Abstract
Endostatin, an inhibitor of angiogenesis and tumor growth, was identified originally in conditioned media of murine hemangioendothelioma (EOMA) cells. N–terminal amino acid sequencing demonstrated that it corresponds to a fragment of basement membrane collagen XVIII. Here we report that cathepsin L is secreted by EOMA cells and is responsible for the generation of endostatin with the predicted N–terminus, while metalloproteases produce larger fragments in a parallel processing pathway. Efficient endostatin generation requires a moderately acidic pH similar to the pericellular milieu of tumors. The secretion of cathepsin L by a tumor cell line of endothelial origin suggests that this cathepsin may play a role in angiogenesis. We propose that cleavage within collagen XVIII's protease-sensitive region evolved to regulate excessive proteolysis in conditions of induced angiogenesis.
Keywords: cathepsin L/collagen XVIII/endostatin/LHVS/metalloproteases
Introduction
The dependence of tumor growth on vascular supply has been studied since the late 1800s. Likewise, the inhibition of metastatic and secondary tumor growth by primary tumor mass has been observed in many experimental models and cancer patients (referenced in O'Reilly et al., 1994). Recent molecular evidence provides an explanation for these two observations: a low metastatic variant of a murine Lewis lung carcinoma (LLC) appears to release an inhibitor of angiogenesis, angiostatin, which prevents neovascularization and, thereby, growth of its metastases (O'Reilly et al., 1994). Angiostatin originally was purified from serum and urine of LLC–bearing mice and identified as a 38 kDa fragment of plasminogen. Co-culture experiments suggest that conversion of plasminogen to angiostatin involves a metalloelastase (MME or MMP–12) secreted by tumor-infiltrating macrophages (Dong et al., 1997). In a second model system, a cell line derived from a non-metastatic murine hemangioendothelioma (EOMA) (Hoak et al., 1971; Obeso et al., 1990) was shown to produce endostatin, an inhibitor of angiogenesis and tumor growth (O'Reilly et al., 1997). Endostatin is generated from collagen XVIII, similarly to other anti-angiogenic factors that are also generated by proteolysis from large precursor proteins (Homandberg et al., 1985; Clapp et al., 1993; Gupta et al., 1995; Brooks et al., 1998; O'Reilly et al., 1999).
Collagen XVIII is the core protein of a heparan sulfate proteoglycan in vascular and epithelial basement membranes (Oh et al., 1994; Rehn and Pihlajaniemi, 1994; Muragaki et al., 1995; Halfter et al., 1998; Saarela et al., 1998). Collagens XVIII and XV form a distinct subgroup within the collagen superfamily characterized by multiple interruptions in the central triple-helical domain and a unique non-triple-helical domain at the C–terminus (NC1). Structural analyses of the recombinant 38 kDa NC1 domain of murine collagen XVIII suggest that it consists of three major segments: a 5 kDa N–terminal association domain that has been implicated in self-assembly of homotrimeric collagen XVIII; a central protease- sensitive hinge region; and the compact 22 kDa C–terminal endostatin domain (Sasaki et al., 1998). Cleavage within the hinge region of collagen XVIII generates several C–terminal fragments ranging from 22 to 32 kDa (O'Reilly et al., 1997; Sasaki et al., 1998; Miosge et al., 1999). Inhibition of endothelial cell proliferation was assigned specifically to a 22 kDa fragment, endostatin, that was molecularly defined based on the N–terminal sequence HTHQD (O'Reilly et al., 1997). How endostatin is released from collagen XVIII has remained unknown.
Matrix metalloproteases (MMPs), serine proteases and cysteine proteases have been implicated in the processing and degradation of native collagens in vitro. Collagenases of the MMP family, the gelatinase MMP–2 (Aimes and Quigley, 1995), the neutrophil serine elastase (Kafienah et al., 1998) and the cysteine protease cathepsin K (Garnero et al., 1998) can attack the triple helix of fibrillar collagens. Other members of the MMP family (referenced in Brown and Whittaker, 1999) and the collagenolytic serine and cysteine proteases (Starkey, 1977; Maciewicz and Etherington, 1988) cleave within non-helical regions located at either end of native collagens, which leads to destabilization of the fibrils.
To identify the protease(s) responsible for endostatin generation, we focused on the original EOMA cell culture model from which the only characterized naturally occurring C–terminal fragment of collagen XVIII with anti-angiogenic activity was isolated (O'Reilly et al., 1997). Furthermore, EOMA cells represent an ideal model to study the processing of collagen XVIII because they produce both basement membrane collagen XVIII and the proteolytic activity that generates endostatin. In this study, we show that the lysosomal cysteine protease cathepsin L is secreted from EOMA cells in large amounts and generates endostatin from collagen XVIII in a moderately acidic extracellular milieu.
Results
Parallel processing of collagen XVIII by MMPs and cysteine-type cathepsins
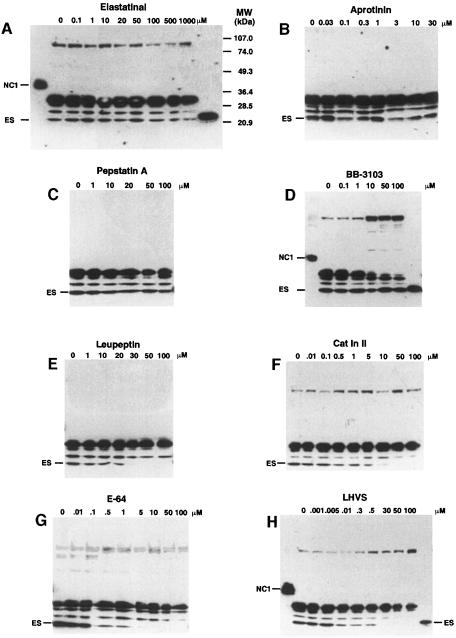
Serum-free conditioned media (SFCM) of EOMA cell cultures were found to contain endostatin when confluent EOMA cells were incubated for 36–48 h. None of the other cell lines tested [murine LLC, B16-BL6 melanoma, F9 embryonal carcinoma, human hepatocellular carcinoma Hep3B, bladder carcinoma ECV304, embryonal kidney (293-EBNA) and umbilical vein endothelial cells (HUVEC)] produced a fragment of the size of endostatin that was reactive with a polyclonal antibody against recombinant human endostatin (data not shown). In addition to endostatin migrating at 22 kDa, a major fragment of 30 kDa and two minor fragments of ∼25 and 29 kDa were detected in SFCM of EOMA cells (Figure 1). All fragments result from cleavage within collagen XVIII's protease-sensitive hinge region, which connects endostatin to the central triple-helical domain (Sasaki et al., 1998).
Fig. 1. Processing of collagen type XVIII by MMPs and cysteine-type cathepsins. SFCM from EOMA cell cultures was collected after 48 h incubation in the presence of increasing amounts of various class-specific protease inhibitors. A 0.5 ml aliquot of SFCM was concentrated, separated on 7.5–17.5% polyacrylamide–SDS gradient gels and electroblotted onto nitrocellulose. Collagen XVIII fragments containing endostatin were detected with a polyclonal antibody against human recombinant endostatin. For comparison, recombinant murine endostatin (ES) and murine NC1 were loaded onto the gels in (A), (D) and (H) on the right and left sides of the gels, respectively.
Several class-specific protease inhibitors were examined for their ability to inhibit generation of endostatin in serum-free EOMA cell culture over 48 h. The serine protease inhibitors elastatinal and aprotinin as well as the aspartic acid protease inhibitor pepstatin A did not inhibit the generation of any of the fragments at concentrations up to 1 mM, 30 μM and 100 μM, respectively (Figure 1A–C). While the hydroxamate MMP inhibitor BB-3103 showed a dose-dependent inhibition of the formation of the 30 kDa fragment, the production of endostatin was not affected (Figure 1D). Inhibition by BB-3103 coincided with the accumulation of a 100 kDa collagen XVIII fragment. These results suggest that although MMPs are not required for the generation of endostatin, they are involved in the processing of collagen XVIII.
The serine and cysteine protease inhibitor leupeptin and the cysteine protease inhibitors cathepsin B inhibitor II (Cat In II, a tripeptide analog of leupeptin) and E-64 (Figure 1E–G) inhibited the formation of endostatin, while the formation of the 25, 29 and 30 kDa fragments was unaffected. A cathepsin-type cysteine protease is therefore responsible for the cleavage of collagen XVIII to endostatin. To distinguish between the cysteine-type cathepsins, N–morpholinurea-leucine-homophenylalanine-vinylsulfone-phenyl (LHVS) was used. LHVS inhibits cathepsins S, L and B at different concentrations (Palmer et al., 1995; Riese et al., 1996). At 1–5 nM, LHVS selectively inhibits cathepsin S, and at this concentration it did not block the generation of endostatin. A reduction in endostatin production was observed at concentrations of 10–500 nM, which inhibit cathepsin L activity. No endostatin was detected at concentrations of 30–50 μM, which also block cathepsin B (Figure 1H). The 100 kDa fragment that was seen with BB-3103 also accumulated at high concentrations of LHVS. Processing of murine collagen XVIII by EOMA cells is therefore due to parallel activities of MMPs and cathepsins. MMPs are responsible for the generation of an endostatin-containing fragment of 30 kDa, while the 22 kDa endostatin is generated by cysteine-type cathepsins. Other proteases could be involved in the generation of the 100 kDa fragment, as accumulation of intact collagen XVIII was not observed with any of the inhibitors tested.
Processing of collagen XVIII's NC1 domain by secreted proteases
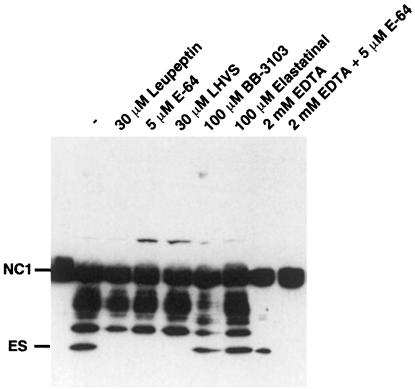
In order to investigate whether collagen type XVIII is cleaved intracellularly or extracellularly, its 38 kDa C–terminal non-triple-helical domain 1 (NC1) was produced in 293-EBNA cells and purified to homogeneity with the help of a C–terminal flag tag (mNC1). mNC1 was added to medium conditioned by EOMA cells for 48 h (SFCM). The SFCM alone was sufficient for processing of mNC1 to endostatin (Figure 2). The generation of endostatin from mNC1 was inhibited by all cysteine protease inhibitors tested, whereas the formation of intermediate fragments was blocked by the MMP inhibitors BB-3103 and EDTA (Figure 2). The combination of EDTA and E-64 completely inhibited the processing of mNC1. These data indicate that processing of mNC1 reproduces the final steps in the processing of collagen XVIII to endostatin and suggest that, in EOMA cell culture, endostatin is generated from collagen XVIII by a secreted cathepsin.
Fig. 2. Processing of recombinant mNC1 by EOMA conditioned medium (SFCM). A 700 ng aliquot of mNC1 containing a flag epitope at the C–terminus was added to 0.5 ml of SFCM from EOMA cell culture and incubated for 48 h at 37°C in the absence or presence of the indicated protease inhibitors. An anti-flag antibody was used to monitor processing by Western blotting.
Cathepsin L generates endostatin
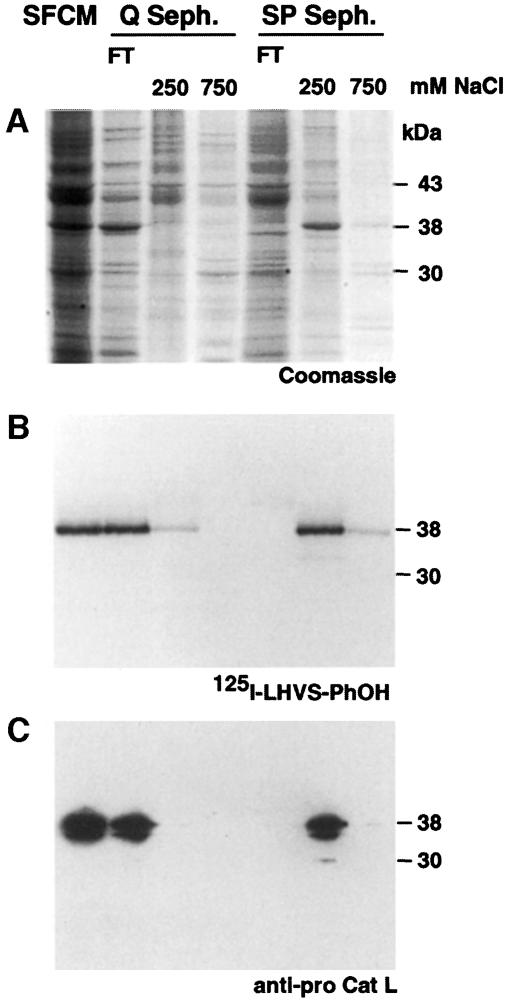
To identify the cysteine-type cathepsin, we took advantage of [125I]LHVS-PhOH, an analog of LHVS, which binds covalently to the active site cysteine of cathepsins S, L and B (Driessen et al., 1999; R.A.R.Bryant, in preparation). When [125I]LHVS-PhOH was incubated with EOMA SFCM, a 38 kDa protein was labeled (Figure 3B). In several test purifications, a correlation between the 38 kDa [125I]LHVS-PhOH-labeled band and one of the major proteins produced by EOMA cells was observed (Figure 3A and B). N–terminal amino acid sequencing established its identity as procathepsin L. An antibody against murine procathepsin L immunoprecipitated the [125I]LHVS-PhOH-labeled protein (data not shown) and identified the same band in Western blots (Figure 3C). Thus, EOMA cells express and secrete large amounts of procathepsin L.
Fig. 3. Identification of procathepsin L in SFCM from EOMA cell cultures. (A) Coomassie Blue staining of SFCM (lane 1) and single fractions of test purifications with Q- and SP-Sepharose columns. N–terminal sequencing of the 38 kDa band in the 250 mM NaCl eluate of SP-Sepharose (S250) gave the sequence TPKFDQTF-A, corresponding to the N–terminus of murine procathepsin L. (B) The fractions from (A) were probed with [125I]LHVS-PhOH and analyzed by SDS–PAGE and autoradiography. Specific labeling was found in the flow-through (FT) of Q-Sepharose and in the S250 fraction. (C) Fractions from (A) and (B) were probed with an antibody against murine procathepsin L (anti-pro Cat L). Some processing of procathepsin L results from repeated freezing and thawing.
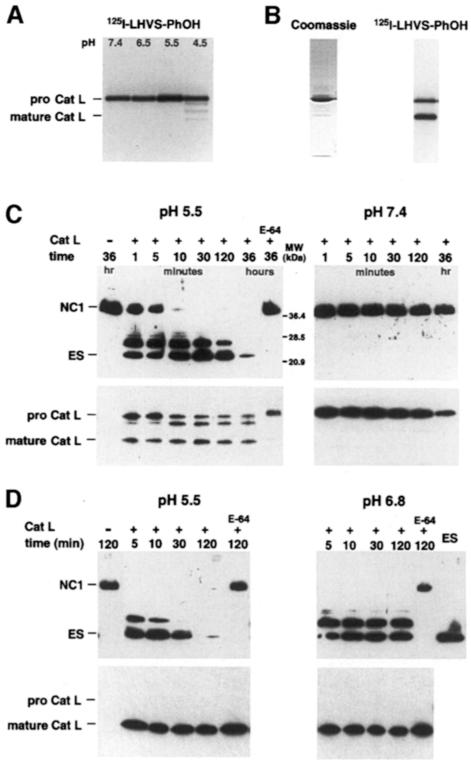
Synthesized as 38 kDa procathepsin L, the proteolytic activity of cathepsin L depends on the conversion into one of the mature forms (30 or 25 kDa) at low pH (Kirschke, 1998). Whether the proform exhibits activity extracellularly is controversial. Small substrates can be digested by the proform at ∼10% the rate of the mature enzyme (Mason et al., 1987). Similarly, the mechanism-based inhibitor [125I]LHVS-PhOH reacts with procathepsin L over a broad pH range, demonstrating activity of procathepsin L against a small peptide analog (Figure 4A).
Fig. 4. Activity of procathepsin L and cathepsin L against a peptide analog and mNC1. (A) SFCM from EOMA cell cultures was adjusted to the pH indicated, probed with the active site-directed reagent [125I]LHVS-PhOH for 1 h at 37°C and analyzed by autoradiography. (B) Coomassie Blue staining and [125I]LHVS-PhOH labeling of the purified enzyme fraction. (C) A 100 ng aliquot of recombinant mNC1 was incubated with 10 ng of the purified enzyme fraction for increasing time periods at pH 5.5 and 7.4. The samples were then probed by Western blotting with an anti-flag antibody (upper panel) as well as an antibody against murine procathepsin L (lower panel). (D) A 10 ng aliquot of procathepsin L was first converted into mature cathepsin L by a 30 min incubation at pH 5.5 and then incubated with 100 ng of recombinant mNC1 at pH 5.5 and 6.8 and analyzed as in (B) (Cat L = cathepsin L).
To test the activity of procathepsin L against mNC1, we purified the enzyme at pH 8.0 with salt concentrations not exceeding 150 mM to avoid conversion of procathepsin L to cathepsin L. Although Coomassie staining showed largely pure procathepsin L, activity-based detection by [125I]LHVS-PhOH labeling revealed significant conversion to mature cathepsin L in the same fraction (Figure 4B). The purified protein fraction contained the proteolytic activity responsible for endostatin production from mNC1. At pH 5.5, endostatin was generated rapidly and, after longer incubation times, completely degraded (Figure 4C). At pH 6.8, variable activity was found (data not shown). No activity was observed at pH 7.4 (Figure 4C). This demonstrates that purified cathepsin L is inactive against mNC1 at neutral pH. Processing of mNC1 to endostatin occurred instantly when procathepsin L was first converted into mature cathepsin L via a 30 min pre-incubation at pH 5.5 (Figure 4D). At pH 6.8, proteolytic activity of mature cathepsin L was observed only in the beginning of the reaction (Figure 4D), probably because of the instability and rapid inactivation of the enzyme above pH 6.5 (Mason et al., 1987). It should be noted that conversion of procathepsin L to cathepsin L was slowed down in the presence of excess substrate (compare lower left subpanels of Figure 4C and D). Taken together, activity of cathepsin L against mNC1 was only observed at low pH under conditions when conversion into the mature form occurred. Thus, endostatin is most probably generated by mature cathepsin L rather than by procath– epsin L.
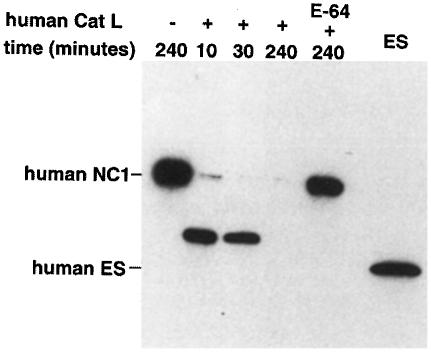
To confirm that the fragment generated by mature cathepsin L is identical to the originally defined endostatin, we sequenced its N–terminus and found that it indeed corresponds to murine endostatin starting with HTHQD (O'Reilly et al., 1997). The murine endostatin cleavage site is not conserved in human collagen XVIII. Therefore, it is not surprising that a C–terminal fragment of collagen XVIII corresponding in size exactly to murine endostatin has not been found in humans. Instead, several similarly sized fragments that were, for example, 12 amino acids smaller or four, nine and 14 amino acids larger than murine endostatin have been identified in human plasma (Ständker et al., 1997; Sasaki et al., 1998). When human recombinant NC1 was incubated with purchased human cathepsin L or with our purified murine cathepsin L, it was processed to a distinct fragment 11 amino acids larger than murine endostatin (Figure 5; data not shown).
Fig. 5. Activity of human cathepsin L against human NC1. A 100 ng aliquot of recombinant human NC1 was incubated with 10 ng of human cathepsin L for the time periods indicated at pH 5.5 and analyzed by Western blotting using the anti-flag antibody. The endostatin-like fragment has the N–terminal sequence LRPAR.
Endostatin generation in EOMA cell culture is facilitated by an acidic extracellular environment
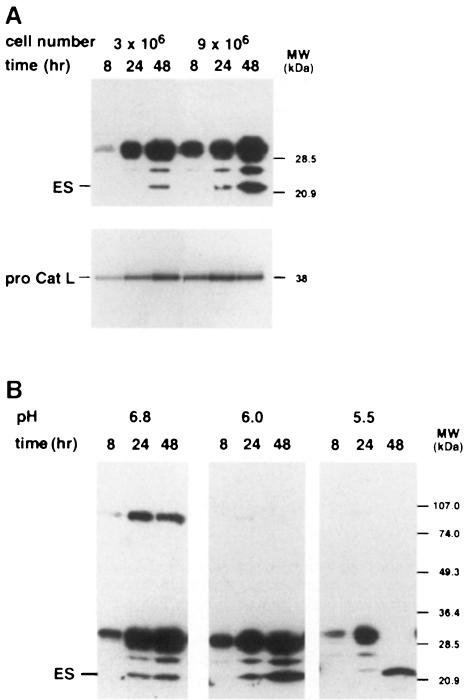
Despite early production of procathepsin L in EOMA cell culture, detectable levels of endostatin were produced only after long incubation periods (Figure 6A), which were paralleled by an acidification of the cellular supernatant to pH 6.8. At higher cell confluencies, endostatin generation as well as acidification occurred earlier. This observation suggests that a lower extracellular pH stimulates the conversion of procathepsin L into the mature form, which then generates endostatin (Figure 6A). An adjustment of the pH to 6.0 and 5.5 resulted in increased endostatin production (Figure 6B). In contrast, when the medium was supplemented with 50 mM HEPES pH 7.4, endostatin production as well as acidification were slowed down (data not shown). As observed in all other incubation experiments with serum-free medium, EOMA cells continued to proliferate even at low pH for at least 48 h.
Fig. 6. Kinetics of endostatin generation in EOMA cell culture. (A) The SFCM from EOMA cell cultures with low or high cell confluencies was harvested after the periods of time indicated, subjected to Western blotting with the anti-endostatin antibody (upper panel) and probed with [125I]LHVS-PhOH (lower panel). (B) The pH of the cell culture medium was buffered to the indicated pH before it was added to confluent EOMA cells (3 × 106 cells/ml). After incubation for the time periods indicated, the media were analyzed by Western blotting with the anti-endostatin antibody.
Discussion
Our data demonstrate that cleavage within the protease-sensitive hinge region of collagen XVIII's NC1 is due to secreted MMPs and cathepsin L in EOMA cell culture. Endostatin production by cathepsin L is independent of MMP activity and does not require the formation of the 30 kDa MMP-generated fragment. Anti-angiogenic activity originally was assigned specifically to the 22 kDa endostatin molecule based on inhibition of proliferation of bovine microvascular endothelial cells in culture (O'Reilly et al., 1997). Recently, human recombinant NC1 was shown to inhibit migration of HUVEC in vitro, suggesting that larger endostatin-containing fragments might also be biologically active (Yamaguchi et al., 1999). However, further processing of NC1 to endostatin during the migration assay was not excluded. Given the possibility that any larger fragment can be processed to endostatin during assays requiring incubation of cells with the test protein for several hours, it is experimentally difficult to assign biological activity to larger fragments.
Endostatin production by cathepsin L in EOMA cell culture is more efficient under conditions of moderate acidification. Secreted as procathepsin L, activity against collagen XVIII apparently depends on conversion into mature cathepsin L. In EOMA cell culture, transient generation of mature cathepsin L probably accounts for the activity found over 48 h. Indeed, traces of mature cathepsin L were observed with [125I]LHVS-PhOH upon long exposures of autoradiographs (data not shown). In vitro, processing of mNC1 to endostatin required conversion of procathepsin L into mature cathepsin L by low pH (Figure 4C and D). Conversion was slowed down in the presence of excess substrate, which may be due to competition between substrate and propeptide. Since no consistent activity was found with purified cathepsin L and mNC1 at pH 6.8, additional factors appear to play a role in the activation of procathepsin L in EOMA cell culture (Mason and Mason, 1992). The stimulation of endostatin generation at low pH is reminiscent of bone collagenolysis by osteoclasts that use acidification of the extracellular microenvironment to enhance the collagenolytic activity of their secreted cathepsins (Delaissé et al., 1991).
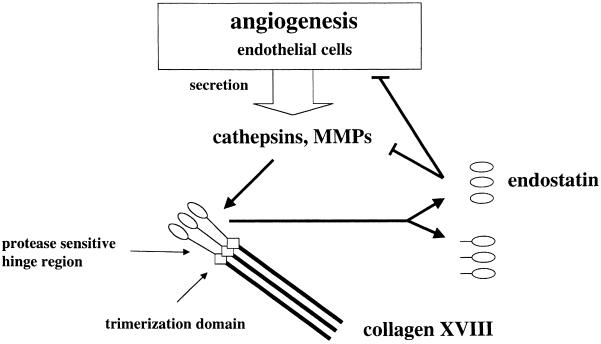
Angiogenesis is an invasive process that requires controlled release and activity of extracellular proteases (Liotta et al., 1991). We therefore hypothesize that the C–terminal portion of collagen XVIII's protease-sensitive hinge region evolved as a sensor of proteolytic activity (Figure 7). Release of fragments with endostatin activity would control angiogenesis in a feedback mechanism. This hypothesis is based on the assumptions that, in addition to MMPs, cathepsin L is actively involved in angiogenesis, and that endostatin interferes with proteolysis either directly or via a signaling pathway leading to down-regulation of proteases. The involvement of MMPs in angiogenesis is documented (Brown and Whittaker, 1999), but a possible role for cathepsin L has not yet been addressed. We suggest that secretion of cathepsin L by EOMA cells reflects a general feature of induced angiogenesis. Derived from a murine hemangioendothelioma, a tumor that is composed predominantly of vascular channels (Hoak et al., 1971), the EOMA cell line maintains characteristic endothelial cell properties (Obeso et al., 1990). In addition, EOMA cells express several factors linked to active angiogenesis: collagen XVIII, which has been reported as a feature of tube-forming endothelial cells (Muragaki et al., 1995), MMPs, which are required for the degradation of subendothelial basement membrane components (Brown and Whittaker, 1999), and BM-40/SPARC/osteonectin (U.Felbor, unpublished data), a multifunctional matricellular protein also involved in regulation of angiogenesis (Motamed and Sage, 1997). Furthermore, the ability of EOMA cells to survive and proliferate at low pH probably reflects the expression of plasmalemmal H+-ATPases that are found specifically on tips of growing vessels (Martínez-Zaguilán, 1999; Moser et al., 1999). Extracellular cathepsin L could, therefore, be an additional factor with a role in angiogenesis, and, as known for MMPs, its inhibitors could function as anti-angiogenic drugs.
Fig. 7. Diagram illustrating the proposed role of collagen XVIII as a regulator of induced angiogenesis. Local secretion of cathepsins and metalloproteases by stimulated endothelial cells leads to cleavage within the protease-sensitive hinge region of collagen XVIII. This results in release of fragments with endostatin activity. Endostatin, in turn, may directly or indirectly inhibit proteolysis associated with angiogenesis.
MMPs and cathepsin L have also been linked to the metastatic potential of tumors. Both angiogenesis and tumor invasion depend on the dissolution of basement membrane components, and the underlying proteolytic principles are identical (Liotta et al., 1991). However, despite the overexpression of cathepsin L, the hemangioendothelioma is a non-metastatic tumor (Hoak et al., 1971). This could be due to cathepsin L-mediated generation of endostatin that counteracts the formation of distant metastases by inhibition of angiogenesis. Likewise, highly vascularized primary tumors could be potent producers of endostatin: cathepsin L secreted by endothelial or tumor cells could produce endostatin from basement membrane collagen XVIII provided by invading vessels. The acidic microenvironment that has been found in many tumors (Warburg, 1926; Gillies et al., 1994) could enhance cathepsin L activity.
Since cathepsin L promotes invasion of tumor cells and has been reported to be associated with metastatic tumor growth and poor prognosis, its inhibitors have been considered for therapeutic use (Liotta et al., 1991; Kolkhorst et al., 1998; Kos and Lah, 1998). The demonstration that cathepsin L can also generate the potent anti-angiogenic molecule endostatin suggests that therapies based on cathepsin inhibitors may have the undesirable effect of blocking endostatin generation. Therefore, therapies with cathepsin inhibitors may be more efficient in combination with exogenous endostatin administration.
Materials and methods
Materials
Aprotinin was purchased from Roche Molecular Biochemicals (Indiana, IN), E-64, elastatinal, leupeptin and pepstatin A from Sigma Chemical Co. (St Louis, MO). BB-3103 was obtained from British Biotech (Oxford, UK). The cathepsin B inhibitor II and human cathepsin L were purchased from Calbiochem Corp. (La Jolla, CA). Aprotinin (7.5 mM), cathepsin B inhibitor II (10 mM), E-64 (10 mM), elastatinal (195 mM) and leupeptin (230 mM) were dissolved in phosphate-buffered saline (PBS). BB-3103 (10 mM), LHVS (100 mM) and pepstatin A (36 mM) stock solutions were dissolved in dimethylsulfoxide (DMSO).
Cell culture
EOMA cells were cultured in Dulbecco's modified Eagle's medium high glucose (DMEM; Irvine Scientific, Santa Ana, CA) supplemented with 10% fetal bovine serum (Summit Biotechnology, Fort Collins, CO) and 1% glutamine–penicillin–streptomycin in a 5% CO2 atmosphere at 37°C. 293-EBNA cells (human embryonic kidney cells) and HUVEC were obtained from Invitrogen (Carlsbad, CA) and Cascade Biologics, Inc. (Portland, OR), respectively, and grown according to specifications. The Hep3B (human hepatocellular carcinoma cells), ECV304 (derivative of the bladder carcinoma cell line T24) and murine F9 embryonal carcinoma cell lines were purchased from the American Type Culture Collection (Rockville, MD). Murine B16-BL6 melanoma and LLC cells were a gift from EntreMed, Inc. and Dr R.Mulligan, respectively.
All subculture procedures of EOMA cells included removal of trypsin/EDTA (Irvine Scientific, Santa Ana, CA) by centrifugation and resuspension of cells in fresh culture medium. The cells were plated at a density of 3 × 106 cells per well in 6-well plates unless otherwise indicated. After 24 h, the cells were washed twice with PBS and then with serum-free DMEM for 1 h. The incubation was started with 1 ml of fresh serum-free DMEM containing 1% glutamine and the respective protease inhibitor. Since BB-3103, pepstatin A and LHVS stocks were prepared in DMSO, 2, 0.5 and 0.12% DMSO were added to the respective controls. All inhibitors were replenished the following day and did not affect cell viability in the concentrations used. SFCM was collected after 48 h and concentrated using microcons YM-10 (Millipore Corporation, Bedford, MA).
SDS–PAGE/Western blotting
Concentrated SFCM corresponding to 0.5 ml of supernatant was separated on 7.5–17.5% gradient SDS–PAGE and electroblotted onto nitrocellulose (Protran; Schleicher & Schuell, Keene, NH) using a Semi-Dry transfer apparatus (Bio-Rad, Hercules, CA). Rabbit polyclonal anti-human endostatin antibody was obtained from Cytimmune Sciences Inc. (College Park, MD) and cross-reacted with all endogenous and recombinant murine endostatin-like fragments. Enhanced chemiluminescence (NEN, Boston, MA) was used for visualization.
Construction, expression and purification of recombinant murine flag-tagged NC1 (mNC1)
The murine α1(XVIII) cDNA clone mc3b (Oh et al., 1994) was used as template to amplify sequences encoding mNC1 by PCR. In addition to the annealing sequences, primer mNC1-Cflag5′ (5′–AGTCAGATATCGCTAGCTGCTGGGCAGGTGAGGATCTGGGCC–3′) contained an EcoRV and an NheI site at the 5′ end, and primer mES-Cflag3′ (5′–CAGGGCGGCCGCCTACTTGTCATCGTCGTCCTTGTAGTCTTTGGAGAAAGAGGTCATGAAGCT–3′) contained the flag peptide sequence and a NotI site at the 3′ end. The EcoRV–NotI fragment was subcloned into pBluescript II SK(+) (Stratagene, La Jolla) for DNA sequence confirmation using Dye Terminator Cycle Sequencing (ABI Prism, Perkin Elmer Corporation, Norwalk, CT) and subsequently cloned into the NheI and NotI sites of the modified episomal expression vector pCEP-Pu/AC7 (Kohfeldt et al., 1997).
293-EBNA cells were transfected according to the SuperFect Transfection Reagent protocol (Qiagen, Valencia, CA) and selected with puro– mycin (1 μg/ml) to generate stable cell lines. Serum-free conditioned media were collected after 48 h, diluted 1:1 in TBS (50 mM Tris–HCl, 150 mM NaCl pH 7.4) and purified with an anti-Flag M2 affinity column (Sigma, St Louis, MO). The protein was dialyzed overnight against TBS and stored at –80°C. The concentration of recombinant mNC1 was determined spectrophotometrically at a wavelength of 280 nm by using an absorption of 0.7 for a 1 mg/ml solution and a molecular weight of 35 569 Da. Recombinant endostatins and human C–flagged NC1 were produced and purified as described (Yamaguchi et al., 1999).
Incubation assays with mNC1
Recombinant mNC1 was added to EOMA cells or to EOMA SFCM for 48 h at 37°C in a 5% CO2 incubator. For this purpose, EOMA SFCM was collected after 48 h, filtered (0.2 μm) and 0.5 ml of SFCM was added to 700 ng of the recombinant protein. These experiments were performed in the presence or absence of protease inhibitors at concentrations known to block endogenous production of endostatin from EOMA cells. Incubation of 100 ng of recombinant mNC1 or human NC1 with 10 ng of purified murine cathepsin L or human cathepsin L was performed in 20 μl of 50 mM Tris–acetate pH 5.5, and 5 mM dithiothreitol (DTT) at 37°C. For detection by Western blotting, the anti-Flag M2 monoclonal antibody (Sigma, St Louis, MO) was used in all assays involving recombinant NC1. For N–terminal amino acid sequencing of the endostatin fragment, 2 μg of mNC1 were incubated with 100 ng of purified mature cathepsin L for 15 min. The 22 kDa band was excised from a PVDF membrane. The sequence was determined by Edman degradation on a 477A protein sequencer (Applied Biosystems, Foster City, CA).
Purification of the proteolytic activity
A 5 ml aliquot of EOMA SFCM was diluted 3–fold in 20 mM sodium phosphate pH 6.0, 1 mM DTT, 1 mM EDTA, and, in parallel, applied to Q-Sepharose Fast Flow and SP-Sepharose Fast Flow (Pharmacia Biotech, Uppsala, Sweden). The flow-through, 250 and 750 mM NaCl fractions were collected, aliquots trichloroacetic acid (TCA) precipitated, loaded onto 7.5–17.5% SDS–PAGE and stained with Coomassie Blue. For N–terminal amino acid sequencing, the 38 kDa band in the 250 mM eluate of SP-Sepharose was excised from a PVDF membrane and sequenced as described above.
To purify procathepsin L, 150 ml of EOMA SFCM buffered with 100 mM HEPES pH 7.4 were harvested from roller bottles after 48 h, diluted 3-fold in 50 mM Tris–HCl pH 8.0, 1 mM DTT and bound to concanavalin A–Sepharose. Bound proteins were eluted with 300 mM α–methylmannoside, 50 mM Tris–HCl pH 8.0, 1 mM DTT and applied to Q-Sepharose. Procathepsin L eluted between 100 and 150 mM NaCl. The diluted material was found in the flow-through of an SP-Sepharose column. Concentrated aliquots appeared pure according to Coomassie and silver staining and were frozen at –80°C. One hundred and fifty milliliters of SFCM contained ∼90 μg of procathepsin L.
Conversion of procathepsin L into mature cathepsin L was performed in 50 mM Tris–acetate pH 5.5, containing 5 mM DTT at 37°C for 30 min and monitored with an antibody against murine procathepsin L and [125I]LHVS-PhOH labeling (see below).
[125I]LHVS-PhOH labeling
LHVS-PhOH was synthesized following a scheme modified from that described by Palmer (Palmer et al., 1995; R.A.R.Bryant, in preparation). [125I]LHVS-PhOH (obtained via an iodogen-catalyzed reaction) was purified by reverse-phase HPLC. The fractions of peak radioactivity were dried down under vacuum and resuspended in DMSO. Concentrated SFCM and purification fractions were brought up in 20 mM phosphate, 0.25 mM DTT pH 6.0, and incubated with 10 nM [125I]LHVS-PhOH for 1 h at 37°C (final DMSO concentration of <3%). Samples were quenched by addition of 4× SDS sample buffer and boiled prior to analysis by 12.5% SDS–PAGE. The pH profile of [125I]LHVS-PhOH activity against procathepsin L in EOMA SFCM was performed in 50 mM Tris–HCl (pH 7.4 and 6.5) or 50 mM Tris–acetate (pH 5.5 and 4.5), 5 mM DTT, 5 mM MgCl2 with 2.5 nM [125I]LHVS-PhOH for 1 h at 37°C.
Acknowledgments
Acknowledgements
We are grateful to T.A.Rapoport for his generous support. We thank C.Alexander for helpful discussions, M.S.O'Reilly for his kind gift of the EOMA cells, L.Tamarkin (Cytimmune Sciences, Inc.) for the anti-human endostatin antibody, A.Erickson for the anti-murine procathepsin L antibody, British Biotech Pharmaceuticals Ltd for the hydroxamate BB-3103, N.Yamaguchi for endostatin-producing 293-EBNA cells, and F.Kusinitz and J.Rush for protein sequencing. This work was supported by the Alexander von Humboldt-Stiftung (U.F.; V-3-FLF-1054223), the Deutsche Forschungsgemeinschaft (U.F.; Fe 432/5-1), the Jane Coffin Childs Memorial Fund for Medical Research (W.M.), the Cancer Research Institute (R.A.R.B.), EntreMed, Inc. (B.R.O.) and NIH (B.R.O. and H.L.P).
References
- Aimes R.T. and Quigley, J.P. (1995) Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J. Biol. Chem., 270, 5872–5876. [DOI] [PubMed] [Google Scholar]
- Brooks P.C., Silletti, S., von Schalscha, T.L., Friedlander, M. and Cheresh, D.A. (1998) Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell, 92, 391–400. [DOI] [PubMed] [Google Scholar]
- Brown P.D. and Whittaker,M. (1999) Matrix metalloproteinase inhibitors. In Teicher,B.A. (ed.), Antiangiogenic Agents in Cancer Therapy. Human Press, Totowa, NJ, pp. 205–223. [Google Scholar]
- Clapp C., Martial, J.A., Guzman, R.C., Rentier-Delrue, F. and Weiner, R.I. (1993) The 16-kilodalton N–terminal fragment of human prolactin is a potent inhibitor of angiogenesis. Endocrinology, 133, 1292–1299. [DOI] [PubMed] [Google Scholar]
- Delaissé J.M., Ledent, P. and Vaes, G. (1991) Collagenolytic cysteine proteinases of bone tissue. Cathepsin B, (pro)cathepsin L and a cathepsin L-like 70 kDa proteinase. Biochem. J., 279, 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z., Kumar, R., Yang, X. and Fidler, I.J. (1997) Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell, 88, 801–810. [DOI] [PubMed] [Google Scholar]
- Driessen C., Bryant, R.A.R., Lennon-Dumenil, A.-M., Villadangos, J.A., Bryant, P.W., Shi, G.-P., Chapman, H.A. and Ploegh, H.L. (1999) Cathepsin S controls the trafficking and maturation of MHC class II molecules in dendritic cells. J. Cell Biol., 147, 775–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnero P., Borel, O., Byrjalsen, I., Ferreras, M., Drake, F.H., McQueney, M.S., Foged, N.T., Delmas, P.D. and Delaissé, J.-M. (1998) The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J. Biol. Chem., 273, 32347–32352. [DOI] [PubMed] [Google Scholar]
- Gillies R.J., Liu, Z. and Bhujwalla, Z. (1994) 31P-MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. Am. J. Physiol., 267, C195–C203. [DOI] [PubMed] [Google Scholar]
- Gupta S.K., Hassel, T. and Singh, J.P. (1995) A potent inhibitor of endothelial cell proliferation is generated by proteolytic cleavage of the chemokine platelet factor 4. Proc. Natl Acad. Sci. USA, 92, 7799–7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W., Dong, S., Schurer, B. and Cole, G.J. (1998) Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J. Biol. Chem., 273, 25404–25412. [DOI] [PubMed] [Google Scholar]
- Hoak J.C., Warner, E.D., Cheng, H.F., Fry, G.L. and Hankenson, R.R. (1971) Hemangioma with thrombocytopenia and microangiopathic anemia (Kasabach–Merritt syndrome): an animal model. J. Lab. Clin. Med., 77, 941–950. [PubMed] [Google Scholar]
- Homandberg G.A., Williams, J.E., Grant, D., Schumacher, B. and Eisenstein, R. (1985) Heparin-binding fragments of fibronectin are potent inhibitors of endothelial cell growth. Am. J. Pathol., 120, 327–332. [PMC free article] [PubMed] [Google Scholar]
- Kafienah W., Buttle, D.J., Burnett, D. and Hollander, A.P. (1998) Cleavage of native type I collagen by human neutrophil elastase. Biochem. J., 330, 897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschke H. (1998) Cathepsin L. In Barrett,A.J., Rawlings,N.D. and Woessner,J.F. (eds), Handbook of Proteolytic Enzymes. Academic Press, San Diego, CA, pp. 617–621. [Google Scholar]
- Kohfeldt E., Maurer, P., Vannahme, C. and Timpl, R. (1997) Properties of the extracellular calcium binding module of the proteoglycan testican. FEBS Lett., 414, 557–561. [DOI] [PubMed] [Google Scholar]
- Kolkhorst V., Stürzebecher, J. and Wiederanders, B. (1998) Inhibition of tumor cell invasion by protease inhibitors: correlation with the protease profile. J. Cancer Res. Clin. Oncol., 124, 598–606. [DOI] [PubMed] [Google Scholar]
- Kos J. and Lah, T.T. (1998) Cysteine proteinases and their endogenous inhibitors: target proteins for prognosis, diagnosis and therapy in cancer. Oncol. Rep., 5, 1349–1361. [DOI] [PubMed] [Google Scholar]
- Liotta L.A., Steeg, P.S. and Stetler-Stevenson, W.G. (1991) Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell, 64, 327–336. [DOI] [PubMed] [Google Scholar]
- Maciewicz R.A. and Etherington, D.J. (1988) A comparison of four cathepsins (B, L, N and S) with collagenolytic activity from rabbit spleen. Biochem. J., 256, 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Zaguilán R. (1999) Angiostatin's partners. Science, 284, 433–434. [DOI] [PubMed] [Google Scholar]
- Mason R.W. and Mason, S.D. (1992) Surface activation of pro-cathepsin L. Biochem. Biophys. Res. Commun., 189, 1659–1666. [DOI] [PubMed] [Google Scholar]
- Mason R.W., Gal, S. and Gottesman, M.M. (1987) The identification of the major excreted protein (MEP) from a transformed mouse fibroblast cell line as a catalytically active precursor form of cathepsin L. Biochem. J., 248, 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miosge N., Sasaki, T. and Timpl, R. (1999) Angiogenesis inhibitor endostatin is a distinct component of elastic fibers in vessel walls. FASEB J., 13, 1743–1750. [DOI] [PubMed] [Google Scholar]
- Moser T.L., Stack, M.S., Asplin, I., Enghild, J.J., Højrup, P., Everitt, L., Hubchak, S., Schnaper, H.W. and Pizzo, S.V. (1999) Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc. Natl Acad. Sci. USA, 96, 2811–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamed K. and Sage, E.H. (1997) Regulation of vascular morphogenesis by the matricellular protein SPARC. Kidney Int., 51, 1383–1387. [DOI] [PubMed] [Google Scholar]
- Muragaki Y., Timmons, S., Griffith, C.M., Oh, S.P., Fadel, B., Quertermous, T. and Olsen, B.R. (1995) Mouse Col18a1 is expressed in a tissue-specific manner as three alternative variants and is localized in basement membrane zones. Proc. Natl Acad. Sci. USA, 92, 8763–8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly M.S., et al. (1994)Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell, 79, 315–328. [DOI] [PubMed] [Google Scholar]
- O'Reilly M.S., et al. (1997)Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell, 88, 277–285. [DOI] [PubMed] [Google Scholar]
- O'Reilly M.S., Pirie-Shepherd, S., Lane, W.S. and Folkman, J. (1999) Antiangiogenic activity of the cleaved conformation of the serpin antithrombin. Science, 285, 1926–1928. [DOI] [PubMed] [Google Scholar]
- Obeso J., Weber, J. and Auerbach, R. (1990) A hemangioendothelioma-derived cell line: its use as a model for the study of endothelial cell biology. Lab. Invest., 63, 259–269. [PubMed] [Google Scholar]
- Oh S.P., Kamagata, Y., Muragaki, Y., Timmons, S., Ooshima, A. and Olsen, B.R. (1994) Isolation and sequencing of cDNAs for proteins with multiple domains of Gly-X-Y repeats identify a novel family of collagenous proteins. Proc. Natl Acad. Sci. USA, 91, 4229–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J.T., Rasnick, D., Klaus, J.L. and Brömme, D. (1995) Vinyl sulfones as mechanism-based cysteine protease inhibitors. J. Med. Chem., 38, 3193–3196. [DOI] [PubMed] [Google Scholar]
- Rehn M. and Pihlajaniemi, T. (1994) α1 (XVIII), a collagen chain with frequent interruptions in the collagenous sequence, a distinct tissue distribution and homology with type XV collagen. Proc. Natl Acad. Sci. USA, 91, 4234–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese R.J., Wolf, P.R., Brömme, D., Natkin, L.R., Villadangos, J.A., Ploegh, H.L. and Chapman, H.A. (1996) Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity, 4, 357–366. [DOI] [PubMed] [Google Scholar]
- Saarela J., Rehn, M., Oikarinen, A., Autio-Harmainen, H. and Pihlajaniemi, T. (1998) The short and long forms of type XVIII collagen show clear tissue specificities in their expression and location in basement membrane zones in humans. Am. J. Pathol., 153, 611–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Fukai, N., Mann, K., Göhring, W., Olsen, B.R. and Timpl, R. (1998) Structure, function and tissue forms of the C–terminal globular domain of collagen XVIII containing the angiogenesis inhibitor endostatin. EMBO J., 17, 4249–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ständker L., Schrader, M., Kanse, S.M., Jürgens, M., Forssman, W.-G. and Preissner, K.T. (1997) Isolation and characterization of the circulating form of human endostatin. FEBS Lett., 420, 129–133. [DOI] [PubMed] [Google Scholar]
- Starkey P.M. (1977) The effect of human neutrophil elastase and cathepsin G on the collagen of cartilage, tendon and cornea. Acta Biol. Med. Ger., 36, 1549–1554. [PubMed] [Google Scholar]
- Warburg O. (1926) Ueber den Stoffwechsel der Tumoren. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Yamaguchi N., et al. (1999)Endostatin inhibits VEGF-induced endothelial cell migration and tumor growth independently of zinc binding. EMBO J., 18, 4414–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]