Abstract
Therapeutic strategies combining the induction of effective antitumor immunity with the inhibition of the mechanisms of tumor-induced immunosuppression represent a key objective in cancer immunotherapy. Herein we demonstrate that effector/memory CD4+ T helper-1 (Th-1) lymphocytes, in addition to polarizing type-1 antitumor immune responses, impair tumor-induced CD4+CD25+FoxP3+ regulatory T lymphocyte (Treg) immunosuppressive function in vitro and in vivo. Th-1 cells also inhibit the generation of FoxP3+ Tregs from naive CD4+CD25−FoxP3− T cells by an interferon-γ–dependent mechanism. In addition, in an aggressive mouse leukemia model (12B1), Th-1 lymphocytes act synergistically with a chaperone-rich cell lysate (CRCL) vaccine, leading to improved survival and long-lasting protection against leukemia. The combination of CRCL as a source of tumor-specific antigens and Th-1 lymphocytes as an adjuvant has the potential to stimulate efficient specific antitumor immunity while restraining Treg-induced suppression.
Introduction
The primary objective of cancer immunotherapy is to promote tumor elimination through the activation of innate and adaptive immune responses. Successful immunotherapy relies on vaccination strategies endowed with the dual capability of inducing tumor-specific lymphocytes while overcoming the mechanisms of immune tolerance. CD4+CD25+FoxP3+ regulatory T lymphocytes (Tregs) critically contribute to the occurrence and persistence of tumor-induced tolerance.1 An increase in the frequency of these immunosuppressive cells in cancer patients has been widely reported. Treg expansion observed during tumor progression may result from the proliferation of naturally occurring Tregs (nTregs) or from the conversion of CD4+CD25−FoxP3− T cells into CD4+CD25+FoxP3+ Tregs (iTregs).2,3 Tregs dampen immune responses by suppressing the function of the effectors CD4+, CD8+, and natural killer (NK) cells4–7 and by inhibiting dendritic cell activation.8–10 Because Tregs are one of the main barriers for the eradication of tumors by immune cells, their therapeutic depletion or their functional inactivation using drugs or antibodies improves responses to cancer immunotherapy, such as dendritic cell–based vaccines.11–16 However, the selective elimination or inactivation of Tregs constitutes a major challenge because these cells share the same surface markers as activated conventional, nonsuppressive T cells. Indeed, antibody-based approaches indistinguishably target both Tregs and activated effector T lymphocytes. Likewise, chemotherapeutic agents such as cyclophosphamide, which are used to eliminate Tregs, do not target these cells selectively.
Several reports have indicated that the adoptive transfer of allogeneic T cells may increase the efficacy of tumor immunotherapy by providing adjuvant/“danger” signals to the host immune cells.17,18 A method has been optimized allowing for the efficient generation in vitro of a large number of allogeneic CD3/CD28 cross-linked T helper-1 (Th-1) memory T cells.19 Adoptive transfer of these Th-1 lymphocytes stimulates anticancer immunity and significantly improves the survival of mice lethally injected with BCL1 leukemia cells.19,20 This effect partly stems from cytokine production by activated T lymphocytes, which foster the establishment of protective type-1 immune responses.18 However, the effects of type I cytokines, including interferon-γ (IFN-γ), on Tregs have been discrepant in previous studies. As an essential effector cytokine for cell-mediated immunity, exogenous or autocrine IFN-γ has been reported to negatively regulate Treg generation.21,22 Other studies have found that IFN-γ enhances activation-induced cell death and that it thereby may regulate the expansion and persistence of effector T cells by promoting apoptosis.23,24
In the present study, we report that effector-memory CD4+ Th-1 (emTh-1) cells are capable not only of fostering the establishment of type-1 immune responses, but also of critically impairing tumor-induced immunosuppressive Tregs in vitro and in vivo. These Th-1 cells inhibit the conversion of naive CD4+CD25-FoxP3− T lymphocytes into CD4+CD25+FoxP3+ Tregs and skew their differentiation toward a Tbet+GATA-3− Th-1 profile. IFN-γ has been identified as being primarily responsible for impairing immunosuppressive Tregs. Unlike conventional approaches aimed at inactivating/depleting Tregs, emTh-1 cells do not hinder effector T lymphocytes, but rather promote their antitumor function. Furthermore, allogeneic emTh-1 cells are potent adjuvants capable of enhancing the in vivo therapeutic efficiency of a tumor-derived chaperone-rich cell lysate (CRCL) vaccine developed in our laboratory.
Methods
Mice
Mice were housed under specific pathogen-free conditions and cared for according to the guidelines of the University of Arizona Institutional Animal Care and Use Committee. Female BALB/c (H2d), C57BL6 (H2b), severe combined immunodeficiency (SCID; H2d), and Nude (H2d) mice were obtained from the National Cancer Institute (Bethesda, MD). IFN-γ-receptor−/− (H2d) mice were purchased from Jackson ImmunoResearch Laboratories. FoxP3EGFP mice that co-express green fluorescent protein (GFP) and FoxP3 under the control of the endogenous promoter were obtained from Jackson ImmunoResearch Laboratories (C.Cg-Foxp3tm2Tch/J). GFP expression allows the accurate identification and isolation of FoxP3+ Tregs. Congenic Thy1.1 mice (CBy.PL(B6)-Thy1a/ScrJ) were obtained from Jackson ImmunoResearch Laboratories. These animals carry the T lymphocyte–specific Thy1.1 allele. Donor T cells from Thy1.2 mice can be distinguished from recipient Thy1.1 mouse T cells using anti-Thy1.2 antibodies. Mice were used at the age of 6-8 weeks.
Preparation of allogeneic emTh-1 cells
emTh-1 lymphocytes were generated and activated in vitro as described by Har-Noy et al.19,20 Spleen cells from C57BL/6 mice were harvested and treated with a hypotonic buffer (150mM NH4Cl, 1mM KHCO3, 0.1mM Na2EDTA) for lysis of red blood cells. CD4+ T cells were then isolated using CD4+ microbeads and an autoMACS separator device (Miltenyi Biotec). Positively and negatively selected cells were routinely analyzed by flow cytometry to assess the purity of each fraction. The percentage of CD4+ cells in the positive fraction was > 95%. These CD4+ T lymphocytes were expanded in RPMI medium (Hyclone) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone), with anti-CD3– and anti-CD28–coated paramagnetic beads (CD3/CD28 T-cell expander beads [Dynabeads]; Invitrogen) at an initial bead:CD4+ cell ratio of 3:1, and in the presence of 20 IU/mL recombinant mouse interleukin-2 (rmIL-2), 20 ng/mL rmIL-7, 10 ng/mL rmIL-12 (Peprotech), and 10 μg/mL anti–mouse IL-4 monoclonal antibody (mAb; Becton Dickinson). Additional complete media containing rmIL-2, rmIL-7, anti–IL-4 mAb, and T-cell expander beads was added to the culture daily from day 3 to day 6 to maintain constant cell density (0.5-1 × 106 cells/mL). The amount of beads added was calculated to maintain a 1:1 bead:cell ratio as the cells expanded. After 6 days in culture, the expansion of CD4+ T cells was approximately 60 to 100 times the initial number of plated cells at day 0. Cells were harvested on day 6 and de-beaded by physical disruption and passage over a magnet. These cells were either used fresh or stored in liquid nitrogen for future use. A similar protocol was followed to generate emTh-1 cells from C57BL/6 mice.
Human emTh-1 cells were generated from healthy donor peripheral blood lymphocytes isolated by density centrifugation with lymphocyte separation medium (1.077; Eurobio). CD4+ T cells were then isolated using human CD4+ microbeads (Miltenyi Biotec) and cultured with human T-cell expander beads (Dynabeads; Invitrogen) in the presence of 20 IU/mL recombinant human IL-2 (rhIL-2), 20 ng/mL rhIL-7, 10 ng/mL rhIL-12 (Peprotech), and 10 μg/mL anti–human IL-4 mAb (BD Biosciences). Human CD4+ T-cell cultures were maintained as described in the previous paragraph for mouse cells. Human studies were approved by the institutional review board (IRB00005448; FWA00004218), with informed consent in compliance with the Declaration of Helsinki.
Magnetic cell sorting
Spleens isolated from BALB/c or C57BL6 mice were harvested and dissociated. CD4+CD62L+, CD4+CD25+, and CD4+CD25− T lymphocytes were purified by magnetic cell sorting using mouse CD4+CD62L+ naive T-cell or CD4+CD25+ T regulatory cell isolation kits and an autoMACS separator according to the manufacturer's instructions (Miltenyi Biotec). We previously reported that CD4+CD25+ T lymphocytes isolated by this technique express high levels of the transcription factor FoxP3 and are endowed with immunosuppressive properties.8,9,14
Conversion of CD4+CD62L+ T cells into CD4+CD25+FoxP3+ Tregs
CD4+CD25−CD62L+ naive T cells were isolated from Balb/c mouse splenocytes as described in “Magnetic cell sorting,” cultured in complete medium in a 96-well plate (1 × 105 cells per well), and activated with T-cell expander beads (T lymphocyte:bead ratio of 1:1) in the presence of transforming growth factor-β1 (TGF-β1; 5 ng/mL) for 72 hours at 37°C. Some wells were treated with emTh-1 supernatant. The percentage of CD4+CD25+FoxP3+ and CD4+CD25+FoxP3− cells was determined by flow cytometric analysis. Blocking antibodies against IFN-γ or tumor necrosis factor-α (TNF-α) were added to the corresponding samples at a concentration of 1 μg/mL.
Flow cytometry analysis and antibodies
Cells (∼ 106) were washed in phosphate-buffered saline (PBS) containing 3% heat-inactivated fetal bovine serum and 0.09% sodium azide (Sigma), and were first incubated with an Fc receptor-blocking Ab (BD Biosciences) for 5 minutes and then with saturating amounts of the appropriate combination of fluorochrome-conjugated antibody for 40 minutes. Cells were then washed and analyzed using an FACSCalibur (Becton Dickinson). A minimum of 10 000 events was collected for each sample, and data analysis was performed with CellQwest Pro 6.0 (Becton Dickinson). For FoxP3 detection, CD4+CD25+ or CD4+CD25− T cells purified by magnetic cell sorting or converted CD4+CD25+ Tregs generated in vitro were fixed, permeabilized, stained using an allophycocyanin anti–mouse FoxP3-staining set following the provider's instructions (clone FJK-16; eBioscience), and analyzed by flow cytometry. For the monitoring of CD4+CD25+ Tregs, cells were first stained with fluorescein isothiocyanate–conjugated anti-CD4 (rat immunoglobulin 2b [IgG2b]; BD Biosciences) and phycoerythrin-conjugated anti-CD25 (rat IgG1; BD Biosciences Pharmingen) antibodies. Cells were then stained using the eBioscience FoxP3 staining set as described. The expression of the transcription factors Tbet and GATA-3 (expressed by Th-1 and Th-2 cells, respectively) was evaluated by intracellular staining using anti–mouse Tbet-phycoerythrin and anti–mouse GATA-3-phycoerythrin monoclonal antibodies (BD Biosciences Pharmingen). Isotype control antibodies were purchased from BD Biosciences (phycoerythrin-conjugated rat IgG1, fluorescein isothiocyanate-conjugated rat IgG2a) or eBioscience (allophycocyanin-conjugated rat IgG1).
T-cell proliferation and suppression assays
CD4+CD25+ and CD4+CD25− T cells were purified from splenocytes and lymph node cells using Miltenyi Biotec isolation kits. The cells were cultured for 48 hours in 96-well plates at 37°C either in complete medium or with emTh-1 supernatant, and activated with plate-bound anti-CD3 (5 ng/mL), soluble anti-CD28 (5 ng/mL), and IL-2 (20 IU/mL).
In other experiments, untreated CD4+CD25− T cells, emTh-1 supernatant–pretreated CD4+CD25− T cells, or freshly isolated CD4+CD25− T cells (1 × 105) were cocultured for 48 hours in round-bottom 96-well plates with CD4+CD25+ T cells (1 × 105) and exposed or not to emTh-1 supernatant. Anti-CD3/CD28 T-cell expander beads (Dynabeads) were added in all cocultures (cell:bead ratio = 1:1). Bromodeoxyuridine (BrdU; Millipore) was then added for an additional 12 hours. The cells were then fixed and the incorporation of BrdU detected by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's procedures (Millipore). Cultures were set up in sixplicates.
Similar experiments were performed using human peripheral blood lymphocytes isolated by density centrifugation with 1.077 lymphocyte separation medium. CD4+CD25+ and CD4+CD25− T cells were purified from total peripheral blood mononuclear cells using a human regulatory T-cell isolation kit (Miltenyi Biotec). Cells were cultured for 24 hours in 96-well plates at 37°C either in complete medium or with human emTh-1 supernatant, and activated with plate-bound anti-CD3 (5 ng/mL), soluble anti-CD28 (5 ng/mL), and IL-2 (20 IU/mL). CD4+CD25− responder T cells were then stained using the CellTrace Violet cell-proliferation kit according to the manufacturer's procedure (Invitrogen). Labeled cells were cocultured with CD4+CD25+ T cells (1 × 105) with human anti-CD3/CD28 T-cell expander beads (cell:bead ratio = 1:1) and cell division was analyzed by flow cytometry after 72 hours, as indicated by the manufacturer.
Detection of cytokine production by ELISA
The concentrations of IFN-γ and TNF-α in cell-culture supernatants were determined using ELISA kits according to the manufacturer's procedures (eBiosciences).
12B1 leukemia cells and tumor generation
The murine leukemia cell line 12B1 was obtained by retroviral transformation of BALB/c bone marrow cells with the human bcr-abl (b3a2) fusion gene. These cells express the p210 bcr-abl protein. This is an aggressive leukemia, with the 100% lethal dose being 100 cells after tail vein injection.25 The cells were cultured (37°C, 5% CO2) in RPMI medium (Gibco/BRL) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone). 12B1 cells, obtained from Dr Wei Chen (Cleveland Clinic, Cleveland, OH), were tested routinely and found to be free of Mycoplasma contamination.
For tumor generation, 12B1 cells were first washed 3 times in PBS (Gibco/BRL), then counted and adjusted to a concentration of 5 × 104 cells/mL. Female BALB/c mice were injected with 0.1 mL (5 × 103 cells) subcutaneously in the right groin and monitored for tumor development.
CRCL preparation
12B1 tumor-derived CRCL vaccines were prepared as described previously.26–30 Briefly, tumor tissues were homogenized in detergent-containing buffers and the high-speed supernatants obtained were subjected to free-solution isoelectric focusing in a Rotofor device (Bio-Rad) at 15W constant power. Fractions were harvested and analyzed for chaperone protein content. Fractions of interest were pooled and prepared as vaccines by dialysis, detergent removal, and centrifugal concentration, as described previously.26–34 The endotoxin level contained in the CRCL preparation was determined using the Limulus Amebocyte Lysate assay kit (Cambrex Bio Science) according to the manufacturer's instructions. The level of endotoxin in CRCL was lower than that in the media control (< 0.01 EU/μg). 12B1 CRCL was used for in vivo vaccination of mice.
Tumor growth in vivo and combination immunotherapy
BALB/c mice were injected with 5 × 103 viable 12B1 cells in the right groin on day 0. Allogeneic (C57BL6) emTh-1 lymphocytes (105 cells/mouse), 12B1-derived CRCL vaccine (25 μg/mouse), or cells plus CRCL were administered in the footpad in a total volume of 100 μL on days +3, +7, and +14. Tumor growth was monitored every other day, and mice were euthanized when tumor volume reached 4000 mm3. Tumor-free survival was compared among the different treatment groups.
Depletion of immune cells in vivo
Mice were depleted of NK cells by intraperitoneal injection with anti-asialo GM1 antibodies (25 μL/mouse, 1/8 diluted with PBS; Wako Chemicals) on days −1, +3, and +5.
Statistics
Kaplan-Meier curves were generated and analyzed by log-rank statistics to determine survival percentages and differences between the treatment groups. In other experiments, Student t tests were used to determine significant differences (P < .05) between groups.
Results
EmTh-1 supernatant impairs the generation of iTregs induced by tumor cells or TGF-β in vitro
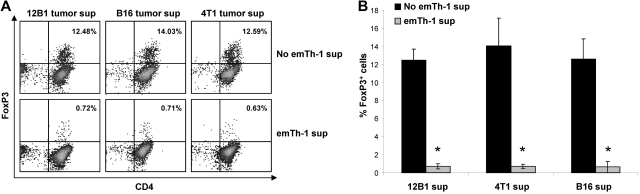
Treg expansion may result from the conversion of CD4+CD25−FoxP3− T cells into CD4+CD25+FoxP3+ cells or from the proliferation of naturally occurring Tregs. The transcription factor FoxP3 is required for the induction of Treg suppressive function, and its expression in nonregulatory cells converts them into immunosuppressive cells. TGF-β1 has been shown to promote the polarization of naive CD4+ T lymphocytes into Tregs.35 We first examined whether soluble factors produced by emTh-1 cells may negatively regulate the generation of Tregs from naive T cells induced by different TGF-β–secreting tumors in vitro. 12B1 leukemia, B16 melanoma, and 4T1 breast cancer cells secrete TGF-β1 in culture (not shown). The culture of naive CD4+CD62L+ T cells with either of these 3 tumor cell lines triggered their differentiation into FoxP3+ T cells (Figure 1A) endowed with immunosuppressive activity (not shown). The presence of the supernatant of emTh-1 during the differentiation process significantly dampened tumor-induced FoxP3 (Figure 1A-B).
Figure 1.
EmTh-1 cells impair tumor-induced iTreg generation. CD4+CD25−CD62L+ naive T cells were isolated from mouse spleens using magnetic activated cell sorting. The cells were activated using T-cell expander beads (cell:bead ratio 1:1), resuspended in the culture medium from different tumor cell lines (12B1, B16, 4T1), and treated with emTh-1 supernatant. (A) FoxP3 expression was determined by flow cytometry. (B) Pooled data from 3 independent experiments are depicted. Student t tests were used to analyze the diagrams. *P < .001, a significant difference compared with the corresponding group without emTh-1 supernatant.
In agreement with these results, the data depicted in Figure 2 indicate that emTh-1 supernatant significantly inhibited the TGF-β–induced conversion of naive T cells into FoxP3+ T lymphocytes (Figure 2A-B). Furthermore, the number of activated effector CD25+FoxP3− cells was significantly augmented by the allogeneic emTh-1 supernatant (Figure 2C). This effect was observed in a dose-dependent manner (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To further evaluate whether the suppressive function of residual FoxP3-expressing cells was affected by the emTh-1 supernatant, TGF-β–induced conversion of naive CD4+CD25−FoxP3− T cells isolated from FoxP3EGFP-transgenic mice was performed in the presence or absence of emTh-1 supernatant. GFP-positive (ie, FoxP3-expressing) cells were then sorted and their suppressive activity evaluated. Our results demonstrate that the function of these residual FoxP3-expressing iTregs was impaired (supplemental Figure 2). In addition, emTh-1 supernatant added to FoxP3+ iTregs that had been previously converted significantly impaired their immunosuppressive function (supplemental Figure 3).
Figure 2.
EmTh-1 supernatant impairs TGF-β–induced iTreg generation and promotes Tbet+ T-lymphocyte differentiation. CD4+CD25−CD62L+ naive T cells were cultured for 72 hours with T-cell expander beads (cell:bead ratio 1:1) with or without TGF-β1 (5 ng/mL) in the presence or absence of the emTh-1 supernatant (emTh-1 sup). Cells were then analyzed by flow cytometry. (A-B) Representative dot plots or histogram plots from 10 independent experiments. (C) Percentage of CD4+CD25+FoxP3−- activated T cells in total CD4+ T lymphocytes. *P < .01, a significant difference compared with cells cultured without emTh-1 supernatant. (D) The expression of the transcription factors FoxP3, Tbet, and GATA-3 was determined in CD4+CD25−CD62L+ T cells cultured for 72 hours with T-cell expander beads with or without TGF-β1 treated or not with emTh-1 supernatant. Results are representative of 3 independent experiments.
We then evaluated whether the emTh-1 supernatant may skew TGF-β–induced Foxp3+ T-cell differentiation toward another T-lymphocyte lineage. The transcription factors Tbet and GATA-3 are predominantly expressed by Th-1 or Th-2 cells, respectively.36 These transcription factors not only play a critical role in promoting the permissive lineage fate, but also actively repress the opposite lineage commitment.36 Flow cytometric analysis indicated that the majority of CD4+ T cells obtained after 72 hours of culture in the presence of TGF-β plus emTh-1 supernatant expressed a low level of GATA-3 and FoxP3, while displaying a Tbet-positive phenotype consistent with Th-1 polarization (Figure 2D). These results indicate that emTh-1 cells produce soluble factors capable of switching TGF-β–dependent polarization of naive T cells from FoxP3+ Tregs to the proinflammatory Th-1 lineage.
Inhibition of iTreg generation by emTh-1 cells depends on IFN-γ
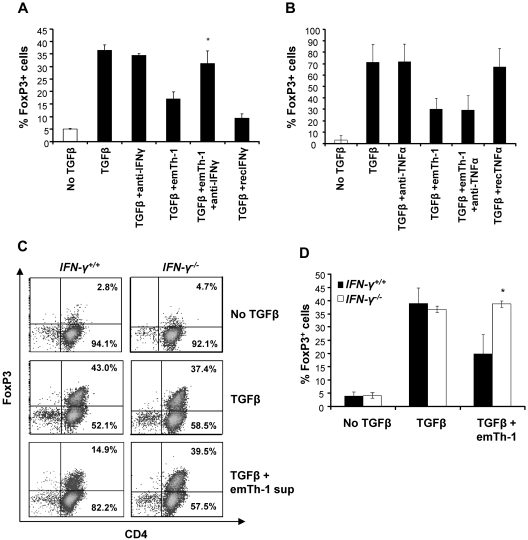
To address the mechanism by which emTh-1 cells suppress Treg generation from naive CD4+ T cells, we examined the role of key cytokines produced by Th-1 cells. Consistent with previous studies,19,20 high levels of IFN-γ and TNF-α were detected in the supernatants from emTh-1 culture (not shown). Neutralization of IFN-γ but not of TNF-α using blocking antibodies abrogated the effects of the emTh-1 supernatant in restoring TGF-β–induced conversion of naive cells into FoxP3-expressing T cells (Figure 3A-B). Consistent with these data, recombinant IFN-γ but not recombinant TNF-α impaired the negative modulation of TGF-β–induced generation of FoxP3+ T cells (Figure 3A-B and data not shown). These results were confirmed using IFN-γR−/− mice. The data depicted in Figure 3C and D indicate that the conversion of CD4+ naive T cells isolated from IFN-γR−/− mice into FoxP3+ T cells was not modified by emTh-1 supernatant (Figure 3C-D). Therefore, IFN-γ produced by Th-1 lymphocytes is primarily responsible for the inhibition of FoxP3+ T-cell generation.
Figure 3.
EmTh-1 cell–mediated inhibition of TGF-β–induced iTreg generation depends on IFN-γ. CD4+CD25−CD62L+ naive T cells were cultured for 72 hours with T-cell expander beads with or without TGF-β1 in the presence or absence of emTh-1 supernatant and with or without blocking antibodies against (A) mouse IFN-γ (*P < .05, a significant difference compared with the TGF-β + emTh-1 group) or (B) mouse TNF-α. (C-D) CD4+CD25−CD62L+ naive T lymphocytes were isolated from mouse spleens (IFN-γR−/−) and cultured for 72 hours with T-cell expander beads with or without TGF-β1 and in the presence or absence of emTh-1 supernatant. Percentage of FoxP3-expressing cells was determined by flow cytometry. Results of 3 independent experiments have been combined. *P < .01, a significant difference compared with cells from wild-type (IFN-γR+/+) mice cultured in the same conditions.
EmTh-1 cells minimally affect nTreg phenotype, but significantly diminish their immunosuppressive function and promote effector T-cell resistance to Treg-mediated suppression
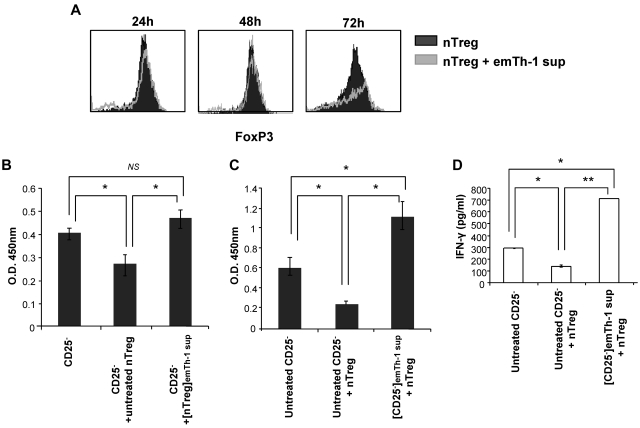
Because the emTh-1 supernatant impaired the generation of FoxP3+ T lymphocytes from non-Treg precursors, we next sought to evaluate their influence on preexisting nTregs. CD4+CD25+FoxP3+ cells were isolated from spleens and lymph nodes and incubated for 24, 48, or 72 hours with supernatant from emTh-1 cells. Our results demonstrated that FoxP3 expression in nTregs was not impaired by the emTh-1 supernatant (Figure 4A).
Figure 4.
EmTh-1 supernatant inhibits nTreg immunosuppressive function. (A) CD4+CD25+ nTregs were isolated from BALB/c mouse lymphoid tissues and cultured for the indicated periods of time with plate-bound anti-CD3 (5 ng/mL), soluble anti-CD28 (5 ng/mL), and IL-2 (20 IU/mL) with or without emTh-1 supernatant. FoxP3 expression was then determined using flow cytometry. (B) CD4+CD25+ nTregs were cultured for 48 hours with plate-bound anti-CD3, soluble anti-CD28, and IL-2 with or without emTh-1 supernatant. Cells were then washed extensively with complete medium. Responder CD4+CD25− T lymphocytes were stimulated with anti-CD3/anti-CD28 T-cell expander beads in the absence (CD25−) or presence of untreated nTregs (CD25− + untreated nTreg) or in the presence of emTh-1 supernatant–treated nTregs (CD25− + [nTreg]emTh-1 sup). Responder CD4+CD25− T-lymphocyte proliferation was determined after 48 hours using BrdU incorporation assays. NS, nonsignificant; *P < .001, a significant difference compared with responder CD25− T cells cultured with untreated Tregs. (C) CD4+CD25− T lymphocytes were first treated ([CD25−]emTh-1 sup) or not (untreated CD25−) for 48 hours with emTh-1 supernatant, washed extensively with complete medium, and cocultured for 48 hours with freshly isolated CD4+CD25+ nTregs (+ nTreg). Proliferation of responder CD25− T cells was then determined using BrdU incorporation assays. *P < .001. (D) IFN-γ concentration was assessed in the cocultures as described for panel C. *P < .001; **P < .0001.
We next investigated the influence of the emTh-1 supernatant on Treg immunosuppressive function in vitro. nTregs isolated from mouse lymphoid tissues were activated with anti-CD3 and anti-CD28 antibodies and IL-2. The cells were cultured in either complete medium or emTh-1 supernatant for 48 hours and then washed before being cocultured with freshly isolated CD4+CD25− cells for an additional 48 hours. The ability of emTh-1 supernatant–treated or untreated nTregs to inhibit the proliferation of CD4+CD25− cells was then analyzed using BrdU incorporation assays. Our data indicate that emTh-1 supernatant (Figure 4B) or IFN-γ (not shown) significantly inhibited the capacity of Tregs to suppress CD4+CD25− conventional T-cell proliferation. Similar results were obtained using human CD4+CD25+ Tregs and CD4+CD25− responder T cells isolated from peripheral blood lymphocytes (supplemental Figure 4A).
To further define whether emTh-1 may modulate the sensitivity of CD4+CD25− T lymphocytes to Tregs, CD4+CD25− T cells were incubated for 48 hours with emTh-1 supernatant and then cocultured with freshly isolated CD4+CD25+FoxP3+ nTregs. Neither the proliferation (Figure 4C) nor the production of IFN-γ (Figure 4D) of CD4+CD25− T cells pretreated with emTh-1 supernatant was suppressed by Tregs. Similar results were obtained when human CD4+CD25− responder T cells pre-incubated with emTh-1 supernatant were exposed to human CD4+CD25+ Tregs (supplemental Figure 4B).
These data indicate that emTh-1 cells not only impair the inhibitory function of Tregs, but also induce resistance of effector T cells to Treg-mediated inhibition.
EmTh-1 cells can be efficiently combined with a tumor-derived CRCL vaccine to treat mice with 12B1 leukemia
We previously reported on the immune stimulatory and protecting effects of the CRCL vaccine against multiple types of cancers, including 12B1 leukemia,8,27–30,37 and have documented that the tumor-derived CRCL vaccination can be efficiently combined with Treg elimination to treat established tumors.8 In the present study, we evaluated whether emTh-1 cell–based immunotherapy can improve CRCL vaccination.
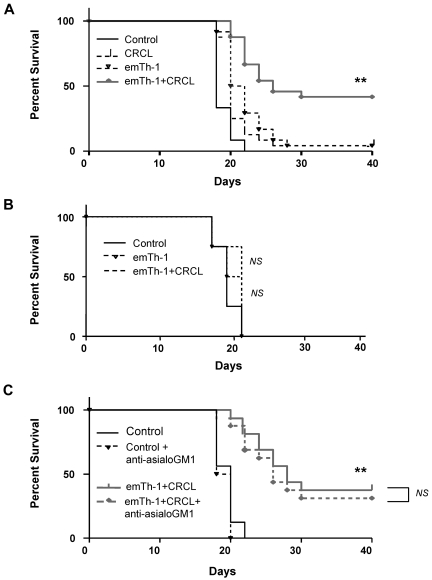
Using a therapeutic approach of established 12B1 tumors in naive Balb/c mice, we confirmed that allogeneic emTh-1 cell–based immunotherapy is safe and can be efficiently combined with CRCL immunization, resulting in a significant tumor-free survival of treated animals (Figure 5A). Similar results were obtained with a B16 melanoma model that consisted of B16 tumor-bearing C57BL/6 mice treated with B16-derived CRCL plus emTh-1 cells generated from Balb/c mice (supplemental Figure 5).
Figure 5.
The combination of emTh-1 and CRCL vaccine improves the survival of mice with 12B1 leukemia. (A) Naive BALB/c mice were injected subcutaneously in the right groin with 5 × 103 12B1 cells on day 0. Animals (8 mice per group) were then administered via footpads on days 3, 7, and 14 with: PBS (Control), 12B1-derived CRCL (CRCL, 25 μg/mouse), emTh-1 lymphocytes (emTh-1, 1 × 105 cells/mouse), or a combination of CRCL plus emTh-1 (emTh-1 + CRCL). **P < .0001. (B) SCID mice were injected subcutaneously in the right groin with 5 × 103 12B1 cells on day 0 and were administered via the footpad on days 3, 7, and 14 with: PBS (control), emTh-1 cells (emTh-1), or the combination of emTh-1 cells plus CRCL (emTh-1 + CRCL). NS, nonsignificant. (C) Immunocompetent Balb/c mice were injected with tumor cells and treated with PBS or with the combination of emTh-1 plus CRCL (emTh-1 + CRCL) on days 3, 7, and 14. In some groups of mice, NK cells were depleted using anti-asialo GM1 antibodies (+anti-asialo GM1) intraperitoneally 25 μg/mouse on days −1, +3 and +5 as described in “Tumor growth in vivo and combination immunotherapy.” In all of the experiments, survival of mice was monitored every other day and is depicted using Kaplan-Meier analysis. NS, nonsignificant; **P < .0001.
The protective effects of emTh-1 cells combined with CRCL vaccine is mediated by host T lymphocytes
We next sought to define the role of T lymphocytes in the antitumor responses induced by CRCL plus allogeneic emTh-1 cells. 12B1 tumor–bearing SCID mice were treated with CRCL plus allogeneic emTh-1 cells on days 3, 7, and 14 after tumor cell inoculation, as described in “Tumor growth in vivo and combination immunotherapy.” CRCL plus allogeneic emTh-1 cells did not improve the survival of 12B1 tumor–bearing SCID mice (Figure 5B). Similar results were obtained using Nude mice (data not shown). Therefore, these data indicate that the protective effects of adoptively transferred allogeneic emTh-1 cells with CRCL immunization are host T-lymphocyte dependent. Consistent with these results, anti–asialo-GM1 did not significantly impair the therapeutic efficacy of the allogeneic emTh-1/CRCL vaccine, indicating that NK cells do not play a major role in the antitumor immune responses induced by this combination immunotherapy (Figure 5C).
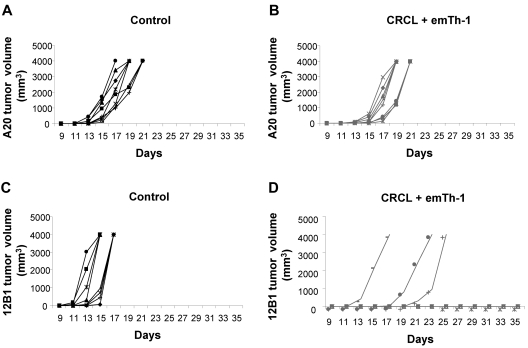
Surviving mice treated with CRCL plus allogeneic emTh-1 cells were rechallenged with the parental 12B1 tumor cells in the right groin and with an unrelated B-cell leukemia (A20, H-2d) in the opposite groin. A20 tumors developed in all 8 mice in both the treated and the control groups (Figure 6A-B), whereas 5 of 8 mice were protected against 12B1 tumor rechallenge in the CRCL plus allogeneic emTh-1 cell group. Two additional mice demonstrated significant tumor growth delay, while all control mice developed 12B1 tumors (Figure 6C-D). Therefore, combination immunotherapy consisting of CRCL plus allogeneic emTh-1 cells can induce long-lasting tumor-specific immunity.
Figure 6.
EmTh-1 plus CRCL immunotherapy induces tumor-specific immunity. Naive BALB/c mice were injected with 5 × 103 12B1 cells (subcutaneously in the right groin) on day 0 and vaccinated as described in the legend to Figure 5. Surviving tumor-free mice were then rechallenged with 5 × 103 12B1 cells given subcutaneously in the right groin and 1 × 106 A20 cells given subcutaneously in the left groin on day 45. Tumor volume was determined every other day. (A) A20 tumor volume monitored in control mice; (B) A20 tumor volume monitored in emTh-1 plus CRCL-treated mice; (C) 12B1 volume monitored in control group; (D) 12B1 tumor volume monitored in emTh-1 plus CRCL-treated animals. Results are representative of 2 independent experiments.
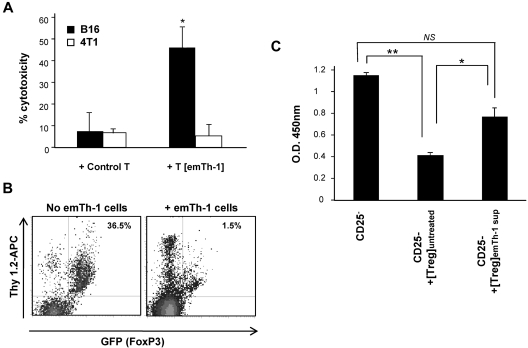
We further analyzed the antitumoral function of T cells isolated from CRCL plus emTh-1 cell–treated mice. Lymphocytes purified from the spleens of animals receiving the combination therapy were capable of specifically killing parental tumor cells but not irrelevant target cancer cells (Figure 7A).
Figure 7.
Effects of emTh-1 cells on antitumoral T lymphocytes and Tregs in vivo. (A) EmTh-1 plus CRCL immunotherapy induces tumor-specific killer T lymphocytes. B16 tumor–bearing mice were injected with control PBS or were treated with B16-derived CRCL and allogeneic emTh-1 cells as indicated in “Tumor growth in vivo and combination immunotherapy.” Seven days after the last vaccination, splenocytes were harvested and incubated for 3 days with CRCL (25 μg/mL) and 50 U/mL IL-2. T lymphocytes were then purified on a nylon wool column and incubated for 36 hours with either B16 tumor cells or irrelevant 4T1 breast cancer cell targets as indicated (effector T cells to target tumor cells ratio = 20:1). Cytotoxicity was determined as previously reported.28,50 + Control T, Tumor cells cultured with T lymphocytes from mice injected with PBS; +T [emTh-1], tumor cells cultured with T lymphocytes from mice treated with CRCL plus emTh-1. *P < .005, a significant difference compared with control T cells cultured with B16 melanoma cells. (B) EmTh-1 cells skew the differentiation of CD4+CD25−FoxP3− naive T lymphocytes toward CD4+CD25+FoxP3− effector T cells rather than Tregs in vivo. CD4+CD25−FoxP3− naive T lymphocytes isolated from Thy1.2 FoxP3EGFP transgenic BALB/c mice (107 cells) were transferred to 12B1 tumor–bearing congenic Thy1.1 BALB/c mice. Animals were treated with emTh-1 cells (+emTh-1 cells) or with control PBS (No emTh-1 cells). Endogenous T cells of recipient Thy1.1 mice express the Thy1.1 but not the Thy1.2 antigen, which allows the specific tracking and identification of Thy1.2 T lymphocytes in Thy1.1 mice. Spleens were harvested and dissociated, and conversion of transferred naive Thy1.2 CD4+CD25−FoxP3− T cells into GFP+ (FoxP3-expressing) Treg in vivo was determined by evaluating the frequency of Thy1.2+GFP+ cells after gating on the CD4+ T-cell population using flow cytometry. (C) EmTh-1 cells impair Treg suppressive function in vivo. Responder CD4+CD25− T lymphocytes were stimulated with anti-CD3/anti-CD28 T-cell expander beads in the absence (CD25−) or presence of Tregs isolated from the draining lymph nodes of untreated tumor-bearing mice (CD25− + [Treg]untreated) or of tumor-bearing mice treated with emTh-1 (CD25− + [Treg]emTh-1). Responder CD4+CD25− T-lymphocyte proliferation was determined after 48 hours using BrdU incorporation assays. NS, nonsignificant; *P < .02; **P < .001.
emTh-1 cells were able to skew the differentiation of naive Thy1.2+CD4+CD25−FoxP3− T lymphocytes transferred to 12B1 tumor–bearing congenic Thy1.1+ mice toward Thy1.2+CD4+CD25+FoxP3− effector T cells rather than Thy1.2+CD4+CD25+FoxP3+ Tregs (Figure 7B). In addition, the suppressive function of Tregs isolated from tumor-bearing animals treated with emTh-1 cells was significantly reduced (Figure 7C). This confirms that the effects of emTh-1 cells on Tregs observed in vitro also occur in vivo, and also demonstrates that the mechanism by which emTh-1 cells augment the efficacy of CRCL vaccination involves the inhibition of tumor-induced Tregs.
Discussion
The advantages of active immunotherapy include its relative lack of side effects, its specificity against target tumor cells, and the generation of memory responses against tumor-specific antigens. However, even if proven clinically safe, immunotherapy has only sparked moderate enthusiasm because of the relatively limited objective clinical responses that have been observed in cancer patients. This modest therapeutic success stems in part from the immunosuppressive environment created by tumors, with CD4+CD25+FoxP3+ Tregs as prominent contributors. The efficacy of current strategies to eliminate/inactivate Tregs, such as antibodies targeting CD25, CTLA-4, or GITR; the immunotoxin LMB-2; OX-40 antibodies; and alkylating agents such as cyclophosphamide or tyrosine kinase inhibitors,9,11,14,38–42 remain limited insofar as they nonspecifically target both Tregs and conventional effector lymphocytes. Our current findings uncovered emTh-1 cell administration as a novel approach to inhibit the suppressive activity of Tregs while simultaneously promoting the function of conventional effector T cells. Our data demonstrate that emTh-1 cells significantly impair the conversion of naive T cells into FoxP3+ iTregs induced by either tumor cells or recombinant TGF-β1, switching their differentiation toward CD4+CD25+Tbet+-activated T lymphocytes. In addition, emTh-1 cells were capable of inhibiting the immunosuppressive function of naturally occurring nTregs. Previous studies have reported opposite effects of IFN-γ on Tregs.23,24 We provide evidence, using anti–IFN-γ blocking antibodies and IFN-gR−/− mice, that Treg inhibition by these emTh-1 cells is dependent on IFN-γ. Moreover, IFN-γ is responsible for the skewing of iTreg differentiation toward activated effector Tbet+ T cells.
Multiple strategies harnessing the adjuvant effect of allogeneic T-cell transfer have been optimized with the goal of skewing the host's immune responses toward the induction of a “graft-versus-tumor” effect.18,43–48 Recent studies have demonstrated that in vitro–generated allogeneic emTh-1 cells can serve as a potent adjuvant for stimulating type-1 antitumor immunity when used together with a source of tumor antigen.19,49 However, significant tumor-free survival of tumor-bearing mice was difficult to achieve with allogeneic emTh-1 cells in association with tumor cell lysate or irradiated cancer cell vaccines. In the current study, we demonstrate that the combination of allogeneic emTh-1 cells with our CRCL vaccine significantly increased the survival of mice bearing established leukemia or melanoma. The demonstrated advantages in efficacy of CRCL over more common vaccine strategies, such as individual tumor-derived chaperone proteins HSP70, gp96, and tumor lysates, have been extensively published by our group.27–32 The combination of tumor-derived CRCL plus allogeneic emTh-1 cells demonstrated a superior therapeutic outcome compared with monotherapy. In addition, combination immunotherapy with CRCL plus allogeneic emTh-1 cells promoted durable, tumor-specific, T cell–dependent adaptive immunity.
We previously documented that the efficacy of tumor-derived CRCL vaccination can be enhanced by Treg elimination.8 The selective negative modulation of Tregs by emTh-1 cells represents an effective mechanism by which these transferred cells improve the therapeutic efficacy of the CRCL vaccine. Allogeneic emTh-1 cells thus represent a powerful adjuvant capable of enhancing the therapeutic potential of the CRCL vaccine, and therefore represent a promising translational approach in cancer immunotherapy clinical trials.
Supplementary Material
Acknowledgments
We thank Paula Campbell (Cytometry Core Facility, Arizona Cancer Center, University of Arizona, Tucson, AZ) for technical assistance.
This work was supported by National Institutes of Health grant R01 CA104926 (to E.K. and N.L.), by Cancer Center Support Grant CA023074 (to E.K. and N.L.), by Leukemia & Lymphoma Society Fellow Award 5188-07 (to N.L.), by Alex's Lemonade Stand Foundation (to N.L.), and by Tee Up for Tots and People Acting Now Discover Answers (PANDA; to E.K. and N.L.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: N.L. and E.K. designed and directed research; N.J., C.J.L., C.L., and M.T. performed research; A.H. and S.B. provided technical assistance; B.B. analyzed and discussed data; N.J., C.J.L., M.H.-N., N.L., and E.K. analyzed data; and N.J., N.L., and E.K. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Emmanuel Katsanis, MD, or Nicolas Larmonier, PhD, University of Arizona, Department of Pediatrics, 1501 N Campbell Ave, PO Box 245073, Tucson, AZ 85724-5073; e-mail: katsanis@peds.arizona.edu or nrlarmon@email.arizona.edu.
References
- 1.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101(5):455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 2.Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25- lymphocytes is thymus and proliferation independent. Cancer Res. 2006;66(8):4488–4495. doi: 10.1158/0008-5472.CAN-05-4217. [DOI] [PubMed] [Google Scholar]
- 3.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 4.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174(5):2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishikawa H, Jager E, Ritter G, Old LJ, Gnjatic S. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood. 2005 Aug 1;106(3):1008–1011. doi: 10.1182/blood-2005-02-0607. [DOI] [PubMed] [Google Scholar]
- 6.Somasundaram R, Jacob L, Swoboda R, et al. Inhibition of cytolytic T lymphocyte proliferation by autologous CD4+/CD25+ regulatory T cells in a colorectal carcinoma patient is mediated by transforming growth factor-beta. Cancer Res. 2002;62(18):5267–5272. [PubMed] [Google Scholar]
- 7.Sutmuller RP, van Duivenvoorde LM, van Elsas A, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194(6):823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larmonier N, Cantrell J, Lacasse C, et al. Chaperone-rich tumor cell lysate-mediated activation of antigen-presenting cells resists regulatory T cell suppression. J Leukoc Biol. 2008;83(4):1049–1059. doi: 10.1189/jlb.0907635. [DOI] [PubMed] [Google Scholar]
- 9.Larmonier N, Marron M, Zeng Y, et al. Tumor-derived CD4(+)CD25 (+) regulatory T cell suppression of dendritic cell function involves TGF-beta and IL-10. Cancer Immunol Immunother. 2007;56(1):48–59. doi: 10.1007/s00262-006-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172(8):4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 11.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34(2):336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 12.Prasad SJ, Farrand KJ, Matthews SA, Chang JH, McHugh RS, Ronchese F. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+CD25+ regulatory T cells. J Immunol. 2005;174(1):90–98. doi: 10.4049/jimmunol.174.1.90. [DOI] [PubMed] [Google Scholar]
- 13.Yu P, Lee Y, Liu W, et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201(5):779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larmonier N, Janikashvili N, LaCasse CJ, et al. Imatinib mesylate inhibits CD4+ CD25+ regulatory T cell activity and enhances active immunotherapy against BCR-ABL- tumors. J Immunol. 2008;181(10):6955–6963. doi: 10.4049/jimmunol.181.10.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janikashvili N, Larmonier N, Katsanis E. Personalized dendritic cell-based tumor immunotherapy. Immunotherapy. 2010;2(1):57–68. doi: 10.2217/imt.09.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larmonier N, Fraszczak J, Lakomy D, Bonnotte B, Katsanis E. Killer dendritic cells and their potential for cancer immunotherapy. Cancer Immunol Immunother. 2010 Jan;59(1):1–11. doi: 10.1007/s00262-009-0736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411(6835):385–389. doi: 10.1038/35077251. [DOI] [PubMed] [Google Scholar]
- 18.Chen BJ, Cui X, Sempowski GD, Liu C, Chao NJ. Transfer of allogeneic CD62L- memory T cells without graft-versus-host disease. Blood. 2004;103(4):1534–1541. doi: 10.1182/blood-2003-08-2987. [DOI] [PubMed] [Google Scholar]
- 19.Har-Noy M, Zeira M, Weiss L, Fingerut E, Or R, Slavin S. Allogeneic CD3/CD28 cross-linked Th1 memory cells provide potent adjuvant effects for active immunotherapy of leukemia/lymphoma. Leuk Res. 2009;33(4):525–538. doi: 10.1016/j.leukres.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Har-Noy M, Zeira M, Weiss L, Slavin S. Completely mismatched allogeneic CD3/CD28 cross-linked Th1 memory cells elicit anti-leukemia effects in unconditioned hosts without GVHD toxicity. Leuk Res. 2008;32(12):1903–1913. doi: 10.1016/j.leukres.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104(46):18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang JH, Kim YJ, Han SH, Kang CY. IFN-gamma-STAT1 signal regulates the differentiation of inducible Treg: potential role for ROS-mediated apoptosis. Eur J Immunol. 2009;39(5):1241–1251. doi: 10.1002/eji.200838913. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Hong J, Sun W, et al. Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25- T cells to CD4+ Tregs. J Clin Invest. 2006;116(9):2434–2441. doi: 10.1172/JCI25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng G, Gao W, Strom TB, et al. Exogenous IFN-gamma ex vivo shapes the alloreactive T-cell repertoire by inhibition of Th17 responses and generation of functional Foxp3+ regulatory T cells. Eur J Immunol. 2008;38(9):2512–2527. doi: 10.1002/eji.200838411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He L, Feng H, Raymond A, et al. Dendritic-cell-peptide immunization provides immunoprotection against bcr-abl-positive leukemia in mice. Cancer Immunol Immunother. 2001;50(1):31–40. doi: 10.1007/PL00006680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graner M, Raymond A, Akporiaye E, Katsanis E. Tumor-derived multiple chaperone enrichment by free-solution isoelectric focusing yields potent antitumor vaccines. Cancer Immunol Immunother. 2000;49(9):476–484. doi: 10.1007/s002620000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graner MW, Zeng Y, Feng H, Katsanis E. Tumor-derived chaperone-rich cell lysates are effective therapeutic vaccines against a variety of cancers. Cancer Immunol Immunother. 2003;52(4):226–234. doi: 10.1007/s00262-002-0359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng Y, Feng H, Graner MW, Katsanis E. Tumor-derived, chaperone-rich cell lysate activates dendritic cells and elicits potent antitumor immunity. Blood. 2003;101(11):4485–4491. doi: 10.1182/blood-2002-10-3108. [DOI] [PubMed] [Google Scholar]
- 29.Zeng Y, Graner MW, Katsanis E. Chaperone-rich cell lysates, immune activation and tumor vaccination. Cancer Immunol Immunother. 2006;55(3):329–338. doi: 10.1007/s00262-005-0694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng Y, Graner MW, Thompson S, Marron M, Katsanis E. Induction of BCR-ABL-specific immunity following vaccination with chaperone-rich cell lysates derived from BCR-ABL+ tumor cells. Blood. 2005;105(5):2016–2022. doi: 10.1182/blood-2004-05-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graner M, Raymond A, Romney D, He L, Whitesell L, Katsanis E. Immunoprotective activities of multiple chaperone proteins isolated from murine B-cell leukemia/lymphoma. Clin Cancer Res. 2000;6(3):909–915. [PubMed] [Google Scholar]
- 32.Zeng Y, Graner MW, Feng H, Li G, Katsanis E. Imatinib mesylate effectively combines with chaperone-rich cell lysate-loaded dendritic cells to treat bcr-abl+ murine leukemia. Int J Cancer. 2004;110(2):251–259. doi: 10.1002/ijc.20115. [DOI] [PubMed] [Google Scholar]
- 33.Li G, Andreansky S, Helguera G, et al. A chaperone protein-enriched tumor cell lysate vaccine generates protective humoral immunity in a mouse breast cancer model. Mol Cancer Ther. 2008;7(3):721–729. doi: 10.1158/1535-7163.MCT-07-2067. [DOI] [PubMed] [Google Scholar]
- 34.Li G, Zeng Y, Chen X, et al. Human ovarian tumour-derived chaperone-rich cell lysate (CRCL) elicits T cell responses in vitro. Clin Exp Immunol. 2007;148(1):136–145. doi: 10.1111/j.1365-2249.2007.03323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dudziak D, Kamphorst AO, Heidkamp GF, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315(5808):107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 37.Kislin KL, Marron MT, Li G, Graner MW, Katsanis E. Chaperone-rich cell lysate embedded with BCR-ABL peptide demonstrates enhanced antitumor activity against a murine BCR-ABL positive leukemia. FASEB J. 2007;21(9):2173–2184. doi: 10.1096/fj.06-7843com. [DOI] [PubMed] [Google Scholar]
- 38.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115(12):3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105(7):2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 40.Ozao-Choy J, Ma G, Kao J, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69(6):2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao H, Lee BN, Talpaz M, et al. Imatinib mesylate suppresses cytokine synthesis by activated CD4 T cells of patients with chronic myelogenous leukemia. Leukemia. 2005;19(11):1905–1911. doi: 10.1038/sj.leu.2403933. [DOI] [PubMed] [Google Scholar]
- 42.Schade AE, Schieven GL, Townsend R, et al. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood. 2008;111(3):1366–1377. doi: 10.1182/blood-2007-04-084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng H, Matte-Martone C, Li H, et al. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood. 2008;111(4):2476–2484. doi: 10.1182/blood-2007-08-109678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson BE, McNiff J, Yan J, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112(1):101–108. doi: 10.1172/JCI17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Joe G, Zhu J, et al. Dendritic cell-activated CD44hiCD8+ T cells are defective in mediating acute graft-versus-host disease but retain graft-versus-leukemia activity. Blood. 2004;103(10):3970–3978. doi: 10.1182/blood-2003-09-3135. [DOI] [PubMed] [Google Scholar]
- 46.Zheng H, Matte-Martone C, Jain D, McNiff J, Shlomchik WD. Central memory CD8+ T cells induce graft-versus-host disease and mediate graft-versus-leukemia. J Immunol. 2009;182(10):5938–5948. doi: 10.4049/jimmunol.0802212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beilhack A, Schulz S, Baker J, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106(3):1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katsanis E, Anderson PM, Filipovich AH, et al. Proliferation and cytolytic function of anti-CD3 + interleukin-2 stimulated peripheral blood mononuclear cells following bone marrow transplantation. Blood. 1991;78(5):1286–1291. [PubMed] [Google Scholar]
- 49.Har-Noy M, Slavin S. The antitumor effect of allogeneic bone marrow/stem cell transplant without graft vs. host disease toxicity and without a matched donor requirement? Med Hypotheses. 2008;70(6):1186–1192. doi: 10.1016/j.mehy.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 50.Larmonier N, Merino D, Nicolas A, et al. Apoptotic, necrotic, or fused tumor cells: an equivalent source of antigen for dendritic cell loading. Apoptosis. 2006;11(9):1513–1524. doi: 10.1007/s10495-006-8765-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.