Abstract
Architecturally defective, leaky blood vessels typify pathologic angiogenesis induced by vascular endothelial growth factor-A (VEGF-A). Such neovascular defects aggravate disease pathology and seriously compromise the therapeutic utility of VEGF. Endothelial cell (EC) transduction with active L61Rac1 strongly improved VEGF-driven angiogenesis in vivo as measured by increased neovascular density, enhanced lumen formation, and reduced vessel leakiness. Conversely, transduction with dominant-negative N17Rac1 strongly inhibited neovascularization. In vitro, active L61Rac1 promoted organization of cortical actin filaments and vascular cords and improved EC-EC junctions, indicating that improved cytoskeletal dynamics are important to the mechanism by which active L61Rac1 rectifies VEGF-driven angiogenesis. SEW2871, a sphingosine 1-phosphate receptor-1 agonist that activates Rac1 in ECs, improved cord formation and EC-EC junctions in vitro similarly to active L61Rac. Moreover, SEW2871 administration in vivo markedly improved VEGF neovessel architecture and reduced neovascular leak. Angiopoietin-1, a cytokine that “normalizes” VEGF neovessels in vivo, activated Rac1 and improved cord formation and EC-EC junctions in vitro comparably to active L61Rac1, and a specific Rac1 inhibitor blocked these effects. These studies distinguish augmentation of Rac1 activity as a means to rectify the pathologic angioarchitecture and dysfunctionality of VEGF neovessels, and they identify a rational pharmacologic strategy for improving VEGF angiogenesis.
Introduction
Rac1, a key member of the Rho guanosine triphosphatase (GTPase) family, regulates the cytoskeleton, lamellipodial extensions, cell adhesion and motility, and cell-cell junctions.1 Activation of Rac1 is mediated by guanine nucleotide exchange factors in response to cytokine stimulation2,3 sphingosine 1-phosphate (S1P) stimulation,4 and integrin ligation.5 After activation, Rac1 is inactivated by GTPase-activating proteins and thereby cycles between active GTP-bound and inactive GDP-bound configurations.1
Rac1 is essential for embryonic angiogenesis; selective deletion of the Rac1 gene in endothelial cells (ECs) caused defective neovascularization and death.6 A Rac1 requirement for angiogenesis in the adult seems controversial. Endothelial-specific Rac1 haplo-insufficient mice exhibited impaired angiogenesis in a hind limb ischemia model,7 whereas another study showed that endothelial Rac1 was not required for tumor angiogenesis unless integrin αvβ3 was absent.8 In vitro, Rac1 activity is generally agreed to be essential for capillary morphogenesis. Dominant negative (DN) N17Rac1 and deletion of Rac1 both inhibited formation of vascular cords6,9,10 and impaired the stability of vascular lumens.11 Thus, Rac1 activity is important for capillary morphogenesis and neovascularization, at least in some settings. However, the consequences of augmenting Rac1 activity for neovascularization in vivo have not been investigated previously.
In this study, we investigated the outcome of augmenting Rac1 activity for angiogenesis in a model that recapitulates pathologic neovascularization in the adult. Paradoxically, vascular endothelial growth factor (VEGF) induces a normal vasculature during embryogenesis but can induce a highly abnormal vasculature in the adult.12,13 Typical abnormalities include aberrant neovascular architecture and leakiness that are attributable to comparatively high VEGF expression in pathologic settings14 and imbalance between VEGF and other important factors.12 Serious neovascular malformations and vascular leak also have been observed in various animal models designed to test proangiogenic therapy with VEGF.15–18 Nonetheless, the underlying fundamentals responsible for abnormal VEGF neovascularization have been largely unexplored. Experiments described here illustrate that active Rac1 markedly improves the angioarchitecture and reduces leakiness of pathologic neovessels induced by VEGF. In addition, they show a pharmacologic strategy involving Rac1 activation that rectifies VEGF-driven angiogenesis in vivo, and they identify important mechanistic parallels with angiopoietin-1 (Ang-1).
Methods
Reagents
Materials and sources were as follows: recombinant human VEGF165 (National Cancer Institute Preclinical Repository, Biological Resources Branch), Rac inhibitor-I, NSC23766 (Calbiochem), Rac1 activation “pull-down” assay (Upstate Biotech), SEW2871 (Cayman Chemical), Ang-1 (BD Biosciences), CD31 antibody (BD Biosciences PharMingen), diaminobenzidine substrate kit (Zymed Laboratory Inc), Matrigel and rat tail collagen-1 (BD Biosciences), Oregon Green–conjugated phalloidin, lysine-fixable Texas Red dextran (molecular weight, 70 kDa) and fluorescein isothiocyanate (FITC)–dextran (molecular weight, 70 kDa) for perfusion and permeability studies (Invitrogen), vascular endothelial (VE)–cadherin goat polyclonal antibody (sc-6458) and secondary antibody: FITC-labeled anti–goat immunoglobulin G (sc-2024; Santa Cruz Biotechnology Inc).
Packaging cells expressing retroviruses encoding Rac1 mutants
Rac1 cDNAs mutants were polymerase chain reaction amplified from a wild-type Rac1 cDNA, cloned from a human fetal kidney cDNA library (Clontech) by polymerase chain reaction. Rac1 mutant cDNAs were subcloned into BamHI sites in a modified retrovirus vector pLCNX2/IRES-EGFP.19 This vector expresses green fluorescent protein (GFP) as a separate protein through an independent ribosome entry site (IRES). The fidelity of all clones was verified by sequencing. PT67 retroviral packaging cells (Clontech), which express the 10A1 viral envelope for production of amphotropic virus, were transfected with pLCNX2/IRES-EGFP vector containing DN N17Rac1, active L61Rac1, or vector without insert (empty vector). Transfectants were cloned, and clones expressing retrovirus at 1 × 105 colony-forming unit/mL were selected.
VEGF-driven angiogenesis, retroviral transduction, and drug administration in vivo, analyses of vascular parameters
Angiogenesis was investigated in vivo with an established method involving both VEGF165-transfected cells and retroviral packaging cells that provide a constant source of VEGF and retrovirus.19,20 All animal research was approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee. Female athymic nude mice (7 weeks old) were injected subcutaneously on the right and left flanks with 0.3 mL of 9 mg/mL Matrigel containing 1 × 106 SK-MEL2 human melanoma cells transfected for stable expression (cytomegalovirus [CMV] promoter) of human VEGF165 together with 1 × 106 retroviral packaging cells, as indicated. Untransfected SK-MEL2 cells do not provoke angiogenesis; therefore, the VEGF–SK-MEL2 transfectants allow for specific investigation of VEGF-driven angiogenesis.21 To analyze perfusion of new blood vessels, animals received tail vein injections (0.2 mL) of 70 kDa of Texas Red dextran (25 mg/mL) in sterile saline at times indicated before harvest. For whole-mount immunofluorescence analysis of tracer-filled vasculature, skin samples were fixed in 4% paraformaldehyde (4 hours), mounted in immersion oil and viewed with a Bio-Rad MRC-1024 Confocal Microscope equipped with an Argon-Krypton Laser. Four to 6 fields per sample were visualized with a 10× objective. For histology, implants together with associated skin were fixed for 1 hour in 10% buffered formalin and embedded in paraffin. Immunohistochemical staining of ECs with CD31 antibody was performed as described.19 Total neovascular density (ie, area of gross images occupied by neovessels) and lumen area were traced through freehand selections on digital images and measured with National Institute of Health (NIH) ImageJ software. Neovessels and ECs per unit area were measured through freehand point selections, and vessel diameters were measured with line selections with the use of NIH ImageJ software.
Finally, for experiments with SEW2871, angiogenesis assays were performed as described in the preceding paragraph but without retroviral packaging cells. SEW2871 was administered by gavage once daily at a dose of 10 mg/kg unless indicated otherwise.
Analyses of vascular leakage
Animals were injected intravenously with FITC-dextran (70 kDa) 20 minutes before harvest (for visualization of vessel perfusion in combination with vessel leakage) and then injected intravenously with Texas Red-dextran (70 kDa) 1 minute before harvest (for visualization of vessel perfusion alone). Excised tissues were fixed in 4% paraformaldehyde, dehydrated in a graded series of alcohols, cleared in methyl salicylate, and subjected to Laser Scanning Confocal Microscopy. FITC and Texas Red fluorescence were excited sequentially at 488 nm and 568 nm, respectively, with the use of a krypton-argon laser. The extent of FITC-dextran tracer leakage was quantified by subtracting the Texas Red signal from the FITC signal with the use of the Image Calculator function of NIH ImageJ software and then integrating the remaining FITC signal intensity values in the resultant histogram with the use of Microsoft Excel. Four images were analyzed for each experimental condition. Relative tracer leak was reported as the percentage of leak observed in the empty vector control.
Microvascular ECs and retroviral transduction
Human dermal microvascular ECs (MVECs) were isolated from neonatal foreskins20 and cultured in the continuous presence of 20 ng/mL VEGF165. Use of human cells was approved by the Beth Israel Deaconess Medical Center Institutional Review Board. All experiments were performed with cells at the fourth to seventh passage. MVECs at passage ≤ 5 were transduced with retroviruses according to a previously established, efficient method.22 The transduction procedure was repeated 3 times on consecutive days before subjecting cells to selection with 300 μg/mL G418. This method yields 100% transduction as indicated with GFP vectors.19,20 Cells were used within 1 week for experiments.
In vitro capillary morphogenesis assays; staining for F-actin and VE-cadherin
Capillary morphogenesis assays were performed by “overlaying” or “sandwiching” confluent MVEC monolayers with rat tail collagen-I.19,20 The sandwich assay was performed in 12-well plates with 1.0 mg/mL collagen-I in full medium (Clonetics EBM-2 or MCDB 131, containing 15% fetal bovine serum and 20 ng/mL VEGF). When indicated, either SEW2871 or Ang-1 was added for overnight incubation before adding the upper layer of collagen-I containing the same concentration of agonist. Capillary morphogenesis was allowed to proceed for 16 hours; the assay plates were fixed with 10% formalin for 1 hour, permeabilized briefly with 0.02% Triton-X100 in phosphate-buffered saline (PBS), and stained for F-actin with fluorescent Oregon Green–conjugated phalloidin (0.5 U/mL) and photographed. For the overlay assay, each well was overlaid with 300 μL of collagen-I (0.5 mg/mL) in serum-free medium containing 20 ng/mL VEGF. Capillary morphogenesis was allowed to proceed for 4 hours; cells were fixed and stained for F-actin (as above). Cells were photographed with a Nikon Eclipse TE300 inverted fluorescent microscope with 20× objective and digital camera (Lieca FireCam v1.5). Cord length, blind ends, and polygons were quantified with NIH ImageJ software; cord length was traced and measured through freehand line selections, and polygons and blind ends were determined with point selections. Measured parameters correspond to actual areas of 0.4 mm2. Cells in monolayer culture also were stained for F-actin (as above) or with VE-cadherin antibody (see “Reagents”).
Analyses of Rac1 activity and VEGF expression
The Rac1 pull-down assay was performed according to the manufacturer's instructions, using 2 × 106 MVECs, and samples were analyzed with immunoblotting. To assay for effects of retroviral transduction or SEW2871 on VEGF expression, VEGF165–SK-MEL2 cells were cocultured with the various Rac1 retroviral packaging cells in the same proportions used in vivo or were treated continuously with SEW2871 at the dose used to improve cord formation (15nM). Medium was harvested daily for 7 days, and VEGF165 was concentrated with heparin-Sepharose chromatography followed by electrophoresis and immunoblotting with VEGF-specific antibody23 and quantification with a digital scanner.
Statistical analyses
Data are presented as mean + SEM. Statistical analyses were performed with InStat 3 software (GraphPad Software Inc) for Macintosh, using the 2-tail Mann-Whitney test, assuming unequal variances between the 2 groups under comparison. In all cases, the experimental group was compared with the corresponding control group; and calculated P values are based on direct comparisons between the 2 groups.
Results
In vivo, active Rac1 promotes assembly of ECs into new blood vessels, improves neovessel architecture, and reduces neovessel leakiness
We used a mouse skin model that uses a constant source of VEGF together with packaging cells expressing retroviruses encoding Rac1 mutants. This model offers the advantage of high retroviral transduction efficiency because transduction is favored in proliferating cells, and ECs divide in response to VEGF stimulation.19,20 Moreover, the packaging cells provide a constant source of freshly produced retrovirus throughout the experimental interval. Previously, we have validated this model with packaging cells expressing retrovirus encoding GFP,19 RhoA mutants,20 and transcription factor Nur77.24 To initiate angiogenesis, transfected SK-MEL2 cells engineered for expression of VEGF165 under the direction of a constitutively active CMV immediate-early gene promoter were mixed with equal numbers of PT67 retroviral packaging cells in basement membrane Matrigel and injected subdermally. The PT67 retrovirus packaging cells expressed retrovirus encoding either an established active Rac 1 mutant (L61Rac1),25 an established DN Rac1 mutant (N17 Rac1),26 or no insert (empty vector).
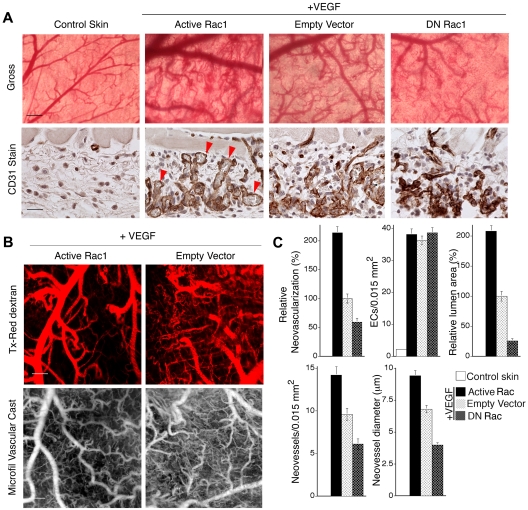
Animals were analyzed on day 7. Neovascularization of the overlying dermis was extensive in the active Rac1 group, intermediate in the control empty vector group, and sharply inhibited in the DN Rac1 group (Figure 1A top panels). Quantification of neovascularization from gross images indicated that active Rac1 increased relative neovascularization by > 100% relative to empty vector controls; conversely, DN Rac1 sharply decreased neovascularization by nearly 50% (Figure 1C). Moreover, as shown with CD31 staining of ECs in paraffin-embedded tissue cross-sections (Figure 1A bottom panels), neovessels with clearly defined lumens were most abundant in the active Rac1 group but nearly absent in the DN Rac1 group. Cumulative lumen area was increased > 100% relative to controls by active Rac1 and decreased 75% by DN Rac1 (Figure 1C), and these differences correlated with the respective differences in neovessel number and average neovessel diameter (Figure 1C). These findings were particularly intriguing, given that the numbers of ECs were comparable in the different experimental groups (Figure 1C). Finally, as shown by perfusion with fluorescent tracer and by perfusion with Microfil, new blood vessels in the active Rac1 group were well perfused and larger than in the empty vector controls (Figure 1B). Thus, these data indicate that, during VEGF-driven angiogenesis in adult skin, Rac1 serves a critical role in supporting the formation of new blood vessels with well-defined lumens. Most importantly, active Rac1 markedly improves neovessel formation.
Figure 1.
Active Rac1 improves neovessel architecture and lumen formation in vivo. (A) VEGF165 transfectants were mixed with packaging cells expressing retrovirus encoding active L61Rac1, dominant-negative N17Rac1, or empty vector (control) and injected together with Matrigel subdermally. Animals were harvested after 7 days. Gross: gross images of dermis overlying the Matrigel implants show that active L61Rac1 improved formation of new blood vessels relative to control, whereas DN Rac1was inhibitory. Scale bar = 375 μm. CD31 Stain: ECs in cross section stained with CD31 antibody (brown color), illustrating that active Rac1 improved lumen formation (arrows) relative to empty vector control, whereas DN Rac1 abolished lumen formation. Scale bar = 15 μm. (B) Tx-Red dextran: perfusion of vessels with 70 kDa, lysine-fixable, Texas-Red dextran (10 minutes), viewed with confocal microscopy (scale bar = 300 μm) confirming that active Rac1 promoted formation of larger, well-perfused, architecturally improved blood vessels. Microfil Vascular Cast: the entire vasculature was perfused with Microfil, illustrating improvement in neovessel diameter and architecture mediated by active Rac1 (scale bar = 250 μm). (C) Quantification of vascular parameters; n > 20 for all groups. From gross images: relative neovascularization (ie, percentage of relative area occupied by neovessels in flat mount; P < .001). From CD31-stained cross-sections: quantification of ECs per 0.015 mm2, relative total lumen area (P < .001), numbers of neovessels per 0.015 mm2 (P < .01), and average internal neovessel diameter (P < .01).
Consistent with observations that active Rac1 promoted and DN Rac1 inhibited blood vessel formation without affecting EC density, coculture of the various retroviral packaging cells with the VEGF–SK-MEL2 cells, in the same proportion used in vivo, did not effect VEGF production (see “Methods”). This was expected because the expression vector was engineered to drive VEGF expression constitutively with a CMV promoter. Thus, the marked differences in blood vessel architecture among the different experimental groups can best be explained by differences in EC organization rather than differences in EC density or VEGF expression.
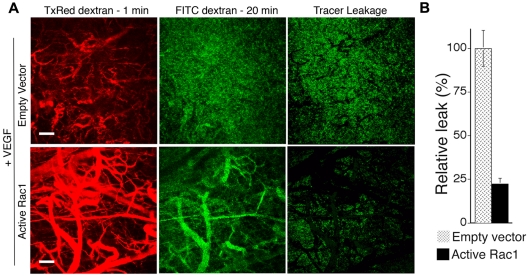
To determine the consequences of active Rac1 for neovessel leakiness, animals were injected intravenously with 70 kDa of FITC-dextran tracer 20 minutes before harvest (for visualization of vessel perfusion in combination with vessel leakage) and injected intravenously with 70 kDa of Texas Red-dextran tracer at 1 minute before harvest (for visualization of vessel perfusion alone). Excised tissues were fixed and subjected to laser scanning confocal microscopy (Figure 2A). The extent of FITC-dextran tracer leakage was quantified by subtracting the signal corresponding to the Texas Red-dextran tracer (intravascular) from the signal corresponding to the FITC-dextran tracer (intravascular + extravascular) to yield the net signal for the extravasated tracer (see “Methods”). Importantly, VEGF neovessels induced in the presence of active Rac1 exhibited a > 4-fold reduction in tracer extravasation in comparison with neovessels induced by VEGF alone (Figure 2B).
Figure 2.
Active Rac1 reduces neovessel leakiness associated with VEGF-driven pathologic angiogenesis. (A) VEGF165 transfectants were mixed with packaging cells expressing retrovirus encoding active L61Rac1 or empty vector (control) and injected together with Matrigel subdermally. Seven days later neovessel leakiness was assessed by confocal microscopy. Intravascular tracer was visualized with Texas Red-dextran (molecular weight, 70 kDa) injected 1 minute before killing (left). FITC-dextran (molecular weight, 70 kDa) injected 20 minutes before killing was used to visualize the sum of (intravascular tracer + extravascular tracer) (center). The extent of tracer extravasation was determined by subtracting the Texas Red signal from the respective FITC-dextran signal to yield the signal for extravascular tracer corresponding to tracer leakage (right). (B) Quantification of tracer leakage was achieved by integrating the signal intensities for the resultant extravasated tracer (n = 4 for both groups). Active Rac1 reduced tracer leakage > 4-fold. Scale bar = 200 μm.
In vitro, active Rac1 promotes capillary morphogenesis, organization of actin cables, and integrity of EC-EC junctions
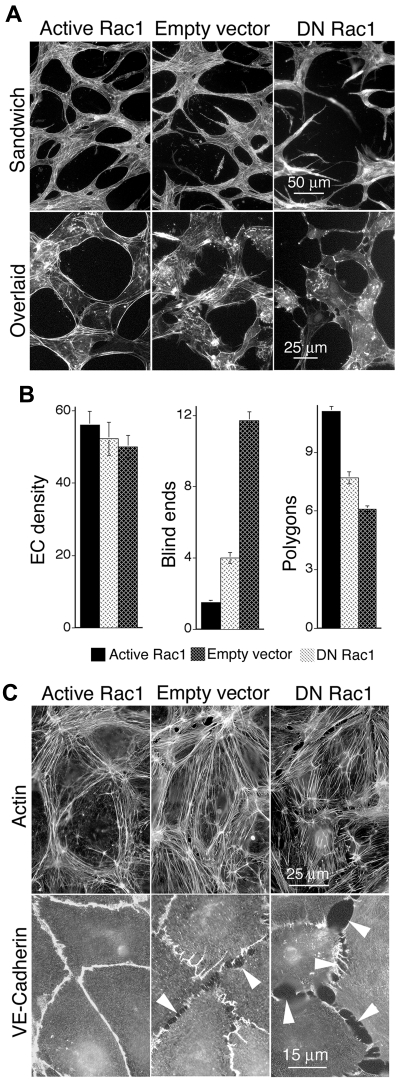
To identify mechanisms through which active Rac1 improves VEGF neovascular architecture and function, dermal MVECs were cultured in the continuous presence of VEGF and transduced with the same active Rac1 and DN Rac1 mutants used in vivo. As determined with a Pak1 binding domain assay for Rac1 activity, MVECs transduced with active Rac1 exhibited modest (∼ 20%-30%) but consistently increased levels of Rac1 activity, whereas DN Rac1 resulted in a ∼ 20%-30% decrease (see “Methods”; data not shown). These changes in Rac1 activity were comparable to those previously achieved with transduction by active and DN mutants of RhoA, which were sufficient to achieve marked reorganization of the cytoskeleton.20 MVECs transduced with active Rac1 and DN Rac1 were sandwiched or overlaid with 3-dimensional collagen-I matrix (see “Methods”), thereby inducing morphogenesis of MVECs into vascular cords,27–29 corresponding to the precapillary cords that form in vivo before the appearance of more mature tubes with lumens.27,30 Similar to findings in vivo, active Rac1 improved formation and integration of capillary cords as measured by a 60% decrease in blind ends and a 40% increase in closed vascular loops (polygons), relative to empty vector control (Figure 3A top panels, B). Staining for F-actin indicated that active Rac1 also strikingly improved the organization of cortical actin cables during cord formation (Figure 3A bottom panels). In contrast to active Rac1, DN Rac1 sharply disrupted formation of capillary cords (Figure 3A top panels, B) and promoted disorganization of actin cables (Figure 3A bottom panels), consistent with our observations that DN Rac1 strongly impaired neovascularization in vivo.
Figure 3.
Active Rac1 improves formation of vascular cords, organizes actin filaments cortically, and improves EC-EC junctions in vitro. (A) Dermal MVECs, transduced with Rac1 mutants or empty vector control, were induced to undergo capillary morphogenesis (cord formation, see “Methods”) by sandwiching between 2 layers of collagen I (top) or overlaying with collagen I (bottom). Subsequently cells were stained for F-actin. Top panels: note abundance of cord blind ends in DN Rac1 and control specimens that are absent in the active Rac1 specimen. Bottom panels (higher magnification): note cortical distribution of actin filaments in MVECs transduced with active Rac1, whereas actin is poorly organized in MVECs transduced with empty vector control or DN Rac1. (B) Quantification of cord parameters; n > 20 for all groups. Relative to controls, active Rac1 strongly reduced cord blind ends (P < .001) and increased cord integration as measured by counting closed polygons (P < .01), whereas DN Rac1 increased cord blind ends and reduced cord integration (polygons). (C) Confluent monolayers of MVECs, cultured in the presence of 20 ng/mL VEGF, and stained for F-actin (top panels) or VE-cadherin (bottom). Relative to empty vector control, active Rac1 strongly organized actin filaments cortically and improved integrity of EC-EC junctions as shown by VE-cadherin staining. DN Rac1 had opposite effects.
In monolayer culture, active Rac1 also strongly organized cortical actin cables (Figure 3C top panels) and improved the integrity of EC-EC junctions as shown by staining for VE-cadherin (Figure 3C bottom panels). These observations are highly indicative of improved barrier function31,32 and probably explain our findings that active Rac1 reduced leak of vascular tracer from neovessels in vivo. In direct contrast, DN Rac1 disrupted cortical actin, induced contractile actin stress fibers, and disrupted EC-EC junctions (Figure 3C).
SEW2871, a S1P receptor-1 agonist and Rac activator, improves capillary morphogenesis and actin dynamics in vitro and improves VEGF-driven neovascularization in vivo similarly to active Rac1
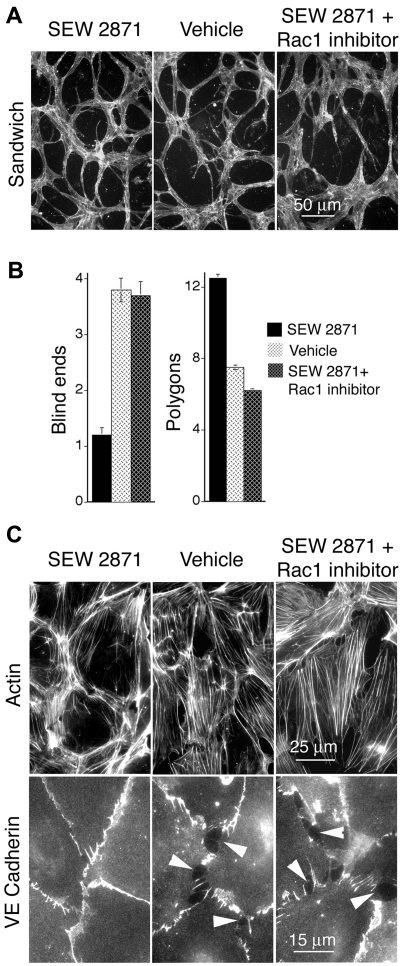
The foregoing observations suggested that activation of Rac1 is a potentially important strategy for improving defective neovessels associated with pathologic angiogenesis. Therefore, to identify a translatable, pharmacologic strategy for improving pathologic VEGF-driven angiogenesis, we proceeded to test compounds known to activate Rac1 in ECs. We specifically investigated SEW287133 because it selectively activates Rac1, rather than other Rho GTPases, through binding to the receptor S1P1.4,33 In vitro, SEW2871 improved capillary morphogenesis, actin organization, and integrity of EC-EC junctions very similarly to active Rac1 (Figure 4), identifying this compound as a good candidate for further investigations in vivo. Moreover, the specific Rac1 inhibitor NSC2376634 neutralized SEW2871-mediated improvement of capillary morphogenesis, cortical actin organization, and EC-EC junctions, indicating that Rac1 was pivotal to the mechanism by which SEW2871 improved these vascular morphogenetic parameters (Figure 4).
Figure 4.
In vitro, S1P1 agonist SEW2871 exerts Rac-dependent improvement of vascular cords, cortical actin, and EC-EC junctions. (A) Dermal MVECs, treated with SEW2871 (15nM, ∼ 1× median effective concentration), vehicle, or SEW2871 + Rac1 inhibitor NSC23766 (50μM, ∼ 1× IC50) were induced to form capillary cords by sandwiching between 2 layers of collagen I (see “Methods”). Subsequently cells were stained for F-actin. Note abundance of cord blind ends in vehicle control and SEW2871 + Rac inhibitor specimens that are absent in the SEW2871 specimen. (B) Quantification of cord parameters; n > 25 for all groups. Relative to controls, SEW2871 strongly reduced cord blind ends (P < .001) and increased cord integration as measured by counting closed polygons (P < .001). Rac1 inhibitor NSC23766 abolished these improvements, indicating that SEW2871-mediated enhancement of cord formation is Rac1 dependent. (C) Confluent monolayers of MVECs cultured in the presence of 20 ng/mL VEGF and stained for F-actin (top) or VE-cadherin (bottom). Relative to empty vector control, SEW2871 (15nM, ∼ 1× median effective concentration, 24 hours) strongly organized actin filaments cortically and improved integrity of EC-EC junctions as highlighted by VE-cadherin staining. Addition of Rac1 inhibitor NSC23766 (50μM, ∼ 1× IC50, 1 hour) abolished SEW2871-mediated enhancement of cortical actin and EC-EC junctions.
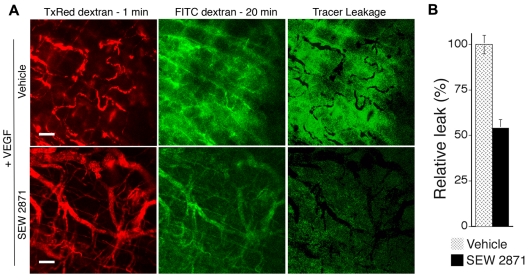
To test the efficacy of SEW2871 in vivo, we used the same angiogenesis model used for the Rac1 retroviral experiments but without retroviral packaging cells. In preliminary experiments, SEW2871 was administered orally by gavage once daily at different doses (5, 10, and 20 mg/kg) beginning on day 2. These doses in mice have been shown previously to be within the pharmacologically active range for immunosuppression.35 As determined grossly at day 7, the daily 10-mg/kg dose most improved the integration of VEGF neovessels; therefore, more extensive experiments and analyses were performed with this dose. As quantified from gross flat mount and cross-sectional images, daily administration of 10 mg/kg SEW2871 markedly increased neovascularization and increased lumen formation and neovessel diameter (Figure 5A-B). Quantitatively, all of these improvements were comparable to those observed with transduction by active Rac1. In addition, similarly to active Rac1, SEW2871 improved blood vessel formation without increasing EC density. In vitro, SEW2871 (10 ng/mL or ∼ 2 × median effective concentration) had no effect on VEGF production by VEGF–SK-MEL2 cells (see “Methods”), consistent with the design of the VEGF vector to express VEGF constitutively with a CMV promoter. Finally, analyses of neovascular leak with fluorescent tracers and confocal microscopy also established that SEW2871 substantially (50%) reduced extravasation of 70 kDa of tracer from VEGF neovessels (Figure 6).
Figure 5.
Administration of SEW2871 improves VEGF-driven angiogenesis in vivo. Mice were treated daily with SEW2871, beginning on day 2 after implantation of the VEGF165-transfected cells in Matrigel. (A) As shown grossly at day 7, SEW2871 improved formation of neovessels (scale bar = 450 μm). In addition, as viewed in cross-sections stained for CD31, neovessels with well-developed lumens were most abundant in the SEW2871 group. S indicates skeletal muscle layer; V, vessels, M, Matrigel; scale bar = 25 μm. (B) Quantification of vascular parameters (n > 20 for all groups). From gross images: relative neovascularization, (ie, percentage of relative area occupied by neovessels in flat mount, P < .001). From CD31-stained cross-sections: quantification of ECs per 0.01 mm2 relative total lumen area (P < .001), numbers of neovessels per 0.015 mm2 and average internal neovessel diameter (P < .001).
Figure 6.
SEW2871 reduces neovessel leakiness associated with VEGF-driven pathologic angiogenesis. (A) Mice were treated daily with SEW2871 or vehicle, beginning on day 2 after implantation of the VEGF165-transfected cells in Matrigel. Six days later vessel leakiness was assessed by confocal microscopy. Intravascular tracer was visualized with Texas Red-dextran (molecular weight, 70 kDa) injected 1 minute before killing (left). FITC-dextran (molecular weight, 70 kDa) injected 20 minutes before killing was used to visualize the sum of (intravascular tracer + extravascular tracer; center). The extent of tracer extravasation was determined by subtracting the Texas Red signal from the respective FITC-dextran signal to yield the signal for extravascular tracer corresponding to tracer leakage (right). (B) Quantification of tracer leakage was achieved by integrating the signal intensities for the resultant extravasated tracer (n = 4 for both groups). Active Rac1 reduced tracer leakage > 2-fold. Scale bar = 200 μm.
Ang-1 promotes capillary morphogenesis and organization of EC-EC junctions in vitro similarly to active Rac1, through a Rac1-dependent mechanism
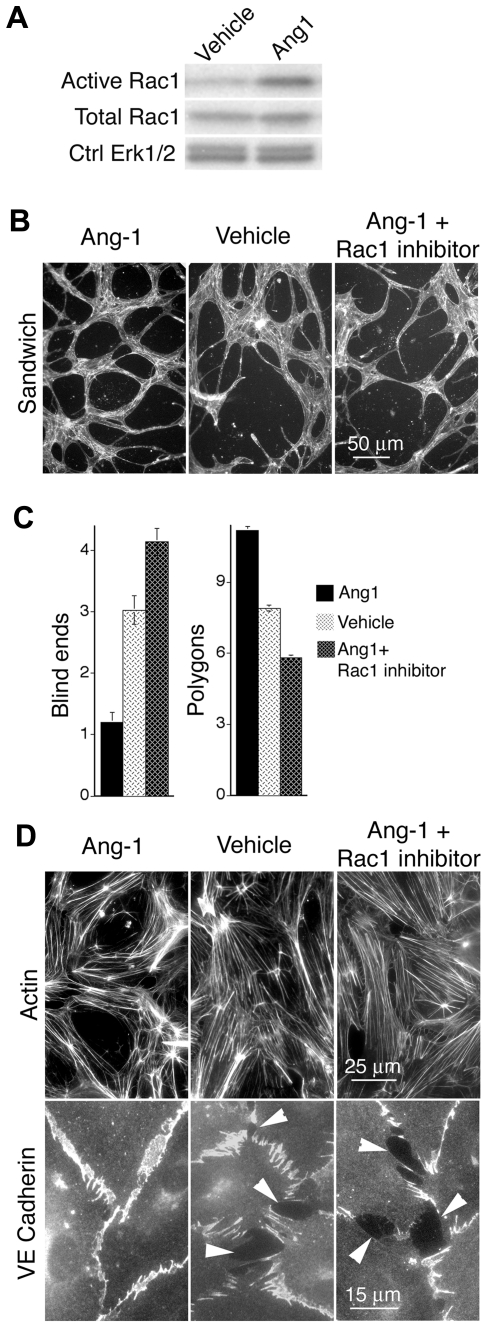
The improvements to pathologic VEGF neovessels mediated by active Rac1 and SEW2871 are reminiscent of previous reports showing that the vascular cytokine Ang-1 “normalizes” VEGF-driven angiogenesis to yield larger, less leaky neovessels.36,37 Moreover, Ang-1 persistently activates Rac1 in dermal MVECs (Figure 7A). This suggested the intriguing hypothesis that Ang-1 improves VEGF-driven angiogenesis through activation of Rac1. To explore this possibility, we analyzed dermal MVECs that were stimulated with Ang-1 in the presence and absence of the specific Rac1 inhibitor, NSC23766.34 Consistent with the established role of Ang-1 in normalizing VEGF neovessels,36 Ang-1 improved formation of precapillary cords very similarly to active Rac1 (Figure 7; compare with Figure 3). Moreover, the specific Rac1 inhibitor NSC23766 blocked Ang-1 improvement of cord formation. Finally, consistent with the role of Ang-1 in reducing vascular leakiness,36 Ang-1 organized cortical actin cables and improved organization of VE-cadherin at EC junctions very similarly to active Rac1 (Figure 7, compare with Figure 3), and these improvements were abolished by the Rac1 inhibitor NSC23766 (Figure 7).
Figure 7.
Ang-1 elevates Rac1 activity in MVECs and exerts Rac1-dependent improvement of cord formation, cortical actin, and EC-EC junctions. (A) Ang-1 (100 ng/mL, 24 hours) elevates Rac1 activity in dermal MVECs. (B) Ang-1 (100 ng/mL) improves cord formation by MVECs sandwiched between 2 layers of collagen I (see “Methods”). Cells were stained for F-actin. Note abundance of cord blind ends in vehicle control and Ang-1 + Rac1 inhibitor (NSC23766 50μM, ∼ 1× median effective concentration) specimens that are absent in the Ang-1 specimen. (C) Quantification of cord parameters; n > 25 for all groups. Relative to controls, Ang-1 reduced cord blind ends (P < .01) and increased cord integration as measured by counting closed polygons (P < .01). Rac1 inhibitor NSC23766 abolished these effects, indicating that Ang-1 improves cord formation through a Rac1-dependent mechanism. (D) Confluent monolayers of MVECs, cultured in the presence of 20 ng/mL VEGF, and stained for F-actin (top) or VE-cadherin (bottom). Relative to empty vector control, Ang-1 (100 ng/mL, 24 hours) strongly organized actin filaments cortically and improved integrity of EC-EC junctions as shown by VE-cadherin staining. Within 1 hour, Rac1 inhibitor NSC23766 (50μM, ∼ 1× median effective concentration) abolished Ang-1–mediated enhancement of cortical actin and EC-EC junctions.
Distinctions between VEGF and Ang-1: regulation of cortical actin and VE-cadherin at EC junctions
Persistent activation of Rac1 by Ang-1 (Figure 7) raises important questions about distinctions between VEGF and Ang-1, regulation of EC junctions, and chronic vascular leakiness. Interestingly, VEGF initially activates Rac1, but this activation quickly declines from 30 to 60 minutes.38 We found that by 30 minutes VEGF induced a cortical actin cytoskeleton similar to active Rac1, Ang-1, and SEW2871 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). However, VEGF-induced organization of cortical actin was only temporary and was replaced with prominent actin stress fibers by 4 hours (supplemental Figure 1). In contrast, Ang-1 and SEW2871 induced cortical actin that was evident at 30 minutes and persisted to 24 hours (supplemental Figure 1; Figures 4,7). Coincident with VEGF induction of stress fibers, by 4 hours VEGF also disrupted the organization of VE-cadherin at cell junctions and induced gaps between adjacent ECs (supplemental Figure 2). These findings are consistent with the association between VEGF and chronic vascular leakiness in vivo. In contrast, Ang-1 and SEW2871 each promoted persistent organization of VE-cadherin and EC junctions, similarly to active Rac1 (supplemental Figure 2; Figures 3, 4, 7) and consistent with reduced vascular leakiness in vivo. Thus, Ang-1, but not VEGF, persistently organizes cortical actin, VE-cadherin junctional localization, and cell junction integrity, key parameters correlated with improved endothelial barrier function.31,32
Discussion
The outcome of enhancing Rac1 activity for adult neovascularization in vivo has not been explored previously. Our studies identify augmentation of Rac1 activity as an important strategy for rectifying neovascular defects typically associated with pathologic angiogenesis. Transduction with active L61Rac1 significantly improved VEGF-driven angiogenesis in vivo, as measured by increased relative neovessel density, enlarged neovessel size, enhanced lumen formation, and reduced neovessel leakiness. Remarkably, active Rac1 improved VEGF-driven angiogenesis without increasing EC density relative to control. Rather, Rac1 improved VEGF-driven angiogenesis by promoting the proper assembly of ECs into new blood vessels with lumens. In vitro, active Rac1 facilitated formation of precapillary cords, as measured by increased cord length and integration; and these improvements in cord formation paralleled a striking improvement in the organization of actin cables. In addition, in monolayer culture, active Rac1 improved organization of cortical actin and EC-EC junctions in vitro, consistent with reduced vascular leak in vivo. Notably, our in vivo experiments do not distinguish possible effects of active Rac1 on cell types other than ECs that may have influenced angiogenesis. However, our in vitro experiments with active Rac1 and isolated ECs in the absence of other cell types showed strong parallels with the in vivo findings that included improved capillary morphogenesis, increased capillary cord length, and improved integrity of EC-EC junctions. These in vitro experiments are consistent with a direct effect of Rac1 on ECs in vivo, resulting in improved formation of neovessels and reduced vascular leak, although they do not exclude possible involvement of other cell types.
Importantly, we found that only a moderate increase in Rac1 activity (∼ 20%-30%) was required to achieve improved capillary morphogenesis and improved organization of EC cortical actin and EC-EC junctions in vitro. Interestingly, modest changes in Rac activity have been shown previously to alter the directionality of cell migration in various cell types,39 and modest changes in RhoA activity have been shown to induce marked reorganization of the cytoskeleton in ECs.20 Although it was not possible to measure Rac1 activity in our in vivo experiments, it is important to consider that moderate increases in Rac1 activity are probably sufficient to achieve marked improvement in neovessel architecture and function.
In direct contrast to active Rac1, DN Rac1 sharply decreased VEGF-induced neovascularization in mouse skin, and neovessels with lumens were virtually absent despite the abundance of ECs in the host skin adjacent to the angiogenic stimulus. As viewed in cross-section, ECs accumulated robustly in skin overlying the angiogenic stimulus; but DN Rac1 prevented ECs from forming new blood vessels with lumens. In vitro, DN Rac1 disrupted formation of precapillary cords; cytoskeletal analyses linked impaired organization of F-actin to poor cord formation. These findings with DN Rac1 are consistent with previous reports that selective deletion of Rac1 in ECs inhibits embryonic angiogenesis,6 that adult mice with EC-specific haplo-insufficiency for Rac1 exhibit impaired angiogenesis in a hind limb ischemia model, and that Rac1 is essential for capillary morphogenesis and lumen formation in vitro.6,9–11 However, they do not seem to correlate with a recent report that endothelial-Rac1 is dispensable for tumor angiogenesis unless integrin αvβ3 is absent,8 although fundamental differences in experimental design and vascular analyses may account for this apparent discrepancy.
Likely mechanisms by which active Rac1 reduced the chronic leakiness of VEGF neovessels in vivo, as measured with 70 kDa of fluorescent tracer, are illustrated by our in vitro findings that active Rac1 reduced actin stress fibers, increased cortical actin, and enhanced EC-EC junctions in dermal MVECs continuously stimulated by VEGF. Previous studies involving analyses of Rac1 and vascular permeability or leakiness have yielded apparently conflicting findings.6,40–43 For example, DN Rac1 has been shown to decrease the barrier function of EC monolayers,40 and a Rac inhibitor increased the baseline permeability of isolated microvessels.41 In contrast, others have shown that DN Rac1 blocked acute, short-term, hyperpermeability induced by VEGF in vitro6,42,43 and in vivo.42 Collectively, these reports underscore the importance of distinguishing between basal permeability, persistent leakiness, and acute or transient hyperpermeability,44 and they suggest that Rac1 probably regulates these parameters by distinctly different mechanisms. In particular, VEGF induces acute hyperpermeability of normal blood vessels transiently, lasting < 30 minutes,23 whereas pathologic VEGF neovessels are persistently leaky, as shown here and previously.12,45 Therefore, it is important to emphasize that the in vivo studies described here address the persistent or chronic leakiness of pathologic VEGF neovessels, as opposed to acute or transient vascular hyperpermeability, and that our studies specifically illustrate that active Rac1 reduces persistent neovessel leakiness. Moreover, our in vitro experiments showed that active Rac1 improved cortical actin and EC-EC junctions in ECs persistently (ie, not acutely) stimulated by VEGF, consistent with our findings that active Rac1 reduced chronic leakiness in vivo.
The vascular cytokine Ang-1 has been shown previously to reduce the chronic leakiness of VEGF neovessels, and Ang-1 also increases VEGF neovessel size and improves VEGF neovessel architecture.36,37 Although we did not directly compare active Rac1 with Ang-1 in our in vivo model of VEGF-driven angiogenesis, our findings with active Rac1 are closely similar to those reported for Ang-1 previously.36,37 Interestingly, Ang-1 provides persistent (ie, long-term) activation of Rac1, but, in contrast, VEGF activates Rac1 with a peak ∼ 30 minutes.38 Consistent with these differences in Rac1 activation, VEGF organized cortical actin and improved the integrity of EC-EC junctions at 30 minutes, but cortical actin was replaced by actin stress fibers and EC-EC barrier integrity was compromised by 4 hours. In contrast, Ang-1 maintained cortical actin and EC-EC junctions persistently through 24 hours. Thus, persistent Rac1 activation by Ang-1 underscores an important distinction with VEGF. Furthermore, VEGF induction of actin stress fibers and disruption of EC-EC junction integrity probably explains the chronic hyperpermeability of VEGF neovessels. Finally, our experiments also show that, even in the presence of VEGF, Ang-1 improves formation of vascular cords and enhances organization of cortical actin and EC-EC junctions comparably to active Rac1 and that a specific Rac1 inhibitor blocks these improvements. Therefore, the data support the intriguing hypothesis that Ang-1 “normalizes” VEGF neovessels, at least in part, by augmenting Rac1 activity. In further support of this hypothesis, others have shown that Ang-1 protects against endotoxin-induced vascular leakage by activating Rac.3
Abnormal angioarchitecture, poor integration of the vascular network, and vessel leakiness limit vascular perfusion and seriously reduce the functionality of VEGF pathologic neovessels, and these vascular defects themselves can aggravate underlying disorders. Moreover, these vascular defects seriously limit the use of VEGF as therapeutic agent for tissue revascularization.15–18 Although proangiogenic therapy with VEGF can increase blood flow in experimental models, the positive effects of VEGF are compromised by vascular malformations and vascular leak.17,18 Accordingly, our studies with SEW2871, an S1P1 agonist, Rac1 activator,33 and persistent inducer of cortical actin and enhanced EC-EC junction integrity, identify a rational drug-based strategy for correcting these neovascular defects. Furthermore, findings with SEW2871 suggest more generally that pharmacologic strategies involving other S1P1 agonists or other Rac1 activators that organize cortical actin and improve EC-EC junction integrity persistently could be used effectively to improve pathologic angiogenesis and promote therapeutic angiogenesis with VEGF. Interestingly, S1P, the natural ligand for S1P1 as well as other S1P receptors,4 has been shown previously to promote capillary morphogenesis in vitro and augment angiogenesis driven by basic fibroblast growth factor and VEGF in vivo.46 In addition, S1P1 is important for stabilization of nascent blood vessels,47 and interactions between S1P and S1P1 are important for maintenance of EC barrier function.46,48–50 Thus, there is a considerable body of evidence linking S1P and S1P1 to angiogenesis and maintenance of vascular barrier function. However, the utility of S1P1 agonists for rectifying neovascular defects typically associated with pathologic angiogenesis has not been shown previously. Thus, our experiments add uniquely to the existing body of information on S1P/S1P1 function by demonstrating that SEW2871 administration in vivo rectifies pathologic angioarchitecture and associated chronic neovascular leakiness comparably to active Rac1. Notably, our experiments do not distinguish possible effects of SEW2871 on cell types other than ECs, and they do not establish that SEW2871 acted exclusively through Rac1 activation. Nonetheless, our in vitro experiments with a specific Rac inhibitor in combination with SEW2871 and isolated ECs in the absence of other cell types demonstrated that SEW2871 improved capillary morphogenesis, increased capillary cord length, and improved integrity of EC-EC junctions through a Rac-dependent mechanism. Thus, these in vitro experiments are consistent with a direct effect of SEW2871 on ECs that results in improved angiogenesis and reduced vascular leak, although additional involvement of other cell types and signaling pathways are not excluded.
Finally, our findings also suggest the more general hypothesis that impaired regulation of the EC cytoskeleton is responsible for vascular defects associated with pathologic angiogenesis. Because multiple signaling molecules in addition to Rac1 regulate the cytoskeleton,1 there are probably other cytoskeletal-targeting strategies, in addition to the one described here, for rectifying neovascular defects.13 In particular, we have shown previously that active RhoA, a key regulator of cytoskeletal dynamics, can also markedly improve VEGF-driven angiogenesis in vivo, although differently from that shown here with active Rac1.20 Therefore, multiple targeting strategies, possibly used in combination may eventually provide optimal rectification of pathologic neovessels.
In summary and conclusion, these experiments show that augmentation of Rac1 activity in a mouse model rectifies key structural and functional defects typically associated with pathologic VEGF-driven angiogenesis. Transduction with active Rac1 improved angiogenesis as measured by increased neovessel density, increased lumen formation, improved angioarchitecture, and reduced vessel leakiness; experiments with isolated human MVECs in vitro suggest that these improvements were mediated through improvement of capillary morphogenesis and integrity of EC-EC junctions. From a translational standpoint, these studies distinguish augmentation of Rac1 activity as a means to rectify the pathologic angioarchitecture and dysfunctionality of VEGF neovessels, and they suggest a rational pharmacologic strategy for achieving this goal.
Supplementary Material
Acknowledgments
We thank Mary Whelan for isolation of MVECs and expert help with experiments.
This work was supported by the National Institutes of Health, National Cancer Institute (grant CA129339) (D.R.S.).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.R.S., J.A.N., and M.V.H. designed studies and wrote the manuscript; M.V.H., J.A.N., and D.R.S. performed research; M.V.H., D.R.S., and J.A.N. analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Donald R. Senger, Department of Pathology, RN 280E, Beth Israel Deaconess Medical Center, 99 Brookline Ave, Boston, MA 02215; e-mail: dsenger@bidmc.harvard.edu.
References
- 1.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9(9):690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 2.Royal I, Lamarche-Vane N, Lamorte L, Kaibuchi K, Park M. Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol Biol Cell. 2000;11(5):1709–1725. doi: 10.1091/mbc.11.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mammoto T, Parikh SM, Mammoto A, et al. Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J Biol Chem. 2007;282(33):23910–23918. doi: 10.1074/jbc.M702169200. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92(5):913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 5.Price LS, Leng J, Schwartz MA, Bokoch GM. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell. 1998;9(7):1863–1871. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan W, Palmby TR, Gavard J, Amornphimoltham P, Zheng Y, Gutkind JS. An essential role for Rac1 in endothelial cell function and vascular development. FASEB J. 2008;22(6):1829–1838. doi: 10.1096/fj.07-096438. [DOI] [PubMed] [Google Scholar]
- 7.Sawada N, Salomone S, Kim HH, Kwiatkowski DJ, Liao JK. Regulation of endothelial nitric oxide synthase and postnatal angiogenesis by Rac1. Circ Res. 2008;103(4):360–368. doi: 10.1161/CIRCRESAHA.108.178897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Amico G, Robinson SD, Germain M, et al. Endothelial-Rac1 is not required for tumor angiogenesis unless alphavbeta3-integrin is absent. PLoS ONE. 2010;5(3):e9766. doi: 10.1371/journal.pone.0009766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connolly JO, Simpson N, Hewlett L, Hall A. Rac regulates endothelial morphogenesis and capillary assembly. Mol Biol Cell. 2002;13(7):2474–2485. doi: 10.1091/mbc.E02-01-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cascone I, Giraudo E, Caccavari F, et al. Temporal and spatial modulation of Rho GTPases during in vitro formation of capillary vascular network. Adherens junctions and myosin light chain as targets of Rac1 and RhoA. J Biol Chem. 2003;278(50):50702–50713. doi: 10.1074/jbc.M307234200. [DOI] [PubMed] [Google Scholar]
- 11.Koh W, Mahan RD, Davis GE. Cdc42- and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. J Cell Sci. 2008;121(Pt 7):989–1001. doi: 10.1242/jcs.020693. [DOI] [PubMed] [Google Scholar]
- 12.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74(2–3):72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagy JA, Senger DR. VEGF-A, cytoskeletal dynamics, and the pathological vascular phenotype. Exp Cell Res. 2006;312(5):538–548. doi: 10.1016/j.yexcr.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Ozawa CR, Banfi A, Glazer NL, et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113(4):516–527. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102(8):898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- 16.Isner JM, Vale PR, Symes JF, Losordo DW. Assessment of risks associated with cardiovascular gene therapy in human subjects. Circ Res. 2001;89(5):389–400. doi: 10.1161/hh1701.096259. [DOI] [PubMed] [Google Scholar]
- 17.Rissanen TT, Korpisalo P, Markkanen JE, et al. Blood flow remodels growing vasculature during vascular endothelial growth factor gene therapy and determines between capillary arterialization and sprouting angiogenesis. Circulation. 2005;112(25):3937–3946. doi: 10.1161/CIRCULATIONAHA.105.543124. [DOI] [PubMed] [Google Scholar]
- 18.von Degenfeld G, Banfi A, Springer ML, et al. Microenvironmental VEGF distribution is critical for stable and functional vessel growth in ischemia. FASEB J. 2006;20(14):2657–2659. doi: 10.1096/fj.06-6568fje. [DOI] [PubMed] [Google Scholar]
- 19.Hoang MV, Senger DR. In vivo and in vitro models of mammalian angiogenesis. Methods Mol Biol. 2005;294:269–285. doi: 10.1385/1-59259-860-9:269. [DOI] [PubMed] [Google Scholar]
- 20.Hoang MV, Whelan MC, Senger DR. Rho activity critically and selectively regulates endothelial cell organization during angiogenesis. Proc Natl Acad Sci U S A. 2004;101(7):1874–1879. doi: 10.1073/pnas.0308525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M. Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins. Proc Natl Acad Sci U S A. 1997;94(25):13612–13617. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Doux JM, Landazuri N, Yarmush ML, Morgan JR. Complexation of retrovirus with cationic and anionic polymers increases the efficiency of gene transfer. Hum Gene Ther. 2001;12(13):1611–1621. doi: 10.1089/10430340152528110. [DOI] [PubMed] [Google Scholar]
- 23.Senger DR, Connolly DT, Van de Water L, Feder J, Dvorak HF. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res. 1990;50(6):1774–1778. [PubMed] [Google Scholar]
- 24.Zeng H, Qin L, Zhao D, et al. Orphan nuclear receptor TR3/Nur77 regulates VEGF-A-induced angiogenesis through its transcriptional activity. J Exp Med. 2006;203(3):719–729. doi: 10.1084/jem.20051523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamarche N, Tapon N, Stowers L, et al. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87(3):519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- 26.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 27.Vernon RB, Sage EH. Between molecules and morphology. Extracellular matrix and creation of vascular form. Am J Pathol. 1995;147(4):873–883. [PMC free article] [PubMed] [Google Scholar]
- 28.Davis GE, Camarillo CW. An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res. 1996;224(1):39–51. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 29.Whelan MC, Senger DR. Collagen I initiates endothelial cell morphogenesis by inducing actin polymerization through suppression of cyclic AMP and protein kinase A. J Biol Chem. 2003;278(1):327–334. doi: 10.1074/jbc.M207554200. [DOI] [PubMed] [Google Scholar]
- 30.Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9. 5 mouse embryo. Blood. 2000;95(5):1671–1679. [PubMed] [Google Scholar]
- 31.Liu F, Schaphorst KL, Verin AD, et al. Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: potential role of glycogen synthase kinase-3beta. FASEB J. 2002;16(9):950–962. doi: 10.1096/fj.01-0870com. [DOI] [PubMed] [Google Scholar]
- 32.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105(5):1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- 33.Jo E, Sanna MG, Gonzalez-Cabrera PJ, et al. S1P1-selective in vivo-active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol. 2005;12(6):703–715. doi: 10.1016/j.chembiol.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101(20):7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanna MG, Liao J, Jo E, et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279(14):13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 36.Thurston G, Suri C, Smith K, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286(5449):2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 37.Chae JK, Kim I, Lim ST, et al. Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arterioscler Thromb Vasc Biol. 2000;20(12):2573–2578. doi: 10.1161/01.atv.20.12.2573. [DOI] [PubMed] [Google Scholar]
- 38.Garrett TA, Van Buul JD, Burridge K. VEGF-induced Rac1 activation in endothelial cells is regulated by the guanine nucleotide exchange factor Vav2. Exp Cell Res. 2007;313(15):3285–3297. doi: 10.1016/j.yexcr.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pankov R, Endo Y, Even-Ram S, et al. A Rac switch regulates random versus directionally persistent cell migration. J Cell Biol. 2005;170(5):793–802. doi: 10.1083/jcb.200503152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114(Pt 7):1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- 41.Waschke J, Baumgartner W, Adamson RH, et al. Requirement of Rac activity for maintenance of capillary endothelial barrier properties. Am J Physiol Heart Circ Physiol. 2004;286(1):H394–401. doi: 10.1152/ajpheart.00221.2003. [DOI] [PubMed] [Google Scholar]
- 42.Eriksson A, Cao R, Roy J, et al. Small GTP-binding protein Rac is an essential mediator of vascular endothelial growth factor-induced endothelial fenestrations and vascular permeability. Circulation. 2003;107(11):1532–1538. doi: 10.1161/01.cir.0000055324.34758.32. [DOI] [PubMed] [Google Scholar]
- 43.Monaghan-Benson E, Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J Biol Chem. 2009;284(38):25602–25611. doi: 10.1074/jbc.M109.009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11(2):109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagy JA, Feng D, Vasile E, et al. Permeability properties of tumor surrogate blood vessels induced by VEGF-A. Lab Invest. 2006;86(8):767–780. doi: 10.1038/labinvest.3700436. [DOI] [PubMed] [Google Scholar]
- 46.Lee MJ, Thangada S, Claffey KP, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99(3):301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 47.Paik JH, Skoura A, Chae SS, et al. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev. 2004 Oct 1;18(19):2392–2403. doi: 10.1101/gad.1227804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia JG, Liu F, Verin AD, et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108(5):689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehta D, Konstantoulaki M, Ahmmed GU, Malik AB. Sphingosine 1-phosphate-induced mobilization of intracellular Ca2+ mediates rac activation and adherens junction assembly in endothelial cells. J Biol Chem. 2005;280(17):17320–17328. doi: 10.1074/jbc.M411674200. [DOI] [PubMed] [Google Scholar]
- 50.Sanna MG, Wang SK, Gonzalez-Cabrera PJ, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol. 2006;2(8):434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.