. 1992;1(3):173–179. [PubMed] [Google Scholar]
- 23.Trainor KJ, Brisco MJ, Wan JH, Neoh S, Grist S, Morley AA. Gene rearrangement in B- and T-lymphoproliferative disease detected by the polymerase chain reaction. Blood. 1991;78(1):192–196. [PubMed] [Google Scholar]
- 24.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: International Agency for Research on Cancer Press; 2008. [Google Scholar]
- 25.Isaacson PG, Chott A, Ott G, Stein H. Enteropathy-associated T-cell lymphoma. In: Swerdlow S, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: International Agency for Research on Cancer Press; 2008. pp. 289–291. [Google Scholar]
- 26.Deleeuw RJ, Zettl A, Klinker E, et al. Whole-genome analysis and HLA genotyping of enteropathy-type T-cell lymphoma reveals 2 distinct lymphoma subtypes. Gastroenterology. 2007:1902–1911. doi: 10.1053/j.gastro.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 27.Meresse B, Curran SA, Ciszewski C, et al. Reprogramming of CTLs into natural killer-like cells in celiac disease. J Exp Med. 2006;203(5):1343–1355. doi: 10.1084/jem.20060028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jabri B, Kasarda DD, Green PH. Innate and adaptive immunity: the yin and yang of celiac disease. Immunol Rev. 2005;206:219–231. doi: 10.1111/j.0105-2896.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- 29.Isaacson PG. Relation between cryptic intestinal lymphoma and refractory sprue. Lancet. 2000;356(9225):178–179. doi: 10.1016/S0140-6736(00)02472-7. [DOI] [PubMed] [Google Scholar]
- 30.Roullet MR, Cornfield DB. Large natural killer cell lymphoma arising from an indolent natural killer cell large granular lymphocyte proliferation. Arch Pathol Lab Med. 2006;130(11):1712–1714. doi: 10.5858/2006-130-1712-LNKCLA. [DOI] [PubMed] [Google Scholar]
- 31.Huang Q, Chang KL, Gaal KK, Weiss LM. An aggressive extranodal NK-cell lymphoma arising from indolent NK-cell lymphoproliferative disorder. Am J Surg Pathol. 2005;29(11):1540–1543. doi: 10.1097/01.pas.0000168510.54867.9a. [DOI] [PubMed] [Google Scholar]
- 32.Long EO. Ready for prime time: NK cell priming by dendritic cells. Immunity. 2007;26(4):385–387. doi: 10.1016/j.immuni.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 33.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7(5):507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 34.Lambiase A, Normando EM, Vitiello L, et al. Natural killer cells in vernal keratoconjunctivitis. Mol Vis. 2007;13:1562–1567. [PubMed] [Google Scholar]
- 35.Buentke E, Heffler LC, Wilson JL, et al. Natural killer and dendritic cell contact in lesional atopic dermatitis skin: Malassezia-influenced cell interaction. J Invest Dermatol. 2002;119(4):850–857. doi: 10.1046/j.1523-1747.2002.00132.x. [DOI] [PubMed] [Google Scholar]
- 36.Ottaviani C, Nasorri F, Bedini C, de Pita O, Girolomoni G, Cavani A. CD56brightCD16(−) NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol. 2006;36(1):118–128. doi: 10.1002/eji.200535243. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi K, Yokoyama M, Ishizawa S, et al. Lymphomatoid gastropathy: a distinct clinicopathological entity of self-limited pseudomalignant NK-cell proliferation. Blood. 2010;116(25):5631–5637. doi: 10.1182/blood-2010-06-290650. [DOI] [PubMed] [Google Scholar]
- 38.Middendorp S, Nieuwenhuis EE. NKT cells in mucosal immunity. Mucosal Immunol. 2009;2(5):393–402. doi: 10.1038/mi.2009.99. [DOI] [PubMed] [Google Scholar]
- 39.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11(3):197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]